Abstract
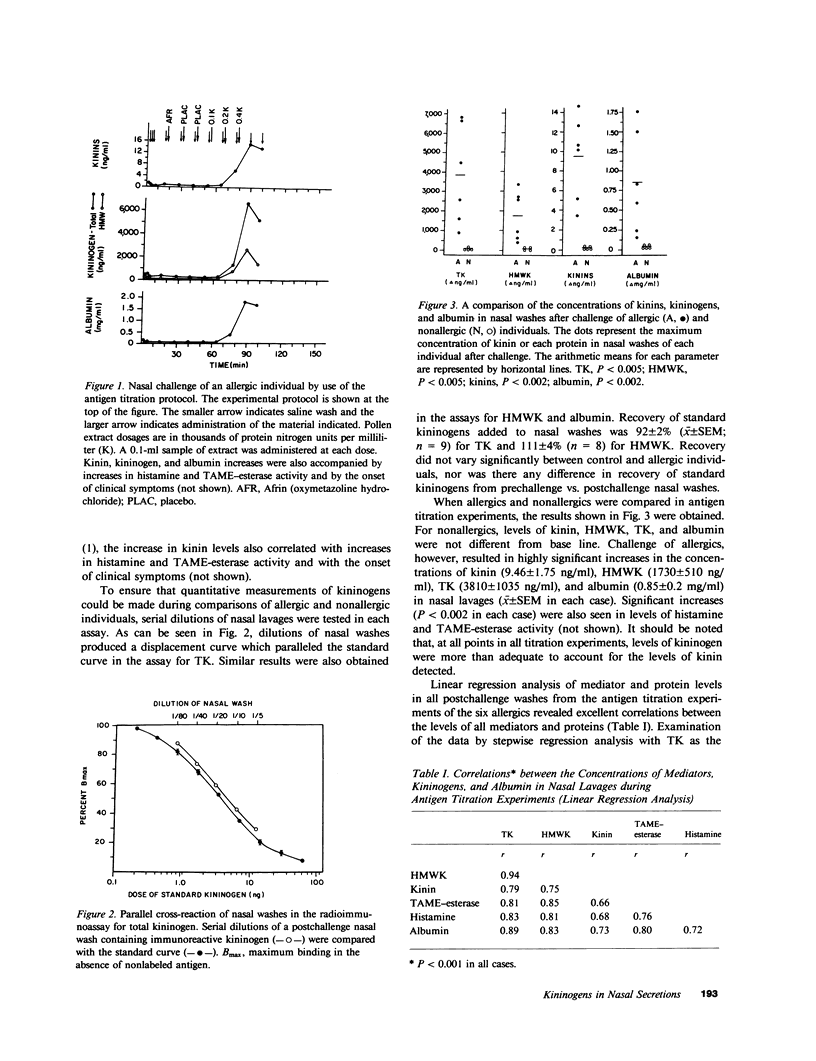
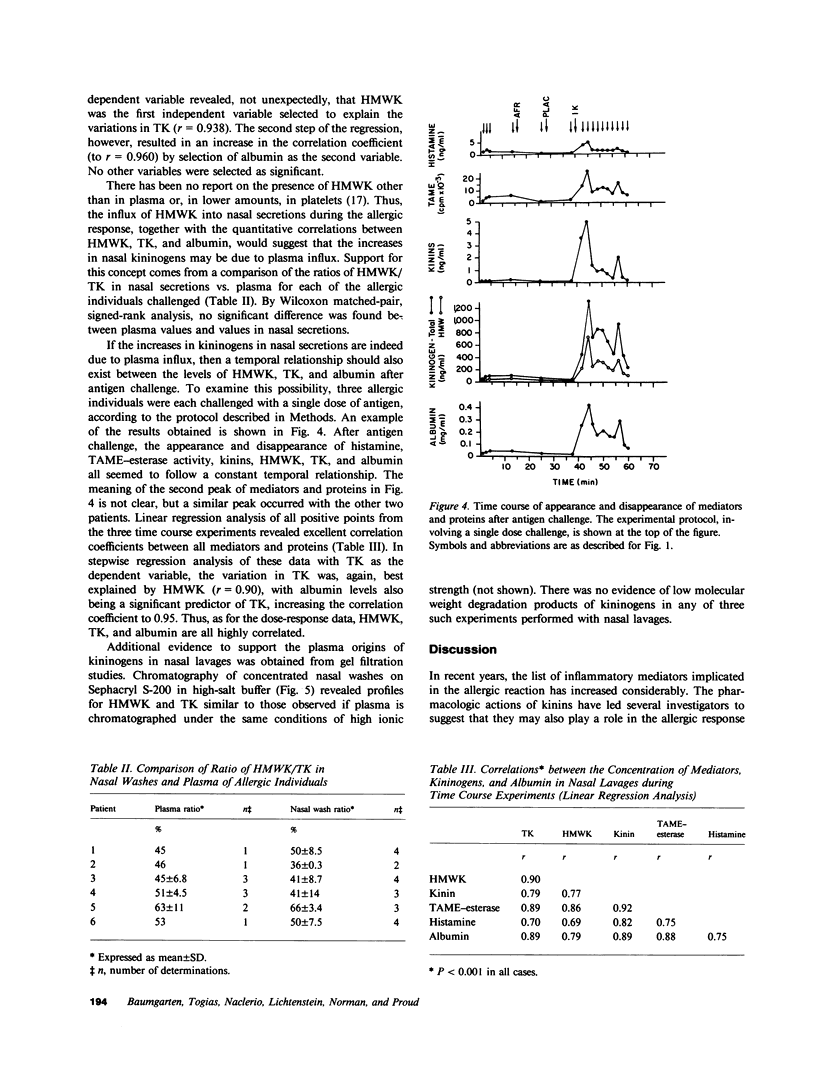
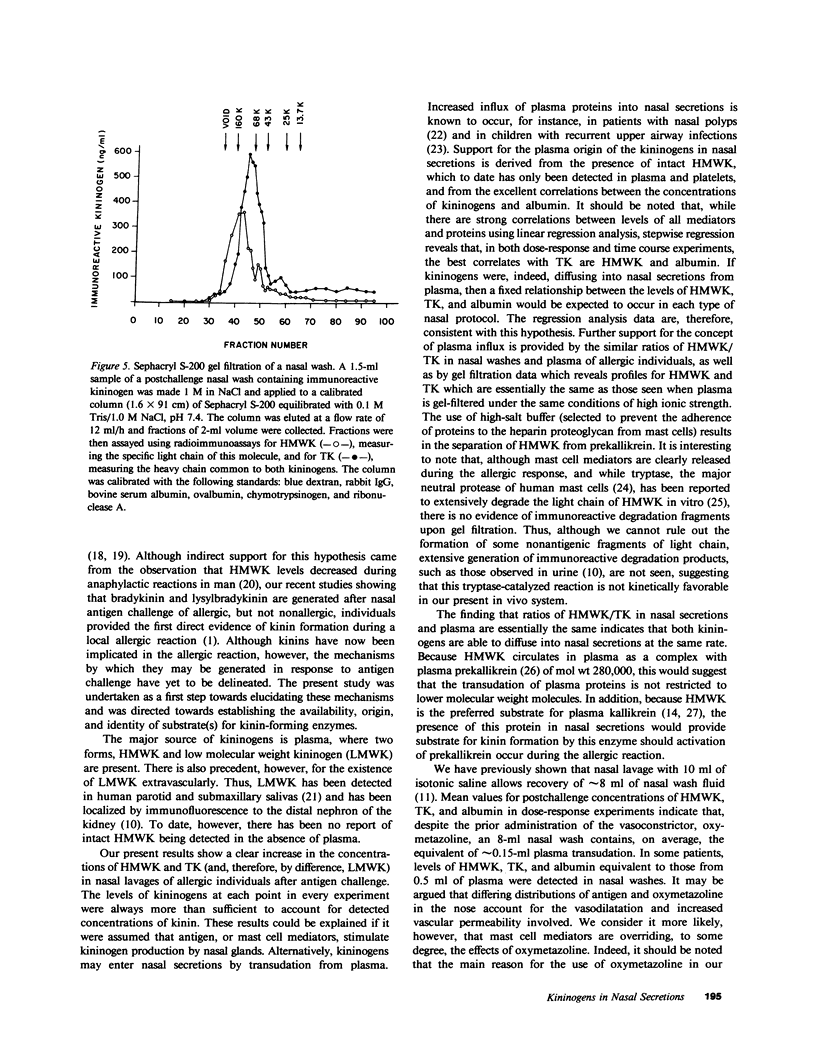
We have recently demonstrated that kinins are generated in vivo after nasal challenge with antigen of allergic, but not nonallergic, individuals. The present study was undertaken as a first step in determining the mechanism(s) of kinin formation during the allergic reaction and was directed towards establishing the availability and origin of kininogens in nasal secretions. Allergic individuals (n = 6) and nonallergic controls (n = 5) were challenged with antigen; and by using specific radioimmunoassays, nasal washes, obtained before and after challenge, were assayed for high molecular weight kininogen (HMWK), total kininogen (TK), albumin, and kinins. Dramatic increases in HMWK (1,730 +/- 510 ng/ml), TK (3,810 +/- 1035 ng/ml), kinin (9.46 +/- 1.75 ng/ml), and albumin (0.85 +/- 0.2 mg/ml) were observed after challenge of allergic individuals which correlated (P less than 0.001) with increases in histamine and N-alpha-tosyl-L-arginine methyl esterase activity and with the onset of clinical symptoms. For nonallergic individuals, levels of kininogens, albumin, and all mediators after antigen challenge were not different from base line. Linear regression analysis revealed excellent correlations (P less than 0.001 in each case) between increases in HMWK, TK, kinin, and albumin during antigen titration experiments and between the time courses of appearance and disappearance of HMWK, TK, kinin, and albumin after antigen challenge. Gel filtration revealed no evidence of degradation products of kininogens in nasal washes. For each allergic individual the ratio of HMWK/TK in postchallenge nasal washes was similar to the ratio of these two proteins in the same individual's plasma. These data suggest that, during the allergic reaction, there is an increase in vascular permeability and a transudation of kininogens from plasma into nasal secretions, where they can provide substrate for kinin-forming enzymes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner M., Baumgarten C., Kunkel G., Rudolph R., Kirchhof E. The possible role of bradykinin as a mediator in bronchial asthma. Allergol Immunopathol (Madr) 1982 Nov-Dec;10(6):453–462. [PubMed] [Google Scholar]
- Cochrane C. G., Griffin J. H. The biochemistry and pathophysiology of the contact system of plasma. Adv Immunol. 1982;33:241–306. doi: 10.1016/s0065-2776(08)60837-8. [DOI] [PubMed] [Google Scholar]
- Guimarães J. A., Borges D. R., Prado E. S., Prado J. L. Kinin-converting aminopeptidase from human serum. Biochem Pharmacol. 1973 Dec 15;22(24):3157–3172. doi: 10.1016/0006-2952(73)90090-7. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Jacobsen S. Separation of 2 different substrates for plasma kinin-forming enzymes. Nature. 1966 Apr 2;210(5031):98–99. doi: 10.1038/210098a0. [DOI] [PubMed] [Google Scholar]
- Kaplan A. P., Silverberg M., Dunn J. T., Ghebrehiwet B. Interaction of the clotting, kinin-forming, complement, and fibrinolytic pathways in inflammation. Ann N Y Acad Sci. 1982;389:25–38. doi: 10.1111/j.1749-6632.1982.tb22123.x. [DOI] [PubMed] [Google Scholar]
- Maier M., Spragg J., Schwartz L. B. Inactivation of human high molecular weight kininogen by human mast cell tryptase. J Immunol. 1983 May;130(5):2352–2356. [PubMed] [Google Scholar]
- Mandle R. J., Colman R. W., Kaplan A. P. Identification of prekallikrein and high-molecular-weight kininogen as a complex in human plasma. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4179–4183. doi: 10.1073/pnas.73.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mygind N., Weeke B., Ullman S. Quantitative determination of immunoglobulins in nasal secretion. Int Arch Allergy Appl Immunol. 1975;49(1-2):99–107. doi: 10.1159/000231383. [DOI] [PubMed] [Google Scholar]
- Mygind N., Wihl J. A. Concentration of immunoglobulins in nasal secretion from children with recurrent infections in the upper airways. Acta Otolaryngol. 1976 Sep-Oct;82(3-4):216–218. doi: 10.3109/00016487609120887. [DOI] [PubMed] [Google Scholar]
- Naclerio R. M., Meier H. L., Kagey-Sobotka A., Adkinson N. F., Jr, Meyers D. A., Norman P. S., Lichtenstein L. M. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis. 1983 Oct;128(4):597–602. doi: 10.1164/arrd.1983.128.4.597. [DOI] [PubMed] [Google Scholar]
- Nustad K., Orstavik T. B., Gautvik K. M., Pierce J. V. Glandular kallikreins. Gen Pharmacol. 1978;9(1):1–9. doi: 10.1016/0306-3623(78)90049-6. [DOI] [PubMed] [Google Scholar]
- Pisano J. J. Observations on the kallikrein-kinin system in the kidney. Adv Exp Med Biol. 1983;156(Pt B):929–938. [PubMed] [Google Scholar]
- Proud D., Perkins M., Pierce J. V., Yates K. N., Highet P. F., Herring P. L., Mangkornkanok/Mark M., Bahu R., Carone F., Pisano J. J. Characterization and localization of human renal kininogen. J Biol Chem. 1981 Oct 25;256(20):10634–10639. [PubMed] [Google Scholar]
- Proud D., Pierce J. V., Pisano J. J. Radioimmunoassay of human high molecular weight kininogen in normal and deficient plasmas. J Lab Clin Med. 1980 Apr;95(4):563–574. [PubMed] [Google Scholar]
- Proud D., Togias A., Naclerio R. M., Crush S. A., Norman P. S., Lichtenstein L. M. Kinins are generated in vivo following nasal airway challenge of allergic individuals with allergen. J Clin Invest. 1983 Nov;72(5):1678–1685. doi: 10.1172/JCI111127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto W., Fukuda H., Nishikaze O. Kininogen and kallikrein in saliva of periodontally-diseased subjects. J Dent Res. 1981 Jan;60(1):6–9. doi: 10.1177/00220345810600011101. [DOI] [PubMed] [Google Scholar]
- Schmaier A. H., Zuckerberg A., Silverman C., Kuchibhotla J., Tuszynski G. P., Colman R. W. High-molecular weight kininogen. A secreted platelet protein. J Clin Invest. 1983 May;71(5):1477–1489. doi: 10.1172/JCI110901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. B., Lewis R. A., Austen K. F. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. 1981 Nov 25;256(22):11939–11943. [PubMed] [Google Scholar]
- Smith P. L., Kagey-Sobotka A., Bleecker E. R., Traystman R., Kaplan A. P., Gralnick H., Valentine M. D., Permutt S., Lichtenstein L. M. Physiologic manifestations of human anaphylaxis. J Clin Invest. 1980 Nov;66(5):1072–1080. doi: 10.1172/JCI109936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen H., Mygind N., Pedersen C. B., Prytz S. [Long-term treatment of nasal polyps with beclomethasone dipropionate aerosol. III. Morphological studies and conclusions]. Acta Otolaryngol. 1976 Sep-Oct;82(3-4):260–262. doi: 10.3109/00016487609120899. [DOI] [PubMed] [Google Scholar]
- WEBSTER M. E., PIERCE J. V. The nature of the kallidins released from human plasma by kallikreins and other enzymes. Ann N Y Acad Sci. 1963 Feb 4;104:91–107. doi: 10.1111/j.1749-6632.1963.tb17655.x. [DOI] [PubMed] [Google Scholar]



