Abstract
Objectives
Cartonectin is a novel adipokine of the C1q complement/TNF-related protein (CTRP) superfamily, with glucose lowering effects, anti-inflammatory and cardio-protective properties. We sought to investigate circulating cartonectin concentrations in subjects with type 2 diabetes mellitus (T2DM) as well as age and BMI matched control subjects. We also examined the effects of a 2 hour 75 g oral glucose tolerance test (OGTT) on serum cartonectin concentrations in T2DM subjects.
Design
Cross-sectional study [newly diagnosed (first discovery, not on any treatments) T2DM (n = 47) and control (n = 63) subjects]. Serum cartonectin was measured by ELISA.
Results
Serum cartonectin concentrations were significantly lower in patients with T2DM compared to controls (P<0.05). Furthermore, serum cartonectin was significantly negatively correlated with glucose and CRP, and significantly positively correlated with leptin, in all subjects (n = 110). When subjected to multiple regression analysis, none of these variables were predictive of serum cartonectin (P>0.05). There were no significant correlations in T2DM subjects (n = 47). In control subjects (n = 63), serum cartonectin was significantly negatively correlated with CRP, and significantly positively correlated with insulin, HOMA-IR and leptin. However, when subjected to multiple regression analysis, none of these variables were predictive of serum cartonectin (P>0.05). Finally, serum cartonectin concentrations were significantly lower in T2DM subjects after a 2 hour 75 g OGTT (P<0.01).
Conclusions
Cartonectin may serve as a novel biomarker for the prediction and early diagnosis of T2DM patients. Furthermore, cartonectin and/or pharmacological agents that increase circulating cartonectin levels can represent a new therapeutic field in the treatment of T2DM patients. Further research is needed to clarify these points.
Introduction
Obesity has now achieved pandemic proportions and left unchecked, leads to atherosclerosis by causing a repertoire of metabolic and cardiovascular perturbations such as type 2 diabetes mellitus (T2DM), dyslipidemia and hypertension [1]. Adipose tissue produces several hormones and cytokines termed ‘adipokines’ that have widespread effects on carbohydrate and lipid metabolism, which appear to play an important role in the pathogenesis of insulin resistance, diabetes, and atherosclerosis [2], [3]. Perturbations in adipokine concentrations have been reported in insulin resistant states such as gestational diabetes mellitus [4] and women with the Polycystic Ovary Syndrome (PCOS) [5].
Presently, there has been intense interest in the adipokines of the C1q complement/TNF-related protein (CTRP) superfamily. Foremost in this group is the adipokine adiponectin that circulates at high concentrations, and has insulin sensitizing, anti-inflammatory and anti-atherogenic properties [6]. Altered circulating adiponectin and adiponectin receptors levels have been described in patients with T2DM and insulin resistant states such as women with PCOS [7], [8]. Recently, CTRP3 (also known as cartonectin, cartducin, CORS-26) was reported as a novel adipokine, concentrations of which were lower in diet induced obese mice, with glucose lowering effects achieved by suppressing hepatic gluconeogenesis [9]. Cartonectin also decreases hepatic steatosis [10]. Cartonectin, similar to adiponectin, has anti-inflammatory properties [11]–[14]; cartonectin stimulates adipocyte adiponectin secretion [15]. Akiyama et al. had shown that cartonectin promotes proliferation and migration of endothelial cells and suggested that cartonectin may be a novel angiogenic factor in the formation of neo-intima following angioplasty [16]. Furthermore, Maeda et al. had demonstrated a biological role of cartonectin in promoting vascular smooth muscle cell proliferation in blood vessel walls after injury [17]. Moreover, Yi et al. had shown that cartonectin is a cardio-protective adipokine [18]; this could be via its protective effects on bone marrow derived mesenchymal stem cells, crucial for tissue regeneration in the ischemic myocardium [19]. More recently, Zhou et al. had reported that cartonectin promotes phosphate-induced smooth muscle vascular calcification [20].
Therefore, we measured circulating cartonectin concentrations in subjects with T2DM as well as age and BMI matched control subjects. We also examined the effects of a 2 hour 75 g oral glucose tolerance test (OGTT) on serum cartonectin concentrations.
Materials and Methods
Ethics
The study was approved by the medical ethics committee of the Affiliated Hospital of Jining Medical University, Shandong, China and written informed consent was obtained from all participants, in accordance with the guidelines in The Declaration of Helsinki 2000.
Subjects
Forty-seven newly diagnosed T2DM (as per the 1999 World Health Organization criteria) [21] and sixty-three non-diabetic subjects participated in this study (Table 1). Newly diagnosed meant first discovery, and all T2DM subjects were not on any treatments at the time when their blood samples were obtained for this study. Exclusion criteria included a history of congestive heart failure, liver or kidney disease, malignancy, signs of inflammation, pregnancy and any drugs influencing body weight like corticosteroids or contraceptives. Subjects were patients of the Affiliated Hospital of Jining Medical University.
Table 1. Clinical, hormonal and metabolic features of study subjects.
| Variable | T2DM (n = 47) | Controls (n = 63) | Significance |
| Sex (female/male) | 22/25 | 28/35 | |
| Age (year) | 55.0 (41.0–60.0) | 47.0 (40.0–54.0) | NS |
| BMI (kg/m2) | 26.0 (22.9–28.4) | 24.2 (22.0–26.4) | NS |
| SBP (mm Hg) | 140.0 (125.0–150.0) | 120.0 (108.0–127.0) | P<0.01 |
| DBP (mm Hg) | 85.0 (75.0–95.0) | 75.0 (68.0–83.0) | P<0.01 |
| TCH (mmol/L) | 5.2 (4.4–5.9) | 4.6 (4.1–5.1) | P<0.01 |
| HDL-cholesterol (mmol/L) | 1.4 (1.1–1.6) | 1.4 (1.2–1.5) | NS |
| LDL-cholesterol (mmol/L) | 3.0 (2.4–3.3) | 2.7 (2.3–3.1) | NS |
| VLDL-cholesterol (mmol/L) | 0.7 (0.4–1.2) | 0.6 (0.4–0.7) | NS |
| Triglycerides (mmol/L) | 1.6 (0.9–2.5) | 1.0 (0.8–1.4) | P<0.01 |
| TCH/HDL | 3.8 (3.0–4.2) | 3.5 (3.1–3.8) | P<0.05 |
| TG/HDL | 0.3 (0.2–0.5) | 0.2 (0.2–0.3) | P<0.05 |
| Glucose (mmol/L) | 7.3 (6.7–9.3) | 4.9 (4.6–5.3) | P<0.01 |
| Insulin (pmol/L) | 69.9 (32.6–92.6) | 54.3 (36.7–72.0) | NS |
| HOMA-IR | 3.1 (1.8–4.4) | 1.7 (1.1–2.3) | P<0.01 |
| HbA1C (%) | 7.3 (6.3–8.8) | ||
| CRP (mg/l) | 4.3 (2.2–8.7) | 1.8 (0.8–2.6) | P<0.01 |
| Leptin (ng/ml) | 2.0 (1.0–5.4) | 8.0 (3.1–12.0) | P<0.01 |
| Adiponectin (µg/ml) | 8.3 (6.1–21.4) | 11.0 (8.4–16.2) | P<0.05 |
| Cartonectin (ng/ml) | 150.2 (72.9–288.7) | 248.9 (115.0–321.2) | P<0.05 |
Data are median (interquartile range). Group comparison by Mann-Whitney U test.
BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure; TCH = total cholesterol; TG = triglycerides; DBP = diastolic blood pressure; NS = not significant.
After an overnight fast, blood samples were collected and immediately centrifuged. A 2 hour 75 g OGTT was also performed in subjects with T2DM. Serum was immediately aliquoted on ice and stored at −80°C. All patients underwent anthropometric measurements. Blood pressure was measured in a sitting position within a quiet and calm environment after a rest of at least 5 minutes. The average of three measurements was obtained.
The primary aim of our study was to investigate serum cartonectin concentrations in newly diagnosed (first discovery) subjects with T2DM.
Biochemical and hormonal analysis
Assays for glucose, HbA1c (only in T2DM patients), total cholesterol (TCH), HDL-cholesterol, LDL-cholesterol, VLDL-cholesterol and triglycerides (TG) [Hitachi 7600 biochemical automatic analyzer] as well as for insulin (Roche electrochemiluminescence analyzer) were performed. The estimate of insulin resistance by HOMA-IR score was calculated as Io×Go/22.5, where Io is the fasting insulin and Go is the fasting glucose, as described by Matthews et al. [22]. The TCH/HDL and TG/HDL ratios were calculated as indices of ischaemic heart disease mortality and morbidity [23], [24]. CRP concentrations in sera were measured using a commercially available ELISA kit (Aviscera, Santa Clara, USA) according to manufacturer's protocol, with an intra-assay coefficient of variation of less than 6%. Leptin concentrations in sera were measured using a commercially available ELISA kit (Aviscera, Santa Clara, USA) according to manufacturer's protocol, with an intra-assay coefficient of variation of less than 8%. Adiponectin concentrations in sera were measured using a commercially available ELISA kit (Biovision, Milpitas, USA) according to manufacturer's protocol, with an intra-assay coefficient of variation of less than 8%. Cartonectin concentrations in sera were measured using a commercially available ELISA kit (USCN Life Science, Inc., Wuhan, China) according to manufacturer's protocol, with an intra-assay coefficient of variation of less than 10%.
Statistical analysis
Data were analysed by Mann-Whitney U test and Wilcoxon matched pairs test. Data are medians (interquartile range). Spearman Rank correlation was used for calculation of associations between variables. Subsequently, if individual bivariate correlations achieved statistical significance, variables were entered into a linear regression model and multiple regression analysis was performed. All statistical analyses were performed using SPSS version 22.0 (SPSS, Inc.). P<0.05 was considered significant.
Results
Table 1 shows the anthropometric, biochemical and hormonal parameters in all subjects. Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), TCH, TG, TCH/HDL ratio, TG/HDL ratio, glucose, HOMA-IR and CRP were significantly higher, whereas leptin and adiponectin were significantly lower in T2DM subjects.
Serum cartonectin concentrations were significantly lower in patients with T2DM compared to controls [150.2 (72.9–288.7) vs. 248.9 (115.0–321.2) ng/ml; P<0.05: Table 1].
Furthermore, women had higher serum adiponectin concentrations [10.6 (7.0–24.8) vs. 7.2 (5.4–12.4)µg/ml; P<0.05] compared to men; however, there were no significant difference with respect to serum cartonectin concentrations [224.1 (98.8–321.2) vs. 188.9 (100.7–304.0) ng/ml; P>0.05].
Effects of a 2 hour 75 g OGTT in T2DM subjects on serum cartonectin concentrations
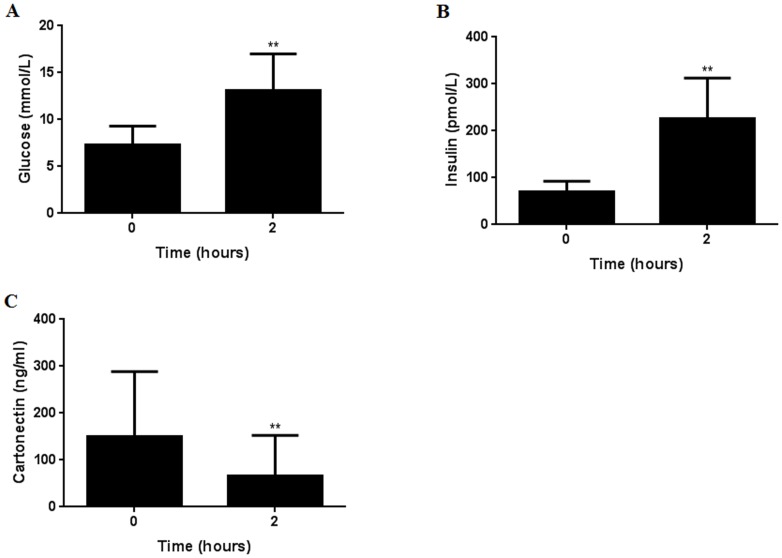
Glucose and insulin concentrations were significantly higher in T2DM subjects after a 2 hour 75 g OGTT (Figure1A: ** P<0.01 and Figure1B: ** P<0.01, respectively).
Figure 1. Effects of a 2 hour 75 g OGTT on serum glucose (A), insulin (B) and cartonectin (C) concentrations in T2DM subjects (n = 47).
Group comparison by Wilcoxon matched pairs test. **P<0·01.
Conversely, serum cartonectin concentrations were significantly lower in T2DM subjects after a 2 hour 75 g OGTT (Figure1C: ** P<0.01).
Correlation of cartonectin with covariates
Spearman Rank analysis showed that serum cartonectin was significantly negatively correlated with glucose and CRP, and significantly positively correlated with leptin, in all subjects (n = 110) [Table 2]. When subjected to multiple regression analysis, none of these variables were predictive of serum cartonectin (P>0.05). There were no significant correlations in T2DM subjects (n = 47) [Table 2]. In control subjects (n = 63), serum cartonectin was significantly negatively correlated with CRP, and significantly positively correlated with insulin, HOMA-IR and leptin [Table 2]. However, when subjected to multiple regression analysis, none of these variables were predictive of serum cartonectin (P>0.05).
Table 2. Linear regression analysis of variables associated with Cartonectin.
| Subjects | All (n = 110) | T2DM (n = 47) | Control (n = 63) | |||
| Variable | r | P | r | P | r | P |
| Age (year) | 0.008 | 0.930 | 0.204 | 0.168 | −0.134 | 0.297 |
| BMI (kg/m2) | −0.053 | 0.580 | 0.020 | 0.893 | −0.013 | 0.918 |
| SBP (mm Hg) | 0.073 | 0.446 | 0.116 | 0.438 | 0.201 | 0.115 |
| DBP (mm Hg) | 0.049 | 0.612 | 0.079 | 0.597 | 0.207 | 0.103 |
| TCH (mmol/L) | −0.158 | 0.099 | −0.004 | 0.977 | −0.169 | 0.185 |
| HDL-cholesterol (mmol/L) | −0.031 | 0.747 | −0.114 | 0.446 | 0.064 | 0.619 |
| LDL-cholesterol (mmol/L) | −0.122 | 0.204 | −0.047 | 0.753 | −0.064 | 0.617 |
| VLDL-cholesterol (mmol/L) | −0.027 | 0.778 | 0.223 | 0.132 | −0.243 | 0.055 |
| Triglycerides (mmol/L) | −0.014 | 0.888 | 0.012 | 0.936 | 0.145 | 0.255 |
| TCH/HDL | −0.099 | 0.305 | 0.075 | 0.618 | −0.136 | 0.289 |
| TG/HDL | 0.045 | 0.642 | 0.005 | 0.974 | 0.229 | 0.070 |
| Glucose (mmol/L) | −0.201* | 0.035 | 0.116 | 0.436 | −0.194 | 0.127 |
| Insulin (pmol/L) | 0.159 | 0.096 | 0.092 | 0.537 | 0.388** | 0.002 |
| HOMA-IR | 0.083 | 0.389 | 0.100 | 0.503 | 0.341** | 0.006 |
| HbA1C (%) | −0.179 | 0.229 | ||||
| CRP (mg/l) | −0.223* | 0.019 | 0.064 | 0.667 | −0.361** | 0.004 |
| Leptin (ng/ml) | 0.274** | 0.004 | 0.196 | 0.187 | 0.263* | 0.037 |
| Adiponectin (µg/ml) | −0.052 | 0.589 | −0.080 | 0.594 | −0.135 | 0.292 |
Spearman Rank correlation was used for calculation of associations between variables. If individual bivariate correlations achieved statistical significance, multiple regression analysis with Cartonectin as dependent variable was performed to test the joint effect of these parameters on Cartonectin. Multiple regression analysis contained glucose, CRP and leptin (all subjects); insulin, HOMA-IR, CRP and leptin (control subjects). *P<0.05; **P<0.01.
Discussion
We report for the first time that serum cartonectin concentrations are significantly lower in newly diagnosed (first discovery) patients with T2DM compared to control subjects. We also found that serum adiponectin concentrations were significantly lower in T2DM compared to control subjects, in keeping with the current research literature [25]. Both cartonectin and adiponectin have apparently similar functions i.e. insulin sensitizing, anti-inflammatory and cardio-protective properties [26], and may function synergistically. Notably, Wolfing et al. had shown that cartonectin stimulates adipocyte adiponectin secretion [15]. Furthermore, we had observed significantly lower leptin concentrations in our newly diagnosed (first discovery) T2DM subjects (median HbA1c: 7.3%). This is similar with previous publications describing lower leptin concentrations in subjects with poorly controlled T2DM [27], [28].
The lower serum cartonectin concentrations in patients with T2DM is bewildering given that Choi et al. had recently reported that plasma cartonectin was significantly higher in patients with T2DM [29], and that a three month combined exercise program significantly decreased cartonectin levels in obese Korean women [30]. Interestingly, the same researchers also reported that serum cartonectin was not significantly lower in subjects with the metabolic syndrome compared to controls [31]. More recently, Choi et al. had published data reporting significantly lower circulating cartonectin concentrations in patients with acute coronary syndrome or stable angina pectoris, compared to control subjects [32]. A possible explanation of elevated cartonectin levels in T2DM observed by Choi et al. [29] could be the effect of medications taken by their study subjects as discussed in their following manuscript [31]. We have reported that metformin significantly increases circulating cartonectin concentrations in insulin resistant women with PCOS [33]. The findings from the current study support this notion.
Also, we present novel data that serum cartonectin concentrations were significantly lower in T2DM patients after a 2 hour 75 g OGTT. In contrast, glucose and insulin concentrations were significantly higher after the 2 hour 75 g OGTT. Therefore, glucose and/or insulin could account for the reduction in serum cartonectin concentrations. Since a recent in vitro study demonstrated that cartonectin concentrations were increased by insulin [34], and that glucose was significantly altered (lower) but not insulin in our T2DM patients, we propose that changes in glucose metabolism are more likely to explain our findings.
A limitation of this study is that it comprised of only Chinese subjects and thus our observations may not apply to other populations. Further studies are needed to elucidate this point. Notwithstanding, our results contribute to the paucity of human studies on cartonectin and raises interesting questions on the factors that regulate cartonectin concentrations in human subjects with metabolic and cardiovascular complications.
Our data supports cartonectin as a potential novel biomarker for the prediction and early diagnosis of T2DM patients. Furthermore, cartonectin and/or pharmacological agents that increase circulating cartonectin levels can represent a new therapeutic field in the treatment of T2DM patients. Further research is needed to clarify these points.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by the National Nature Science Foundation of China (31271243 and 81070961), http://www.nsfc.gov.cn/publish/portal1/BBai, and the Natural Science Foundation of Shandong province (ZR2009CZ006 and ZR2011CM027), http://www.sdnsf.gov.cn/portal/BBan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kaul K, Tarr JM, Ahmad SI, Kohner EM, Chibber R (2012) Introduction to diabetes mellitus. Adv Exp Med Biol 771: 1–11. [DOI] [PubMed] [Google Scholar]
- 2. Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89: 2548–2556. [DOI] [PubMed] [Google Scholar]
- 3. Hallschmid M, Randeva H, Tan BK, Kern W, Lehnert H (2009) Relationship between cerebrospinal fluid visfatin (PBEF/Nampt) levels and adiposity in humans. Diabetes 58: 637–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewandowski KC, Stojanovic N, Bienkiewicz M, Tan BK, Prelevic GM, et al. (2008) Elevated concentrations of retinol-binding protein-4 (RBP-4) in gestational diabetes mellitus: negative correlation with soluble vascular cell adhesion molecule-1 (sVCAM-1). Gynecol Endocrinol 24: 300–305. [DOI] [PubMed] [Google Scholar]
- 5. Randeva HS, Tan BK, Weickert MO, Lois K, Nestler JE, et al. (2012) Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev 33: 812–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, et al. (1999) Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100: 2473–2476. [DOI] [PubMed] [Google Scholar]
- 7. Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, et al. (2001) Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86: 1930–1935. [DOI] [PubMed] [Google Scholar]
- 8. Tan BK, Chen J, Digby JE, Keay SD, Kennedy CR, et al. (2006) Upregulation of adiponectin receptor 1 and 2 mRNA and protein in adipose tissue and adipocytes in insulin-resistant women with polycystic ovary syndrome. Diabetologia 49: 2723–2728. [DOI] [PubMed] [Google Scholar]
- 9. Peterson JM, Wei Z, Wong GW (2010) C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem 285: 39691–39701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peterson JM, Seldin MM, Wei Z, Aja S, Wong GW (2013) CTRP3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am J Physiol Gastrointest Liver Physiol 305: G214–G224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kopp A, Bala M, Weigert J, Büchler C, Neumeier M, et al. (2010) Effects of the new adiponectin paralogous protein CTRP-3 and of LPS on cytokine release from monocytes of patients with type 2 diabetes mellitus. Cytokine 49: 51–57. [DOI] [PubMed] [Google Scholar]
- 12. Kopp A, Bala M, Buechler C, Falk W, Gross P, et al. (2010) C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology 151: 5267–5278. [DOI] [PubMed] [Google Scholar]
- 13. Hofmann C, Chen N, Obermeier F, Paul G, Büchler C, et al. (2011) C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm Bowel Dis 17: 2462–2471. [DOI] [PubMed] [Google Scholar]
- 14. Murayama MA, Kakuta S, Maruhashi T, Shimizu K, Seno A, et al. (2014) CTRP3 plays an important role in the development of collagen-induced arthritis in mice. Biochem Biophys Res Commun 443: 42–48. [DOI] [PubMed] [Google Scholar]
- 15. Wölfing B, Buechler C, Weigert J, Neumeier M, Aslanidis C, et al. (2008) Effects of the new C1q/TNF-related protein (CTRP-3) "cartonectin" on the adipocytic secretion of adipokines. Obesity (Silver Spring) 16: 1481–1486. [DOI] [PubMed] [Google Scholar]
- 16. Akiyama H, Furukawa S, Wakisaka S, Maeda T (2007) CTRP3/cartducin promotes proliferation and migration of endothelial cells. Mol Cell Biochem 304: 243–248. [DOI] [PubMed] [Google Scholar]
- 17. Maeda T, Wakisaka S (2010) CTRP3/cartducin is induced by transforming growth factor-beta1 and promotes vascular smooth muscle cell proliferation. Cell Biol Int 34: 261–266. [DOI] [PubMed] [Google Scholar]
- 18. Yi W, Sun Y, Yuan Y, Lau WB, Zheng Q, et al. (2012) C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation 125: 3159–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hou M, Liu J, Liu F, Liu K, Yu B (2014) C1q tumor necrosis factor-related protein-3 protects mesenchymal stem cells against hypoxia- and serum deprivation-induced apoptosis through the phosphoinositide 3-kinase/Akt pathway. Int J Mol Med 33: 97–104. [DOI] [PubMed] [Google Scholar]
- 20. Zhou Y, Wang JY, Feng H, Wang C, Li L, et al. (2014) Overexpression of c1q/tumor necrosis factor-related protein-3 promotes phosphate-induced vascular smooth muscle cell calcification both in vivo and in vitro. Arterioscler Thromb Vasc Biol 34: 1002–1010. [DOI] [PubMed] [Google Scholar]
- 21. Borch-Johnsen K (2001) The new classification of diabetes mellitus and IGT: a critical approach. Exp Clin Endocrinol Diabetes 109 Suppl 2: S86–S93. [DOI] [PubMed] [Google Scholar]
- 22. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 23. Prospective Studies Collaboration (2007) Lewington S, Whitlock G, Clarke R, Sherliker P, et al. (2007) Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 370: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 24. da Luz PL, Favarato D, Faria-Neto JR Jr, Lemos P, Chagas AC (2008) High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics (Sao Paulo) 63: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shehzad A, Iqbal W, Shehzad O, Lee YS (2012) Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens) 11: 8–20. [DOI] [PubMed] [Google Scholar]
- 26. Villarreal-Molina MT, Antuna-Puente B (2012) Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie 94: 2143–2149. [DOI] [PubMed] [Google Scholar]
- 27. Buyukbese MA, Cetinkaya A, Kocabas R, Guven A, Tarakcioglu M (2004) Leptin levels in obese women with and without type 2 diabetes mellitus. Mediators Inflamm 13: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clément K, Lahlou N, Ruiz J, Hager J, Bougnères P, et al. (1997) Association of poorly controlled diabetes with low serum leptin in morbid obesity. Int J Obes Relat Metab Disord 21: 556–561. [DOI] [PubMed] [Google Scholar]
- 29. Choi KM, Hwang SY, Hong HC, Yang SJ, Choi HY, et al. (2012) C1q/TNF-related protein-3 (CTRP-3) and pigment epithelium-derived factor (PEDF) concentrations in patients with type 2 diabetes and metabolic syndrome. Diabetes 61: 2932–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi HY, Park JW, Lee N, Hwang SY, Cho GJ, et al. (2013) Effects of a combined aerobic and resistance exercise program on C1q/TNF-related protein-3 (CTRP-3) and CTRP-5 levels. Diabetes Care 36: 3321–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoo HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, et al. (2013) Implication of progranulin and C1q/TNF-related protein-3 (CTRP3) on inflammation and atherosclerosis in subjects with or without metabolic syndrome. PLoS One 8: e55744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi KM, Hwang SY, Hong HC, Choi HY, Yoo HJ, et al. (2014) Implications of C1q/TNF-related protein-3 (CTRP-3) and progranulin in patients with acute coronary syndrome and stable angina pectoris. Cardiovasc Diabetol 13: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tan BK, Chen J, Hu J, Amar O, Mattu HS, et al. (2013) Metformin increases the novel adipokine cartonectin/CTRP3 in women with polycystic ovary syndrome. J Clin Endocrinol Metab 98: E1891–E1900. [DOI] [PubMed] [Google Scholar]
- 34. Schmid A, Kopp A, Hanses F, Bala M, Müller M, et al. (2012) The novel adipokine C1q/TNF-related protein-3 is expressed in human adipocytes and regulated by metabolic and infection-related parameters. Exp Clin Endocrinol Diabetes 120: 611–617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.