Abstract
Glioblastoma multiforme (GBM) is the deadliest form of brain tumor with a more than 90% 5-year mortality. GBM has a paltry median survival of 12.6 months attributed to the unique treatment limitations such as the high average age of onset, tumor location, and poor current understandings of the tumor pathophysiology. The resection techniques, chemotherapic strategies, and radiation therapy currently used to treat GBM have slowly evolved, but the improvements have not translated to marked increases in patient survival. Here, we will discuss the recent progress in our understanding of GBM pathophysiology, and the diagnostic techniques and treatment options. The discussion will include biomarkers, tumor imaging, novel therapies such as monoclonal antibodies and small-molecule inhibitors, and the heterogeneity resulting from the GBM cancer stem cell population.
Keywords: biomarkers, brain imaging, cancer stem cells, epigenetics, glioblastoma multiforme (GBM)
Introduction
Gliomas are the most commonly occurring form of brain tumor. Glioblastoma multiforme (GBM) is the most malignant form of glioma causing 3–4% of all cancer-related deaths (Louis et al, 2007). The World Health Organization defines GBM as a grade IV cancer characterized as malignant, mitotically active, and predisposed to necrosis. GBM has a very poor prognosis with a 5-year survival rate of 4–5%, which perhaps is an overestimation (McLendon & Halperin, 2003). GBM has a paltry median survival of 12.6 months attributed to unique treatment limitations such as a high average age of onset, tumor location, and poor current understandings of the tumor pathophysiology (Louis et al, 2007). Current standard of care for GBM includes tumor resection with concurrent radiotherapy and chemotherapy. However, no marked improvements have been achieved that increase survival rates close to other glioma subtypes (Stewart, 2002). The development of proteomic, genetic, and epigenetic tools highlighted here may hold the potential to improve survival rates for GBM patients.
The diagnosis of GBM
Medical imaging
For the last 20 years, magnetic resonance imaging (MRI) has been the standard in brain tumor imaging to define lesion boundaries including size, shape, and location of the tumors. There are, however, a number of emerging MRI developments with the potential to provide more detail about the structural changes that differentiate the higher-grade glioma subtypes. For example, advanced diffusion-weighted imaging can differentiate soft tissue populations based on cellular density, thus discriminating GBM from malignant lymphoma (Yamasaki et al, 2005). Such techniques provide useful information beyond the initial tumor diagnosis. Perfusion weight imaging can be used to monitor the clinical effectiveness of anti-angiogenic drugs like bevacizumab through the use of post-treatment parametric response map comparisons (Aquino et al, 2014). Relative cerebral blood volume (rCBV), a measure of microvascular density, is decreased in patients that responded to the anti-angiogenic treatments. Elevated rCBV values also correlate with EGFR amplification which may have prognostic and treatment monitoring applications in the future (Gupta et al, 2014). Despite the well-characterized diagnostic and treatment applications of MRI, tumor assessment is still largely confined to the evaluation of changes in brain anatomy and structure, which does not profile real-time tumor dynamics.
Methods of identifying solid tumors based on alterations of metabolism are now being actively developed. As first observed by Otto Warburg in 1927, cancer cells switch glucose metabolism favoring glycolysis followed by lactic acid production over oxidative phosphorylation, even in the presence of sufficient oxygen (Warburg et al, 1927). Although this aerobic glycolysis is less efficient than oxidative phosphorylation, higher biomass incorporation through glycolysis is advantageous to proliferating cancer cells by providing the necessary organic substrates for nucleic acid and lipid synthesis. About 90% of all glucose consumed in glioblastoma cells is converted to lactate or alanine, which may be useful to differentiate GBM tumors from surrounding tissue (DeBerardinis et al, 2007). In this respect, the emergence of positron–emission tomography (PET) has been critical. High-grade glioma can be differentiated from CNS lymphoma using the tumor's ability to avidly uptake the glucose analog deoxy-2-(18F)fluoro-D-glucose (FDG) (Kosaka et al, 2008). Increased FDG uptake negatively correlates with patient survival in glioma patients (Pardo et al, 2004). In GBM and in general brain tumors, however, the poor signal-to-noise ratio due to the elevated glucose uptake in the brain compared to other tissues somewhat limits the usefulness of FDG. In fact, radiolabeled amino acids such as C-methionine (C-MET) are preferred over FDG due to their lower uptake in normal brain cells (Stolc et al, 2011). C-MET is actively transported into proliferating cells through the carrier-mediated transport activity of LAT1, which is upregulated in malignant glioma (Kobayashi et al, 2008). C-MET and not FDG was found to be a significant prognostic factor for GBM based on uptake in proliferative cells (Kim et al, 2005). As far back as 1986, pathological histology has defined GBM boundaries beyond those determined through MRI (Lunsford et al, 1986). While C-MET is likely a better imaging technique than FDG, the use of C-MET may be advantageous over conventional MRI as well. MET PET imaging substantially enlarges the three-dimensional volume of an active tumor in comparison with gadolinium–DTPA-enhanced MRI (Galldiks et al, 2010). In conclusion, while MRI remains the standard for GBM brain imaging, advances in technology and wider availability support the use of PET as a complementary technology.
Biomarkers
While GBM tumors may present similar or overlapping phenotypes, differences in tumor progression and molecular mechanisms require diagnosing beyond histological profiling. Histological profiling per se presents a challenge as the required resection or biopsy can be problematic given the location and sensitive nature of brain tissue. Clearly, non-invasive diagnostic measures able to better identify and differentiate GBM tumors would expedite non-resection therapeutic methods ultimately improving patient survival.
Biomarkers are potentially a non-invasive and universal diagnostic tool that focuses on molecular markers over the phenotypic differences described through biopsy. GBM microvesicles may prove to be useful in this respect. Microvesicles are plasma membrane-derived membrane-enclosed particles that are released from cells through membrane fission and can carry mRNA, miRNA, and proteins from the parent cell (Cocucci et al, 2009). When these microvesicles are GBM derived, the tumor-specific contents can adjust the nearby microenvironment to be more hospitable to tumor growth (Skog et al, 2008). One example is the GBM driver mutant form of the EGF receptor EGFRvIII, which can induce neighboring cells to transform into GBM-like phenotypes. Microvesicles carrying EGFRvIII can transfer the oncogenic receptor to induce EGFRvIII activity in the receiving cell (Al-Nedawi et al, 2008). Patient serum may provide prognostic information as it has also been used to detect a small non-coding RNA, RNU6-1, which is an independent predictor of GBM (Manterola et al, 2014). Clearly, the serum composition of GBM patients should be further studied as it may non-invasively provide highly valuable prognostic information for treating GBM beyond the current paradigms.
Microfluidic chips can be used to detect microvesicles in the bloodstream and have been shown to detect a significant dose-dependent post-temozolomide (TMZ) treatment decline in total microvesicle populations (Shao et al, 2012). In addition, microvesicles can accurately model the profile of the tumor cell including changes in IDH-1, EGFR, and EGFRvIII. The information provided by microvesicle detection may provide a quick and non-invasive biomarker of GBM status using patient blood samples. Microvesicles and mRNA are not the only potential biomarkers; however, a number of peptides have been found to change in CSF samples between normal and GBM patients (Schuhmann et al, 2010). Elevations in albumin, osteopontin, and others, although not GBM specific, might suggest that peptide levels in the CSF reflect changes in the nervous system environment that could be used to determine the status of a GBM tumor. Routine blood sampling after GBM resection may allow early detection of recurrence, thus reducing the time from tumor regrowth to second-line treatment.
GBM molecular pathophysiology
Epidermal growth factor receptor
A common driver of GBM progression is epidermal growth factor receptor (EGFR) amplification, found in nearly 40% of all GBM cases (Hatanpaa et al, 2010). EGFR phenotypic changes in GBM can occur by overexpression, amplification, and mutation. Amplification of EGFR can occur by reverse transcription from RNA or insertion, for example. Essentially, all cases of EGFR amplification in GBM are accompanied by EGFR overexpression, contrasted to the 97.7% of non-amplified EGFR GBMs that instead have no EGFR overexpression (Shinojima et al, 2003).
Amplification of EGFR is associated with the presence of EGFR protein variants. In 68% of EGFR mutants, there is a deletion in the N-terminal ligand-binding region between amino acids 6 and 273 termed EGFRvIII. Deletion in the ligand-binding domains of EGFR can lead to ligand-independent activation of EGFR (Yamazaki et al, 1990). Due to the specific nature of these exon 2–7 deletions in EGFRvIII, common tyrosine kinase inhibitors such as gefitinib have limited therapeutic use (Schulte et al, 2013). Therefore, approaches to address the lack of extracellular receptor are being pursued. EGFRvIII is implicated in the PKA-dependent phosphorylation of DOCK180, a guanine exchange factor for Rac1. Overexpression of mutant DOCK180 lacking the phosphorylation site at S1280 in an EGFRvIII-containing cell line inhibited receptor-stimulated proliferation and survival (Feng et al, 2014). This EGFRvIII/PKA/DOCK180 interaction may offer a unique therapeutic target if EGFRvIII-specific PKA phosphorylation can be inhibited. However, EGFRvIII is not prognostic of overall median survival except in cases of survivors of ≥ 1 year which may limit the therapeutic value of this target (Heimberger et al, 2005).
p53 and PTEN
p53 is a well-known tumor suppressor protein that plays a fundamental role in the formation of high-grade tumors (England et al, 2013). p53 initiates DNA repair, or apoptosis if DNA damage is irreparable. There is a strong correlation between the presence of mutant p53 and the transition from low-grade astrocytoma to the high-grade glioblastoma (Sidransky et al, 1992). p53 mutant cells are more likely to expand to high-grade glioma as these cells outgrow and overtake the non-p53 mutant cell population (Sidransky et al, 1992). There is evidence that nuclear localization is correlated with long-term survival rates as nuclear p53 is responsible for apoptotic induction limiting tumor cell expansion. Long-term survivors (> 3 years) have tumors with high levels of nuclear p53 compared to short-term survivors. This is not caused by differences in the mutation rate (Burton et al, 2002). Recent gene therapy experiments with nanoparticle delivery of the p53 gene targeting glioblastoma and cancer stem cells showed induction of apoptosis after standard chemotherapy (Kim et al, 2014b) and improved survival in a mouse model. To date, this has not been tested in a clinical trial.
Multiple concurrent tumor suppressor mutations are common in GBM progression. One study found that primary tumors expressing mutant p53 had concomitant PTEN mutations or deletions in 6 out of the 10 samples (Zheng et al, 2008). PTEN is a phosphatase tumor suppressor critical in cellular homeostasis that is mutated in between 5 and 40% of GBMs and can be a prognostic indicator in patients > 45 years old (Srividya et al, 2011). Under normal conditions, PTEN facilitates homeostasis by preventing cell cycle entry, thus maintaining the neural stem cell population. Unsurprisingly, PTEN null mutants are more sensitive to growth factors and more prone to proliferation than wild-type neural stem cells (Groszer et al, 2006). Diagnostically, PTEN may turn out to be a valuable marker as PTEN levels are positively correlated with patient survival (Ermoian et al, 2002). The Cancer Genome Atlas (TCGA), a collaborative effort between the National Cancer Institute and the National Human Genome Institute, has elucidated PTEN mutations that may influence GBM development using genomic sequencing. The presence of PTEN non-sense mutations resulted in lower survival than wild-type in a murine xenograft model (Xu et al, 2014). Bryostatin, an inhibitor of PKC downstream of PTEN, suppressed tumor growth in the non-sense PTEN background suggesting that PTEN non-sense mutations can be indirectly targeted for treatment of GBM.
Isocitrate dehydrogenase
The transition from low-grade gliomas to secondary GBM relies on the convergence of many pro-oncogenic events. In addition to the critical roles of PTEN and p53, isocitrate dehydrogenase (IDH-1) mutations are now considered to be a fundamental step in this transition. Although rare in primary GBM at a rate of 5%, this mutation is found in 83% of all secondary GBM cases (Kloosterhof et al, 2011). IDH-1 mutations are, in fact, now believed to be one the earliest events in the formation of low-grade gliomas preceding any mutation that may occur in the p53 gene (Watanabe et al, 2009). Mutations in IDH-1 exist as somatic point mutations and can simultaneously result in a reduction in enzyme efficiency or enzymatic gain of function depending on the substrate. The known point mutation resides in the active site, which prevents the enzyme from successfully converting isocitrate to alpha-ketoglutarate. More importantly, the arginine at codon 132 is replaced with a histidine in 90% of cases (Yan et al, 2009). The R132H mutation causes IDH-1 to gain the ability to convert alpha-ketoglutarate to 2-hydroxyglutarate (2HG), an onco-metabolite. Since IDH-1 mutations occur on one allele, this allows both normal and mutant IDH-1 to co-dimerize or act in cis to produce this onco-metabolite by converting isocitrate to 2HG in a two-step metabolism (Fig 1). Loss of wild-type IDH-1 when the R132H mutation is present on the other allele results in a 14-fold lower level of 2HG suggesting that both isoforms must be active for onco-metabolite production (Jin et al, 2013). 2HG may prove to be a suitable biomarker for the presence of IDH-1 mutations as 2HG levels can be detected using magnetic resonance (Kalinina et al, 2012). However, IDH-1 mutations have not been shown to effect median survival rate or progression-free survival in secondary GBM (Juratli et al, 2012). IDH-1 is now being targeted for therapeutic use for instance by Agios Pharmaceuticals, who are currently moving forward with the drug candidate AG-120 after their tool compound inhibitors were found to be successful in glioma xenografts (Rohle et al, 2013). AG-120 is currently undergoing a phase I trial (clinicaltrials.gov; NCT02073994).
Figure 1. Inhibition of 2-hydroxyglutarate production in heterozygotic IDH-1 cells.
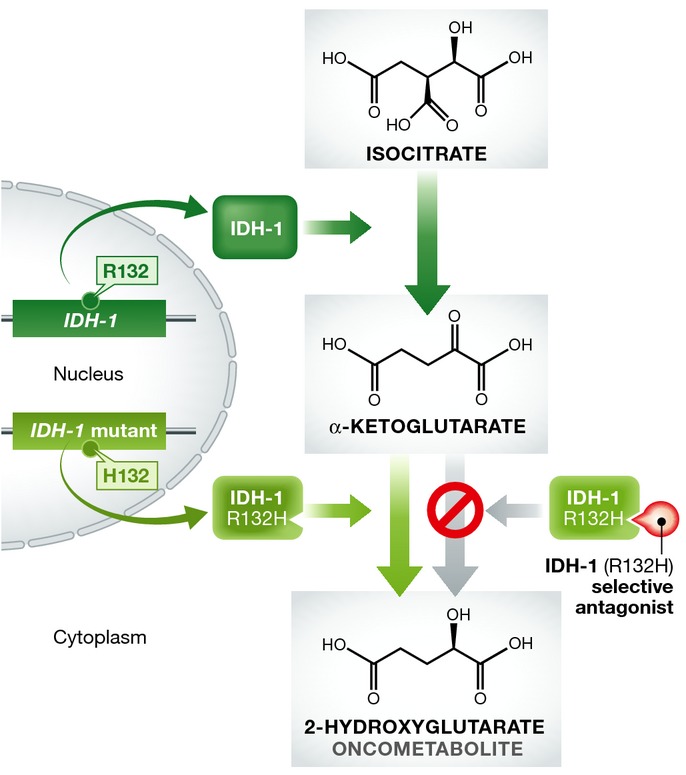
Wild-type IDH-1 converts isocitrate to alpha-ketoglutarate. Heterozygotic IDH-1 (R132H) mutant protein can convert alpha-ketoglutarate to 2-hydroxyglutarate, an onco-metabolite. Selective therapeutics against IDH-1 (R132H) prevent 2-hydroxyglutarate production while leaving normal IDH-1 enzymatic function intact.
Genomics
Personalized medicine for GBM is evolving in part due to progress in genomic sequencing. TCGA has made the genomic databases of over 20 cancers public in an effort to enhance integrated analysis on a common data set. In 2008, TCGA detailed a multi-dimensional analysis of 216 cases of GBM that identified genetic changes that include inactivation of the Neurofibromin 1 gene, ERRB2 mutations, and MGMT methylation (Cancer Genome Atlas Research Network, 2008). TCGA GBM data set continues to provide new insights in the development of GBM. A recent evaluation of TCGA RNA-Seq data revealed a neurotrophic tyrosine kinase receptor type 1–neurofascin gene fusion in GBM. In vitro, this gene fusion showed increased proliferation of 3T3 cells, suggesting an oncogenic-like role (Kim et al, 2014a). Oncogenic gene fusion analysis is a growing field, made possible through the exploitation of large databases like TCGA.
However, TCGA GBM data set has use beyond targets of oncogenesis and has potential prognostic value. For example, although miRNA does not appear to add prognostic power to multidimensional genomic models in GBM (Zhao et al, 2014), the involvement of miRNAs in GBM is evident and has been greatly elucidated due to TCGA. Loss of MIR-491 has been recently implicated in the proliferation and invasion of GBM. MIR-491, the gene encoding for miR-491-5p and miR-491-3p, is frequently deleted in GBM. This deletion is inversely correlated with the overexpression of EGFR, CDK6, and IGFBP2 (Li et al, 2014). In addition, the presence of functional MIR-491 impairs the propagation of GBM cancer stem cells through the EGFR, CDK6, and IGFBP2 proliferative pathways. Reintroduction of MIR-491 has promising therapeutic potential through the suppression of the proliferative pathways listed above. This depth of analysis would not have been possible without the volume of data available through TCGA.
miRNA pathways in GBM can influence the effectiveness of current treatments as with TMZ. For instance, miR-455-3p and miR-10a* confer cellular resistance to TMZ. Knockdown of either miR did not lead to cell death, but enhanced sensitivity to TMZ (Ujifuku et al, 2010). Other miRs such as miR-21 are differentially upregulated in GBM compared to lower-grade gliomas (Berthois et al, 2014). miR-21 is upregulated in response to TMZ and confers a certain degree of resistance to the drug, while the loss of miR-21 in TMZ-resistant cell lines resensitized them to the drug (Wong et al, 2012). Hence, while miR-21 inhibition or TMZ alone may be insufficient, their combination may significantly enhance cancer stem cell death (Zhang et al, 2012).
Cancer stem cells
GBM, similarly to others, is a heterogeneous tumor comprised of many cell types. A 2004 study first identified a small CD133+ stem cell-like population in GBM responsible for the maintenance and proliferation of the tumor (Yuan et al, 2004). Transplantation of CD133-positive, but not CD133-negative, cells from patient biopsies in severely compromised immunodeficient mice produced a phenocopy of the patient's original tumor (Choy et al, 2012). Targeting CD133 is a potential therapeutic strategy to eliminate the cancer stem cell population. CD133 silencing in GBM-derived stem cells was shown to increase post-implantation survival in an in vivo mouse model (Brescia et al, 2013).
Normal neural stem cells rely on NOTCH signaling for cellular homeostasis (Alexson et al, 2006). Gamma-secretase-mediated inhibition of the NOTCH pathway depletes CD133 and blocks tumor growth in vivo and neurosphere formation in culture (Fan et al, 2010). CD133 cells can also be depleted by knockdown of BMI1, a transcription repressor that prevents stem cells from altering pluripotency, indicating the importance of CD133 for cancer stem cells.
There is a clear negative relationship between GBM progression and tumor location in the adult subventricular zone (SVZ). Forty-seven percent of patients with SVZ-located tumors experienced progression-free survival after 6 months after treatment compared to 69% of non-SVZ-contacted tumor patients (Jafri et al, 2013). Over 95% of GBMs expressed SSEA-1, a known stem cell marker in the SVZ (Son et al, 2009). SSEA-1+ cells are capable of differentiation and expansion in neurospheres similarly to CD133+ cells (Son et al, 2009). In addition to recurrence, SVZ-associated GBM cases also progressed more than their non-SVZ-located counterparts.
A new pathway has been discovered that links GBM stem cells to the development of GBM-specific endothelial cell-related pericytes (G-pericytes). Pericytes are core components of the neurovasculature critical to the maintenance of the blood–brain barrier (BBB) and microvasculature regulation. Pericyte-deficient mice have extensive endothelial cell hyperplasia in addition to morphological changes such as increased vessel diameter (Hellstrom et al, 2001). In addition, 14- to 16-month-old pericyte-deficient mice have a 20- to 25-fold plasma-derived immunoglobulin buildup in the cortex and hippocampus compared to age-matched controls suggesting blood–brain barrier breakdown (Bell et al, 2010). While these pericytes are beneficial to normal brain function, TGFβ induction of G-pericyte differentiation from the cancer stem cell lineage has been shown to preserve mutations present in the stem cell population, thus facilitating in tumor-specific growth (Cheng et al, 2013a). Pericyte signaling pathways are stimulated by hypoxic-specific exosomes released from GBM cells that trigger paracrine stimulation of pericytes by endothelial cells (Kucharzewska et al, 2013). This signaling pathway promotes pericytic release of regulatory factors such as VEGF-A to maintain proper vascular function. Thus, G-pericyte prevention or elimination may rescue ineffective angiogenic therapies for GBM that modulate VEGF-A.
Metastasis
While lung or breast cancers often metastasize to the brain, GBM distal metastases are exceedingly rare and have only been reported in 0.44% of all cases (Robert & Wastie, 2008) The metastatic potential of GBM has nevertheless been recorded. For example, a case study found that two patients who had received organs from a single GBM donor eventually developed GBM metastases that led to their death. (Armanios et al, 2004). Most probably, the relative paucity of documented extracranial metastasis for GBM is in part due to the short lifespan of GBM patients. It is also possible that GBM cell escape is limited by the lack of lymphatic transport in the brain (Robert & Wastie, 2008). Indeed, the few reported metastasis cases were postulated to have been initiated by surgical resection causing BBB disruption (Schonsteiner et al, 2011). Clearly, GBM does have metastatic potential, similar to most other solid tumors, but this does not appear to be a significant factor in patient survival rates; as GBM-directed therapeutics improve patient life span, however, this may become an emerging issue.
Current standard of care
Resection
The current standard of care for GBM is maximally possible surgical resection of the tumor followed by combination radiotherapy and chemotherapy. Maximal resection for each tumor is case-specific based on tumor size, shape, and location of blood vessels, arteries, or sensitive brain regions. Surgical resection is generally classified as gross total resection (GTR), and subtotal resection (STR) when complete removal of the tumor is not met. Not surprisingly, one-year survival is significantly higher for patients with more than 90% tumor resection compared to those with less than 90% tumor resection (Orringer et al, 2012). It is thus essential that the surgeon's ability to resect the tumor as much as possible is improved to increase GBM patient survival. A current clinical trial is testing tumor-specific fluorescent staining to help surgeons differentiate between tumor and non-tumor cell tissue. 5-aminolevulinic acid (ALA) was the first attempt to show how this approach could aid in tumor resection (Stummer et al, 2000). ALA induces the accumulation of porphyrins specific to GBM that fluoresce under violet-blue light. The contrast in color between the porphyrin-containing tumor and adjacent normal tissue allows for more specific and thorough resection of tumor cells. ALA is likely to be very safe, with no associated deaths and only 1% of patients experiencing any neurological effects. Sixty-five percent of resections using ALA obtained GTR, while only 36% met GTR criteria using conventional methods (Stummer et al, 2006). Other fluorescent compounds are being tested to provide better resolution for optimal resection such as sodium fluorescein, which is both safe and feasible to use (Schebesch et al, 2013).
Radiotherapy
When GTR is unfeasible, radiotherapy has been used in conjunction with surgery as early as 1979 when increasing levels of radiation applied to tumors were found to be dose-dependently correlated with median survival rates (Walker et al, 1979). Radiation therapy induces severe DNA damage causing the cells to undergo apoptosis due to double-strand breaks. Standard external beam radiation therapy includes six weeks of localized radiation therapy five times per week. Resistance to radiotherapy can be problematic in GBM as EGFRvIII confers cellular resistance to such treatment options by upregulating the DNA double-stranded break repair machinery (Mukherjee et al, 2009). Therefore, EGFRvIII inhibitors may increase overall tumor sensitivity to radiation therapy. While external beam radiation therapy (EBRT) is the standard of care, radio-surgical techniques have been developed to increase radiation therapy effectiveness in patients experiencing GBM recurrence. Gamma knife therapy delivers stereotactic high doses of radiation that confine treatment to the targeted GBM area. Gamma knife is considered as ineffective in the treatment of primary tumors due to the excessively large tumor volume. However, gamma knife monotherapy in a GBM mouse xenografts model increased survival (Skeie et al, 2013). This may be advantageous in future treatment regimens if routine biomarker analysis for recurrence is implemented. Quick identification of recurrence would allow for gamma knife treatment while the tumor remains volumetrically small.
Chemotherapy
The current standard for chemotherapy for GBM is TMZ. First described in 2005, concurrent TMZ and radiotherapy increased median survival rates to 26.5% at 24 months, a vast improvement over the 10.4% with radiotherapy alone (Stupp et al, 2005). TMZ is a brain-penetrant alkylating agent that methylates purines (A or G) in DNA and induces apoptosis. TMZ is effective in a rechallenge setting where TMZ is reintroduced after a TMZ-free time period. TMZ rechallenge maintained similar progression-free rates seen in constant administration paradigms (Wick et al, 2009). However, as previously described, the genetic background of a given GBM greatly affects drug effectiveness. TMZ sensitivity was found to be correlated with the methylation state of O6-methylguanine-DNA methyltransferase (MGMT) promoter in cancer cells committed to differentiation and not in the stem-like progenitors. MGMT is a mediator of DNA mismatch repair that corrects TMZ-induced damage (Villalva et al, 2012). Lower cellular concentrations of MGMT due to gene silencing are correlated with higher sensitivity to TMZ and longer overall survival; this may be useful as a biomarker (Hegi et al, 2005; Esteller et al, 2000). Indeed, patients with MGMT gene silencing had survival rates of 21.5 versus 15.3 months. TMZ efficacy in stem cells may also be dependent upon the presence of MGMT. GBM cancer stem cells expressing MGMT did not respond as well as non-MGMT expressing cancer stem cells at the same dosage (Beier et al, 2008). The downside to TMZ use is the significant risk arising from TMZ-dependent DNA damage in healthy cells. This risk, combined with the possible inefficacy on GBM cells, strongly indicates that additional chemotherapy options are urgently required to improve both targeting of treatment to GBM cells and improving efficacy.
Novel therapies
Although current therapy regimens have improved over the past 20 years, overall patient survival has not risen to the levels obtained for other solid tumors. New therapies with novel empirical designs are currently in clinical trials (Table1). All such therapies are designed for use in combination with the current standard of care as a means to improve treatment efficacy, and range from personalized medicine approaches targeting the tumor cells to the disruption of the tumor microenvironment.
Table 1.
Potential GBM treatments currently in clinical trial.
| Treatment | Intervention | Molecular target | Clinical phase |
|---|---|---|---|
| TRC105 + Bevacizumab (Avastin) | Antibody + Drug | Endoglin/VEGF | 1 |
| Amgen386 | Antibody | Angiopoietin-1 and -2 | 1 |
| AMG595 | Antibody Drug Conjugate | EGFRvIII | 1 |
| PSMA ADC MMAE | Antibody Drug Conjugate | PSMA/Tubulin | 2 |
| Ketogenic Diet | Dietary Adjustment | N/A | 1 |
| Bevacizumab (Avastin) + TPI 287 | Drug | VEGF/Tubulin | 2 |
| AR-67 | Drug | Topoisomerase 1 | 2 |
| PD 0332991 (Palbociclib) | Drug | Cyclin-dependent Kinase 4/6 | 2 |
| Pazopanib (Votrient) + Topotecan (Hycamtin) | Drug | Tyrosine Kinase Receptors (multiple) + Topoisomerase 1 | 2 |
| G-202 | Drug | Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase (SERCA) pump | 2 |
| Aldoxorubicin | Drug | DNA | 2 |
| Dovitinib (TKI258) | Drug | FGFR/VEGFR/PDGFR | 1 |
| AG-120 | Drug | IDH-1 R132H | 1 |
| Axitinib (Inlyta) + Radiation Therapy | Drug + Radiation | Tyrosine Kinase Receptors (multiple) | 2 |
| NovoTTF-100A device + TMZ | Electrical device + Drug | N/A | 3 |
| DC-Vax L | Immunotherapy | N/A | 3 |
| HER2 Chimeric Antigen Receptor Expressing CMV-Specific Cytotoxic T Cells | Immunotherapy | N/A | 1 |
| Rindopepimut | Immunotherapy | EGFRvIII | 3 |
| Parvovirus H-1 (ParvOryx) | Virus | N/A | 1 |
| Live attenuated, oral (Sabin) serotype 1 poliovirus vaccine | Virus | N/A | 1 |
| DNX2401 and Temozolomide | Virus + TMZ | N/A | 1 |
Monoclonal antibodies
One of the leading new classes of therapeutic agents is based on the use of monoclonal antibodies that recognize cell surface receptors and ligands, to prevent receptor signaling through the disruption of receptor–ligand interactions and downstream receptor activation. FDA approved Avastin (bevacizumab), an antibody against vascular endothelial growth factor (VEGF), currently leads the way. GBM tumors, as they grow, secrete VEGF to promote neoangiogenesis. Systemic injection of Avastin aims to block the response to VEGF and thus prevent neovascularization of the tumor and consequently decrease its size (Ferrara et al, 2005). This treatment destabilizes the tumor microenvironment and does not target tumor-specific receptors or antigens, which is beneficial as the treatment is not restricted to a specific tumor type. There are, of course, side effects caused by a broad blockage of VEGF signaling, such as deep vein thrombosis (Hosokawa et al, 2010). Indeed, recent data show that Avastin in combination with the standard treatment did not improve overall patient survival compared to standard treatment alone (Gilbert et al, 2014); in addition, the group receiving Avastin experienced a significant impact in terms of angiogenic side effects. Avastin did improve progression-free survival to 10.6 months up from 6.2 months as reported by Genentech as part of the AVAglio phase III study. Prevention of GBM progression while not improving overall survival suggests that Avastin is more a means to contain GBM growth, rather than eliminate the tumor. As such, Avastin may be more useful for mitigating early-stage progression. An independent investigation showed that VEGF blockade reduced tumor size as expected, but unfortunately bolstered tumor invasiveness in human and mouse models (de Groot et al, 2010), possibly due to starvation-induced stimulation of tumor cell escape. Avastin is nevertheless still considered one of the best new treatments for GBM due to the relatively limited added toxicity compared to standard treatment of care.
In contrast to tumor agnostic antibodies like Bevacizumab, AMG595 specifically targets EGFRvIII and is currently being tested in phase I clinical trials. AMG595 is a non-cleavable linker immunoconjugate between a human monoclonal antibody directed against EGFRvIII and the cytotoxic agent mertansine (DM1). Once AMG595 engages EGFRvIII, receptor-mediated internalization occurs, thus targeting cytotoxic DM1 to the tumor cells expressing EGFRvIII. AMG595 comes with its own set of limitations. As described previously, EGFR is mutated in roughly 40% of GBM cases. Of these, 65% have EGFRvIII mutations, which thus leaves only a limited percentage of the total GBM cases that can potentially benefit from AMG595 as a possible treatment option and only in those cases that can be easily identified through immunohistological staining of tumor biopsies or microvesicle detection.
While therapeutic antibodies carry great potential due to the inherent specificity of binding and the multitude of surface proteins, there are specific issues in the case of GBM (and other brain tumors). In fact, any drug administered systemically would require transport across the blood–brain barrier, which normally impedes access to the vast majority of drugs. There are, however, various endothelial uptake mechanisms, which may be exploited to make antibody delivery to brain tumors possible. The transferrin receptor mediates the transfer of ligands via iron-mediated endocytosis (Qian et al, 2002). Antibodies might be adapted to use this system for brain delivery by enhancing their affinity for the transferrin receptor and thus increase passage across the BBB.
Innate immunotherapy
As an alternative approach, some groups are attempting to reengineer the patient innate immune system in order to combat their own GBM tumor. DCVax-L by Northwest Biotherapeutics is currently in phase III trial for newly diagnosed GBM cases. The phase I/II clinical trials showed that median life expectancy for DCVax-L-treated patients increased to nearly 3 years. DCVax-L uses patient-derived tumor and healthy dendritic cell tissues to educate the innate immune response to recognize GBM tissue for elimination and has been shown to be safe (Yu et al, 2004) (Prins et al, 2011). Differentiated dendritic cells are presented with the tumor biopsy and then reintroduced in the patient, thereby promoting T-cell aggregation and elimination of tumor cells. Prepared DCVax-L is administered intradermally three times with 2 week intervals between each administration. This is a clear example of personalized medicine as it requires immunization against a person's own tumor. DCVax-L immunization also requires tissue samples to be shipped to the DCVax-L laboratory for vaccine manufacturing.
A recent press release from Agenus on their Prophage G100 vaccine details the positive outcomes of a phase II trial (http://www.agenusbio.com). As with DCVax-L, clinicians use tumor biopsies to develop a personalized vaccine which induces the patient's T-cell population to eliminate the tumor. Heat-shock protein glycoprotein 96 and bound tumorigenic peptides partners are extracted from a patient's tumor and reintroduced intradermally to activate innate antigen-presenting cells, which expands the T-cell population. This vaccine is used in conjunction with the standard treatment of care. The released results of the phase II study indicate that the median survival rate had increased to 23.3 months from the 14.6 months with standard treatment alone. The positive outcome of the multi-institutional phase II study has clear promise as a combination therapy, but Prophage G100 has not entered a randomized phase III trial as of yet.
Oncolytic viruses
Oncolytic viruses have potential use as a treatment for GBM. These viruses are replication incompetent except in specific cell populations such as tumors. Once the selected viruses find their host cell through surface marker identification, the viruses undergo lytic expansion, thus destroying the cell population, and remain replicative incompetent once the cell population is eradicated. After the tumor cell population is eliminated, patients can be treated with anti-viral medication to remove excess virus. These viruses are readily genetically manipulated and are effective unless the patient has a pre-existing immunity to the viral type used. Selectivity of these viruses depends on the cell surface expression of targeted receptors. EGFRvIII, PDGFR, and IL-13R have all been used as selectivity receptors for GBM in oncolytic virus production.
Oncolytic viruses are under investigation for use in GBM, including Herpes Simplex 1 as it contains double-stranded DNA and is a common infectious human pathogen. HSV-1 M032 is being explored as it lacks the y134.5 neurovirulence loci which prevents virus latency and has appropriate bio-distribution after intracerebral injection in non-human primates with no adverse clinical signs (Roth et al, 2014). HSV-1 M032 is in phase I trial, which has not been opened for recruitment to date. GBM Adenovirus trials have also begun using DNX-2401. Formerly known as Delta-24 this adenovirus is selective for GBM due to the deregulation of retinoblastoma protein in several cancers like GBM. Delta-24 replication is dependent on functionally inactive retinoblastoma protein (Fueyo et al, 2000). The addition of an RGD-4C peptide gives the virus high affinity for integrins αvβ3 and αvβ5 and increases oncolytic activity against GBM compared to non-RGD-containing analogs (Fueyo et al, 2003). Although the mechanism remains unclear, the adenovirus may promote cell death through autophagic activity as noted through the appearance of autophagic vesicles. In mouse xenograft models, Delta-24-RGD-4C therapy decreased tumor size and increased mouse survival. Preliminary results indicate that Delta-24-RGD-4C single-dose injections resulted in either stable, partial, or complete regression in 52% of GBM cases (Pol et al, 2013).
Small-molecule inhibitors
Targeted screening has led to potential GBM small-molecule inhibitors such as NSC-154829 which selectively upregulates caspases 3 and 7 in EGFRvIII-expressing GBM cells promoting apoptotic death (Trembath et al, 2007). NSC-154829 does not have any downstream pathway effect and does not elicit a response in secondary GBM cell lines which may suggest caspases 3 and 7 as markers of primary GBM (Trembath et al, 2007). There is limited public data on NSC-154829 to estimate the feasibility of this small-molecule inhibitor as a treatment.
The WNT pathway inhibitor SEN461 is another potential small-molecule therapeutic target for GBM. The WNT pathway is not traditionally considered a GBM-relevant one; however, SEN461 was found to inhibit GBM growth both in vitro and in vivo (De Robertis et al, 2013). The compound interferes with β-catenin phosphorylation, which is required for the anchorage-dependent growth of GBM, and although its efficacy has been shown both in patient-derived cells and in a Xenopus embryo model in vivo, no clinical progress has been reported to date.
Screening of a NIH diversity set of 1364 compounds identified Vacquinol-1 as an inducer of non-apoptotic cell death in glioma cells (Kitambi et al, 2014). Cell death was the result of micropinocytotic vacuole accumulation, which led to redistribution of the cytoplasm causing cell membrane rupture. The effect of Vacquinol-1 appears glioma cell specific. While the exact mechanism is unknown, shRNA knockdown of MMK4, a factor critical in micropinocytosis, rendered glioma cells resistant to Vacquinol-1. Of relevance, the compound crossed the blood–brain barrier in a murine xenograft model of GBM, where it significantly increased survival providing positive preclinical validation of the compound. This novel and potentially effective compound may in turn provide a unique therapeutic strategy given its mode of action.
While the two small molecules described above are pathway inhibitors, small-molecule epigenetic modulators are also receiving considerable attention as a possible therapeutic option. Such compounds alter the epigenetic landscape and may impact many downstream pathways simultaneously. For instance, epigenetic drugs may affect tumor growth by regulating gene expression through the availability of heterochromatin. Bromodomain (BRD)-containing proteins are sensors that bind to acetylated lysines on histone residues and recruit protein complexes to alter gene expression by modulating heterochromatin (Sanchez & Zhou, 2009). The inhibition of epigenetic readers can prohibit complex formation and subsequent transcription (Fig 2). JQ1, an inhibitor of the Bromodomains and extra terminal (BET) domain family of proteins, has been shown to reduce GBM size in mice, which might be of clinical relevance even though JQ1 is unlikely to be useful in the clinic due to its short half-life and low CNS delivery (Cheng et al, 2013b). JQ1, however, is but one of many BET inhibitors that are currently under investigation. For instance, IBET-151 or IBET-762 is currently being investigated in GBM as a possible alternative, although they are not brain penetrant (Dawson et al, 2011). For instance, Bromodomain 4 disruption using the small-molecule inhibitor IBET-151 led to GBM cell cycle arrest in cell line models (Pastori et al, 2014). Epigenetix Inc. appears to have brain-penetrant long-lasting BRD inhibitors useful for GBM, though no clinical trials have been initiated yet (epigenetix.com, personal communication). No epigenetic small-molecule inhibitor is currently under clinical trial for GBM; it is therefore impossible to assess this approach in terms of treatment outcomes. It is, however, clear that epigenetic regulation plays an important role in tumorigenesis, and thus, this approach is one of the most exciting potential developments in GBM therapy development, and we eagerly anticipate future clinical trials in this space.
Figure 2. Drug modification of epigenetic regulation.
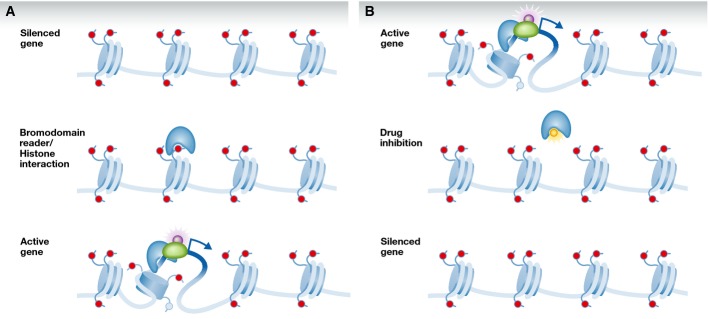
(A) Bromodomain readers recognize modified residues on histone tails which can lead to unraveling of the DNA/histone complex contingent on the bromodomain-containing complex composition. Unwound DNA is available for transcription complex interaction and transcription. (B) Unwound, transcriptionally active DNA reliant on bromodomain-containing complexes can be therapeutically targeted. Drugs blocking the bromodomain/histone tail modification interaction can prevent the helicase activity by bromodomain-containing complexes, thus stereohindering transcription regulators and silencing genes.
Conclusion
Current GBM treatments have not improved overall patient survival rates to the levels achieved for other brain tumors. From the basic science standpoint, there is a critical need to understand how GBM arises or is evolved from earlier gliomas. Targeted therapies may prove to have limited efficacy as GBM can arise from a variety of mutations. Early diagnosis may be the key to improving patient survival rates through the prevention of tumor growth, and therefore, the identification of early biomarkers is critical. Non-invasive blood monitoring of tumor microvesicles may provide quick, accurate, and early detection of GBM. Early subtyping of GBM tumors could occur before patients undergo tumor resection to identify treatment regimens that may reduce tumor volume pre-resection. The combination of treatment approaches detailed here may prove an effective regime for the treatment of GBM tumors.
We suggest, for example, that early detection of an EGFRvIII-presenting GBM, diagnosed through blood microvesicle screening, could perhaps one day allow a therapeutic regimen that would extend patient survival well beyond current levels. Such a patient could also be given epigenetic drugs to arrest differentiating GBM tumor cells and prevent tumor growth and development in a neoadjuvant setting. Indeed, should the tumor in such a patient be located distally from the SVZ and the patient undergoes dye-assisted resection, followed by a personalized vaccine, then prognosis is likely to be even better. Current TMZ and radiotherapy treatment options to kill the remaining tumor cells will continue to be used to prevent any possibility of recurrence. Considering all the possible new treatments that are under investigation, we posit that we are on the verge of a watershed moment in GBM management.
Glossary
- 2-hydroxyglutarate
Isocitrate is metabolized to alpha-ketoglutarate by IDH-1. Mutated IDH-1 (with a histidine substitution at arginine 132) can further metabolize alpha-ketoglutarate to 2-hydroxyglutarate. This onco-metabolite has been associated with a dysregulation in reactive oxygen species.
- Aerobic Glycolysis
The conversion of glucose to lactic acid even in the presence of oxygen
- Adenovirus
Non-enveloped virus that contains single-stranded double DNA capable of using host machinery to propagate
- Biomarker
Cellular component capable of predicting the biological state of a cell population
- Bromodomain
Alpha helical protein domain that recognizes acetylation marks on histone tails
- Epigenetics
The study of transcriptional regulation not caused by DNA sequence. Epigenetic changes can lead to transient or sustained alterations to gene expression based on the type of modification such as histone acetylation or DNA methylation.
- Pericyte
Vasculature-wrapping cells critical in maintaining the blood–brain barrier through interactions with endothelial cells, neurons, and astrocytes. G-pericytes are those derived from glioblastoma multiforme.
- Gamma Knife
Stereotactic high-dose radiation targeting the brain region of interest
- Immunoconjugate
Antibody conjoined to a second molecule, usually cytotoxic, in order to direct the cytotoxin to the target of interest
- Microvesicle
Plasma membrane-derived membrane-enclosed bodies that may contain miRNA, mRNA, or protein, and are capable of intercellular communication.
- Perfusion Weight Imaging
Contrast dye visualization of cerebral blood flow in vessels using magnetic resonance imaging
Conflict of interest
The authors declare that they have no conflict of interest.
Pending issues
Tumor heterogeneity is a major impediment to successful GBM treatment.
Creating near-term solutions to bridge current diagnostic techniques will inform therapeutic development to combat heterogeneity.
GBM cell models rarely recapitulate actual tumor heterogeneity.
Patient-derived xenograft (PDX) models may address heterogeneity as a personalized medicine investigative technique.
Epigenetic therapy may limit tumor heterogeneity if identified with early diagnosis.
References
- Alexson TO, Hitoshi S, Coles BL, Bernstein A, van der Kooy D. Notch signaling is required to maintain all neural stem cell populations–irrespective of spatial or temporal niche. Dev Neurosci. 2006;28:34–48. doi: 10.1159/000090751. [DOI] [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- Aquino D, Di Stefano AL, Scotti A, Cuppini L, Anghileri E, Finocchiaro G, Bruzzone MG, Eoli M. Parametric response maps of perfusion MRI may identify recurrent glioblastomas responsive to bevacizumab and irinotecan. PLoS One. 2014;9:e90535. doi: 10.1371/journal.pone.0090535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios MY, Grossman SA, Yang SC, White B, Perry A, Burger PC, Orens JB. Transmission of glioblastoma multiforme following bilateral lung transplantation from an affected donor: case study and review of the literature. Neuro-Oncol. 2004;6:259–263. doi: 10.1215/S1152851703000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier D, Rohrl S, Pillai DR, Schwarz S, Kunz-Schughart LA, Leukel P, Proescholdt M, Brawanski A, Bogdahn U, Trampe-Kieslich A, et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008;68:5706–5715. doi: 10.1158/0008-5472.CAN-07-6878. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y, Delfino C, Metellus P, Fina F, Nanni-Metellus I, Al AH, Pirisi V, Ouafik L, Boudouresque F. Differential expression of miR200a-3p and miR21 in grade II-III and grade IV gliomas: evidence that miR200a-3p is regulated by O -methylguanine methyltransferase and promotes temozolomide responsiveness. Cancer Biol Ther. 2014;15:938–950. doi: 10.4161/cbt.28920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brescia P, Ortensi B, Fornasari L, Levi D, Broggi G, Pelicci G. CD133 is essential for glioblastoma stem cell maintenance. Stem Cells. 2013;31:857–869. doi: 10.1002/stem.1317. [DOI] [PubMed] [Google Scholar]
- Burton EC, Lamborn KR, Forsyth P, Scott J, O'Campo J, Uyehara-Lock J, Prados M, Berger M, Passe S, Uhm J, et al. Aberrant p53, mdm2, and proliferation differ in glioblastomas from long-term compared with typical survivors. Clin Cancer Res. 2002;8:180–187. [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013a;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Gong Y, Ma Y, Lu K, Lu X, Pierce LA, Thompson RC, Muller S, Knapp S, Wang J. Inhibition of BET bromodomain targets genetically diverse glioblastoma. Clin Cancer Res. 2013b;19:1748–1759. doi: 10.1158/1078-0432.CCR-12-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy W, Nagasawa DT, Trang A, Thill K, Spasic M, Yang I. CD133 as a marker for regulation and potential for targeted therapies in glioblastoma multiforme. Neurosurg Clin N Am. 2012;23:391–405. doi: 10.1016/j.nec.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C, Savitski MM, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis A, Valensin S, Rossi M, Tunici P, Verani M, De Rosa A, Giordano C, Varrone M, Nencini A, Pratelli C, et al. Identification and characterization of a small-molecule inhibitor of wnt signaling in glioblastoma cells. Mol Cancer Ther. 2013;12:1180–1189. doi: 10.1158/1535-7163.MCT-12-1176-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England B, Huang T, Karsy M. Current understanding of the role and targeting of tumor suppressor p53 in glioblastoma multiforme. Tumour Biol. 2013;34:2063–2074. doi: 10.1007/s13277-013-0871-3. [DOI] [PubMed] [Google Scholar]
- Ermoian RP, Furniss CS, Lamborn KR, Basila D, Berger MS, Gottschalk AR, Nicholas MK, Stokoe D, Haas-Kogan DA. Dysregulation of PTEN and protein kinase B is associated with glioma histology and patient survival. Clin Cancer Res. 2002;8:1100–1106. [PubMed] [Google Scholar]
- Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Hu B, Vuori K, Sarkaria JN, Furnari FB, Cavenee WK, Cheng SY. EGFRvIII stimulates glioma growth and invasion through PKA-dependent serine phosphorylation of Dock180. Oncogene. 2014;33:2504–2512. doi: 10.1038/onc.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, Liu TJ, Jiang H, Lemoine MG, Suzuki K, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- Galldiks N, Ullrich R, Schroeter M, Fink GR, Jacobs AH, Kracht LW. Volumetry of [(11)C]-methionine PET uptake and MRI contrast enhancement in patients with recurrent glioblastoma multiforme. Eur J Nucl Med Mol Imaging. 2010;37:84–92. doi: 10.1007/s00259-009-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro-Oncol. 2010;12:233–242. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, Geschwind DH, Liu X, Kornblum HI, Wu H. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci USA. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Young RJ, Shah AD, Schweitzer AD, Graber JJ, Shi W, Zhang Z, Huse J, Omuro AM. Pretreatment Dynamic Susceptibility Contrast MRI Perfusion in Glioblastoma: Prediction of EGFR Gene Amplification. Clin Neuroradiol. 2014 doi: 10.1007/s00062-014-0289-3. doi: 10.1007/s00062-014-0289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanpaa KJ, Burma S, Zhao D, Habib AA. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia. 2010;12:675–684. doi: 10.1593/neo.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, Sawaya R, Aldape K. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462–1466. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T, Tokita H, Matsuyama T, Sakamoto K, Nishida K, Iwao Y, Koshiishi H, Okamura T, Koshinaga T. Two cases of venous thrombosis confirmed during the bevacizumab combination chemotherapy for colorectal cancer. Gan To Kagaku Ryoho. 2010;37:2520–2522. [PubMed] [Google Scholar]
- Jafri NF, Clarke JL, Weinberg V, Barani IJ, Cha S. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro-Oncol. 2013;15:91–96. doi: 10.1093/neuonc/nos268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Reitman ZJ, Duncan CG, Spasojevic I, Gooden DM, Rasheed BA, Yang R, Lopez GY, He Y, McLendon RE, et al. Disruption of wild-type IDH1 suppresses D-2-hydroxyglutarate production in IDH1-mutated gliomas. Cancer Res. 2013;73:496–501. doi: 10.1158/0008-5472.CAN-12-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juratli TA, Kirsch M, Geiger K, Klink B, Leipnitz E, Pinzer T, Soucek S, Schrock E, Schackert G, Krex D. The prognostic value of IDH mutations and MGMT promoter status in secondary high-grade gliomas. J Neurooncol. 2012;110:325–333. doi: 10.1007/s11060-012-0977-2. [DOI] [PubMed] [Google Scholar]
- Kalinina J, Carroll A, Wang L, Yu Q, Mancheno DE, Wu S, Liu F, Ahn J, He M, Mao H, et al. Detection of “oncometabolite” 2-hydroxyglutarate by magnetic resonance analysis as a biomarker of IDH1/2 mutations in glioma. J Mol Med. 2012;90:1161–1171. doi: 10.1007/s00109-012-0888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee Y, Cho HJ, Lee YE, An J, Cho GH, Ko YH, Joo KM, Nam DH. NTRK1 fusion in glioblastoma multiforme. PLoS One. 2014a;9:e91940. doi: 10.1371/journal.pone.0091940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Rait A, Kim E, Pirollo KF, Nishida M, Farkas N, Dagata JA, Chang EH. A Nanoparticle Carrying the p53 Gene Targets Tumors Including Cancer Stem Cells, Sensitizes Glioblastoma to Chemotherapy and Improves Survival. ACS Nano. 2014b:5494–5514. doi: 10.1021/nn5014484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Chung JK, Im SH, Jeong JM, Lee DS, Kim DG, Jung HW, Lee MC. 11C-methionine PET as a prognostic marker in patients with glioma: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2005;32:52–59. doi: 10.1007/s00259-004-1598-6. [DOI] [PubMed] [Google Scholar]
- Kitambi SS, Toledo EM, Usoskin D, Wee S, Harisankar A, Svensson R, Sigmundsson K, Kalderen C, Niklasson M, Kundu S, et al. Vulnerability of glioblastoma cells to catastrophic vacuolization and death induced by a small molecule. Cell. 2014;157:313–328. doi: 10.1016/j.cell.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Kloosterhof NK, Bralten LB, Dubbink HJ, French PJ, van den Bent MJ. Isocitrate dehydrogenase-1 mutations: a fundamentally new understanding of diffuse glioma? Lancet Oncol. 2011;12:83–91. doi: 10.1016/S1470-2045(10)70053-X. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ohnishi A, Promsuk J, Shimizu S, Kanai Y, Shiokawa Y, Nagane M. Enhanced tumor growth elicited by L-type amino acid transporter 1 in human malignant glioma cells. Neurosurgery. 2008;62:493–504. doi: 10.1227/01.neu.0000316018.51292.19. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Tsuchida T, Uematsu H, Kimura H, Okazawa H, Itoh H. 18F-FDG PET of common enhancing malignant brain tumors. AJR Am J Roentgenol. 2008;190:W365–W369. doi: 10.2214/AJR.07.2660. [DOI] [PubMed] [Google Scholar]
- Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu Y, Granberg KJ, Wang Q, Moore LM, Ji P, Gumin J, Sulman EP, Calin GA, Haapasalo H, et al. Two mature products of MIR-491 coordinate to suppress key cancer hallmarks in glioblastoma. Oncogene. 2014 doi: 10.1038/onc.2014.98. doi: 10.1038/onc.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunsford LD, Martinez AJ, Latchaw RE. Magnetic resonance imaging does not define tumor boundaries. Acta Radiol Suppl. 1986;369:154–156. [PubMed] [Google Scholar]
- Manterola L, Guruceaga E, Gallego Perez-Larraya J, Gonzalez-Huarriz M, Jauregui P, Tejada S, Diez-Valle R, Segura V, Sampron N, Barrena C, et al. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro-Oncol. 2014;16:520–527. doi: 10.1093/neuonc/not218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLendon RE, Halperin EC. Is the long-term survival of patients with intracranial glioblastoma multiforme overstated? Cancer. 2003;98:1745–1748. doi: 10.1002/cncr.11666. [DOI] [PubMed] [Google Scholar]
- Mukherjee B, McEllin B, Camacho CV, Tomimatsu N, Sirasanagandala S, Nannepaga S, Hatanpaa KJ, Mickey B, Madden C, Maher E, et al. EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res. 2009;69:4252–4259. doi: 10.1158/0008-5472.CAN-08-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C, Chaudhary N, Sagher O. Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg. 2012;117:851–859. doi: 10.3171/2012.8.JNS12234. [DOI] [PubMed] [Google Scholar]
- Pardo FS, Aronen HJ, Fitzek M, Kennedy DN, Efird J, Rosen BR, Fischman AJ. Correlation of FDG-PET interpretation with survival in a cohort of glioma patients. Anticancer Res. 2004;24:2359–2365. [PubMed] [Google Scholar]
- Pastori C, Daniel M, Penas C, Volmar CH, Johnstone AL, Brothers SP, Graham RM, Allen B, Sarkaria JN, Komotar RJ, et al. BET bromodomain proteins are required for glioblastoma cell proliferation. Epigenetics. 2014;9:611–620. doi: 10.4161/epi.27906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol JG, Marguerie M, Arulanandam R, Bell JC, Lichty BD. Panorama from the oncolytic virotherapy summit. Mol Ther. 2013;21:1814–1818. doi: 10.1038/mt.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- Robert M, Wastie M. Glioblastoma multiforme: a rare manifestation of extensive liver and bone metastases. Biomed Imaging Intervention J. 2008;4:e3. doi: 10.2349/biij.4.1.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B, Komisopoulou E, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JC, Cassady KA, Cody JJ, Parker JN, Price KH, Coleman JM, Peggins JO, Noker PE, Powers NW, Grimes SD, et al. Evaluation of the safety and biodistribution of M032, an attenuated herpes simplex virus type 1 expressing hIL-12, after intracerebral administration to aotus nonhuman primates. Hum Gene Ther Clin Dev. 2014;25:16–27. doi: 10.1089/humc.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez R, Zhou MM. The role of human bromodomains in chromatin biology and gene transcription. Curr Opin Drug Discov Devel. 2009;12:659–665. [PMC free article] [PubMed] [Google Scholar]
- Schebesch KM, Proescholdt M, Hohne J, Hohenberger C, Hansen E, Riemenschneider MJ, Ullrich W, Doenitz C, Schlaier J, Lange M, Brawanski A. Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery—a feasibility study. Acta neurochirurgica. 2013;155:693–699. doi: 10.1007/s00701-013-1643-y. [DOI] [PubMed] [Google Scholar]
- Schonsteiner SS, Bommer M, Haenle MM, Klaus B, Scheuerle A, Schmid M, Mayer-Steinacker R. Rare phenomenon: liver metastases from glioblastoma multiforme. J Clin Oncol. 2011;29:e668–e671. doi: 10.1200/JCO.2011.35.9232. [DOI] [PubMed] [Google Scholar]
- Schuhmann MU, Zucht HD, Nassimi R, Heine G, Schneekloth CG, Stuerenburg HJ, Selle H. Peptide screening of cerebrospinal fluid in patients with glioblastoma multiforme. Eur J Surg Oncol. 2010;36:201–207. doi: 10.1016/j.ejso.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Schulte A, Liffers K, Kathagen A, Riethdorf S, Zapf S, Merlo A, Kolbe K, Westphal M, Lamszus K. Erlotinib resistance in EGFR-amplified glioblastoma cells is associated with upregulation of EGFRvIII and PI3Kp110delta. Neuro-oncology. 2013;15:1289–1301. doi: 10.1093/neuonc/not093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, Hochberg FH, Breakefield XO, Weissleder R, Lee H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012;18:1835–1840. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, Oka K, Ishimaru Y, Ushio Y. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–6970. [PubMed] [Google Scholar]
- Sidransky D, Mikkelsen T, Schwechheimer K, Rosenblum ML, Cavanee W, Vogelstein B. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature. 1992;355:846–847. doi: 10.1038/355846a0. [DOI] [PubMed] [Google Scholar]
- Skeie BS, Wang J, Dodoo E, Heggdal JI, Gronli J, Sleire L, Bragstad S, Ganz JC, Chekenya M, Mork S, et al. Gamma knife surgery as monotherapy with clinically relevant doses prolongs survival in a human GBM xenograft model. Biomed Res Int. 2013;2013:139674. doi: 10.1155/2013/139674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srividya MR, Thota B, Shailaja BC, Arivazhagan A, Thennarasu K, Chandramouli BA, Hegde AS, Santosh V. Homozygous 10q23/PTEN deletion and its impact on outcome in glioblastoma: a prospective translational study on a uniformly treated cohort of adult patients. Neuropathology. 2011;31:376–383. doi: 10.1111/j.1440-1789.2010.01178.x. [DOI] [PubMed] [Google Scholar]
- Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- Stolc S, Jakubikova L, Kukurova I. Body distribution of C-methionine and FDG in rat measured by microPET. Interdiscip Toxicol. 2011;4:52–55. doi: 10.2478/v10102-011-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93:1003–1013. doi: 10.3171/jns.2000.93.6.1003. [DOI] [PubMed] [Google Scholar]
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. Group AL-GS ( [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent BM, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Trembath DG, Lal A, Kroll DJ, Oberlies NH, Riggins GJ. A novel small molecule that selectively inhibits glioblastoma cells expressing EGFRvIII. Mol Cancer. 2007;6:30. doi: 10.1186/1476-4598-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujifuku K, Mitsutake N, Takakura S, Matsuse M, Saenko V, Suzuki K, Hayashi K, Matsuo T, Kamada K, Nagata I, et al. miR-195, miR-455-3p and miR-10a(*) are implicated in acquired temozolomide resistance in glioblastoma multiforme cells. Cancer Lett. 2010;296:241–248. doi: 10.1016/j.canlet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Villalva C, Cortes U, Wager M, Tourani JM, Rivet P, Marquant C, Martin S, Turhan AG, Karayan-Tapon L. O6-Methylguanine-Methyltransferase (MGMT) Promoter Methylation Status in Glioma Stem-Like Cells is Correlated to Temozolomide Sensitivity Under Differentiation-Promoting Conditions. Int J Mol Sci. 2012;13:6983–6994. doi: 10.3390/ijms13066983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick A, Pascher C, Wick W, Jauch T, Weller M, Bogdahn U, Hau P. Rechallenge with temozolomide in patients with recurrent gliomas. J Neurol. 2009;256:734–741. doi: 10.1007/s00415-009-5006-9. [DOI] [PubMed] [Google Scholar]
- Wong ST, Zhang XQ, Zhuang JT, Chan HL, Li CH, Leung GK. MicroRNA-21 inhibition enhances in vitro chemosensitivity of temozolomide-resistant glioblastoma cells. Anticancer Res. 2012;32:2835–2841. [PubMed] [Google Scholar]
- Xu J, Li Z, Wang J, Chen H, Fang JY. Combined PTEN mutation and protein expression associate with overall and disease-free survival of glioblastoma patients. Transl Oncol. 2014;7:196–205.e1. doi: 10.1016/j.tranon.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki F, Kurisu K, Satoh K, Arita K, Sugiyama K, Ohtaki M, Takaba J, Tominaga A, Hanaya R, Yoshioka H, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235:985–991. doi: 10.1148/radiol.2353031338. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Ohba Y, Tamaoki N, Shibuya M. A deletion mutation within the ligand binding domain is responsible for activation of epidermal growth factor receptor gene in human brain tumors. Jpn J Cancer Res. 1990;81:773–779. doi: 10.1111/j.1349-7006.1990.tb02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wan Y, Pan T, Gu X, Qian C, Sun G, Sun L, Xiang Y, Wang Z, Shi L. MicroRNA-21 inhibitor sensitizes human glioblastoma U251 stem cells to chemotherapeutic drug temozolomide. J Mol Neurosci. 2012;47:346–356. doi: 10.1007/s12031-012-9759-8. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Shi X, Xie Y, Huang J, Shia B, Ma S. Combining multidimensional genomic measurements for predicting cancer prognosis: observations from TCGA. Brief Bioinform. 2014 doi: 10.1093/bib/bbu003. doi: 10.1093/bib/bbu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]


