Abstract
Pharmacometric characterization studies of liquiritigenin have historically overlooked its chiral nature. To achieve complete characterization, an analytical method enabling the detection and quantification of the individual enantiomers of racemic (±) liquiritigenin is necessary. Resolution of the enantiomers of liquiritigenin was achieved using a simple high-performance liquid chromatographic method. A Chiralpak® ADRH column was employed to perform baseline separation with UV detection at 210 nm. The standard curves were linear ranging from 0.5 to 100 μg/mL for each enantiomer. Limit of quantification was 0.5 μg/mL. The assay was applied successfully to stereoselective serum disposition of liquiritigenin enantiomers in rats. Liquiritigenin enantiomers were detected in serum as both aglycones and glucuronidated conjugates. Both unconjugated enantiomers had a serum half-life of ~15 min in rats. The volume of distribution (Vd) for S- and R-liquiritigenin was 1.49 and 2.21 L/kg, respectively. Total clearance (Cltotal) was 5.12 L/h/kg for S-liquiritigenin and 4.79 L/h/kg for R-liquiritigenin, and area under the curve (AUC0-inf) was 3.95 μg h/mL for S-liquiritigenin and 4.23 μg h/mL for R-liquiritigenin. The large volume of distribution coupled with the short serum half-life suggests extensive distribution of liquiritigenin into tissues.
Keywords: reversed-phase HPLC, liquiritigenin, stereospecific, pharmacokinetics, flavonoid
Introduction
(±)-Liquiritigenin (4′,7-dihydroxyflavanone) is a chiral flavonoid present in liquorice. It is a potent bioactive compound, demonstrating anti-cancer and hepatoprotective activity as well as attenuation of the acute effects of cocaine administration (Zhang et al., 2009; Kim et al., 2009; Jang et al., 2011). Liquiritgenin has also been shown to be an estrogen receptor β-agonist (Mersereau et al., 2008). These properties highlight the potential therapeutic application of liquiritigenin in human health.
The pharmacokinetics of racemic liquiritigenin via achiral high-performance liquid chromatography (HPLC) methods has been studied extensively (Li et al., 2007; Kang et al., 2009a, b, 2010a, b). However, chirality is an aspect often overlooked in the pharmacometric characterization of phytochemicals, including flavonoids. Very few studies have been published on the stereospecific separation of liquiritigenin (Li et al., 1998; Fliegmann et al., 2010).
Differences in the disposition and activity of individual stereoisomers can sometimes cause significant or harmful effects in humans (Shah et al., 1998; Hutt, 2007). This study is the first to develop a stereoselective, isocratic, reversed-phase HPLC assay of liquiritigenin for detection in rat biological matrices with application to a pharmacokinetic study.
Experimental
Chromatographic system and conditions
The HPLC system used was a Shimadzu LC-2010A (Kyoto, Japan). Data collection and integration were accomplished using Shimadzu EZ Start 7.4 software (Kyoto, Japan). The analytical column used was a Chiralpak® AD-RH (150 × 4.6 mm i.d., 5 μm particle size, Chiral Technologies Inc., West Chester, PA, USA). The mobile phase consisted of acetonitrile, water and acetic acid (50:50:0.05 v:v:v) filtered and degassed under reduced pressure, prior to use. Separation was carried out isocratically at ambient temperature (25 ± 1°C), with a flow rate of 0.6 mL/min and ultraviolet (UV) detection at 210 nm.
Sample preparation
To the working standards or samples (0.1 mL), 25 μL of (±)-pinocembrin (internal standard) solution (100 μg/mL) was added into 2.0 mL Eppendorf tubes. Next, 1 mL of cold acetonitrile (−20°C) was added, immediately followed by 1 min of vortexing (Vortex Genie-2, VWR Scientific, West Chester, PA, USA). Samples were then centrifuged at 5000 rpm for 5 min (Beckman Microfuge Centrifuge, Beckman Coulter, Inc., Fullerton, CA, USA). The supernatant was collected into 2.0 mL Eppendorf tubes and evaporated to dryness under compressed nitrogen gas. The residue was reconstituted with 200 μL of mobile phase, vortexed for 30 s and centrifuged at 5000 rpm for 5 min. The supernatant was then transferred to HPLC vials and 100 μL of it was injected into the HPLC system.
Pharmacokinetic disposition of liquiritigenin in rats
Male Sprague–Dawley rats (n = 3, average weight 250 g) were anesthesized using isoflurane and a silastic catheter was cannulated into the right jugular vein. Rats were dosed intravenously with 20 mg/kg racemic (effectively 10 mg/kg S-liquiritigenin and 10 mg/kg R-liquiritigenin) liquiritigenin (Extrasynthèse, Genay Cedex, France) in saline and polyethylene glycol (PEG) 400 (60:40 v:v). A series of blood samples (0.30 mL) was collected at 0, 1, 15 and 30 min, and at 1, 2, 4, 6, 24, 48, 72, 96 and 120 h. Following centrifugation of the blood samples in microcentrifuge tubes, serum was collected and stored at −20°C until analysis. Serum samples (0.1 mL) were run in duplicate with or without the addition of 40 μL of 500 U/mL β-glucuronidase from Escherichia coli type IX-A and incubated in a shaking water bath at 37°C for 2 h to liberate any glucuronide conjugates.
Data analysis
Quantification was based on calibration curves constructed using the peak area ratio of liquiritigenin to internal standard, against liquiritigenin concentrations using unweighted least squares linear regression. Pharmacokinetic analysis was performed using data from individual rats for which the mean and standard error of the mean (SEM) were calculated for each group. Pharmacokinetic modeling was completed using WinNonlin® software (version 5.1).
Results and discussion
Chromatography
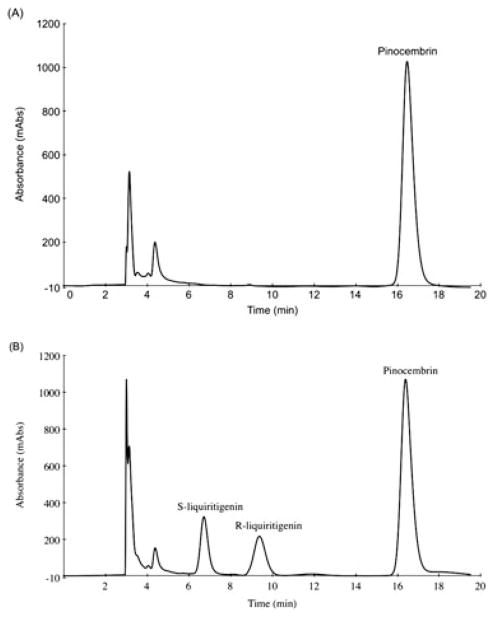
Separation of liquiritigenin enantiomers and the internal standard, (±)-pinocembrin, in serum (Fig. 1) was achieved. There were no interfering peaks co-eluting with the peaks of interest. The retention times of S- and R-liquiritigenin were approximately 9 and 12 min, respectively. The internal standard (±)-pinocembrin eluted at approximately 16 min (Fig. 1).
Figure 1.
(A) Blank serum with the internal standard, pinocembrin. (B) Liquiritigenin enantiomers in serum (5 μg/mL) and the internal standard, pinocembrin.
The performance of the HPLC assay was assessed using the following parameters: peak shape and purity, interference from endogenous substances in biological fluid, linearity and limit of quantitation (LOQ). Various compositions of mobile phase were tested to achieve the best resolution between liquiritigenin enantiomers. Optimal separation was achieved with acetonitrile, water and acetic acid (50:50:0.05 v:v:v) with a flow rate of 0.6 mL/min. The present assay is practical to use in pre-clinical applications of liquiritigenin analysis in which small sample volumes are obtained.
Linearity and LOQ
Excellent linear relationships (r2 = 0.998) were demonstrated between the peak area ratio of S- and R-liquiritigenin to the internal standard and the corresponding serum concentrations of liquiritigenin enantiomers over a range of 0.5–100 μg/mL. The LOQ of this assay was 0.5 μg/mL in biological fluids.
Stereospecific pharmacokinetics of liquiritigenin in rats
The HPLC method was applied to the determination of liquiritigenin enantiomers in pharmacokinetic studies in rats (n = 3) (Table 1). Liquiritigenin enantiomers were detected in serum as both aglycones and glucuronidated conjugates. The Rand S-glucuronidated conjugates had a serum half-life of around 9 min (t1/2 S-liquiritigenin-glucuronidate, 0.159 h; t1/2, R-liquiritigenin–glucuronidate, 0.141 h) and an area under the curve of 1.202 and 1.589 μg h/mL, respectively. Both unconjugated enantiomers had a serum half-life of ~15 min. R-Liquiritigenin had a slightly larger volume of distribution (Vd S-liquiritigenin, 1.492 L/kg; Vd R-liquiritigenin, 2.213 L/kg) than did S-liquiritigenin. Total clearance (Cltotal S-liquiritigenin, 5.123 L/h/kg; Cltotal R-liquiritigenin, 4.787 L/h/kg) and area under the curve (AUC0–∞, S-liquiritigenin, 3.951 μg h/mL; AUC0–∞ R-liquiritigenin, 4.226 μg h/mL) were similar between the two enantiomers. The relatively large volume of distribution coupled with the short serum half-life suggests extensive distribution of liquiritigenin into tissues.
Table 1.
Stereospecific pharmacokinetic parameters of unconjugated liquiritigenin
| Parameter | S-Liquiritigenin (mean ± SEM) | R-Liquiritigenin (mean ± SEM) |
|---|---|---|
| AUC0–∞ (h μg/mL) | 3.951 ± 0.267 | 4.226 ± 0.278 |
| Vd (L/kg) | 1.492 ± 0.103 | 2.213 ± 0.340 |
| Cltotal (L/h/kg) | 5.123 ± 0.349 | 4.787 ± 0.316 |
| Fe (%) | 31.49 ± 1.00 | 5.02 ± 0.97 |
| t1/2 serum (h) | 0.202 ± 0.0002 | 0.33 ± 0.07 |
Conclusions
In summary, the developed HPLC method for liquiritigenin is stereospecific. It has been applied successfully in the study of the pharmacokinetics of liquiritigenin in rats for the first time. Further studies are on-going in our laboratory to further characterize the pharmacometric profiles of liquiritigenin as well as other flavonoid enantiomers.
Acknowledgments
The authors would like to acknowledge support from the Pfizer Canada Centennial Pharmacy Research Award and University of Manitoba Graduate Fellowship to C.L.S.
References
- Fliegmann J, Furtwangler K, Malterer G, Cantarello C, Schuler G, Ebel J, Mithofer A. Flavonesynthase II (CYP93B16) from soybean (Glycinemax L.) Phytochemistry. 2010;71(5–6):508–514. doi: 10.1016/j.phytochem.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Hutt AJ. Chirality and pharmacokinetics: an area of neglected dimensionality? Drug Metabolism and Drug Interactions. 2007;22(2–3):79–112. doi: 10.1515/dmdi.2007.22.2-3.79. [DOI] [PubMed] [Google Scholar]
- Jang EY, Hwang M, Yoon SS, Lee JR, Kim KJ, Kim HC, Yang CH. Liquiritigenin decreases selective molecular and behavioral effects of cocaine in rodents. Current Neuropharmacology. 2011;9(1):30–34. doi: 10.2174/157015911795017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HE, Cho YK, Jung HY, Choi KY, Sohn SI, Baek SR, Lee MG. Pharmacokinetics and first-pass effects of liquiritigenin in rats: low bioavailability is primarily due to extensive gastrointestinal first-pass effect. Xenobiotica. 2009a;39(6):465–475. doi: 10.1080/00498250902890151. [DOI] [PubMed] [Google Scholar]
- Kang HE, Jung HY, Cho YK, Kim SH, Sohn SI, Baek SR, Lee MG. Pharmacokinetics of liquiritigenin in mice, rats, rabbits, and dogs, and animal scale-up. Journal of Pharmaceutical Sciences. 2009b;98(11):4327–4342. doi: 10.1002/jps.21702. [DOI] [PubMed] [Google Scholar]
- Kang HE, Kim YW, Sohn SI, Baek SR, Lee JW, Kim SG, Lee I, Lee MG. Pharmacokinetics of liquiritigenin and its two glucuronides, M1 and M2, in rats with acute hepatitis induced by D-galactosamine/lipopolysaccharide or CCl(4) Xenobiotica. 2010a;40(6):424–436. doi: 10.3109/00498251003734251. [DOI] [PubMed] [Google Scholar]
- Kang HE, Sohn SI, Baek SR, Lee JW, Lee MG. Liquiritigenin pharmacokinetics in a rat model of diabetes mellitus induced by streptozotocin: greater formation of glucuronides in the liver, especially M2, due to increased hepatic uridine 5′-diphosphoglucuronic acid level. Metabolism. 2010b;59(10):1472–1480. doi: 10.1016/j.metabol.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Kim YW, Kang HE, Lee MG, Hwang SJ, Kim SC, Lee CH, Kim SG. Liquiritigenin, a flavonoid aglycone from licorice, has a choleretic effect and the ability to induce hepatic transporters and phase-II enzymes. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2009;296(2):G372–381. doi: 10.1152/ajpgi.90524.2008. [DOI] [PubMed] [Google Scholar]
- Li C, Homma M, Oka K. Chiral resolution of four major flavanones in post-administrative urine of Chinese herbal medicines by HPLC on macroporous silica gel coated with cellulose tris(3,5 dimethylphenylcarbamate) Biomedical Chromatography. 1998;12(4):199–202. doi: 10.1002/(SICI)1099-0801(199807/08)12:4<199::AID-BMC735>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Li L, Liang S, Du F, Li C. Simultaneous quantification of multiple licorice flavonoids in rat plasma. Journal of the American Society for Mass Spectrometry. 2007;18(4):778–782. doi: 10.1016/j.jasms.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Mersereau JE, Levy N, Staub RE, Baggett S, Zogovic T, Chow S, Ricke WA, Tagliaferri M, Cohen I, Bjeldanes LF, Leitman DC. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Molecular and Cellular Endocrinology. 2008;283(1–2):49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RR, Midgley JM, Branch SK. Stereochemical origin of some clinically significant drug safety concerns: lessons for future drug development. Adverse Drug Reactions and Toxicological Reviews. 1998;17(2–3):145–190. [PubMed] [Google Scholar]
- Zhang SP, Zhou YJ, Liu Y, Cai YQ. Effect of liquiritigenin, a flavanone existed from Radix glycyrrhizae on pro-apoptotic in SMMC-7721 cells. Food and Chemical Toxicology. 2009;47(4):693–701. doi: 10.1016/j.fct.2008.12.015. [DOI] [PubMed] [Google Scholar]