Abstract
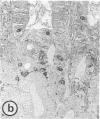
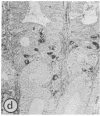
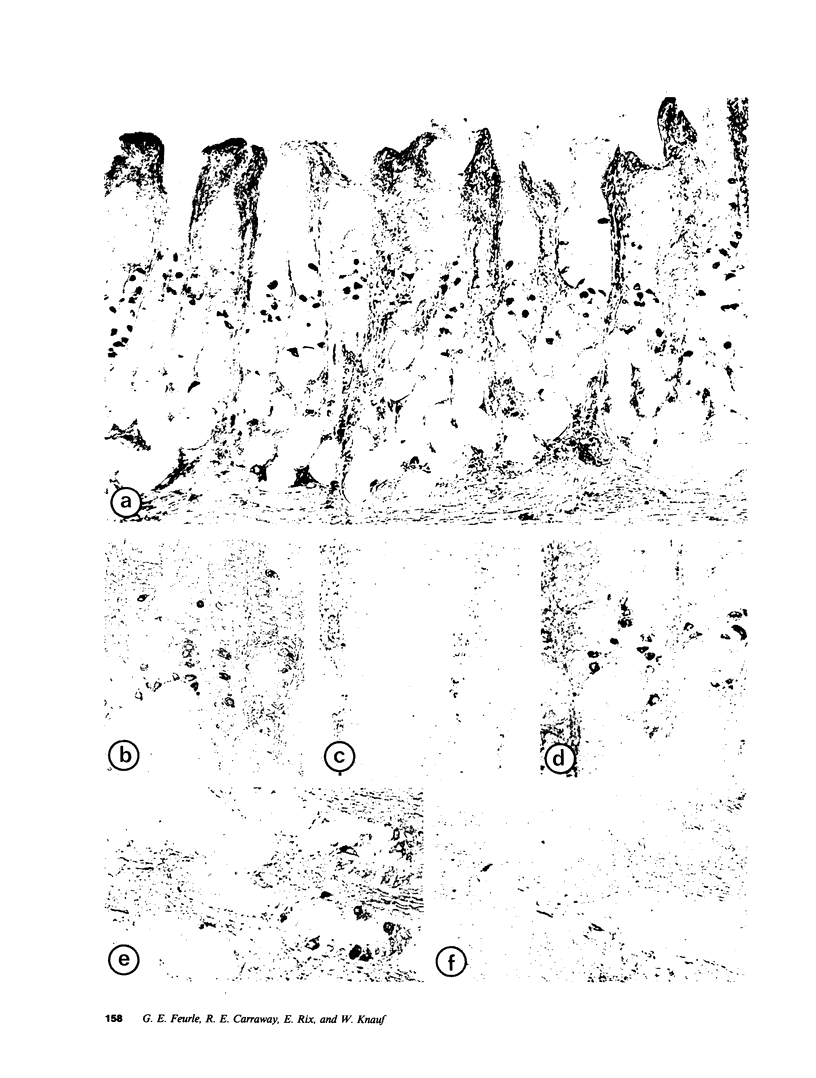
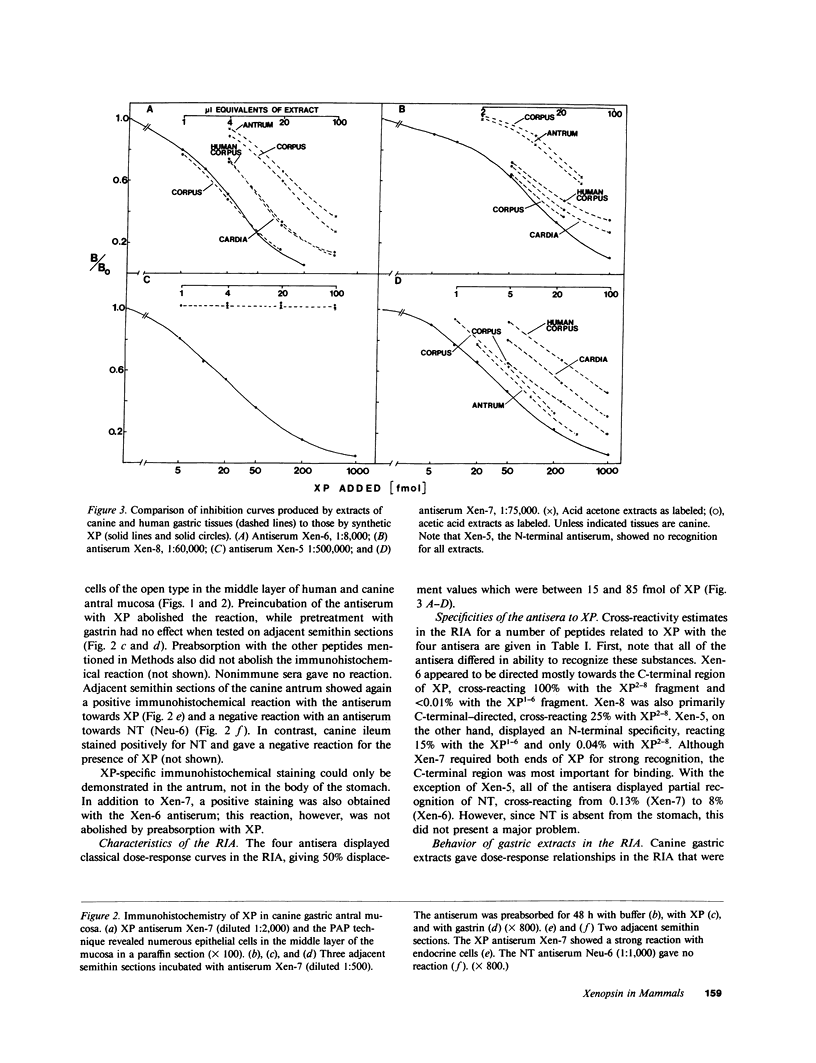
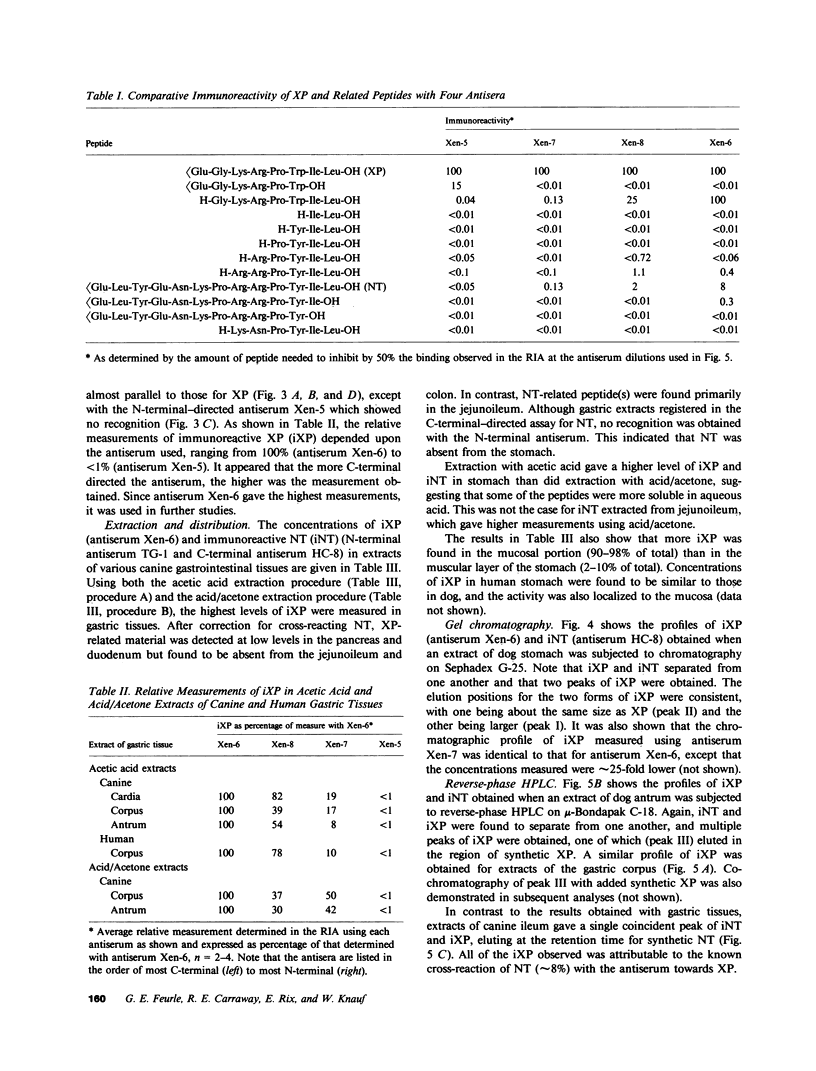
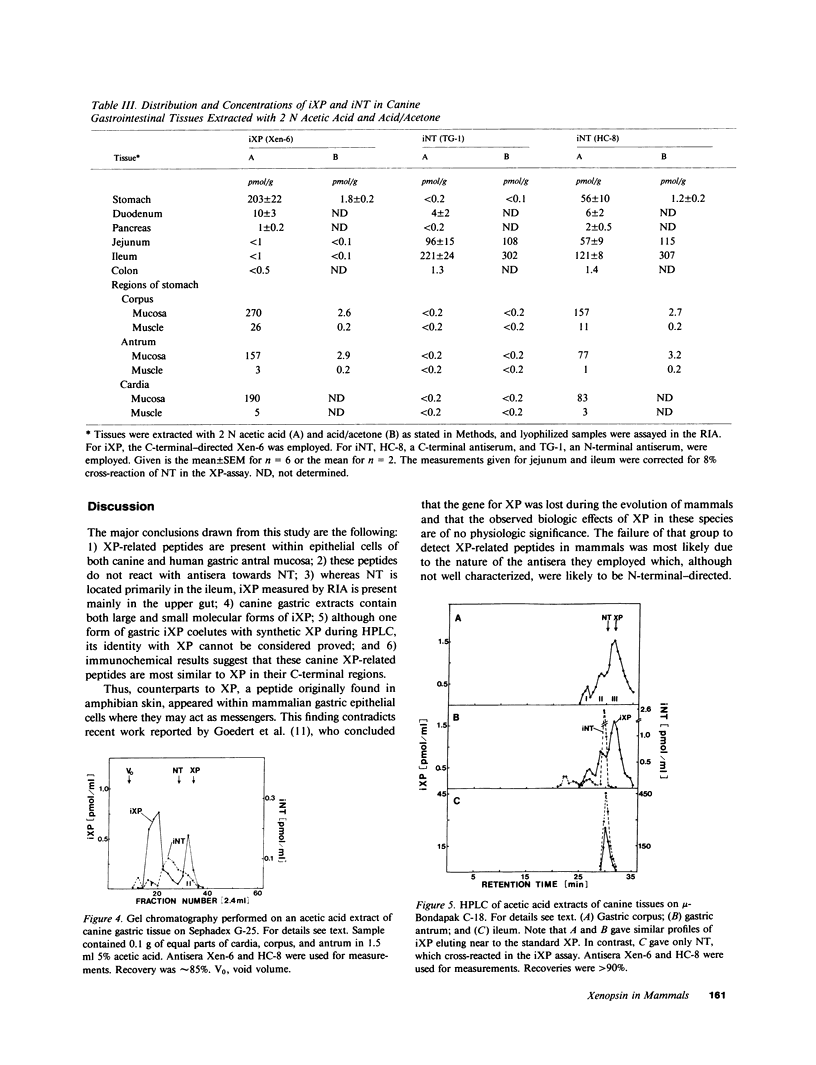
Using immunohistochemistry and radioimmunoassay, substance(s) related to the amphibian octapeptide xenopsin (XP) were demonstrated in the gastric mucosa of humans and dogs. Immunohistochemistry localized XP-immunoreactive epithelial cells in the gastric antral mucosa. The reaction was abolished by preabsorption of the antiserum with XP but not by neurotensin or other peptides. Immunoreactive XP (iXP) was found by radioimmunoassay in extracts of both the antrum and body of the stomach prepared with acid/acetone or acetic acid. A study of its distribution in the dog indicated that the level of iXP was highest in the stomach, lower in the pancreas and duodenum, and not measurable in the jejunoileum and colon. Gel chromatography on Sephadex G-25 indicated the presence of at least two forms of iXP, one larger and the other about the same size as XP. Reverse-phase high pressure liquid chromatography on mu-Bondapak C-18 yielded several peaks of iXP, one of which eluted at the position of synthetic XP. The results of immunochemical analyses using four different antisera towards XP were consistent with structures for canine iXPs that were closely related to XP only in their C-terminal regions. These results suggest that mammalian counterparts to amphibian XP reside within endocrine cells of the gastric mucosa. It seems possible that these peptides function as gastrointestinal signals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki K., Tachibana S., Kato Y., Tajima T. [Comparative studies of xenopsin and neurotensin on some biological activities (author's transl)]. Yakugaku Zasshi. 1979 May;99(5):466–470. doi: 10.1248/yakushi1947.99.5_466. [DOI] [PubMed] [Google Scholar]
- Araki K., Tachibana S., Uchiyama M., Nakajima T., Yasuhara T. Isolation and structure of a new active peptide xenopsin on rat stomach strip and some biogenic amines in the skin of Xenopus laevis. Chem Pharm Bull (Tokyo) 1975 Dec;23(12):3132–3140. doi: 10.1248/cpb.23.3132. [DOI] [PubMed] [Google Scholar]
- Carraway R., Leeman S. E. Characterization of radioimmunoassayable neurotensin in the rat. Its differential distribution in the central nervous system, small intestine, and stomach. J Biol Chem. 1976 Nov 25;251(22):7045–7052. [PubMed] [Google Scholar]
- Carraway R., Leeman S. E. Radioimmunoassay for neurotensin, a hypothalamic peptide. J Biol Chem. 1976 Nov 25;251(22):7035–7044. [PubMed] [Google Scholar]
- Carraway R., Ruane S. E., Feurle G. E., Taylor S. Amphibian neurotensin (NT) is not xenopsin (XP): dual presence of NT-like and XP-like peptides in various amphibia. Endocrinology. 1982 Apr;110(4):1094–1101. doi: 10.1210/endo-110-4-1094. [DOI] [PubMed] [Google Scholar]
- Feurle G. E., Baća I., Knauf W., Schwab A., Araki T., Carraway R. Xenopsin stimulates exocrine pancreatic secretion in the dog. Experientia. 1982 Jun 15;38(6):679–680. doi: 10.1007/BF01964091. [DOI] [PubMed] [Google Scholar]
- Goedert M., Sturmey N., Williams B. J., Emson P. C. The comparative distribution of xenopsin- and neurotensin-like immunoreactivity in Xenopus laevis and rat tissues. Brain Res. 1984 Aug 13;308(2):273–280. doi: 10.1016/0006-8993(84)91066-7. [DOI] [PubMed] [Google Scholar]
- Hackenthal E., Metz J., Poulsen K., Rix E., Taugner R. Renin in the uterus of non-pregnant mice. Immunocytochemical, ultrastructural and biochemical studies. Histochemistry. 1980;66(3):229–238. doi: 10.1007/BF00495736. [DOI] [PubMed] [Google Scholar]
- Hammer R. A., Leeman S. E., Carraway R., Williams R. H. Isolation of human intestinal neurotensin. J Biol Chem. 1980 Mar 25;255(6):2476–2480. [PubMed] [Google Scholar]
- Helmstaedter V., Taugner C., Feurle G. E., Forssmann W. G. Localization of neurotensin-immunoreactive cells in the small intestine of man and various mammals. Histochemistry. 1977 Jul 18;53(1):35–41. doi: 10.1007/BF00511208. [DOI] [PubMed] [Google Scholar]
- Ishida T., Kawamura K., Goto A., Nishina Y., Takahara J. Comparison studies of neurotensin and xenopsin upon pancreatic secretion in the dog [proceedings]. Metabolism. 1976 Nov;25(11 Suppl 1):1467–1467. doi: 10.1016/s0026-0495(76)80168-0. [DOI] [PubMed] [Google Scholar]
- Kawanishi K., Goto A., Ishida T., Kawamura K., Nishina Y., Machida S., Yamamoto S., Ofuji T. The effects of xenopsin of endocrine pancreas and gastric antrum in dogs. Horm Metab Res. 1978 Jul;10(4):283–286. doi: 10.1055/s-0028-1093415. [DOI] [PubMed] [Google Scholar]
- Reichlin M., Schnure J. J., Vance V. K. Induction of antibodies to porcine ACTH in rabbits with nonsteroidogenic polymers of BSA and ACTH. Proc Soc Exp Biol Med. 1968 Jun;128(2):347–350. doi: 10.3181/00379727-128-33011. [DOI] [PubMed] [Google Scholar]
- Rix E., Hackenthal E., Hilgenfeldt U., Taugner R. Neuropeptides in the pineal gland? A critical immunocytochemical study. Histochemistry. 1981;72(1):33–38. doi: 10.1007/BF00496776. [DOI] [PubMed] [Google Scholar]
- Zinner M. J., Kasher F., Modlin I. M., Jaffe B. M. Effect of xenopsin on blood flow, hormone release, and acid secretion. Am J Physiol. 1982 Sep;243(3):G195–G199. doi: 10.1152/ajpgi.1982.243.3.G195. [DOI] [PubMed] [Google Scholar]