Abstract
The REGγ-proteasome serves as a short-cut for the destruction of certain intact mammalian proteins in the absence of ubiquitin-and ATP. The biological roles of the proteasome activator REGγ are not completely understood. Here we demonstrate that REGγ controls degradation of protein kinase A catalytic subunit-α (PKAca) both in primary human umbilical vein endothelial cells (HUVECs) and mouse embryonic fibroblast cells (MEFs). Accumulation of PKAca in REGγ-deficient HUVECs or MEFs results in phosphorylation and nuclear exclusion of the transcription factor FoxO1, indicating that REGγ is involved in preserving FoxO1 transcriptional activity. Consequently, VEGF-induced expression of the FoxO1 responsive genes, VCAM-1 and E-Selectin, was tightly controlled by REGγ in a PKA dependent manner. Functionally, REGγ is crucial for the migration of HUVECs. REGγ−/− mice display compromised VEGF-instigated neovascularization in cornea and aortic ring models. Implanted matrigel plugs containing VEGF in REGγ−/− mice induced fewer capillaries than in REGγ+/+ littermates. Taken together, our study identifies REGγ as a novel angiogenic factor that plays an important role in VEGF-induced expression of VCAM-1 and E-Selectin by antagonizing PKA signaling. Identification of the REGγ–PKA–FoxO1 pathway in endothelial cells (ECs) provides another potential target for therapeutic intervention in vascular diseases.
Keywords: Angiogenesis, E-Selectin, FoxO1, PKAca, REGγ, VCAM-1
1. Introduction
Angiogenesis is a physiological process and essential for reproduction, development, and wound repair. It also plays an important role in pathological conditions, such as tumor growth and metastasis, rheumatoid arthritis, and diabetic retinopathy [1, 2]. Angiogenesis is a highly ordered process which is tightly controlled by key angiogenic factors, including vascular endothelial growth factor (VEGF). There is consensus that VEGF signaling to ECs occurs by engagement with its receptors and represents the crucial rate-limiting step during normal and pathophysiological angiogenesis [3]. However, VEGF-targeted therapies have been limited by insufficient efficacy, development of resistance, and inherent toxicities [4].
Known angiogenic mediators include soluble forms of vascular cell adhesion molecule-1 (VCAM-1) and endothelial-Selectin (E-Selectin) [5]. Both VCAM-1 and E-Selectin are subject to regulation by VEGF and play a pro-angiogenic role, contributing to the cross-regulation of angiogenesis and inflammation [6, 7]. Elevated levels of soluble E-selectin have been found in patients with vasculoproliferative disorders such as rheumatoid arthritis [8] and in malignant tumors [9]. Serum VCAM-1 has been proposed as a surrogate marker for angiogenesis in breast cancer [10]. VCAM-1 gene expression is effectively controlled by an upstream regulator — Forkhead box O1 (FoxO1), a key modulator of numerous genes that govern a wide array of cellular functions [11]. FoxO1 function is regulated by post-translational modifications [12], phosphorylation of FoxO1 at specific sites promotes FoxO1 nuclear exclusion and impairs its transcriptional activity [13]. PI3K-Akt-mediated phosphorylation of FoxO1 is the most characterized signaling pathway to regulate intercompartmental FoxO1 shuttling [14]. However, there are additional kinases that phosphorylate FoxO1 to regulate its function in a similar manner, including SGK (serum and glucocorticoid-regulated kinase) and CK1 (casein kinase 1) [15]. Recently, the protein kinase A catalytic subunit-α (PKAca) was demonstrated to interact with FoxO1 and directly phosphorylate FoxO1 to regulate VCAM-1 gene expression in human aortic endothelial cells, suggesting an involvement of the PKA–FoxO1 pathway in angiogenesis [16].
PKA has been linked to angiogenesis as both a positive [17–20] and negative [21–25] regulator. So far, there is no detailed analysis on the regulation of PKA degradation. Forskolin-induced PKA stimulation enhances angiogenesis through PKA-dependent VEGF expression [18]. PKA inhibitor H89 blocks VIP-induced VEGF production to inhibit proliferation of ECs [17]. On the contrary, PKA antagonizes angiogenesis when the exogenous VEGF stimulation is involved [21–23]. Pharmacologic or genetic activation of PKA inhibits cell migration in endothelial and other cells [22, 26, 27]. Indeed, PKA plays a key role in the regulation of vascular sprouting by stimulating ECs adhesion and inhibiting cell migration [23], yet the molecular mechanism is only partially understood.
Recently, emerging mechanisms point to regulation of angiogenesis by the proteasome system. The ubiquitin proteasome pathway has been suggested to have potential therapeutic value in angiogenesis-associated diseases [28], but no linkage is known between angiogenesis and the ubiquitin independent proteasome pathway. The REGγproteasome, a representative of this proteolytic mode, is drawing considerable attention nowadays. REGγ (also known as PA28γ; PSME3) is a member of the 11S family of proteasome activators and has been shown to promote the degradation of several intact cellular proteins, including SRC-3 and p21 [29–32], in a ubiquitin independent manner. Apart from previous studies showing reduced body size in REGγ−/− mice and decreased cell proliferation in REGγ−/− MEFs [33, 34], REGγ has been related to cancer progression [35, 36]. In tumor development, angiogenesis is a necessary and required step in tumor growth and metastasis [37]. Understanding the precise regulation of angiogenesis is fundamental for the identification of new therapeutic strategies for cancers [38] and vascular abnormalities. Previous studies indicated anti-angiogenic and anti-tumor properties of proteasome inhibitors, such as PS-341 and MG132, indicating that limiting proteasome activity restricted capillary growth [39, 40]. Thus, it is of interest to explore whether regulation of the ubiquitinin-dependent proteasome pathway can also benefit patients with vascular diseases.
Here we report, for the first time, that the proteasome activator REGγ is required for VCAM-1 and E-Selectin gene expression through PKA-FoxO1 pathway and controls EC migration as well as VEGF-induced angiogenesis. Mechanistically, REGγ interacts with PKA catalytic subunit α (PKAca) and reduces PKAca intracellular stability. In HUVECs and MEFs depleted of REGγ, PKAca accumulation accelerated phosphorylation of the transcription factor FoxO1 and its subsequent nuclear exclusion. As a result, REGγ positively regulated VEGF-mediated expression of the FoxO1 downstream genes, VCAM-1 and E-Selectin, promoting HUVECs migration. In the mouse models, REGγ−/− mice had poor responses to VEGF-induced angiogenesis in the cornea and the aortic ring, as well as during hypoxia-induced angiogenesis in the retina. VEGF-matrigels implanted in REGγ−/− mice induced fewer capillaries than in REGγ+/+ control mice. Collectively, our results identify REGγ as a new regulatory mediator for VEGF-induced expression of VCAM-1 and E-Selectin genes via the PKAFoxO1 pathway and substantiate an important role for REGγ in ECs migration and angiogenesis.
2. Materials and methods
2.1. Animals
REGγ+/+ and REGγ−/− mice were kindly provided by Dr. John Monaco at the University of Cincinnati College of Medicine [33]. Animal procedures were carried out according to the rules of the American Association for the Accreditation of Laboratory Animal Care International and approved by the Animal Center of the school.
2.2. Cell lines and cell culture
Primary HUVECs were purchased from ScienCell Research Laboratories and cultured in ECM (ScienCell, Carlsbad, CA, USA). Primary REGγ+/+ and REGγ−/− MEFs were previously generated [29]. Immortalized REGγ+/− (which behaves similar to REGγ+/+) and REGγ−/− MEFs were kindly provided by James M. Roberts (Division of Basic Sciences, Fred Hutchinson Cancer Research Center, Seattle) [41]. 293T, A549, HMEC-1 and HeLa cells were purchased from ATCC and maintained at the Cell culture Core in our department. All cells were cultured under standard conditions described by the ATCC.
2.3. Plasmids and reagents
Flag-REGγ was previously generated [36]. GFP-PKAca was generated in pEGFP-n1-vector (Addgene). Antibodies were purchased from Invitrogen (PA28γ), Cell Signaling (FoxO1, Phospho-FoxO1/Ser256, Thr24, Ser319), Sigma (β-actin, M2 anti-Flag), Santa Cruz (PKAα cat, HA, GFP), GeneTex (E-Selectin, VCAM-1). Other purchased reagents include VEGF-A165 (PeproTech Inc), cycloheximide (Amresco). All the experiments shown in the study were repeated at least three times.
2.4. In vivo and ex vivo experiments
REGγ+/+ and REGγ−/− mice were prepared for aortic ring assay [42], oxygen-induced retinopathy assay [43], mouse corneal micropocket assay [44] and matrigel plug assay [45] following the procedure (see Supplementary methods online).
2.5. Wound healing, transwell assay, MTT assay and pulse-chase labeling experiment
See supplementary methods online [30, 44].
2.6. siRNA transfection
All siRNA duplexes were purchased from Dharmacon (Lafayette, CO). siRNA targeting REGγ (catalog #L-012133-00-0010), PKAca (catalog #L-004649-00), FoxO1 (catalog #M-003006-01-05) or control siRNA (catalog #D-001810-10-20) were transfected into cells by 48–72 h using Lipofectamine 2000 (Invitrogen) following the instructions provided by the company.
2.7. Immunoprecipitation, Western blot, immunostaining and real-time qPCR
Immunoprecipitation, Western blot, immunostaining and qPCR were performed as described [29, 30].
2.8. Data collection and statistical analysis
The intensity of the Western blot results was analyzed by densitometry using Bio-Rad Quantity One 4.4.0 software and normalized to the band with the least intensity which was arbitrarily set as 1. The results were expressed as the mean ± SD. Statistical analyses of all experimental data in this study were performed using the two-tailed Student' t-test or one-way ANOVA (Tukey's Multiple Comparison Test). A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. REGγ mediates the degradation of PKAca
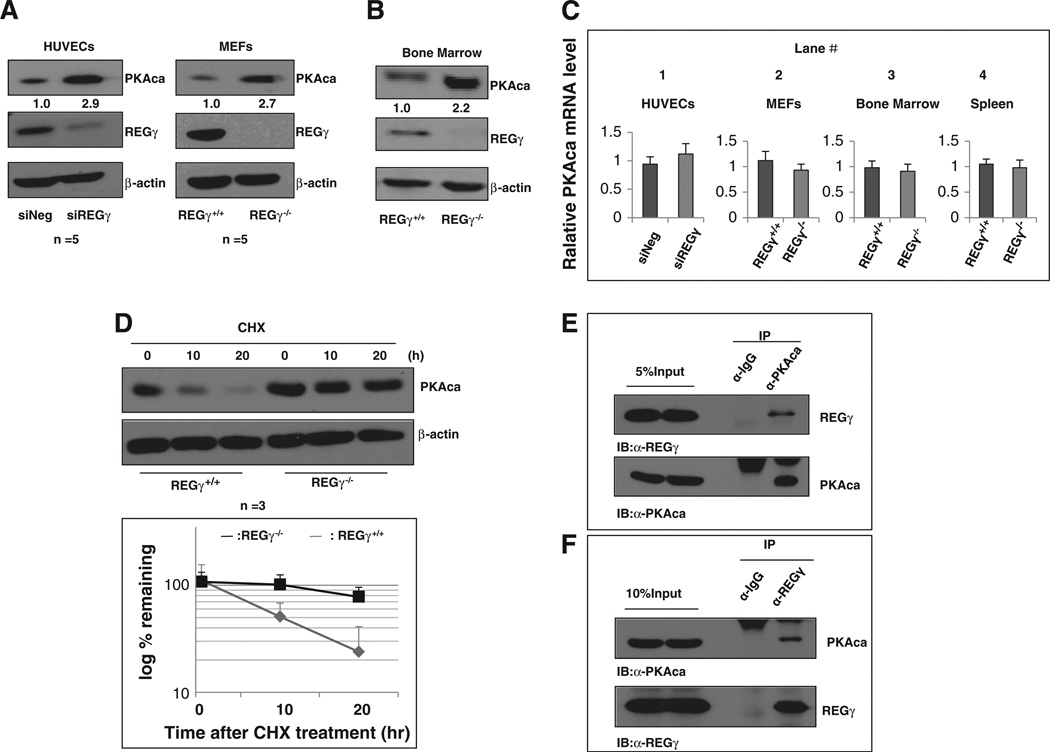
Majority of the proteins that are degraded by the REGγ-proteasome pathway remain unknown. In an effort to search for REGγ target proteins, our large-scale antibody microarray analysis on whole cell lysates of REGγ+/+ and REGγ−/− MEFs indicated that PKAca was negatively correlated with REGγ [31]. We then validated REGγ-mediated regulation of PKAca in different cells/tissues in vitro and in vivo. Silencing REGγ in HUVECs resulted in an almost 3-fold increase in PKAca protein level compared with samples treated with a control siRNA (Fig. 1A). Similar results were observed in immortalized Human Microvascular Endothelial Cell-1 (HMEC-1 cells) (Fig. S1A). To evaluate in vivo effects of REGγ on PKAca expression, we isolated primary MEFs from REGγ−/− and REGγ+/+ mice. Consistent with our expectation, PKAca protein level was elevated in REGγ−/− MEFs compared to REGγ+/+ MEFs (Fig. 1A, right panel). Similar results were observed in immortalized REGγ−/− MEFs which showed the increase of PKAca (Fig. 2A). Furthermore, we measured PKAca expression in several mouse tissues. As shown in Fig. 1B and Fig. S1B, expression of PKAca proteins in REGγ−/− bone marrow and spleen (2-week-old) was more abundant than that from REGγ+/+ littermates. We also noticed that the regulation of PKAca by REGγ appears to be tissue/cell specific, since REGγ had no effect on PKAca in A549 cells (Fig. S1C) or in mouse liver (Fig. S1D). To exclude a potential mechanism involving transcriptional regulation by REGγ, expression of PKAca mRNA was assessed by real-time RT-PCR analyses. As shown in Fig. 1C, REGγ-depletion in HUVECs (lane 1), MEFs (lane 2), bone marrow and spleen (lane 3 and 4) had no effect on PKAca mRNA levels, indicating that REGγ likely regulates PKAca by enhancing turnover of PKAca protein.
Fig. 1.
REGγ controls PKAca stability. (A–B) REGγ negatively regulated PKAca level in multiple cell types. Primary HUVECs were treated with 20 nM siREGγ or a negative control (siNeg) (A, left panel). Primary REGγ+/+ and REGγ−/− MEFs from the third passage were used for Western blot analysis (A, right panel). Bone marrows (B, left panel) collected from day 14 REGγ+/+ and REGγ−/− mice were prepared for Western blot analysis. Relative PKAca levels were quantitated and normalized to β-actin as indicated by the numbers. (C) REGγ deficiency had no effect on PKAca mRNA levels. Total RNA extracted from primary HUVECs treated with 20 nM siREGγ or siNeg, primary MEFs, bone marrows, or spleens from REGγ+/+ and REGγ−/− mice, were prepared for real-time RT-PCR analysis. Data are shown as mean ± SD of three independent experiments. (D) REGγ dictated degradation kinetics of PKAca. Primary REGγ+/+ and REGγ−/− MEFs were treated with 100 µg/ml cycloheximide for different periods of time as indicated. Total cell lysates were extracted for Western blot (upper panel). Quantitated results of the Western blot analysis were plotted against indicated time course (lower panel). (E–F) Intracellular interactions between REGγ and PKAca. Immunoprecipitation was performed with whole cell lysates of HUVECs in the presence of MG132 using an anti-PKAca (E) or an anti-REGγ (F) antibody. The immuno-precipitated complexes were separated by SDS-PAGE and detected by an antibody against PKAca or REGγ.
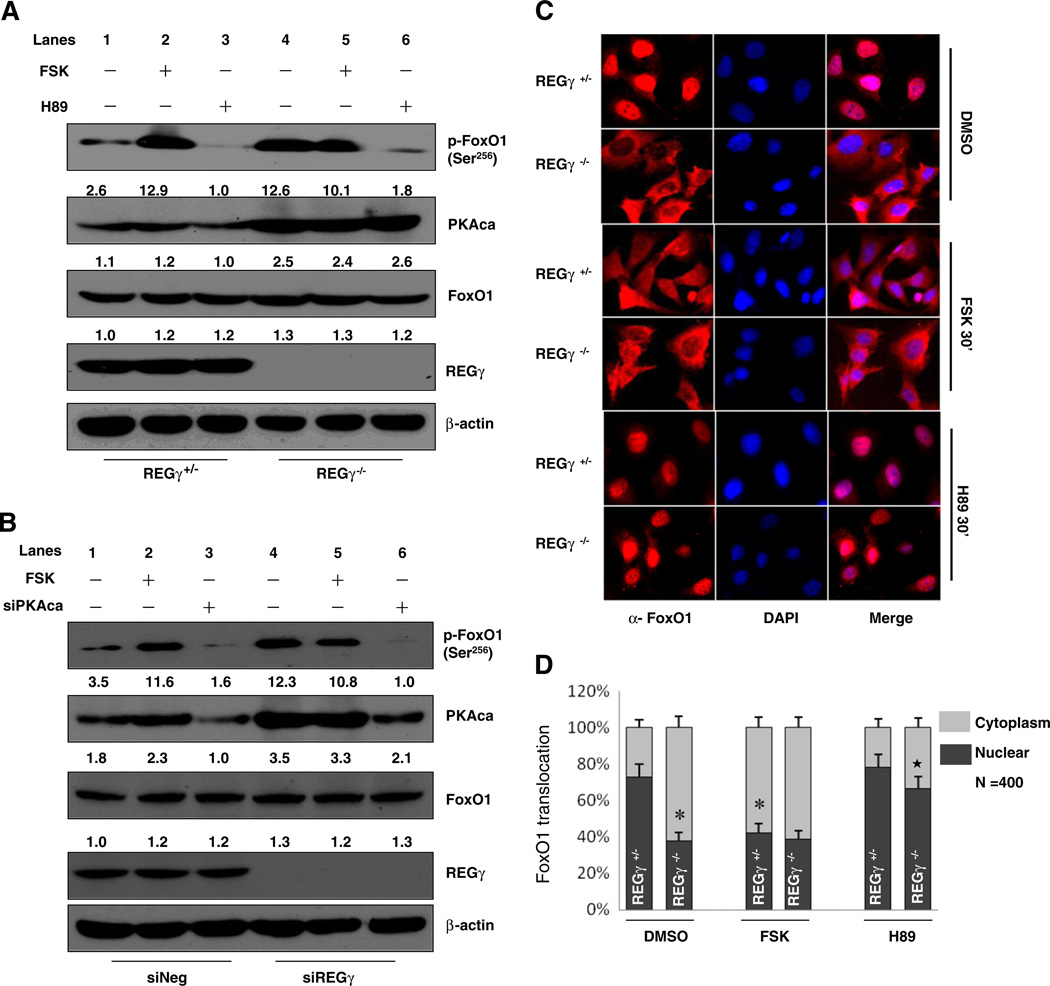
Fig. 2.
REGγ modulates phosphorylation and subcellular distribution of FoxO1. (A–B) REGγ depletion enhanced FoxO1 phosphorylation through activating PKA signaling. Immortalized REGγ+/− and REGγ−/− MEFs were serum-starved overnight and treated with DMSO, 20 uM forskolin (FSK) or 20 uM H89 for 30 min (A). Primary HUVECs were treated with/without siREGγ alone or in combination with siPKAca, then serum-starved overnight following incubating with 20 uM forskolin or DMSO for 30 min (B). Protein expression of endogenous p-FoxO1(Ser256), PKAca, FoxO1, REGγ, β-actin was detected by Western blot. (C) REGγ deficiency promoted FoxO1 nuclear export by activating PKA. Immunofluorescence staining was performed in REGγ+/− and REGγ−/− MEFs. Cells were serum-starved overnight before treatment with DMSO, 20 uM forskolin, or 20 uM H89, for 30 min. Cells were immunostained with anti-FoxO1 antibody (red) and the nuclei were counterstained with DAPI (blue). (D) The bar graph shows the corresponding quantitative analyses of the subcellular localization of FoxO1 and was generated by manually counting nuclear and cytoplasmic localization of FoxO1 in 400 FoxO1-positive staining MEFs for each condition. The area of the nucleus was determined using DAPI staining (nuclear staining). Data are shown as mean ± SD of quantitated results from three independent experiments (*p < 0.05 relative to DMSO-treated REGγ+/− MEFs; ★, p < 0.05 relative to DMSO-treated REGγ−/− MEFs).
To further understand if REGγ patrols PKAca stability, we tested the degradation kinetics of PKAca. Following cycloheximide treatment for various periods of time, the degradation of PKAca in REGγ−/− MEFs was nearly blocked, indicating that the REGγ-proteasome could be the major mechanism controlling PKAca protein turnover in test cells (Fig. 1D). By pulse-chase experiments, we verified that REGγ indeed is responsible for accelerated degradation of PKAca protein (Fig. S1E).
All of the REGγ target proteins identified to date can interact with this proteasome activator. Therefore, we performed a co-immunoprecipitation assay to define the intracellular interactions between REGγ and PKAca. Endogenously expressed REGγ and PKAca in HUVECs were immunoprecipitated using either anti-PKAca or anti-REGγ antibody. Western blotting detected co-precipitation of REGγ (Fig. 1E) or PKAca (Fig. 1F), respectively. We also validated the interaction by over-expression and reciprocal immunoprecipitation of REGγ and PKAca in 293T cells (Fig. S1F&G). Taken together, our results suggest that REGγ directly mediates PKAca turnover.
3.2. REGγ controls PKAca-mediated FoxO1 phosphorylation and cellular localization
Since the role of PKA signaling in ECs involves phosphorylation of one or more of the factors responsible for regulating the expression of downstream genes, we next wished to explore the effectors downstream of PKA that are also regulated by REGγ. We screened the expression of a panel of genes in HUVECs before and after silencing REGγ (Fig. S2A). Among the differentially expressed factors were VCAM-1 and E-Selectin, whose expressions were negatively regulated by cAMP/PKA signaling [46]. More recent studies linked PKA-FoxO1 to the regulation of VCAM-1 and E-Selectin in vascular ECs [16]. Therefore, we hypothesized that the PKA-FoxO1 pathway might be required for REGγ-dependent regulation of VCAM-1 and E-Selectin gene expression. To test our hypothesis, we examined PKA-dependent phosphorylation of FoxO1 in immortalized REGγ−/− MEFs and HUVECs. As shown in Fig. 2A, REGγ knockout caused a dramatic increase in the phosphorylation of FoxO1 at Ser256 along with marked elevation of PKAca (Fig. 2A, lane4). Similar results were observed at Ser319 and Thr24 (Fig. S2B). To determine whether the increases are mediated by PKA signaling, we monitored phosphorylation of FoxO1 following treatment with forskolin or H89. Consistent with previous reports, forskolin-mediated PKA activation dramatically elevated phosphorylation of FoxO1 by 30 min in REGγ+/− cells (Fig. 2A, lane 2). In contrast, there was no further phosphorylation of FoxO1 observed in REGγ−/− MEFs upon forskolin treatment (Fig. 2A, lane 5, compare with lane 4). Similar results were observed in HUVECs (Fig. 2B, lanes 2 and 5), suggesting that PKA signaling has been activated and phosphorylation of FoxO1 is already saturated in cells lacking REGγ. We next examined the phosphorylation of FoxO1 following H89 treatment. Elevated phosphorylation of FoxO1 in REGγ−/− MEFs was almost eradicated by inhibiting PKA activity (Fig. 2A, lane 6), indicating that enhanced phosphorylation of FoxO1 in REGγ deficient cells was mostly mediated by a sustained and activated PKA signaling. To ensure the specificity of REGγ-PKA regulation of FoxO1 phosphorylation, both REGγ and PKAca were simultaneously silenced by RNA interference in HUVECs. Knocking down PKAca in HUVECs (Fig. 2B, lanes 3 and 6) dramatically attenuated phosphorylation of FoxO1 regardless of REGγ levels, validating that REGγ modulates FoxO1 phosphorylation in a PKA-dependent manner.
Post-translational modification of FoxO1 has been known to regulate its translocation between the nucleus and cytoplasm [15]. To determine whether REGγ-PKA pathway regulates subcellular localization of FoxO1, immunofluorescence studies were performed in immortalized REGγ+/− and REGγ−/− cells in the presence or absence of PKA modulators. As shown in Fig. 2C, FoxO1 was mainly located in the nucleus in REGγ+/− MEFs, whereas FoxO1 was mostly cytoplasmic in REGγ−/− cells (Fig. 2C, top panels), indicating that REGγ is a key factor regulating FoxO1 subcellular localization. Activation of PKA by forskolin induced nuclear exclusion of FoxO1 in REGγ+/− MEFs but had minimal effects on FoxO1 nuclear export in REGγ−/− MEFs (Fig. 2C, middle panels), consistent with the changes in FoxO1 phosphorylation status in Fig. 2A. However, blockage of PKA signaling by H89 prevented FoxO1 export to cytoplasm in REGγ−/− MEFs (Fig. 2C, lower panels), substantiating that the cytoplasmic localization of FoxO1 in the absence of REGγ was mediated by the action of PKA. All of the experimental results for subcellular localization were quantitatively analyzed (Fig. 2D). We further confirmed our observation of FoxO1 distribution by cell fractionation and Western blot analysis (Fig. S2C). The influence of PKA signaling on the dynamic changes in FoxO1 subcellular localization in HUVECs also was validated in the presence of forskolin (Fig. S2D). Taken together, our results demonstrate that REGγ regulates FoxO1 phosphorylation and cellular localization in a PKAca-dependent manner.
3.3. REGγ affects the expression of VCAM-1 and E-Selectin genes through the PKA-FoxO1 pathway
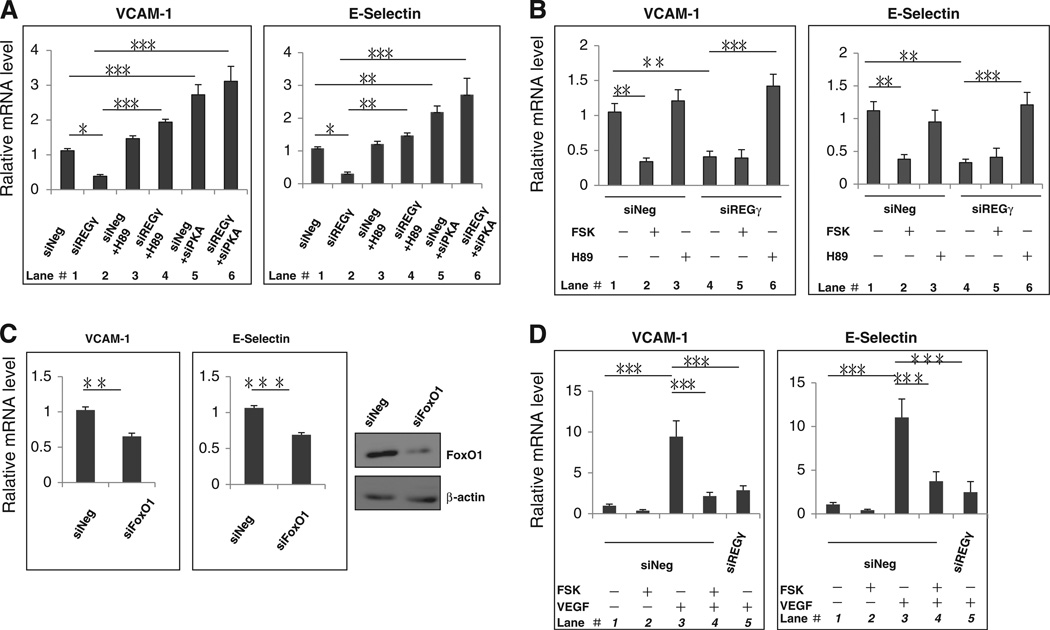
As described above, VCAM-1 and E-Selectin were among the genes differentially regulated in HUVECs with or without REGγ silencing (Fig. S2A). Similar results were observed in HUVECs (Fig. 3A, lanes 1&2) and HMEC-1 cells (Fig. 3B, lanes 1&4). We next sought to understand how REGγ regulates VCAM-1 and E-Selectin mechanistically. Given that REGγ regulates FoxO1 in a PKA dependent manner, we tested whether REGγ mediated regulation of VCAM-1 and E-Selectin could be affected by manipulation of PKAca levels. Interestingly, ablation of PKA activity by H89 in siREGγ-treated HUVECs (Fig. 3A, lanes 4) or HMEC-1 cells (Fig. 3B, lanes 6) enhanced the expression of VCAM-1 and E-Selectin genes, indicating an inhibitory role for PKA pathway in the regulation of these FoxO1 responsive genes [47]. Similarly, simultaneous silencing REGγ and PKAca in HUVECs resulted in derepression of VCAM-1 and E-Selectin genes (Fig. 3A, compare lanes 2 and 6). We also diminished forkhead activity by RNA interference against FoxO1 and demonstrated that VCAM-1 and E-Selectin indeed requires FoxO1 for their transcription in HUVECs (Fig. 3C). To further ensure that FoxO1 dependent transcription of VCAM-1 and E-Selectin can be modulated by REGγ-PKA signaling, we used a PKA activator to promote FoxO1 nuclear export (Fig. 2C) and to “switch off” FoxO1 activity. Upon forskolin treatment, expression of VCAM-1 and E-Selectin genes was reduced in HMEC-1 cells (Fig. 3B, lane 2) and HUVECs (Fig. S3C), whereas no significant effects were noted in cells depleted of REGγ (Fig. 3B, compare lanes 4 and lane 5; Fig. S3A). In addition, there was corresponding regulation of VCAM-1 and E-Selectin protein expression by REGγ (Fig. S3B). In summary, these results demonstrate that REGγ regulates the expression of VCAM-1 and E-Selectin genes through the PKA-FoxO1 pathway.
Fig. 3.
REGγ tightly controls the expression of VCAM-1 and E-Selectin genes. (A) Cells lacking REGγ prohibited optimal expression of VCAM-1 and E-Selectin via activation of PKA in HUVECs. Total RNA extracted from primary HUVECs separately treated with control siNeg (lane 1), siREGγ (lane 2), siNeg + H89 (20 uM, 6 h) (lane 3), siREGγ + H89 (20 uM, 6 h) (lane 4), siNeg + siPKAca (lane 5) or siREGγ + siPKAca (lane 6), was prepared for real-time RT-PCR analysis. Cells were serum-starved overnight before H89 treatment. Statistical analyses were performed using the one-way ANOVA. Data are shown as mean ± SD of three independent experiments (*, p < 0.05;; ***, p < 0.001). (B) Depleted of REGγ prohibited optimal expression of VCAM-1 and E-Selectin via activation of PKA in HMEC-1 cells. HMEC-1 cells separately treated with 20 nM siNeg or siREGγ, following incubated with DMSO (lanes 1&4), 20 uM forskolin (lanes 2&5) or 20 uM H89 (lanes 3&6) for 6 h. Cells were serum-starved overnight before forskolin or H89 treatment. Total RNA extracted from HMEC-1 was prepared for real-time RT-PCR analysis. Statistical analyses were performed using the one-way ANOVA. Data are shown as mean ± SD of three independent experiments (**, p < 0.01; ***, p < 0.001). (C) FoxO1 is required for the expression of VCAM-1 and E-Selectin genes. Total RNA from primary HUVECs treated with 20nM siNeg or siFoxO1 for 48 h was prepared for real-time RT-PCR analysis. Cells were serum-starved overnight before RNA extraction. Data are shown as mean ± SD of three independent experiments (**, p < 0.01). (D) REGγ is responsible for VEGF-induced expression of VCAM-1 and E-Selectin genes. Primary HUVECs treated with 20 nM control siRNA (siNeg) were incubated with either vehicle (lane 1), 20uM forskolin (lane 2) for 6 h, 20 µg/ml VEGF (lane 3) for 2 h, or a combination of 20 uM forskolin (6 h) and 20 µg/ml VEGF treatment (2 h) (lane 4). HUVECs transfected with 20nM siREGγ were treated with 20ug/ml VEGF for 2 h (lane 5). Cells were serum-starved overnight before forskolin and VEGF treatment. Total RNA extracted from HUVECs was prepared for real-time RT-PCR analysis. Statistical analyses were performed using the one-way ANOVA. Data are shown as mean ± SD of three independent experiments (*, p < 0.05; ***, p < 0.001).
3.4. REGγ facilitates VEGF-induced expression of pro-angiogenic genes by repressing PKA signaling
VEGF-mediated up-regulation of VCAM-1 can be inhibited by a PI3K-Akt agonist that facilitates FoxO1 phosphorylation and nuclear exclusion [48]. Given the similar effect of PI3K-Akt and PKA in promoting FoxO1 phosphorylation, we analyzed whether PKA signaling may also interfere with VEGF-mediated expression of downstream responsive genes. As expected, VEGF stimulation induced robust expression of VCAM-1 and E-Selectin genes in HUVECs (Fig. 3D, lanes 3). Pretreatment with forskolin interrupted VEGF-mediated gene transcription (Fig. 3D, lanes 4), demonstrating an inhibitory role for activated PKA in VEGF-induced expression of VCAM-1 and E-Selectin genes. Likewise, VEGF-mediated induction of VCAM-1 and E-Selectin genes expression was also impaired in REGγ deficient HUVECs both on mRNA and protein levels (Fig. 3D, lanes 5 & Fig. S3C), substantiating that REGγ is required for efficient VEGF-stimulated expression of FoxO1 responsible genes. To test if there is a feedback regulation by VEGF signaling, we examined the effect of VEGF stimulation on REGγ expression. As shown in Fig. S3D, REGγ expression was not altered upon VEGF treatment, suggesting that a steady expression of REGγ may be required to balance the VEGF signaling and the suppressive PKAca activity.
Numerous studies have shown that VEGF activates PI3K/Akt, which can lead to phosphorylation and nuclear export of FoxO proteins [48]. To investigate the seemingly contradictory actions between the VEGFAKT and the PKA signalings, we compared the effects of VEGF-stimulation vs. PKA-activation on FoxO1 phosphorylation in HUVECs (Fig. S3E). VEGF-treatment induced FoxO1 phosphorylation (about 1.3-fold increase) in 15 min, and then quickly decreased to basal level in 45 min; however, forskolin induced a late onset but higher phosphorylation in FoxO1 at 30 min, which was prolonged to 45 min. Based on these observations, we believe that the effect of VEGF on FoxO1 nuclear localization is minor and transient; however, activation of PKA promotes more intense and prolonged FoxO1 phosphorylation. Given that VEGF-dependent regulation of VCAM-1 and E-Selectin is likely mediated by TNFalpha [49], only stronger and sustained phosphorylation in FoxO1 may be sufficient to antagonize VEGF action on VCAM-1 and E-Selectin expression.
3.5. REGγ is involved in ECs migration
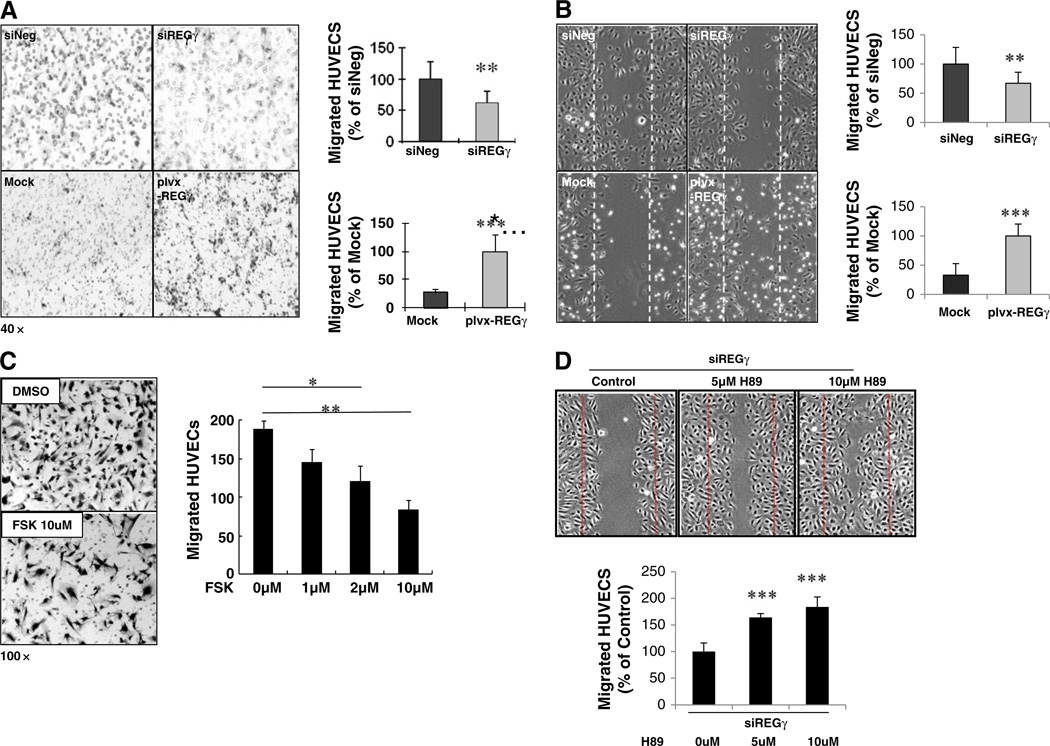
Both VCAM-1 and E-Selectin genes are known to play a role in angiogenic process [50, 51]. Based on the observation that REGγ regulates the expression of VCAM-1 and E-Selectin genes via PKA, whose activity is tightly associated with ECs movement [23], we carried out transwell and wound-healing assays to assess REGγ's capability in regulating ECs migration. Silencing REGγ in HUVECs with transient expression of specific siRNAs resulted in a significant reduction in cell migration in both the transwell and wound-healing assays (Fig. 4A and B, upper panels). In contrast, overexpressing REGγ in HUVECs by lenti-viral (plvx-REGγ) infection remarkably enhanced cell migration (Fig. 4A and B, lower panels). To better understand the biological functions of the REGγ-PKA pathway in ECs migration, a HUVECs transwell assay was performed in the absence or presence of forskolin. Indeed, the number of migrated cells was decreased significantly by forskolin in a dose-dependent manner, substantiating that PKA signaling inhibits migration of ECs (Fig. 4C). Furthermore, inhibition of PKA by H89 restored HUVECs migration when REGγ was depleted (Fig. 4D), demonstrating that PKA is the major effector in REGγ-regulated HUVECs migration. To test whether the ECs proliferation was affected in the above experiments, we analyzed proliferation of HUVECs at the same condition in the cell migration assays. Neither REGγ knockdown nor manipulation of PKA activity affected ECs proliferations by MTT assays (Fig. S4B). Taken together, the above experimental results demonstrate that REGγ is a novel modulatory factor in ECs migration.
Fig. 4.
REGγ regulates migration of HUVECs. (A–B) REGγ is crucial to HUVECs migration. Transwell assays (A) and wound healing experiments (B) were performed in HUVECs following REGγ overexpression by virus infection (plvx-REGγ lentivirus) or transient knockdown of REGγ by siRNA. The migrated HUVECs were pictured using an Olympus inverted microscope and counted. Data are shown as mean ± SD of three independent experiments (**, p < 0.01; ***, p < 0.001). The upper y-axis represents migrated HUVECs (% of siNeg), and the lower y-axis refers to migrated HUVECs (% of Mock) in the quantitation bar graphs. (C) Activation of PKA by forskolin inhibited the migration of HUVECs. HUVECs were seeded in the upper chamber containing different doses of forskolin in the presence of 20 ng/ml VEGF. Migration of HUVECs to the other surface in the lower chamber of the transwell was recorded in 24 h (left panel) and statistically analyzed (right panel). Statistical analyses were performed using the one-way ANOVA. Data are shown as mean ± SD of three independent experiments (*, p < 0.05; **, p < 0.01). (D) Inhibition of PKA by H89 restored the HUVECs migration during REGγ depletion. Wound healing initiated 48 h after REGγ siRNA transfection in HUVECs, following incubation with different doses of H89 by 5 h. The migrated HUVECs were pictured using an Olympus inverted microscope and quantified. Statistical analyses were performed using the one-way ANOVA. Data are shown as mean ± SD of three independent experiments (**, p < 0.01).
3.6. REGγ ablation attenuates VEGF-induced angiogenesis in vivo/ex vivo
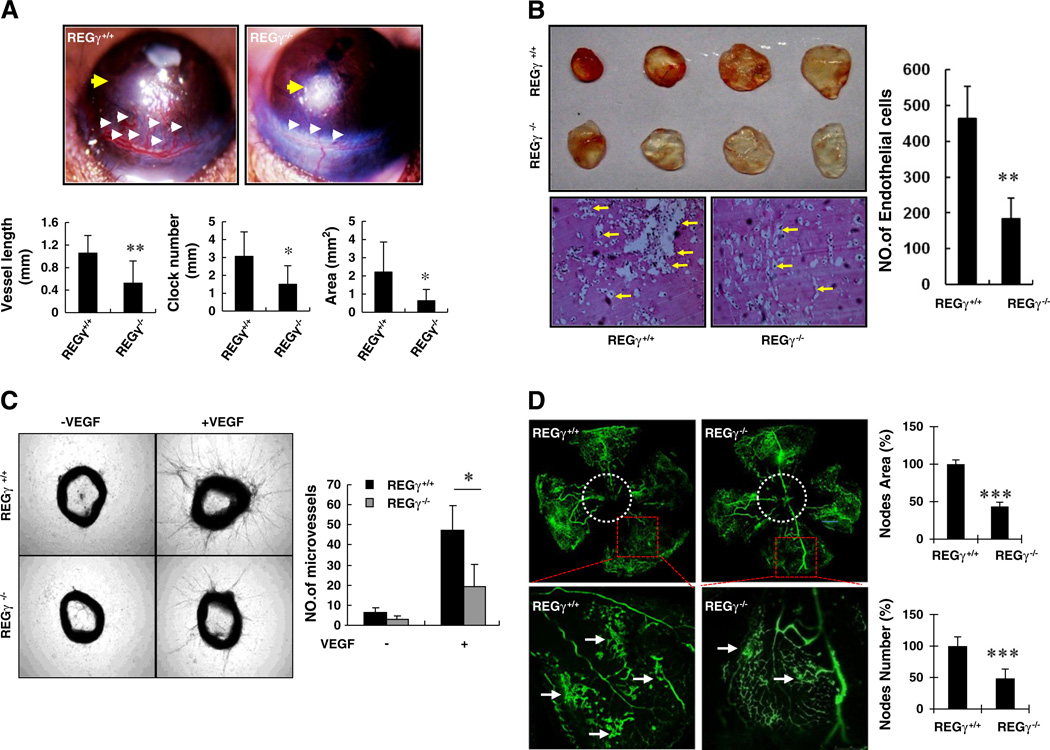
The discovery of REGγ's ability in the regulation of VEGF-induced VCAM-1 and E-Selectin gene expression in vascular ECs prompted us to test if REGγ may be involved in VEGF-induced angiogenesis in our mouse models. The mouse corneal micropocket assay has been widely accepted as a gold standard for in vivo study of angiogenic factors. Hence, we inserted a VEGF-containing slow-release pellet into a preformed mouse cornea micropocket, and watched for the outgrowth of new limbus vasculature. Seven days later, we visualized robust VEGF-induced corneal angiogenesis in the REGγ+/+ eyes whereas this phenomenon was restrained in the REGγ−/− groups (Fig. 5A). Significant and consistent differences between REGγ+/+ and REGγ−/− corneal angiogenesis appeared by all of the three criteria employed in this model, including vessel length, clock number and coverage area (Fig. 5A, lower panel), thereby reflecting an important role for REGγ in VEGF-induced angiogenesis.
Fig. 5.
REGγ plays a crucial role in VEGF-induced angiogenesis in vivo. (A) REGγ depletion compromised VEGF-induced neovascularization in corneal pocket model. VEGF-containing pallets were placed into cornea in REGγ+/+ and REGγ−/− mice (indicated by yellow arrows) for 7 days and then the corneal angiogenesis were imaged (Upper panel). White arrow indicates the VEGF-induced microvessels. The bar graphs show the results of quantitative analysis including vessel length, clock number and vessel area (Lower panel). Data are shown as mean ± standard deviation (SD) of four pairs of individual animals (*, p < 0.05). (B) REGγ−/− mice revealed poor angiogenesis in VEGF-matrigel plug assays. A total of 500 µl matrigel containing 50 ng VEGF was injected subcutaneously into the ventral abdomen of REGγ+/+ and REGγ−/− mice. Pictures of matrigel plugs were taken 8 days after the plugs were removed (left panel). The embedded matrigels were cut into 5 uM slides and were analyzed by H&E staining to visualize and quantify the number of invaded ECs (lower panel and right panel). Data are shown as mean ± SD of four independent experiments (**, p < 0.01). (C) REGγ−/− mice had impaired aortic angiogenesis. Three aortic rings for each treatment in total from 3 pairs of REGγ+/+ and REGγ−/− mice were cut into rings. The rings was starved overnight and then fed with opti-MEM with/without 30 ng/ml VEGF. Pictures were taken at day 7 (left panel). The number of microvessels emanating from aortic rings were counted (right panel). Statistical analyses were performed using the one-way ANOVA. Data are shown as mean ± SD of the results using three pairs of mice (*, p < 0.05). (D) REGγ−/− mice displayed attenuated oxygen-induced retinopathy. Postnatal day 7 REGγ+/+ and REGγ−/− mice were exposed to hyperoxia for 5 days followed by another 5 days of room air exposure. Neovascular proliferative retinopathy was imaged. Red squares represent invisible areas in the original scale and are amplified in lower panels. Arrows points to twisted vascular plexuses. Quantitated results of three pairs of animals for nodes area/nodes numbers as percentage of REGγ+/+ are shown as mean ± SD.
Next, we injected VEGF-containing matrigels into REGγ+/+ and REGγ−/− mice and analyzed the results 7 days later. As shown in Fig. 5B, there were more blood vessels in the gel-plugs peeled from REGγ+/+ mice than found in REGγ−/− mice. Quantification of migrated ECs within the plugs embedded in paraffin [45] also disclosed inhibition of VEGF-induced angiogenesis in the absence of REGγ (Fig. 5B, right panel). To further evaluate the blockade of REGγ deficiency on physiological angiogenesis, we performed a mouse aortic ring assay, a classical and reliable ex vivo angiogenesis model [42]. Freshly isolated REGγ+/+ and REGγ−/− aortas were explanted into VEGF-soaked or control collagen. The outgrowth of micro-vessels from REGγ+/+ aortas were more prominent than that in REGγ−/− aortas in the presence of VEGF (Fig. 5C). To verify that the outgrowth emanating from aortic rings contains differential endothelial-cell numbers in WT and KO samples, we immunostained aorta with endothelial-cell-specific marker vWF (Fig. S5A). The result showed that the ECs stimulated by VEGF are more abundant in the aorta from REGγ+/+ mice. Furthermore, to test the role for REGγ in pathological angiogenesis, we carried out experiments using the model of oxygen-induced retinopathy, which has been well-documented for the study of pathological angiogenesis in mouse Retinopathy of prematurity (ROP) [43]. As expected, pathological neovascularization (Fig. 5D, mid-upper panel) was induced in REGγ+/+ retina when the mice were shifted from high-oxygen cages back to “relative hypoxia” (room air) cages. In contrast, REGγ ablation reduced the pathological vascular plexuses characteristic of excessive angiogenesis (Fig. 5D, mid-lower panel) occurring during the relative hypoxic stress. These results above identify a novel function for REGγ in VEGF-mediated angiogenesis.
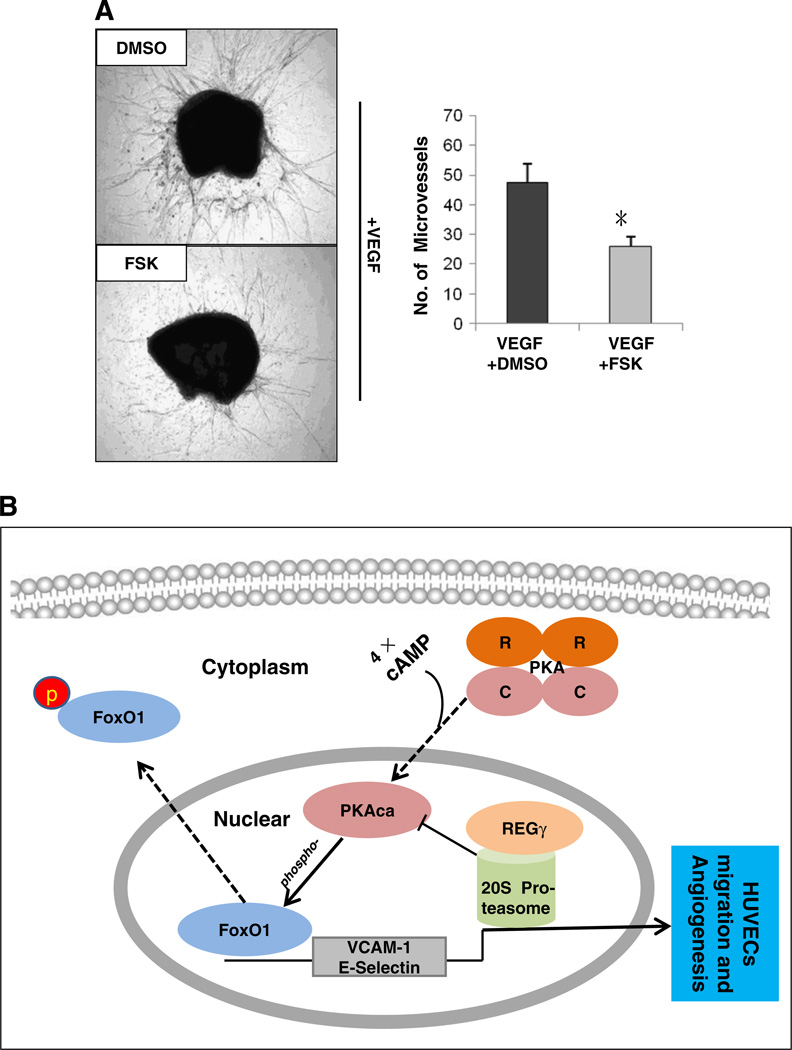
Finally, we performed aortic ring assays to characterize if PKA signaling antagonizes VEGF-induced angiogenesis. In the presence of VEGF, we observed a vigorous growth of endothelial-cell-specific microvessels (Fig. 6A and Fig. S5B, upper panel,). However, co-culture of the aortic ring with VEGF in the presence of forskolin attenuated VEGF-induced microvessel growth (Fig. 6A and Fig. S5B, lower panel). These results demonstrate that PKA signaling negatively regulates VEGF-induced angiogenesis.
Fig. 6.
PKA signaling antagonizes VEGF-induced angiogenesis. (A) Activation of PKA by forskolin inhibited VEGF-induced aortic angiogenesis. Three aortic rings for each treatment in total from 3 pairs of REGγ+/+ mice were cut into rings and fed by opti-MEM containing 30 ng/ml VEGF followed by treated with DMSO or 20 uM forskolin. Three pairs of aortic rings (forskolin treated vs. controls) were quantified by Image-Pro Plus 6.0. Data are shown as mean ± SD of three independent experiments (*, p < 0.05). (B) A working model for the role of REGγ in regulating VCAM-1 and E-Selectin genes expression. PKA catalytic subunit α (PKAca) released from inactive PKA complex is translocated to the nucleus in the presence of cAMP. Activated PKA directly phosphorylates FoxO1 and induces its nuclear exclusion, which negatively regulates the expression of VCAM-1 and E-Selectin. REGγ down-regulates PKAca levels by decreasing its protein stability, which in turn preserves FoxO1 transcriptional activity and facilitates down-stream responsive genes expression.
4. Discussion
The proteasome activator REGγ has emerged as a master regulator in certain biological processes [29, 36]. Yet the vast majority of its biological functions remain unknown. In this study, we present evidence that REGγ is required for VCAM-1 and E-Selectin genes expression via the PKA-FoxO1 pathway and crucial for ECs migration, as well as VEGF-induced angiogenesis. This study has elaborated a regulatory signaling cascade emanating from REGγ-proteasome mediated regulation of PKA, which turns on/off FoxO1 activity by phosphorylation-dependent subcellular localization, thereby promoting or attenuating the transcription of downstream pro-angiogenic genes. Our results reveal a previously unrecognized regulatory mechanism upstream of PKA and uncover new molecular links in a REGγ-PKA-FoxO1 angiogenic pathway (Fig. 6B).
VCAM-1 and E-Selectin genes are well known to be associated with pathological angiogenic responses involving inflammation [52]. Expression of VCAM-1 is effectively controlled through PKA-FoxO1 pathway [16]. Due to limited prior studies, the effects of PKA-FoxO1 pathway on angiogenesis are largely unknown. FoxO1 plays roles in the regulation of vascular homeostasis in response to insulin and other growth factors [53]; FoxO1 knockout mice die around embryonic day 11 because of defects in their bronchial arches and remarkably impaired vascular development of embryos and yolk sacs due to an abnormal response of ECs to VEGF stimulation [54]. Consistent with the biochemical evidence for PKA-dependent phosphorylation of FoxO1 [16], we recapitulated the regulation of sub-cellular localization of FoxO1 in response to the REGγ-PKA activities. In addition to VCAM-1, a well-known target of FoxO1, we found that REGγ also is required for the expression of two other endothelial adhesion molecules, E-Selectin and ICAM-1 (Fig. S2A). Our results are endorsed by the fact that endothelial adhesion molecules facilitate the entry of leukocytes into inflamed tissues and thereby promote angiogenesis [55]. To our surprise, regulation of ICAM-1 by REGγ is distinct from VCAM-1 and E-Selectin since this regulation does not require PKA signaling (Fig. S4A), indicating involvement of additional mechanisms for REGγ-mediated angiogenesis.
Numerous in vivo studies have shown that expression of VEGF is up-regulated during angiogenesis under physiological and pathological processes [56, 57]. Different mechanisms appear to participate in the regulation of VEGF mRNA expression, including PKA-mediated signals [58]. Thus, activated PKA may facilitate angiogenesis by promoting endogenous expression of VEGF, or through Epac-dependent ERK activation and PI3K/Akt/eNOS/NO signaling [17, 18]. Furthermore, activated PKA may promote angiogenesis during early developmental. Two studies have implicated PKA activity in postnatal angiogenesis and ischemic angiogenesis [19, 20]. However, there are also several mechanisms involved in PKA-mediated negative regulation of angiogenesis. Activation of PKA signaling can not only attenuate VEGF signaling in physiological conditions by blocking Raf activation [21] or through their actions on pp60Src [23], but also prohibit VEGF-induced increase in vascular permeability during cancer progression [22]. Paradoxically, distinct from previous demonstration that PKA promotes VEGF secretion, PKA may inhibit VEGF production by antagonizing Rap1 to regulate tumorstromal induction of angiogenesis in prostate cancer [24]. Besides the inhibitory role of PKA on VEGF signaling, direct activation of PKA signaling can induce EC apoptosis, resulting in the inhibition of angiogenesis in vivo [25]. It has been reported more recently that PKA phosphorylates FoxO1 directly in vascular ECs [16], whose effect is quite similar to hepatocyte growth factor to promote FoxO1 nuclear exclusion [48], suggesting that PKA may also prohibit the effect of VEGF on FoxO1-dependent gene expression. In our present study, since the post-translational modification of FoxO1 and the subsequent activation/inactivation of cell adhesion molecule is the major mechanism affected by REGγ-mediated regulation of PKA, we propose that the major PKA function may be to inhibit angiogenesis under a physiological condition. Nevertheless, the role of PKA on angiogenesis can be altered, depending upon different cell context and various signal cascades in physiological or pathological environments.
Protein degradation has been documented previously to play an important role in angiogenesis [28]. However, prior studies represent an incomplete understanding of the impact that protein degradation pathways play in the regulation of angiogenesis. The pro-angiogenic role of REGγ-proteasome further substantiates the importance of the proteasome system for fine-tuning the functions of core angiogenic proteins. The discovery that REGγ negatively regulates the PKA pathway in a cell specific manner highlights a novel function of this proteasome pathway and demonstrates the complexity existing in the regulation of angiogenesis. Although we are aware of no publication that describes the ubiquitin proteasome system as a key mediator of degradation of PKAca, we can't exclude the possibility that REGγ-proteasome is not the only pathway required for decay of PKAca. Since we observed that elevated PKAca levels in REGγ deficient cells are coupled with a dramatic increase of PKA activity, perhaps the REGγ-proteasome has been designed by nature to guard against nuclear accumulation of activated PKAca molecules that are either “leaked” or prematurely released from their regulatory subunits upon inadvertent cAMP surges (Fig. 6B). Despite the identification of PKAca as a primary target for REGγ's regulation of angiogenesis, our study does not exclude the possibility of additional REGγ substrates involved in this function. In fact, we recently found casein kinase 1 (CK1) to be a novel REGγ substrate for its effect on cellular aging [31]. CK1 also has been reported to phosphorylate FoxO1 within several intramolecular domains to promote nuclear exclusion of FoxO1 [15]. In addition, a recent study suggests that the ubiquitin E3 ligase SCFβ-TRCP promotes ubiquitination and destruction of VEGF2 in a CK1-dependent manner and subsequently suppresses angiogenesis [59]. The totality of these data indicates that diverse mechanisms underlie the regulation of angiogenesis by REGγ.
In conclusion, the present study that provides evidence that REGγ is a novel positive regulator for VCAM-1 and E-Selectin gene expression, plays an important role in ECs migration and VEGF-induced angiogenesis. REGγ antagonizes the PKA pathway and facilitates VEGF-induced expression of pro-angiogenic genes by modulating FoxO1 phosphorylation and sub-cellular localization (Fig. 6B). Our findings provide the missing upstream links for PKA regulation of its downstream angiogenic effectors. This novel angiogenic function of REGγ provides another potential molecular basis for future therapeutic applications.
Supplementary Material
Acknowledgments
This work was supported by the National Basic Research Program (2011CB504200) and in part by the National Natural Science Foundation of China (81261120555, 30870503, 81071657, 31100946); the Science and Technology Commission of Shanghai Municipality (11DZ2260300, 11ZR1410000). This manuscript was also funded by the National Institutes of Health (1R01CA131914 and HD08818), the Norman Hackerman Advanced Research Program (1082318401; PN004949-0012-2009), the NCI Cancer Center Support Grant (P30CA125123) to Protein & Antibody Array Core (SH & MC), and the Pilot/Feasibility Program of the Diabetes & Endocrinology Research Center (P30-DK079638) at Baylor College of Medicine.
Footnotes
Conflict of interest
None declared.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yjmcc.2014.02.007.
Contributor Information
Zhengfang Yi, Email: yizhengfang@gmail.com.
Jianru Xiao, Email: jianruxiao@163.com.
Xiaotao Li, Email: xiaotaol@bcm.edu.
References
- 1.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- 3.Costa C, Soares R, Schmitt F. Angiogenesis: now and then. APMIS. 2004;112:402–412. doi: 10.1111/j.1600-0463.2004.apm11207-0802.x. [DOI] [PubMed] [Google Scholar]
- 4.Roodhart JM, Langenberg MH, Witteveen E, Voest EE. The molecular basis of class side effects due to treatment with inhibitors of the VEGF/VEGFR pathway. Curr Clin Pharmacol. 2008;3:132–143. doi: 10.2174/157488408784293705. [DOI] [PubMed] [Google Scholar]
- 5.Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature. 1995;376:517–519. doi: 10.1038/376517a0. [DOI] [PubMed] [Google Scholar]
- 6.Kim W, Moon SO, Lee S, Sung MJ, Kim SH, Park SK. Adrenomedullin reduces VEGF-induced endothelial adhesion molecules and adhesiveness through a phosphatidylinositol 3′-kinase pathway. Arterioscler Thromb Vasc Biol. 2003;23:1377–1383. doi: 10.1161/01.ATV.0000081740.65173.D1. [DOI] [PubMed] [Google Scholar]
- 7.Schweighofer B, Testori J, Sturtzel C, Sattler S, Mayer H, Wagner O, et al. The VEGF-induced transcriptional response comprises gene clusters at the crossroad of angiogenesis and inflammation. Thromb Haemost. 2009;102:544–554. doi: 10.1160/TH08-12-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch AE, Turkiewicz W, Harlow LA, Pope RM. Soluble E-selectin in arthritis. Clin Immunol Immunopathol. 1993;69:29–35. doi: 10.1006/clin.1993.1146. [DOI] [PubMed] [Google Scholar]
- 9.Hebbar M, Peyrat JP. Significance of soluble endothelial molecule E-selectin in patients with breast cancer. Int J Biol Markers. 2000;15:15–21. doi: 10.1177/172460080001500103. [DOI] [PubMed] [Google Scholar]
- 10.Byrne GJ, Ghellal A, Iddon J, Blann AD, Venizelos V, Kumar S, et al. Serum soluble vascular cell adhesion molecule-1: role as a surrogate marker of angiogenesis. J Natl Cancer Inst. 2000;92:1329–1336. doi: 10.1093/jnci/92.16.1329. [DOI] [PubMed] [Google Scholar]
- 11.Ferdous A, Morris J, Abedin MJ, Collins S, Richardson JA, Hill JA. Forkhead factor FoxO1 is essential for placental morphogenesis in the developing embryo. Proc Natl Acad Sci U S A. 2011;108:16307–16312. doi: 10.1073/pnas.1107341108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 13.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 14.Kops GJ, Burgering BM. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J Mol Med (Berl) 1999;77:656–665. doi: 10.1007/s001099900050. [DOI] [PubMed] [Google Scholar]
- 15.Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JW, Chen H, Pullikotil P, Quon MJ. Protein kinase A-alpha directly phosphorylates FoxO1 in vascular endothelial cells to regulate expression of vascular cellular adhesion molecule-1 mRNA. J Biol Chem. 2011;286:6423–6432. doi: 10.1074/jbc.M110.180661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Shi QD, Song TB, Feng GF, Zang WJ, Zong CH, et al. Vasoactive intestinal peptide increases VEGF expression to promote proliferation of brain vascular endothelial cells via the cAMP/PKA pathway after ischemic insult in vitro. Peptides. 2013;42C:105–111. doi: 10.1016/j.peptides.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Namkoong S, Kim CK, Cho YL, Kim JH, Lee H, Ha KS, et al. Forskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell Signal. 2009;21:906–915. doi: 10.1016/j.cellsig.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Xiong Y, Huo Y, Han J, Yang X, Zhang R, et al. Grb-2-associated binder 1 (Gab1) regulates postnatal ischemic and VEGF-induced angiogenesis through the protein kinase A-endothelial NOS pathway. Proc Natl Acad Sci U S A. 2011;108:2957–2962. doi: 10.1073/pnas.1009395108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkatesh PK, Pattillo CB, Branch B, Hood J, Thoma S, Illum S, et al. Dipyridamole enhances ischaemia-induced arteriogenesis through an endocrine nitrite/nitric oxide-dependent pathway. Cardiovasc Res. 2010;85:661–670. doi: 10.1093/cvr/cvq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Angelo G, Lee H, Weiner RI. cAMP-dependent protein kinase inhibits the mitogenic action of vascular endothelial growth factor and fibroblast growth factor in capillary endothelial cells by blocking Raf activation. J Cell Biochem. 1997;67:353–366. [PubMed] [Google Scholar]
- 22.Kim SH, Cho YR, Kim HJ, Oh JS, Ahn EK, Ko HJ, et al. Antagonism of VEGF-A-induced increase in vascular permeability by an integrin alpha3beta1-Shp-1-cAMP/PKA pathway. Blood. 2012;120:4892–4902. doi: 10.1182/blood-2012-05-428243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin H, Garmy-Susini B, Avraamides CJ, Stoletov K, Klemke RL, Varner JA. A PKA-Csk-pp60Src signaling pathway regulates the switch between endothelial cell invasion and cell–cell adhesion during vascular sprouting. Blood. 2010;116:5773–5783. doi: 10.1182/blood-2010-07-296210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menon J, Doebele RC, Gomes S, Bevilacqua E, Reindl KM, Rosner MR. A novel interplay between Rap1 and PKA regulates induction of angiogenesis in prostate cancer. PLoS One. 2012;7:e49893. doi: 10.1371/journal.pone.0049893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, Bakre M, Yin H, Varner JA. Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J Clin Invest. 2002;110:933–941. doi: 10.1172/JCI14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clegg CH, Correll LA, Cadd GG, McKnight GS. Inhibition of intracellular cAMP-dependent protein kinase using mutant genes of the regulatory type I subunit. J Biol Chem. 1987;262:13111–13119. [PubMed] [Google Scholar]
- 27.Laudanna C, Campbell JJ, Butcher EC. Elevation of intracellular cAMP inhibits RhoA activation and integrin-dependent leukocyte adhesion induced by chemoattractants. J Biol Chem. 1997;272:24141–24144. doi: 10.1074/jbc.272.39.24141. [DOI] [PubMed] [Google Scholar]
- 28.Rahimi N. The ubiquitin-proteasome system meets angiogenesis. Mol Cancer Ther. 2012;11:538–548. doi: 10.1158/1535-7163.MCT-11-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Amazit L, Long W, Lonard DM, Monaco JJ, O'Malley BW. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol Cell. 2007;26:831–842. doi: 10.1016/j.molcel.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Lonard DM, Jung SY, Malovannaya A, Feng Q, Qin J, et al. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell. 2006;124:381–392. doi: 10.1016/j.cell.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Zhao D, Wei H, Yao L, Dang Y, Amjad A, et al. REGgamma deficiency promotes premature aging via the casein kinase 1 pathway. Proc Natl Acad Sci U S A. 2013;110:11005–11010. doi: 10.1073/pnas.1308497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong S, Jia C, Zhang S, Fan G, Li Y, Shan P, et al. The REGgamma proteasome regulates hepatic lipid metabolism through inhibition of autophagy. Cell Metab. 2013;18:380–391. doi: 10.1016/j.cmet.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barton LF, Runnels HA, Schell TD, Cho Y, Gibbons R, Tevethia SS, et al. Immune defects in 28-kDa proteasome activator gamma-deficient mice. J Immunol. 2004;172:3948–3954. doi: 10.4049/jimmunol.172.6.3948. [DOI] [PubMed] [Google Scholar]
- 34.Murata S, Kawahara H, Tohma S, Yamamoto K, Kasahara M, Nabeshima Y, et al. Growth retardation in mice lacking the proteasome activator PA28gamma. J Biol Chem. 1999;274:38211–38215. doi: 10.1074/jbc.274.53.38211. [DOI] [PubMed] [Google Scholar]
- 35.Roessler M, Rollinger W, Mantovani-Endl L, Hagmann ML, Palme S, Berndt P, et al. Identification of PSME3 as a novel serum tumor marker for colorectal cancer by combining two-dimensional polyacrylamide gel electrophoresis with a strictly mass spectrometry-based approach for data analysis. Mol Cell Proteomics. 2006;5:2092–2101. doi: 10.1074/mcp.M600118-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Yu G, Zhao Y, Zhao D, Wang Y, Wang L, et al. REGgamma modulates p53 activity by regulating its cellular localization. J Cell Sci. 2010;123:4076–4084. doi: 10.1242/jcs.067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 38.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 39.Matsuo Y, Sawai H, Ochi N, Yasuda A, Sakamoto M, Takahashi H, et al. Proteasome inhibitor MG132 inhibits angiogenesis in pancreatic cancer by blocking NF-kappaB activity. Dig Dis Sci. 2010;55:1167–1176. doi: 10.1007/s10620-009-0814-4. [DOI] [PubMed] [Google Scholar]
- 40.Nawrocki ST, Bruns CJ, Harbison MT, Bold RJ, Gotsch BS, Abbruzzese JL, et al. Effects of the proteasome inhibitor PS-341 on apoptosis and angiogenesis in orthotopic human pancreatic tumor xenografts. Mol Cancer Ther. 2002;1:1243–1253. [PubMed] [Google Scholar]
- 41.Chen X, Barton LF, Chi Y, Clurman BE, Roberts JM. Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Mol Cell. 2007;26:843–852. doi: 10.1016/j.molcel.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D'Amico G, et al. Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc. 2012;7:89–104. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- 43.Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai L, Liu J, Zhai D, Lin Q, He L, Dong Y, et al. Plumbagin inhibits tumour angiogenesis and tumour growth through the Ras signalling pathway following activation of the VEGF receptor-2. Br J Pharmacol. 2012;165:1084–1096. doi: 10.1111/j.1476-5381.2011.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruzinova MB, Schoer RA, Gerald W, Egan JE, Pandolfi PP, Rafii S, et al. Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell. 2003;4:277–289. doi: 10.1016/s1535-6108(03)00240-x. [DOI] [PubMed] [Google Scholar]
- 46.Ghersa P, Hooft van Huijsduijnen R, Whelan J, Cambet Y, Pescini R, De Lamarter JF. Inhibition of E-selectin gene transcription through a cAMP-dependent protein kinase pathway. J Biol Chem. 1994;269:29129–29137. [PubMed] [Google Scholar]
- 47.Abid MR, Shih SC, Otu HH, Spokes KC, Okada Y, Curiel DT, et al. A novel class of vascular endothelial growth factor-responsive genes that require forkhead activity for expression. J Biol Chem. 2006;281:35544–35553. doi: 10.1074/jbc.M608620200. [DOI] [PubMed] [Google Scholar]
- 48.Abid MR, Nadeau RJ, Spokes KC, Minami T, Li D, Shih SC, et al. Hepatocyte growth factor inhibits VEGF-forkhead-dependent gene expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:2042–2048. doi: 10.1161/ATVBAHA.108.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stannard AK, Khurana R, Evans IM, Sofra V, Holmes DI, Zachary I. Vascular endothelial growth factor synergistically enhances induction of E-selectin by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 2007;27:494–502. doi: 10.1161/01.ATV.0000255309.38699.6c. [DOI] [PubMed] [Google Scholar]
- 50.Dong A, Shen J, Zeng M, Campochiaro PA. Vascular cell-adhesion molecule-1 plays a central role in the proangiogenic effects of oxidative stress. Proc Natl Acad Sci U S A. 2011;108:14614–14619. doi: 10.1073/pnas.1012859108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh IY, Yoon CH, Hur J, Kim JH, Kim TY, Lee CS, et al. Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood. 2007;110:3891–3899. doi: 10.1182/blood-2006-10-048991. [DOI] [PubMed] [Google Scholar]
- 52.Fukushi J, Ono M, Morikawa W, Iwamoto Y, Kuwano M. The activity of soluble VCAM-1 in angiogenesis stimulated by IL-4 and IL-13. J Immunol. 2000;165:2818–2823. doi: 10.4049/jimmunol.165.5.2818. [DOI] [PubMed] [Google Scholar]
- 53.Chen H, Lin AS, Li Y, Reiter CE, Ver MR, Quon MJ. Dehydroepiandrosterone stimulates phosphorylation of FoxO1 in vascular endothelial cells via phosphatidylinositol 3- kinase- and protein kinase A-dependent signaling pathways to regulate ET-1 synthesis and secretion. J Biol Chem. 2008;283:29228–29238. doi: 10.1074/jbc.M802906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, et al. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 55.van Wetering S, van den Berk N, van Buul JD, Mul FP, Lommerse I, Mous R, et al. VCAM-1-mediated Rac signaling controls endothelial cell–cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol. 2003;285:C343–C352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 56.Shweiki D, Itin A, Neufeld G, Gitay-Goren H, Keshet E. Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J Clin Invest. 1993;91:2235–2243. doi: 10.1172/JCI116450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 58.Hattori Y, Yamamoto S, Matsuda N. Sympathetic control of VEGF angiogenic signaling: dual regulations by alpha 2-adrenoceptor activation? Circ Res. 2007;101:642–644. doi: 10.1161/CIRCRESAHA.107.161855. [DOI] [PubMed] [Google Scholar]
- 59.Shaik S, Nucera C, Inuzuka H, Gao D, Garnaas M, Frechette G, et al. SCF(beta-TRCP) suppresses angiogenesis and thyroid cancer cell migration by promoting ubiquitination and destruction of VEGF receptor 2. J Exp Med. 2012;209:1289–1307. doi: 10.1084/jem.20112446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.