Abstract
OBJECTIVE
We previously reported that cardiac-restricted deletion of focal adhesion kinase (FAK) exacerbated myocyte death following ischemia/reperfusion (I/R). Here we interrogated whether targeted elevation of myocardial FAK activity could protect the heart from I/R injury.
METHODS AND RESULTS
Transgenic mice were generated with myocyte-specific expression of a FAK variant (termed SuperFAK) that conferred elevated allosteric activation. FAK activity in unstressed transgenic hearts was modestly elevated, but this had no discernable effect on anabolic heart growth or cardiac function. Importantly, SuperFAK hearts exhibited a dramatic increase in FAK activity and a reduction in myocyte apoptosis and infarct size 24–72 hrs following I/R. Moreover, serial echocardiography revealed that the transgenic mice were protected from cardiac de-compensation for up to 8 weeks following surgery. Mechanistic studies revealed that elevated FAK activity protected cardiomyocytes from I/R-induced apoptosis by enhancing NF-κB-dependent survival signaling during the early period of reperfusion (30 and 60 minutes). Moreover, adenoviral-mediated expression of SuperFAK in cultured cardiomyocytes attenuated H2O2 or hypoxia/re-oxygenation-induced apoptosis, whereas blockade of the NF-κB pathway using a pharmacological inhibitor or small interfering RNAs completely abolished the beneficial effect of SuperFAK.
CONCLUSIONS
Enhancing cardiac FAK activity attenuates I/R-induced myocyte apoptosis through activation of the pro-survival NF-κB pathway and may represent a novel therapeutic strategy for ischemic heart diseases.
Keywords: integrin, myocardial infarction, apoptosis, remodeling, reactive oxygen species, FAK, NF-κB
Introduction
Cardiovascular diseases are the number one cause of death globally and current estimates indicate that as many as 1 out of 6 deaths/year in the United States can be attributed to coronary disease and associated myocardial ischemia1. While rapid reperfusion is necessary to reduce ischemia-dependent myocyte necrosis, it can also result in intracellular calcium overload and oxidative stress which can initiate apoptosis2. Importantly, while apoptosis can account for up to 60% of myocyte death within infarcted tissue3, this programmed cell death cascade (unlike necrosis) can be reversed by activation of pro-survival signals4, 5. Therefore defining the mechanisms that govern the transition between cardiomyocyte apoptosis and survival will undoubtedly have a significant impact on the generation of promising treatments to reduce ischemia-induced myocardial dysfunction.
Attachment of cells to the extracellular matrix results in the clustering of integrin receptors and initiates the recruitment of numerous structural and catalytically active signaling proteins to the adhesion complexes that are necessary for maintaining tissue integrity, conveying tensile strength, and for the transduction of growth and survival signals6. Several recent studies indicate that integrin signaling may play a pivotal role in preserving the myocardium from pathological stressors. For example, myocardial-restricted deletion of β1 integrin in mice led to myocardial dysfunction in postpartum females and in male mice subjected to hemodynamic overload 7, 8. As well, mice with myocyte-restricted depletion of β3 integrin exhibited elevated cardiomyocyte apoptosis and concomitant heart failure following isoproterenol infusion 9. Moreover, the finding that human ischemic cardiomyopathy was associated with down-regulation of the muscle selective β1D-integrin and the integrin-activated kinase, focal adhesion kinase (FAK) supports the possibility that targeting this pathway may be beneficial in the prevention of ischemic human heart failure10.
Nearly two thirds of the known integrin heterodimers including all β1, β3 or β5 containing integrins couple to the non-receptor protein tyrosine kinase, FAK 11, 12 as do several growth factor receptors, including those activated by VEGFs and FGFs13, 14. FAK activation proceeds by a two-step process that involves dimerization and autophosphorylation of Y397 which creates a high affinity SH2 binding site for the tyrosine kinase, Src. Once bound, Src phosphorylates FAK on two additional sites within the activation loop (Y576 and Y577) leading to further enhancement of FAK catalytic activity. FAK binding partners/substrates include the adapter proteins paxillin, CAS, and/or GRB 215–18 that can activate the ERK and JNK growth promoting Map Kinases. FAK also coordinates signaling to the pro-survival NF-κB19, 20 and Akt19 pathways through interactions with receptor-interacting protein and Pi3 kinase respectively. While FAK activity is necessary for myocyte proliferation and mid-gestational heart growth, we recently showed that myocyte-specific deletion of FAK in the adult myocardium did not affect anabolic growth or basal contractility21. However, upon challenge, FAK-depleted hearts progressed to profound cardiac decompensation following pressure overload 21 and exhibited markedly increased myocyte apoptosis and infarct size relative to wt mice following ischemia/reperfusion (I/R) 22. Because our mechanistic studies supported a critical role for myocardial FAK in promoting NF-κB-induced survival signaling following I/R, we predicted that enhanced myocardial FAK activity might provide cardioprotection from an ischemic insult.
To date, attempts to enhance pro-survival signaling in the myocardium by expression of up-stream kinases have met with limited success, likely due to strategies that often lead to supra-physiological expression of constitutively active kinases. In these cases over-expression alone can lead to striking changes in myocardial remodeling that culminate in phenotypes ranging from hypertrophy to heart failure and sudden death as has been observed in several transgenic mouse models with targeted expression of active Akt variants23–27. Since we hypothesized that transient elevation of FAK-dependent survival signals would be cardioprotective, while un-controlled activation could lead to detrimental remodeling, we sought to express a variant of FAK that requires signal-dependent activation but exhibits enhanced catalytic activity. Thus we took advantage of known mutations within the FAK activation loop (K578E/K581E) that mimic the charge transfer (and enhanced catalytic activity) induced by Src phosphorylation28. Herein, we found that transgenic mice with myocyte-restricted expression of this super-activatable FAK variant (termed SuperFAK) exhibited remarkable protection from I/R-dependent cell death. These studies provide the first evidence that FAK could be a tractable target for gene therapy and provide proof-of-concept for a methodological approach to exploit post-translational modifications to enable spatial- and temporal control of kinase activation in vivo.
Methods
An expanded Methods section is available in the Online Data Supplement.
Generation of SuperFAK transgenic mice
The SuperFAK cDNA variant was kindly provided by Dr. Michael Schaller28. Cardiac-specific transgenic mice with SuperFAK overexpression was achieved with a 3.4 kb piece of the cardiac βMHC promoter that was modified to prevent down-regulation at birth 29. All animals were housed in a University Animal Care Facility accredited by the American Association for Accreditation of Laboratory Animal Care and all procedures were approved by the University of North Carolina Institutional Animal Care and Use Committee.
Statistical analysis
All values are presented as means±SEM. Comparisons were performed by using unpaired Student t-test or one-way analysis of variance (ANOVA) with Tukey’s post-hoc test as appropriate. All tests were two-tailed and significance was accepted at p < 0.05.
Results
Generation of transgenic mice that confer enhanced allosteric FAK activity in the myocardium
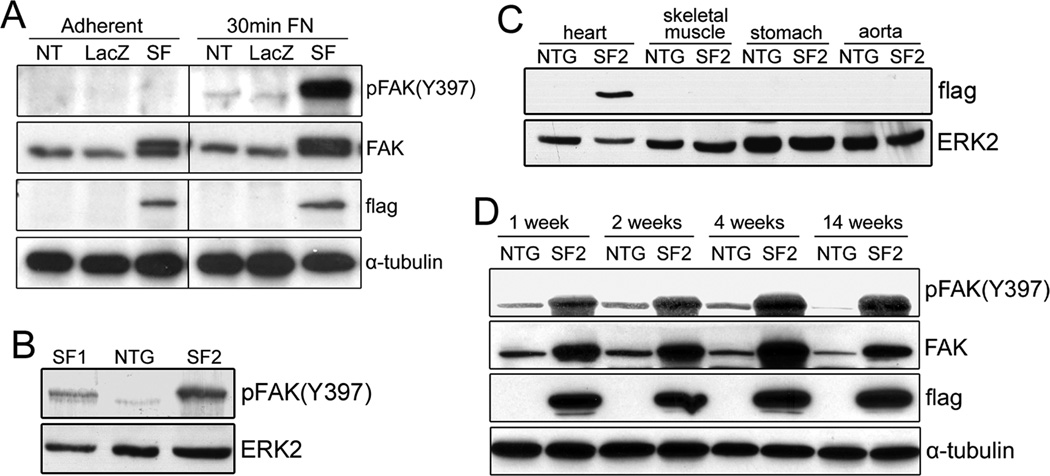
We previously demonstrated that cardiac-restricted deletion of FAK exacerbates ischemia-reperfusion (I/R) induced apoptosis and leads to enhanced cardiac decompensation following I/R or prolonged pressure overload21, 22. Since the toggling between pro-survival and pro-apoptotic signals remains central to preventing irreversible damage to the heart30, we strove to determine whether enhanced FAK activity could salvage "at risk" myocytes in the ischemic heart. To address this critical issue, we generated transgenic mice that expressed a “Super-activatable” variant of FAK (SuperFAK) in cardiomyocytes. SuperFAK contains glutamic acid substitutions for two lysine residues in the activation loop of FAK (K578E, K581E) that renders the protein “primed” for allosteric activation (Supplemental Figure IA) 28, 31. SuperFAK has substantially increased catalytic activity in comparison to wild type FAK when expressed at comparable levels (Supplemental Figure IB). Nonetheless, SuperFAK is not constitutively active. Indeed, cells transfected with SuperFAK for 48 hr and maintained on tissue culture plastic exhibited comparable levels of FAK activity as non-transfected or LacZ-transfected cells (Figure 1A, left). However, when re-plated on a fibronectin-coated surface for 30 min, the SuperFAK transfected cardiomyocytes exhibited much more pronounced FAK activity (Figure 1A, right), indicating that SuperFAK confers enhanced allosteric activation.
Figure 1. Generation of cardiomyocyte-specific SuperFAK transgenic mice.
(A) COS7 cells were non-transfected (NT), transfected with LacZ control or SuperFAK (SF) and grown to confluence (adherent) or re-plated on fibronectin (FN)-coated dishes for 30 min. Western blotting was performed with indicated antibodies. (B) Levels of basal myocardial FAK activity in 1-month-old SuperFAK transgenic (SF1 and SF2) and non-transgenic (NTG) mice. (C) Detection of flag-tagged SuperFAK in various tissues revealed myocardial-restricted expression in SF2 mice. (D) SF2 mice exhibit elevated levels of FAK activity during development.
Two novel mouse lines were generated in which a flag-epitope tagged SuperFAK (SF) transgene was expressed under the control of a truncated β-MHC promoter that was modified to drive cardiomyocyte-specific expression into adulthood 29. The so-named SF1 and SF2 mice exhibited a persistent and myocardial-restricted increase (2- to 6-fold respectively) in basal cardiac FAK activity relative to age-matched non-transgenic (NTG) littermate control hearts (Figure 1B–D). Both SF1 and SF2 mice were born in the expected Mendelian frequency and no noticeable morbidity or mortality was observed for up to 18 months of age.
Enhanced cardiomyocyte-specific FAK activity does not influence cardiac growth or basal cardiac function
We carefully assessed the consequence of elevated myocyte-restricted FAK activity on post-natal myocardial growth in SF1 and SF2 mice. No significant differences were observed in myocardial shape or size (Supplemental Figure IIA), heart weight:body weight ratios (Supplemental Figure IIB) or cardiomyocyte cross-sectional area (Supplemental Figure IIC,D, III) when SF mice 8 weeks to 12 months of age were compared to age-matched littermate controls. To determine if elevated myocyte-specific FAK activity affected cardiac performance, we first measured left ventricular function by conscious echocardiography in young adult male SF2 and NTG control mice. No significant differences in either ejection fraction (EF) or fractional shortening (FS) were observed between these lines (Supplemental Figure IIE,F). As well, serial echocardiography in a second cohort of anesthetized mice revealed comparable LV wall thickness, chamber diameter, mass and function between NTG and SF2 mice from 1 to 12 months of age (Supplemental Figure IV). Hemodynamic measurements by cardiac catheterization also revealed no significant differences in intrinsic cardiac contractility or diastolic function between 12 month old NTG and SF2 mice (Supplemental Figure V). Taken together with our previous findings21, 32, these data indicate that FAK activity is neither necessary nor sufficient to promote anabolic cardiac growth. Moreover, while we and others previously found that FAK was necessary for the promotion of pathological cardiac remodeling following pressure overload21, 33, we found no signs of pathological hypertrophy in either SF line. qRT-PCR for the canonical hypertrophy marker, ANF revealed no significant difference between SF2 and NTG (Supplemental Figure IIG). Levels of myocardial fibrosis as assessed by Masson’s Trichrome staining was also comparable between control and SF2 mice for up to 1 yr of age, consistent with our findings that systolic function was maintained in these mice (Supplemental Figure VI). In accordance with these findings, postnatal SF2 and NTG hearts exhibited comparable levels of cardiac Akt and ERK activity, signals that regulate anabolic and pathological hypertrophic growth of the post-natal heart (Supplemental Figure IIH).
Elevated FAK activity ameliorates adverse cardiac remodeling and dysfunction following I/R
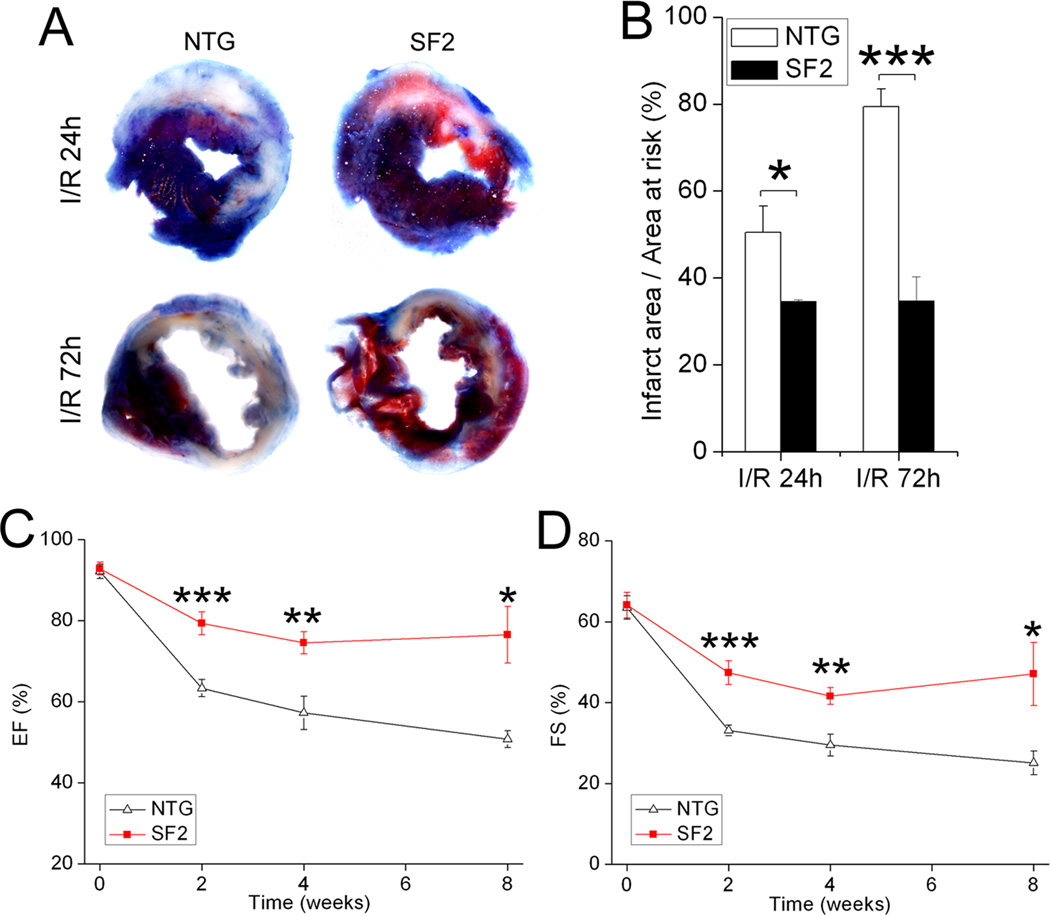
Since we previously showed that cardiac-restricted deletion of FAK exacerbated I/R-induced myocardial infarction22, we hypothesized that elevated FAK activity would confer cardioprotection in this setting. To this end, we subjected NTG and SF2 mice to 30 min of ischemia (induced by transient ligation of the LAD coronary artery) followed by reperfusion for 24 hr to 8 weeks and assessed infarct size and myocardial function. Evans blue/TTC staining showed that the relative area at risk (non-blue/total LV area) was comparable between the two groups, indicating a similar level of ischemia was induced following surgical ligation (data not shown). However, 24 hr following reperfusion, the SF2 mice exhibited a significantly decreased relative infarct size (white/non-blue area) when compared to control mice (control, 50.5±6.0% vs. SF2, 34.6±3.2%; p<0.05; n=6 for control and n=7 for SF2, Figure 2A top, B). A much greater difference was observed between the 2 groups after 72 hr of reperfusion (control, 79.4±4.1% vs. SF2, 34.7±5.6%; p<0.001; n=5 for control and n=4 for SF2, Figure 2A bottom, B). Notably, in the NTG controls the relative infarct size increased dramatically from 24 to 72 hrs following I/R, while the SF2 mice exhibited no significant increase in infarct size during this critical time period. To determine whether SuperFAK expression promoted long term cardio-protection, cardiac function was monitored by conscious echocardiography at 2, 4 and 8 weeks post I/R in a third cohort of NTG and SF2 mice. Both interventricular septal and posterior wall thickness were much better preserved in the SF2 hearts (Supplemental Figure VII). Concomitantly, while both groups exhibited a decline in EF and FS following I/R, the SF2 hearts maintained significantly greater function than the NTG controls (Figure 2C,D). Ultimately, I/R-induced chamber dilatation was observed in the NTG but not SF2 hearts at the 4 and 8 week time points (Supplemental Figure VII). Collectively, these data indicate that enhanced myocardial FAK activity protects the myocardium from I/R-dependent cell death and promotes functional recovery following an ischemic insult.
Figure 2. Targeted FAK activation provided protection from I/R injury.
Non-transgenic (NTG) littermate control and SF2 mice at 8–12 weeks of age were subjected to 30min of ischemia followed by reperfusion. (A) Representative heart slices at 24h or 72h post I/R stained with Evans blue/TTC showing infarct area (Evans blue and TTC unstained, white) and area at risk (Evans blue unstained, shown as red and white). (B) Infarct size (ratio of infarct area to area at risk) was significantly decreased in the SF2 mice compared with NTG mice 24h (NTG, n=6; SF2, n=7) or 72h (NTG, n=5; SF2, n=4) post I/R. * p < 0.05; *** p < 0.001. (C,D) LV ejection fraction (EF) and fractional shortening (FS) were evaluated by conscious echocardiography before, 2, 4, and 8 weeks post I/R. NTG, n=6; SF2, n=5. * p < 0.05 vs. NTG; ** p < 0.01 vs. NTG; *** p < 0.001 vs. NTG. Data are expressed as mean ± SEM.
Elevated FAK activation attenuates cardiomyocyte apoptosis following ischemia/reperfusion
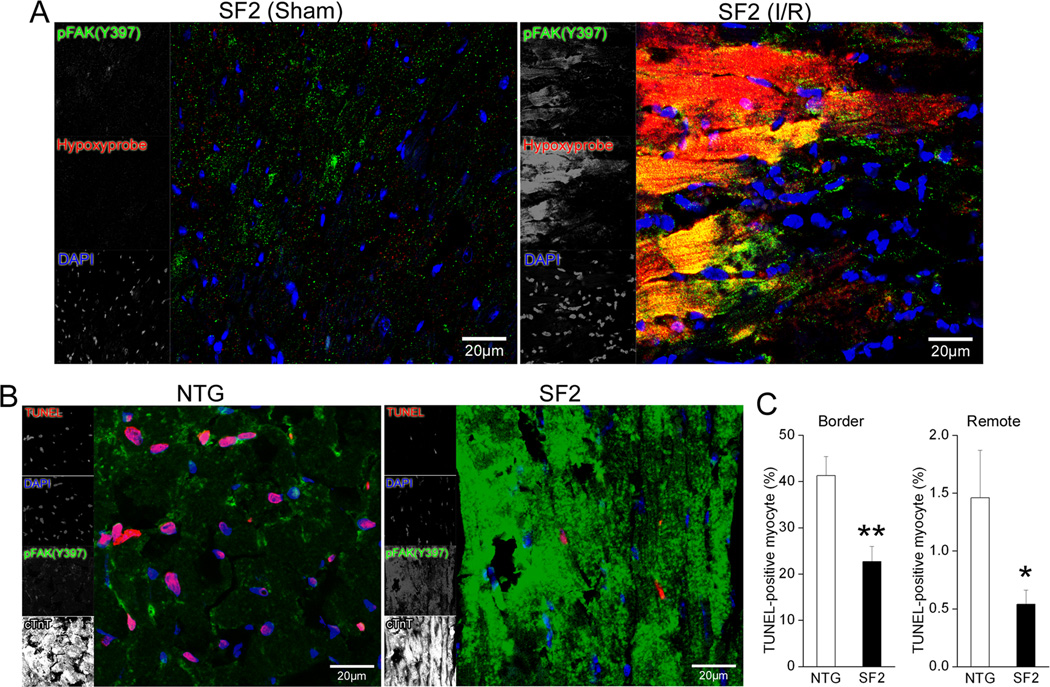
Ischemia/reperfusion induced myocyte death results from irreversible (necrotic) and reversible (apoptotic) signals. Since our previous studies indicated that FAK depletion renders cardiomyocytes more susceptible to ischemia-induced apoptosis, we reasoned that enhanced FAK activity might limit cardiomyocyte apoptosis following I/R. We first sought to determine the extent to which SuperFAK is activated in ischemic myocytes. To aid in the demarcation of the ischemic zone, I/R-treated mice were injected with hypoxyprobe-1 (pimonidazole hydrochloride) which forms protein adducts in cells with a pO2 of 10 mm Hg or less that can be detected by immunostaining34. As shown in figure 3A, sham SF2 hearts contained little immunoreactivity for hypoxyprobe-1, while those subjected to I/R for 24 hr revealed intense focal reactivity, typical of hypoxia. Consistent with previous studies from us and others indicating that wt FAK was activated following I/R 22 or ischemic pre-conditioning 35, co-staining with the pFAK(Y397) antibody revealed that FAK activity was relatively low in sham SF2 hearts, but was markedly induced within the hypoxic myocytes in I/R treated SF2 hearts (Figure 3A). As expected, the magnitude of ischemia-induced FAK activation was much higher in SF2 hearts than control hearts subjected to I/R despite a similar extent of hypoxyprobe staining (Figure 3B and data not shown), indicating that SuperFAK is primed for robust activation in response to myocyte hypoxia. We next asked whether ischemic myocytes with elevated FAK activity were more resistant to I/R-induced apoptosis. As shown in figure 3B–C, the number of TUNEL-positive myocytes 24 hr following I/R was significantly lower in the SF2 hearts than in NTG controls in both the ischemic border zone and in remote regions. Moreover, SF2 hearts exhibited a stark reduction in I/R induced cleaved caspase 3 levels as assessed by immunohistochemistry (Supplemental Figure VIII). Taken together with our results that infarcts in SF2 hearts did not increase between 24 and 72 hrs following I/R (time points in which apoptosis contributes significantly to myocyte death) these studies provide strong evidence that enhanced FAK activity can protect myocytes from ischemia-induced apoptosis. Importantly, capillary and coronary vascular density was comparable between control and SF2 hearts (Supplemental Figure IX), indicating that the beneficial effect of elevated FAK activity was likely myocyte autonomous and was not due to enhanced collateral blood flow.
Figure 3. FAK was activated in hypoxic cardiomyocytes and myocyte FAK activation conferred protection from I/R induced apoptosis.
(A) SF2 mice were injected with 60 mg/kg (i.p.) hypoxyprobe-1 and sacrificed at 24h post I/R or sham operation. Heart sections were stained with mouse anti-hypoxyprobe-1 antibody to label hypoxic cells (red), and rabbit anti-pFAK (Y397) to show FAK activation (green). Signals for hypoxyprobe-1 and pFAK (Y397) were dramatically increased and co-localized (yellow-orange) following I/R compared with sham-operated SF2 hearts, indicative of FAK activation in hypoxic myocardial cells. Nuclei were labeled with DAPI (blue). Scale bar = 20µm. (B) At 24h post I/R, the ischemic border zone of SF2 hearts displayed elevated FAK activity (green) and decreased TUNEL staining (red) when compared with non-transgenic (NTG) littermate control hearts. Nuclei were labeled with DAPI (blue) and cardiomyocytes labeled with cardiac troponin T (cTnT, white). Scale bar = 20µm. (C) Quantification of TUNEL-positive myocytes in the infarct border (left) and remote (right) region showed significantly less apoptotic cardiomyocytes in the SF2 hearts than in the control hearts. NTG, n=3; SF2, n=4 (100–200 cells/heart). * p < 0.05 vs. NTG; ** p < 0.01 vs. NTG. Data are expressed as mean ± SEM.
Elevated FAK activity protects cultured cardiomyocytes from oxidative stress-induced apoptosis
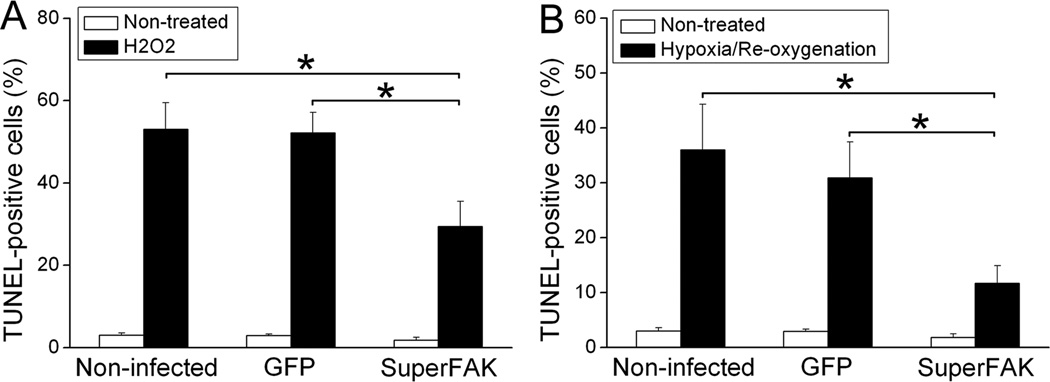
We developed an in vitro assay to further test whether elevated FAK activity confers protection from ischemia-induced programmed cell death in a cell autonomous fashion. To this end, we infected primary neonatal rat cardiomyocytes (NRCM) with adenoviruses expressing GFP or SuperFAK and subjected these cells to oxidative stress by treatment with 10µM H2O2 or by incubation in low O2 as previously described22. Oxidative stress is known to play a critical role in I/R-induced cardiomyocyte apoptosis2, and we found that treatment of cultured cardiomyocytes with 10µM H2O2 for 5 hr induced marked apoptosis in non-infected or GFP-infected cardiomyocytes (as assessed by TUNEL labeling) while significantly fewer TUNEL-positive cells were found in SuperFAK-infected cultures (Figure 4A, Supplemental Figure X). SuperFAK also decreased TUNEL labeling in NRCM subjected to hypoxia (1% O2) for 2 hr followed by re-oxygenation for 1 hr, which closely mimics the hypoxia observed following I/R in vivo (Figure 4B) 22. As a secondary measure of apoptosis, we evaluated cleavage of caspase 3 in cell lysates by Western analysis. As shown in supplemental figure X, H2O2 treatment led to cleavage of caspase 3 in GFP- infected but not SuperFAK-infected NRCM. Taken together, these data indicate that FAK acts in a cell autonomous fashion to protect myocytes from oxidative stress-induced apoptosis.
Figure 4. Overexpression of SuperFAK protected cultured cardiomyocytes from oxidative stress-induced apoptosis.
(A) Quantification of TUNEL-positive myocyte nuclei showing a significant decrease of apoptosis following H2O2 treatment in SuperFAK-infected neonatal rat cardiomyocytes (NRCM) when compared with non-infected or GFP-infected NRCM. Results are mean ± SEM of 4 independent experiments. * p < 0.05. (B) Non-infected, GFP- or SuperFAK-infected NRCM were subjected to hypoxia for 2h followed by re-oxygenation for 1h. Cellular apoptosis assessed by TUNEL staining was significantly decreased by overexpression of SuperFAK compared with intact or GFP-infected cells. Data represent mean ± SEM of 3 independent experiments. * p < 0.05.
SuperFAK enhanced nuclear translocation and transcriptional activity of NF-κB in cardiac myocytes
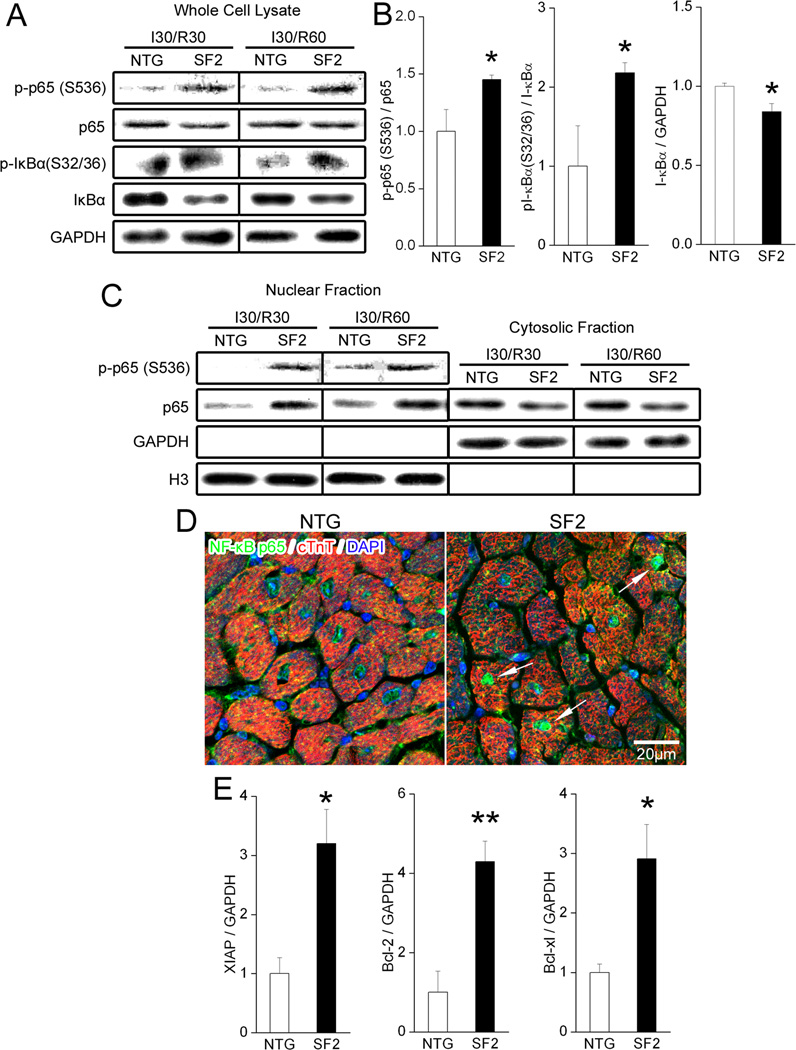
Since we recently showed that FAK was required for I/R–mediated activation of NF-κB, and some studies indicate that NF-κB protects cardiomyocytes from I/R-induced apoptosis 22, 36–38, we interrogated the activity of this pathway in NTG and SF2 hearts. As shown in figure 5, SF2 hearts subjected to 30 min of ischemia followed by 30 or 60 min of reperfusion exhibited increased levels of phosphorylated Ser32/36 IκBα relative to control hearts with a concomitant decrease in IκBα levels, and enhanced phosphorylation of p65 (Ser536) that returned to baseline by 24 hr of reperfusion (Figure 5A,B, Supplemental Figure XI). As well, SF2 hearts exhibited enhanced p65 NF-κB nuclear localization, enhanced DNA binding activity at 60 min post I/R and higher levels of the NF-κB-transcriptional targets XIAP, Bcl-2 and Bcl-xl 39 at 24 hr post I/R (Figure 5C–E, Supplemental Figure XII). Collectively, these data indicate that elevated FAK activity transiently augmented NF-κB-dependent survival signaling. Of note, while I/R also induced transient activation AKT and ERK (as assessed by Western blotting with activation-specific antibodies), the levels of activation did not differ between NTG and SF2 hearts (Supplemental Figure XI).
Figure 5. FAK enhanced nuclear translocation and transcriptional activation of NF-κB in cardiac myocytes.
(A–D) Non-transgenic (NTG) littermate control mice and SF2 mice were subjected to 30min of ischemia followed by 30min or 60min of reperfusion. Border zone of the infarcted myocardium was collected for subcellular fractionation or immunohistochemistry. (A) Representative immunoblots of whole cell lysates 30min and 60min post I/R with GAPDH as a loading control. (B) Quantification of protein expression in whole cell lysate at 60min post I/R showed a significant increase of phospho-NF-κB p65 (S536), phospho-IκBα (S32/36) and a decrease of total IκBα in the SF2 group when compared with control hearts. NTG, n=3; SF2, n=4. * p < 0.05 vs. NTG. (C) Representative immunoblots of nuclear and cytosolic fractions 30min and 60min post I/R. Histone H3 served as a nuclear loading control and GAPDH as a cytosolic loading control. See supplemental figure XII for quantification. (D) Confocal images of NTG and SF2 hearts 60min post I/R immunolabeled for NF-κB p65 (green), cardiac troponin T (cTnT, red) and DAPI (blue). SF2 hearts exhibited increased NF-κB p65 signal in the nuclei of cardiac myocytes (arrows). Scale bar = 20µm. (E) Protein levels of XIAP, Bcl-2 and Bcl-xl were significantly higher in the SF2 heart than in controls at 24h post I/R. See supplemental figure XII for Western images. NTG, n=3; SF2, n=4. * p < 0.05 vs. NTG. ** p < 0.01 vs. NTG. Data are mean ± SEM.
FAK-dependent resistance to oxidative stress requires NF-κB signaling
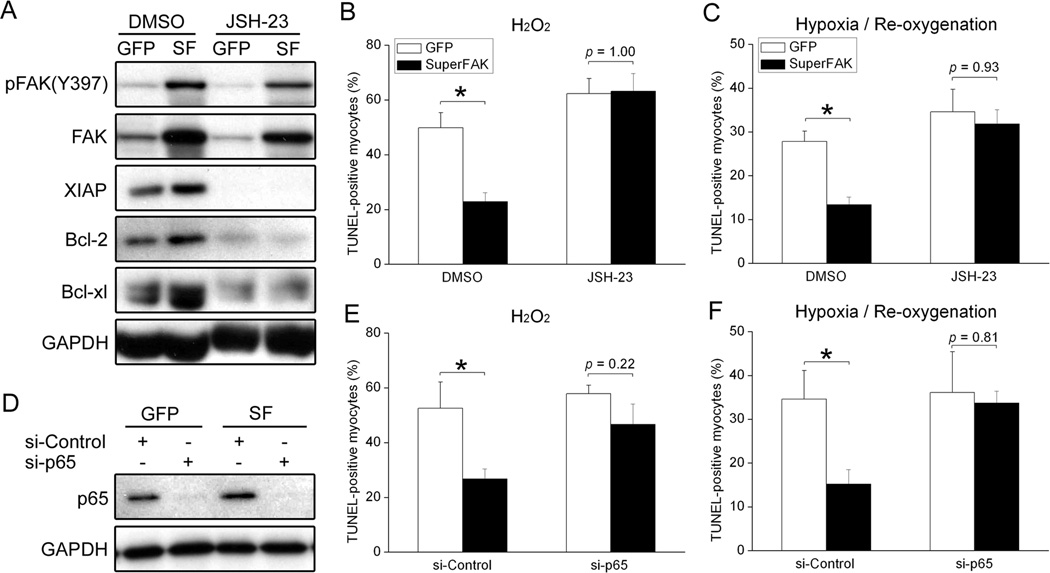
To confirm that regulation of NF-κB was attributable to a primary change in myocyte FAK activity, we next examined activation of this survival pathway in cultured cardiomyocytes. As expected, SuperFAK expressing cardiomyocytes that were plated on FN to induce FAK activity exhibited a decrease in IκBα and a concomitant increase in NF-κB activity (Supplemental Figure XIII) and expression of XIAP, Bcl-2 and Bcl-xl (Figure 6A) when compared to non-infected or GFP-infected cells. To determine the functional importance of this survival pathway with respect to SuperFAK-dependent protection from oxidative stress, we next explored whether inhibition of NF-κB signaling would restore myocyte apoptosis in SuperFAK-expressing cardiomyocytes. As shown in figure 6A, treatment with JSH-23, a cell-permeable inhibitor of NF-κB p65 nuclear translocation, suppressed the capacity of SuperFAK to induce XIAP, Bcl-2 and Bcl-xl expression. Moreover, while treatment of JSH-23 had little effect on H2O2- or hypoxia/re-oxygenation-induced apoptosis in GFP expressing cells, it completely abolished the protection conferred by SuperFAK overexpression (Figure 6B, C). Indeed, in the vehicle control group, the number of TUNEL positive SuperFAK expressing cardiomyocytes was significantly lower than GFP-expressing cardiomyocytes exposed to oxidative stress, while the numbers of TUNEL positive SuperFAK- and GFP-expressing cardiomyocytes were comparable in cultures pre-treated with JSH-23. We next utilized siRNAs targeted towards the p65 NF-κB transcript to confirm a role for this pathway in SuperFAK-mediated myocyte survival. As shown in figure 6D–F, treatment of cultured cardiomyocytes with NF-κB siRNAs led to an approximate 90% reduction of p65 levels as assessed by Western blotting, and nearly completely reversed the beneficial effect of SuperFAK expression when cells were exposed to H2O2 or hypoxia/re-oxygenation. In combination, these results indicate that induction of the NF-κB pathway is a major mechanism by which elevated FAK activity confers cardioprotection.
Figure 6. Blockade of NF-κB pathway abrogated the anti-apoptotic effect of FAK activation.
(A) Neonatal rat cardiomyocytes (NRCM) overexpressing GFP or SuperFAK were treated with DMSO or the NF-κB inhibitor JSH-23 (25µM) for 12h. Cell lysates were probed with indicated antibodies by Western blotting, with GAPDH as a loading control. SF, SuperFAK. (B,C) NRCM were infected with adenoviruses overexpressing GFP or SuperFAK for 48h. Cells were then incubated with DMSO or the NF-κB inhibitor JSH-23 (25µM) for 2h before stimulation with H2O2 (B) or hypoxia/re-oxygenation (C) as described in Methods. TUNEL staining revealed that overexpression of SuperFAK significantly decreased myocyte apoptosis in response to oxidative stress. However, inhibition of NF-κB completely abolished the protective effect of SuperFAK. Data represent mean ± SEM of 4 independent experiments (200–400 cells/experiment). * p < 0.05. (D) NRCM were transfected with control siRNA (si-Control) or NF-κB p65 siRNA (si-p65) for 24h followed by infection with GFP or SuperFAK adenoviruses for 48h. Western blotting showed >90% knockdown of NF-κB p65. Images are representatives of 3 independent experiments. (E,F) NRCM in (D) were treated with H2O2 (E) or hypoxia/re-oxygenation (F). TUNEL staining revealed that knockdown of NF-κB completely abolished the protective effect of SuperFAK. Data represent 4 independent experiments. * p < 0.05.
Discussion
Ischemic heart disease is a significant cause of morbidity and mortality worldwide, thus an enormous interest exists for the development of cardioprotective gene therapies. One potentially promising avenue involves attempts to enhance the activity of intrinsic pro-survival signals that are hard-wired in post-mitotic cardiomyocytes to impart relatively high levels of resistance to oxidative stress. Under physiological conditions, most signaling molecules exhibit transient waves of activation to achieve the appropriate biological response and one of the current challenges of this therapeutic approach is how to achieve such spatial and temporal control of pro-survival signals, since prolonged activation can lead to maladaptive cardiac remodeling 25–27. We previously reported that FAK plays an important role in the induction of intrinsic survival pathways in cardiomyocytes following ischemia/reperfusion and sought to explore whether targeted FAK activation might ameliorate ischemia-induced apoptosis. To this end, we utilized the knowledge that mutations in the activation loop of FAK enhanced catalytic activity but also required signal-dependent activation for maximal induction. We found that transgenic mice that expressed this “super-activatable” variant of FAK (SuperFAK) in the myocardium exhibited elevated FAK activity in un-stressed hearts without consequence on cardiac growth or function. Importantly, FAK activity was profoundly increased in SuperFAK expressing hearts following I/R and these hearts displayed remarkable cardioprotection. Indeed, hearts with transiently elevated FAK activity exhibited a dramatic reduction in myocyte apoptosis and infarct size and were protected from myocardial infarction-induced cardiac de-compensation. We reason that the attenuation of myocyte apoptosis and initial infarct size by FAK activation leads to a long-standing benefit in contractile performance.
It is important to note that our studies reflect a beneficial effect of FAK when activated solely in cardiomyocytes. Interestingly, a recent study revealed that germline deletion of the ECM component fibronectin-EDA, a major fibronectin splice variant, did not affect infarct size, but prevented adverse ventricular remodeling following myocardial infarction; an effect attributed to reduced activation of leukocytes and cardiac fibroblasts that resulted in lower levels of inflammation and metalloproteinase 2 and 9 activities 40. While FAK activity was not directly evaluated in these hearts, a separate study revealed that treatment of fibroblasts with fibronectin-EDA induced myofibroblast differentiation by a mechanism that involved binding to the α4β7 integrin and inducing integrin-dependent activation of FAK41. These studies corroborated earlier findings that FAK can induce inflammatory cytokine production in fibroblasts42. While myofibroblasts (i.e. activated cardiac fibroblasts) are important for the initial healing responses following myocardial infarction, these cells also contribute to pathologic fibrosis and detrimental cardiac remodeling. Thus, one might expect that wide-spread activation of FAK throughout the myocardium would not likely provide as beneficial an effect as was observed herein following specific cardiomyocyte activation and caution should be taken when devising therapeutic strategies to activate this molecule for cardioprotective purposes.
Our mechanistic studies revealed that elevated FAK activity protected cardiomyocytes from oxidative-stress induced apoptosis, at least in part, through enhanced intrinsic NF-κB-dependent survival signaling. These findings confirm and extend our previous loss-of-function studies indicating that FAK was necessary for NF-κB activation in cardiomyocytes exposed to oxidative stress and studies from others indicating that FAK was required for NF-κB activation and survival of fibroblasts exposed to cytokines20 and endothelial cells exposed to shear stress43. FAK has been shown to induce NF-κB activation by forming a complex with the TNF receptor-associated factor 2 (TRAF2) and RIP and facilitating the phosphorylation/activation of the upstream kinase IKK44. Since, we observed increased phosphorylation of the IKK substrate, IκBα in the SF2 hearts relative to controls, it is likely that a similar mechanism resulted in FAK-dependent activation of NF-κB signaling in these hearts. However, recent findings by Petzold et al. indicate that FAK may facilitate NF-κB activation by different mechanisms in cytokine vs. shear stress-induced stress signaling43. These authors found that following mechanical signals, FAK acts in a direct fashion to promote NF-κB phosphorylation and activation43. Although we did find elevated activity of the IKK kinase and decreased levels of IκB in the SF2 hearts, our data do not rule out the possibility that I/R-dependent activation of FAK also directly augments NF-κB activity.
Cardiomyocytes are known to be particularly sensitive to the levels of NF-κB-dependent survival genes. Indeed studies in cultured myocytes indicated that NF-κB suppressed mitochondrial defects and apoptosis through transcriptional up-regulation of survival genes (including Bcl-2, Bcl-xl and XIAP) or by silencing pro-apoptotic genes such as BNIP3 36, 45, 46. However, the function of NF-κB in the intact heart is controversial as it has been reported to induce both pro-survival and pro-apoptotic signals37, 38, 47, 48. More recent studies indicate that timing of activation may be causal for this disparity 36, 47, 48. Thus, while acute myocyte NF-κB activation promoted cardioprotection, chronic myocyte NF-κB activation led to an exacerbated inflammatory response and resulted in maladaptive remodeling 36. Our studies are consistent with a FAK-dependent cardioprotective effect of transient NF-κB activation as we found elevated NF-κB activity in the SF2 hearts 30 to 60 minutes following I/R and a return to baseline by the 24 hr time point. To determine which cell type was responsible for elevated NF-κB activity in SF2 hearts, immunohistochemistry was performed with an NF-κB p65-specific antibody. Our results showed that enhanced nuclear translocation of NF-κB p65 was present in cardiac myocytes of SF2 hearts when compared with NTG hearts (Figure 5D), though we cannot exclude the possibility that NF-κB is activated in additional resident heart cells, like the endothelium, in which I/R has been reported to lead to activation of NF-κB49. Moreover, although we did not observe enhanced activation of other major survival signals such as ERK1/2 or AKT in the SF2 hearts, it is formally possible that other FAK-dependent survival pathways contribute to the cardioprotective effects of FAK. Nonetheless, our finding that blockade of NF-κB-dependent signals reverses the protection induced by SuperFAK expression in cardiomyocytes exposed to oxidative stress indicates that intrinsic NF-κB activation does play a causative role in FAK-mediated myocyte survival.
We and others have previously identified a significant role for FAK in mediating the robust growth responses necessary for both mid-gestational cardiac development and for the promotion of pressure-overload induced myocyte hypertrophy 21, 33, 50 but not for anabolic cardiac growth. Subsequent mechanistic studies indicated that basal FAK activity in embryonic hearts was necessary to repress p38 kinase activity and to promote cardiomyocyte cell cycle progression through G2/M 32. However, in terminally differentiated cardiomyocytes, we found that FAK activity was necessary for ERK-dependent promotion of pathological hypertrophic growth32. While postnatal SF2 hearts exhibited elevated levels of FAK activity, no change in cardiac ERK activity was observed, consistent with previous findings that SuperFAK did not enhance adhesion- or serum-stimulated ERK activation in cultured cells 28. Concomitantly, adult SF2 hearts exhibited no signs of pathological hypertrophic remodeling. Indeed, SF2 and littermate control NTG mice had comparable heart weight/body weight ratios, cardiomyocyte cross-sectional area, ANF expression, and cardiac function. These results confirm and extend previous studies indicating that overexpression of wt FAK in NRCM did not increase stretch-induced ANF expression 51. Taken together, these data indicate that FAK is necessary but not sufficient to promote ERK activation and pathological hypertrophic growth.
In sum, there are two major findings from the studies presented herein 1) they support the feasibility of exploiting mutations in the activation domain of kinases to augment the successes of gene therapy approaches and 2) they indicate that methodologies to enhance FAK activity (including administration of upstream agonists52, 53, or possibly via ischemic pre- and post-conditioning35, 53 or heat shock35) may represent a novel therapeutic strategy for ischemic heart diseases. Since several recently developed chemotherapeutic agents that target receptor tyrosine kinases upstream of FAK, including sunitinib and imatinib, induce myocyte apoptosis and cardiomyopathy 54, 55, it will be of future importance to evaluate whether enhancing FAK activation might also be an effective strategy to preserve myocardial function in this setting.
Supplementary Material
Acknowledgments
The authors thank C. Robert Bagnell, Jr., Steven J. Ray (Microscopy Services Laboratory, UNC-CH), Jackie Kylander and Tayler Kopple (Mouse Cardiovascular Models Core Lab, UNC-CH), Morgan V. Cameron, and Devin Bailey for excellent technical assistance as well as Dr. Thomas J. O’Neill, Zhigang Zhou, and Kaitlin Lenhart for helpful discussions and critical comments throughout the duration of these studies.
Sources of Funding
This work was supported by grants from NIH-NHLBI (HL-081844 and HL-071054 to J.M.T.) and the American Heart Association (AHA. 0355776U to J.M.T.). Z.C. was supported by an American Heart Association Postdoctoral Fellowship (#11POST7600008).
Abbreviations
- SF1
Cardiac-specific SuperFAK transgenic mice line 1
- SF2
Cardiac-specific SuperFAK transgenic mice line 2
- SuperFAK
Super-activatable variant of FAK
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 3.Zhao ZQ, Vinten-Johansen J. Myocardial apoptosis and ischemic preconditioning. Cardiovasc Res. 2002;55:438–455. doi: 10.1016/s0008-6363(02)00442-x. [DOI] [PubMed] [Google Scholar]
- 4.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 5.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 6.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 7.Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 8.Elsherif L, Huang MS, Shai SY, Yang Y, Li RY, Chun J, Mekany MA, Chu AL, Kaufman SJ, Ross RS. Combined deficiency of dystrophin and beta1 integrin in the cardiac myocyte causes myocardial dysfunction, fibrosis and calcification. Circ Res. 2008;102:1109–1117. doi: 10.1161/CIRCRESAHA.108.173153. [DOI] [PubMed] [Google Scholar]
- 9.Johnston RK, Balasubramanian S, Kasiganesan H, Baicu CF, Zile MR, Kuppuswamy D. Beta3 integrin-mediated ubiquitination activates survival signaling during myocardial hypertrophy. FASEB J. 2009;23:2759–2771. doi: 10.1096/fj.08-127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfister R, Acksteiner C, Baumgarth J, Burst V, Geissler HJ, Margulies KB, Houser S, Bloch W, Flesch M. Loss of beta1D-integrin function in human ischemic cardiomyopathy. Basic Res Cardiol. 2007;102:257–264. doi: 10.1007/s00395-006-0640-1. [DOI] [PubMed] [Google Scholar]
- 11.LaFlamme SE, Thomas LA, Yamada SS, Yamada KM. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol. 1994;126:1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akiyama SK, Yamada SS, Yamada KM, LaFlamme SE. Transmembrane signal transduction by integrin cytoplasmic domains expressed in single-subunit chimeras. J Biol Chem. 1994;269:15961–15964. [PubMed] [Google Scholar]
- 13.Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty JT, Conlon FL, Mack CP, Taylor JM. Focal adhesion kinase is essential for cardiac looping and multichamber heart formation. Genesis. 2010;48:492–504. doi: 10.1002/dvg.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 16.Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishibe S, Joly D, Zhu X, Cantley LG. Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol Cell. 2003;12:1275–1285. doi: 10.1016/s1097-2765(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 19.Sonoda Y, Matsumoto Y, Funakoshi M, Yamamoto D, Hanks SK, Kasahara T. Anti-apoptotic role of focal adhesion kinase (FAK). Induction of inhibitor-of-apoptosis proteins and apoptosis suppression by the overexpression of FAK in a human leukemic cell line, HL-60. J Biol Chem. 2000;275:16309–16315. doi: 10.1074/jbc.275.21.16309. [DOI] [PubMed] [Google Scholar]
- 20.Huang D, Khoe M, Befekadu M, Chung S, Takata Y, Ilic D, Bryer-Ash M. Focal adhesion kinase mediates cell survival via NF-kappaB and ERK signaling pathways. Am J Physiol Cell Physiol. 2007;292:C1339–C1352. doi: 10.1152/ajpcell.00144.2006. [DOI] [PubMed] [Google Scholar]
- 21.DiMichele LA, Doherty JT, Rojas M, Beggs HE, Reichardt LF, Mack CP, Taylor JM. Myocyte-restricted focal adhesion kinase deletion attenuates pressure overload-induced hypertrophy. Circ Res. 2006;99:636–645. doi: 10.1161/01.RES.0000240498.44752.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakim ZS, DiMichele LA, Rojas M, Meredith D, Mack CP, Taylor JM. FAK regulates cardiomyocyte survival following ischemia/reperfusion. J Mol Cell Cardiol. 2009;46:241–248. doi: 10.1016/j.yjmcc.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J., Jr. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 26.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniyama Y, Ito M, Sato K, Kuester C, Veit K, Tremp G, Liao R, Colucci WS, Ivashchenko Y, Walsh K, Shiojima I. Akt3 overexpression in the heart results in progression from adaptive to maladaptive hypertrophy. J Mol Cell Cardiol. 2005;38:375–385. doi: 10.1016/j.yjmcc.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Gabarra-Niecko V, Keely PJ, Schaller MD. Characterization of an activated mutant of focal adhesion kinase: 'SuperFAK'. Biochem J. 2002;365:591–603. doi: 10.1042/BJ20020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rindt H, Gulick J, Knotts S, Neumann J, Robbins J. In vivo analysis of the murine beta-myosin heavy chain gene promoter. J Biol Chem. 1993;268:5332–5338. [PubMed] [Google Scholar]
- 30.Masaki M, Izumi M, Oshima Y, Nakaoka Y, Kuroda T, Kimura R, Sugiyama S, Terai K, Kitakaze M, Yamauchi-Takihara K, Kawase I, Hirota H. Smad1 protects cardiomyocytes from ischemia-reperfusion injury. Circulation. 2005;111:2752–2759. doi: 10.1161/CIRCULATIONAHA.104.490946. [DOI] [PubMed] [Google Scholar]
- 31.Sayers RL, Sundberg-Smith LJ, Rojas M, Hayasaka H, Parsons JT, Mack CP, Taylor JM. FRNK Expression Promotes Smooth Muscle Cell Maturation During Vascular Development and After Vascular Injury. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.175455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiMichele LA, Hakim ZS, Sayers RL, Rojas M, Schwartz RJ, Mack CP, Taylor JM. Transient expression of FRNK reveals stage-specific requirement for focal adhesion kinase activity in cardiac growth. Circ Res. 2009;104:1201–1208. doi: 10.1161/CIRCRESAHA.109.195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clemente CF, Tornatore TF, Theizen TH, Deckmann AC, Pereira TC, Lopes-Cendes I, Souza JR, Franchini KG. Targeting focal adhesion kinase with small interfering RNA prevents and reverses load-induced cardiac hypertrophy in mice. Circ Res. 2007;101:1339–1348. doi: 10.1161/CIRCRESAHA.107.160978. [DOI] [PubMed] [Google Scholar]
- 34.Lavine KJ, Kovacs A, Ornitz DM. Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Invest. 2008;118:2404–2414. doi: 10.1172/JCI34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei H, Vander Heide RS. Ischemic preconditioning and heat shock activate Akt via a focal adhesion kinase-mediated pathway in Langendorff-perfused adult rat hearts. Am J Physiol Heart Circ Physiol. 2010;298:H152–H157. doi: 10.1152/ajpheart.00613.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res. 2011;108:1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 37.Misra A, Haudek SB, Knuefermann P, Vallejo JG, Chen ZJ, Michael LH, Sivasubramanian N, Olson EN, Entman ML, Mann DL. Nuclear factor-kappaB protects the adult cardiac myocyte against ischemia-induced apoptosis in a murine model of acute myocardial infarction. Circulation. 2003;108:3075–3078. doi: 10.1161/01.CIR.0000108929.93074.0B. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Guo Y, Tan W, Ou Q, Wu WJ, Sturza D, Dawn B, Hunt G, Cui C, Bolli R. Cardioprotection afforded by inducible nitric oxide synthase gene therapy is mediated by cyclooxygenase-2 via a nuclear factor-kappaB dependent pathway. Circulation. 2007;116:1577–1584. doi: 10.1161/CIRCULATIONAHA.107.689810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 40.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–592. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 41.Kohan M, Muro AF, White ES, Berkman N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J. 2010;24:4503–4512. doi: 10.1096/fj.10-154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlaepfer DD, Hou S, Lim ST, Tomar A, Yu H, Lim Y, Hanson DA, Uryu SA, Molina J, Mitra SK. Tumor necrosis factor-alpha stimulates focal adhesion kinase activity required for mitogen-activated kinase-associated interleukin 6 expression. J Biol Chem. 2007;282:17450–17459. doi: 10.1074/jbc.M610672200. [DOI] [PubMed] [Google Scholar]
- 43.Petzold T, Orr AW, Hahn C, Jhaveri KA, Parsons JT, Schwartz MA. Focal adhesion kinase modulates activation of NF-kappaB by flow in endothelial cells. Am J Physiol Cell Physiol. 2009;297:C814–C822. doi: 10.1152/ajpcell.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funakoshi-Tago M, Sonoda Y, Tanaka S, Hashimoto K, Tago K, Tominaga S, Kasahara T. Tumor necrosis factor-induced nuclear factor kappaB activation is impaired in focal adhesion kinase-deficient fibroblasts. J Biol Chem. 2003;278:29359–29365. doi: 10.1074/jbc.M213115200. [DOI] [PubMed] [Google Scholar]
- 45.Regula KM, Baetz D, Kirshenbaum LA. Nuclear factor-kappaB represses hypoxia-induced mitochondrial defects and cell death of ventricular myocytes. Circulation. 2004;110:3795–3802. doi: 10.1161/01.CIR.0000150537.59754.55. [DOI] [PubMed] [Google Scholar]
- 46.Baetz D, Regula KM, Ens K, Shaw J, Kothari S, Yurkova N, Kirshenbaum LA. Nuclear factor-kappaB-mediated cell survival involves transcriptional silencing of the mitochondrial death gene BNIP3 in ventricular myocytes. Circulation. 2005;112:3777–3785. doi: 10.1161/CIRCULATIONAHA.105.573899. [DOI] [PubMed] [Google Scholar]
- 47.Brown M, McGuinness M, Wright T, Ren X, Wang Y, Boivin GP, Hahn H, Feldman AM, Jones WK. Cardiac-specific blockade of NF-kappaB in cardiac pathophysiology: differences between acute and chronic stimuli in vivo. Am J Physiol Heart Circ Physiol. 2005;289:H466–H476. doi: 10.1152/ajpheart.00170.2004. [DOI] [PubMed] [Google Scholar]
- 48.Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte NF-kappaB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc Res. 2011;89:129–138. doi: 10.1093/cvr/cvq274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canty TG, Jr., Boyle EM, Jr., Farr A, Morgan EN, Verrier ED, Pohlman TH. Oxidative stress induces NF-kappaB nuclear translocation without degradation of IkappaBalpha. Circulation. 1999;100:II361–II364. doi: 10.1161/01.cir.100.suppl_2.ii-361. [DOI] [PubMed] [Google Scholar]
- 50.Peng X, Kraus MS, Wei H, Shen TL, Pariaut R, Alcaraz A, Ji G, Cheng L, Yang Q, Kotlikoff MI, Chen J, Chien K, Gu H, Guan JL. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J Clin Invest. 2006;116:217–227. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torsoni AS, Constancio SS, Nadruz W, Jr., Hanks SK, Franchini KG. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ Res. 2003;93:140–147. doi: 10.1161/01.RES.0000081595.25297.1B. [DOI] [PubMed] [Google Scholar]
- 52.Del Re DP, Miyamoto S, Brown JH. Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection. J Biol Chem. 2008;283:35622–35629. doi: 10.1074/jbc.M804036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vessey DA, Li L, Honbo N, Karliner JS. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol. 2009;297:H1429–H1435. doi: 10.1152/ajpheart.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 55.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan JA, Harris DM, Ismail NS, Chen JH, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T, Chen MH. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.