Abstract
Sorghum/millet-based beverages, Obiolor and Pito, were evaluated for their nutritional and antioxidant dispositions. Analyzed Obiolor and Pito contained 96% and 97% moisture; 7.8% and 3.7% crude protein; 8.9% and 5.6% available carbohydrate; 0.39% and 0.31% crude fat; 0.3% and 0.2% crude fiber; 2.4% and 1.5% ash; and 459.3 and 164 kJ/g energy value, respectively. Obiolor and Pito (1.0 mL) scavenged 2,2-diphenyl-1-picrylhydrazyl by 87% and 81%; superoxide ion by 65% and 59%; hydrogen peroxide by 79% and 76%; and hydroxyl radical by 82% and 85%, respectively. The beverages significantly reduced ferric ion. Aflatoxin B1-mediated increase in lipid peroxidation products (conjugated dienes, lipid hydroperoxides, and malondialdehydes) and protein carbonyl in the microsomes were significantly (P < 0.05) reduced by the beverages. The data obtained from this study show that the sorghum-based beverages (Obiolor and Pito) can serve as functional foods, as evident from their antioxidant capabilities in addition to their gross energy content.
Keywords: Lipid peroxidation, Obiolor, Pito, protein carbonyl, reactive oxygen species
Introduction
Consumer's interests in the health-enhancing roles of specific foods or physiologically active food components (functional foods) have gained rapid increase (Hasler 1998). The physiologically active components of functional foods, which include polyphenols, micronutrients, and macronutrients, proffer physiological benefit beyond basic nutritional requirements (Pang et al. 2012). Functional foods play an important role in the prevention of diseases of metabolic imbalances such as obesity, type 2 diabetes, hypertension, food allergies and intolerances, gastrointestinal and inflammatory disorders as well as cancer. Cereals, vegetables, fruits, pulses, and other plant foods are major sources of antioxidants (e.g., isothiocyanates, phytic acids, flavonoids, phenolics, sterols). Thus, any sorghum/millet-based food products could possess functional food capability. In Nigeria and some other African countries, sorghum/millet is an important component of staple foods.
The nutritional composition of Sorghum bicolor grain includes energy (193 cal), moisture content (52%), protein (7.1 g), fat (0.6 g), carbohydrates (39.8 g), fiber (0.9 g), calcium (10 mg), iron (3.5 mg), and niacin (1.7 mg). Phytochemical constituents include phenolics, polyflavonols, thiols, anthocyanins, tannins, 3-deoxyanthocyanidin, flavone, and flavanone (Dykes et al. 2009). Ajiboye et al. (2013a,b) recently reported that S. bicolor grains extract protected N-nitrosodiethylamine-induced oxidative stress in rat microsomes in vitro and in vivo. Millets are the most widely grown cereals and used in preparation of various kinds of beverages (e.g., koko sour water, Obiolor, kunun-zaki) and porridges (koko and kirario). Millet has nutritive value, which resemble that of sorghum. However, pearl millet has slightly lower starch contents, higher protein and lipid content than sorghum and most other common cereals. This makes the energy yield of millet higher than that of sorghum. Millet contains lysine and sulfur containing amino acids, threonine and tryptophan (Matz 1991), and therefore millet has a better amino acid balance than sorghum. Indigenous sorghum-based beverages widely consumed in Nigeria are Obiolor and Pito.
Pito is a light brown alcoholic beverage made from S. bicolor grains with sweet–sour taste (Ekundayo 1969). It is widely consumed in Ghana and Nigeria. Traditionally, Pito is considered a nutritious source of instant energy for work by many Nigerian tribes. It is also offered to ancestors by pouring it on the ground, a ritual practiced by Nigerians (Tamang and Samuel 2010). It contains lactic acid, sugars, and amino acids and has an alcohol content of 3% (Ekundayo 1969). Organisms responsible for souring include Geotrichum candidum and Lactobacillus sp. (Odunfa and Oyewole 1998), while Candida sp. are responsible for the alcoholic fermentation. Pito is an excellent source of calories and also contributes valuable protein to consumers (Ekundayo 1969).
Obiolor is a nonalcoholic beverage produced from fermented sorghum and millet malts in Nigeria. Obiolor is consumed daily by the Igala tribe in Nigeria and highly associated with good health. It is a thin gruel with sweet taste. The sweet taste is attributed to sorghum and millet malt (Achi 1990). The microorganisms involved in the fermentation of Obiolor are Lactobacillus plantarum and Lactococcus lactis, which are capable of producing organic acids contributing to the acidity, taste, and aroma of the end product. Presence of Bacillus species is also encountered during the fermentation of Obiolor (Achi 1990).
Despite the arrays of information available on the nutritional and antioxidant constituents of sorghum and millet, none exist for the sorghum/millet-based beverages (Obiolor and Pito). This study, thus investigates the nutritional and antioxidant dispositions of Obiolor and Pito.
Materials and Methods
Plant materials
Red variety of S. bicolor and millet were obtained from Igbona market, Osogbo, Nigeria. The identification of the sorghum and millet were done at Forestry Research Institute of Nigeria, Ibadan, Nigeria.
Chemicals and assay kits
Folin–Ciocalteu's reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), hydrogen peroxide (H2O2), iron (II) tetraoxosulfate (IV) (FeSO4), 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), nitroblue tetrazolium (NBT), phenazine methosulfate (PMS), epicatechin, nicotinamide adenine dinucleotide (NADH), diphenylamine, guanidine hydrochloride, and salicylic acid were procured from Research Organics, Cleveland, OH. All other reagents used were supplied by Sigma-Aldrich Inc., St. Louis, MO.
Preparation of Obiolor
Obiolor was prepared in the laboratory using the procedure described by Achi (1990). Briefly, sorghum and millet grains were steeped in water overnight, after which the grains were wrapped in fresh banana leaves and allowed to germinate for 3 days. The germinated grains (80% sorghum + 20% millet) were wet milled and prepared into slurry. The slurry was mixed with boiled water (ratio 1:4 v/v), cooled, filtered, and the residue was discarded, while the filtrate was concentrated by boiling for 30 min with continuous stirring. The resulting gruel was cooled rapidly and allowed to spontaneously ferment for 24 h at ambient temperature and kept frozen.
Preparation of Pito
Pito was brewed in the laboratory using the procedure described by Ekundayo (1969). Briefly, sorghum grains were soaked in water for 2 days, followed by malting, and allowing to sit for 5 days in baskets lined with moistened banana leaves. The malted grains were ground, mixed with water, and boiled. The resulting mash was allowed to cool and later filtered through a fine mesh basket. The filtrate obtained was allowed to stand overnight and boiled to a concentrate. A starter from the previous brew was added to the cooled concentrate, which was again allowed to ferment overnight.
Proximate composition
Moisture contents
Moisture was determined using the AACC Method 44–15A (American Association of Cereal Chemists [AACC] 1999). Crude protein was determined using the thermal combustion (Dumas) method with the Leco FP–528 Protein/Nitrogen Analyzer (Leco Corporation, St. Joseph, MI). The nitrogen content was converted to percentage protein by using a protein conversion factor of 6.25. Crude fat was determined by extraction of 3 g of the sample with 40 mL petroleum ether (boiling point 40–60°C) for 4 h according to AACC method 30–25 (1983) using a Soxhlet test apparatus. The AACC method 08–01 (1999) was used to determine the ash content. The total carbohydrate was calculated by difference.
Gross energy
Gross energy (kJ/g) was estimated by multiplying the percentages of protein, lipid, and carbohydrate by the factors 17, 37, and 16, respectively (FAO/WHO/UNU 1981).
Quantitative phenolics and flavonoids analysis
Total phenolics
The concentrations of phenolic compounds in Obiolor and Pito were determined using the method described by Spanos and Wrolstad (1991). Briefly, 2.5 mL of 10% Folin–Ciocalteu reagent and 2 mL of Na2CO3 (2% w/v) were added to 0.5 mL Obiolor and Pito. The resulting mixtures were incubated at 45°C with constant shaking for 15 min. The absorbance of the samples was read at 765 nm. This was done in triplicate. The total phenolic content in the beverages was expressed as mg of epicatechin (0–0.5 mg/mL) dissolved in distilled water.
Total flavonoids
The concentration of total flavonoids in the beverages (Obiolor and Pito) was determined using the procedure described by Zhishen et al. (1999). Briefly, Obiolor and Pito (1 mL each) was mixed with 3 mL of methanol, 0.2 mL of 10% aluminum chloride, 0.2 mL of 1 mol/L potassium acetate, and 5.6 mL of distilled water. The mixture was allowed to stand at room temperature for 30 min. The absorbance of the reaction mixture was read at 420 nm. The concentration of flavonoids in mg/mL was obtained from the calibration curve of epicatechin solution (0–0.8 mg/mL) in distilled water.
Free radical and reactive oxygen species scavenging assays
DPPH radical scavenging assay
The antioxidant activities of Obiolor and Pito were determined by measuring the capacity of bleaching a purple-colored ethanol solution of DPPH, as described by Turkoglu et al. (2007), with a slight modification. Briefly, 2 mL of various concentrations (20–100%) of the samples in ethanol were added to 2 mL of 0.2 mmol/L sample of DPPH in ethanol. After 30-min incubation period at room temperature, the absorbance was read against blank at 517 nm. Inhibition rate (%I) on the DPPH radical was calculated using the expression:
Superoxide anion radical (O2•−) scavenging assay
The scavenging effects of Obiolor and Pito on superoxide anion radical were examined by the spectrophotometric measurement of the product formed on reduction in NBT (Yen and Chen 1995). Briefly, superoxide anion was generated in a nonenzymic system. The reaction mixture contained 1 mL of the beverages (20–100% v/v) in distilled water, 1 mL of 60 μmol/L of PMS in phosphate buffer (0.1 mol/L, pH 7.4), 1 mL of 468 μmol/L of NADH in phosphate buffer, and 1 mL of 150 μmol/L of NBT in phosphate buffer and was incubated at ambient temperature for 5 min, and the color was read at 560 nm against blank samples.
Hydrogen peroxide scavenging assay
Hydrogen peroxide (H2O2) scavenging activities of Obiolor and Pito were determined as described by Ruch et al. (1989). Briefly, 3.4 mL of varying concentrations (20–100% v/v) of Obiolor and Pito were mixed with 0.6 mL H2O2 (40 mmol/L). The absorbance was read at 230 nm after 10 min of incubation at room temperature. The percentage H2O2 scavenging activities of Obiolor and Pito were calculated using the following expression:
where Acontrol is the absorbance of the mixture without beverage, Asample is the absorbance of the mixture with the beverage, and Abeverage is the absorbance of the beverage alone.
Hydroxyl radical (OH•−) scavenging assay
OH•− scavenging activities of Obiolor and Pito were measured as described by Smirnoff and Cumbes (1989) with slight modifications. Briefly, 2 mL of Obiolor and Pito at concentrations ranging from 20% to 100%, 0.6 mL of 8 mmol/L ferrous sulfate, 0.5 mL of 20 mmol/L hydrogen peroxide, and 2 mL of 3 mmol/L salicylic acid were mixed and incubated at 37°C for 30 min. Then, 0.9 mL of distilled water was added to each vial. The final solution was centrifuged at 4472 g for 10 min. After centrifugation, the absorbance was measured at 510 nm. The percentage OH•− scavenging activities of Obiolor and Pito were calculated using the following expression:
where Acontrol is the absorbance of the mixture without beverage, Asample is the absorbance of the mixture with the beverage, and Abeverage is the absorbance of the beverage alone.
Reducing power
The reducing power of Obiolor and Pito was evaluated by adopting the method of Oyaizu (1986). Varying concentrations of the beverages (20–100%) were suspended in 1 mL of distilled water and mixed with 2.5 μL of 0.2 mol/L phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide [K3Fe(CN)6]. The mixture was incubated at 50°C for 20 min, after which 2.5 μL of trichloroacetic acid (TCA) was added to the mixture. Following centrifugation at 402 g for 10 min, 2.5 μL of the supernatant was mixed with an equal amount of distilled water and 0.5 mL of 0.1% FeCl3. The absorbance of the resulting solution was measured at 700 nm.
Inhibition of reactive oxygen species generation, lipid peroxidation, and protein oxidation
Preparation of hepatic microsomes
Liver was homogenized in sucrose–Tris buffer (0.25 mol/L sucrose, 10 mmol/L Tris–HCl, pH 7.4) as described by Ajiboye et al. (2014). Liver microsomes were isolated by the calcium chloride precipitation method described by Kondraganti et al. (2005). The protein concentration of the microsomal fraction was determined according to Gornall et al. (1949) and the microsomes were finally stored at −80°C.
Induction of microsomal lipid peroxidation and protein oxidation
Oxidation of microsomal lipids and proteins was done as described by Ajiboye et al. (2013a) with little modifications. The reaction mixture consisted of 1.25 mg microsomal proteins, 0.1 mol/L MgCl2, 0.2 mol/L bovine serum albumin (BSA), 2 mmol/L NADPH, and AFB1 with or without Obiolor, Pito, or vitamin C in sucrose–Tris buffer (0.25 mmol/L sucrose, 10 mmol/L Tris-HCl, pH 7.4). The incubation was carried out for 1 h at 37°C with intermittent shaking and the reaction was terminated by chilling the mixture on ice after which the resulting mixtures were assayed for superoxide anion radical, lipid peroxidation products, and protein carbonyl.
Reactive oxygen species (superoxide anion radical) production
The generation of ROS by cells (respiratory burst) was measured by the formation of colored formazan due to reduction in NBT. Briefly, appropriately diluted microsomes from incubation mixtures (final volume 1 mL) was mixed with 1 mL of 150 μmol/L of NBT in phosphate buffer and was incubated at ambient temperature for 5 min, and the color was read at 560 nm.
Lipid peroxidation and protein oxidation assay
The levels of lipid peroxidation products (conjugated dienes, lipid hydroperoxides, and malondialdehyde) in the microsomes were determined using the procedures described by Bus et al. (2001). Protein carbonyl, a marker of protein oxidation, was assessed in the microsomes using the procedure described by Levine et al. (1990).
Statistical analysis
All experimental values were represented as mean ± SD (n = 3). Free radical and ROS scavenging activities were expressed in percentage. Analysis of variance (ANOVA) followed by Tukey–Kramer test for differences between means was used to account for any significant differences (P < 0.05) between the variables in this study using StatPlus, 2011 (AnalystSoftInc, Alexandria, VA).
Results and Discussion
Wise use of fruits, medicinal plants, and vegetables requires investigations into the phytochemicals and antioxidants as well as the possible medicinal properties and prospective products, such as nutraceuticals and phytomedicines (Oloyede et al. 2013). This study provides the nutritional and antioxidants disposition of sorghum/millet-based beverages (Obiolor and Pito).
Proximate composition
Proximate analysis is an important tool in the evaluation of nutritional status of foods and food products. The proximate and nutritional composition of Obiolor and Pito are presented in Table 1. The very high level of moisture content of the beverages (Table 1) is expected, as they are liquid-based beverages. Thus, the beverages would be better stored airtight and in refrigerator. Protein is an essential macronutrient for growth and maintenance of body tissues (WHO/FAO Report 1985). The protein content of sorghum grains is within the range of 5.44–12.9% (Salinas et al. 2006). The level of protein in the beverages (Table 1) indicates its potential to complement protein-based meal. The higher protein content of Obiolor could be due to the millet component of the beverage, as millet is a better source of protein than sorghum.
Table 1.
Nutritional composition of sorghum/millet-based beverages.
| Nutrients | Obiolor | Pito |
|---|---|---|
| Moisture content (%) | 80.21 ± 0.24 | 88.69 ± 0.48 |
| Crude protein (%) | 7.80 ± 0.12 | 3.70 ± 0.02 |
| Available carbohydrate (%) | 8.90 ± 0.05 | 5.60 ± 0.31 |
| Crude fat (%) | 0.39 ± 0.01 | 0.31 ± 0.01 |
| Crude fiber (%) | 0.30 ± 0.01 | 0.20 ± 0.01 |
| Ash (%) | 2.40 ± 0.03 | 1.50 ± 0.01 |
| Energy value (kJ/g) | 459.30 ± 0.42 | 164.00 ± 3.12 |
Available carbohydrates are digestible, absorbable, and hence utilizable nutritionally (Oloyede 2005). These include sugars like glucose, fructose (found in fruits, vegetables, honey, and sugarcane), starch (which is the storage form of carbohydrates in plants and contains amylopectin and amylose) as found in cereals, and tubers like yam, cassava, potatoes, etc. (Oloyede 2005). Thus, the appreciable amount of available carbohydrates in the beverages (Table 1) could serve as source of energy derivable as adenosine triphosphate. The low level of unavailable carbohydrates (dietary fibers) is not a surprise, as majority of the fibers (pectin, cellulose, hemicellulose, and lignin) in the sorghum and millet could have been hydrolyzed to simple sugar during fermentation.
Gross energy (kJ/g) establishes the relationship between food composition and available energy (FAO/WHO/UNU 1981). The gross energy (kJ/g) of Obiolor is appreciable higher than that of Pito (Table 1). This could have resulted from the lower amount in the nutritional composition of Pito brought about by longer day of fermentation. Thus, Obiolor is a better source of energy.
Free radical and reactive oxygen species scavenging activities
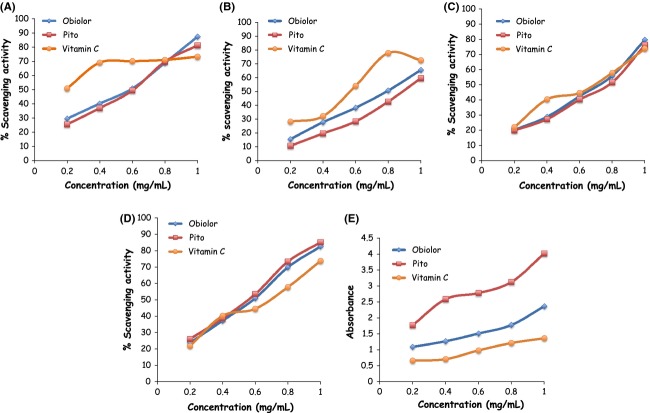
Free radicals are highly reactive substances capable of giving rise to chain reactions, that is, reactions that involve a number of steps, each of which forms a free radical that triggers the next step. Studies have indicated the free radical scavenging properties of sorghum extracts and sorghum-based products in vitro (Awika et al. 2003; Ajiboye et al. 2013a). Thus, the DPPH radical scavenging activity produced by Obiolor and Pito (Fig. 1A) indicates hydrogen ion donating capability of the beverages. This could be important in the prevention of free radical-induced lipid peroxidation.
Figure 1.
(A) DPPH scavenging; (B) superoxide anion radical scavenging; (C) hydrogen peroxide scavenging; (D) hydroxyl radical scavenging; and (E) reducing activity of the polyphenolic extract of Obiolor and Pito beverages.
ROS (•OH, O2•−, and H2O2) scavenging activities of Obiolor and Pito (Fig. 1B–D) show potentials of the beverages in complementing ROS detoxifying enzymes in vivo, if effectively absorbed. It could also help terminate lipid peroxidation that could arise from O2•−, and the stronger ROS (O•,•OH and H2O2) (Yen and Der Duh 1994). Also, the capability of beverages to significantly reduce K3Fe(CN)6 (Fig. 1E) indicates effectiveness to halt oxidation of cellular macromolecules by oxidizing molecules that could arise from the metabolism of either drugs or toxins. The free radical and ROS scavenging activity recorded in this study could be due to the high level of flavonoids and phenolics present in the beverages (Table 2).
Table 2.
Total phenols and total flavonoids content of sorghum/millet-based beverages.
| Total phenols | Total flavonoids | |
|---|---|---|
| Obiolor | 582 ± 2.50 | 537 ± 7.81 |
| Pito | 310 ± 1.06 | 275 ± 0.70 |
The results are mean ± SD for three replicates.
ROS production in the AFB1-treated microsomes increased significantly (P < 0.05) when compared with no treatment (Table 3). Obiolor and Pito significantly reduced AFB1-mediated generation of ROS as does by the reference antioxidants, vitamin C and compared with the control (Table 3).
Table 3.
Levels of lipid peroxidation products, protein carbonyl, and superoxide anion radical following treatment of sorghum/millet-based beverages to aflatoxin B1-treated microsome.
| Treatments | Conjugated dienes (nmol mg protein−1) | Lipid hydroperoxide (nmol mg protein−1) | Malondialdehyde (nmol mg protein−1) | Protein carbonyl (nmol mg protein−1) | Superoxide anion radical (μmol NBT reduce mg protein−1) |
|---|---|---|---|---|---|
| Untreated microsomes | 51.58 ± 0.86a | 49.81 ± 0.45a | 5.69 ± 0.43a | 4.81 ± 0.11a | 3.26 ± 0.52a |
| AFB1-treated microsomes | 118.59 ± 3.51b | 123.92 ± 0.48b | 15.70 ± 0.23b | 8.05 ± 0.18b | 13.23 ± 0.42b |
| Obiolor | 51.86 ± 1.26a | 50.23 ± 0.60a | 5.62 ± 0.18a | 4.18 ± 0.03a | 3.23 ± 0.12a |
| Pito | 50.98 ± 1.34a | 47.83 ± 0.15a | 5.40 ± 0.21a | 4.61 ± 0.02a | 3.83 ± 0.30a |
| Obiolor + aflatoxin B1 | 59.24 ± 1.06c | 57.42 ± 0.12c | 6.49 ± 0.28c | 4.17 ± 0.03a | 6.22 ± 0.24c |
| Pito + aflatoxin B1 | 55.42 ± 0.61c | 58.27 ± 0.70c | 6.22 ± 0.46c | 4.54 ± 0.05a | 7.27 ± 0.70c |
| Vitamin C + aflatoxin B1 | 52.58 ± 0.84a | 59.01 ± 0.26c | 5.89 ± 0.32a | 4.76 ± 0.04a | 5.01 ± 0.12c |
The results are mean ± SD for three replicates. Values carrying superscripts different for each parameter are significantly different (P < 0.05).
Lipid peroxidation and protein oxidation
Lipid peroxidation, which is constantly taking place in vivo, induces disturbances of membrane organization and functional loss and modification of proteins and DNA bases (Niki 2009). AFB1 has been reported to induce lipid peroxidation in numerous in vitro and in vivo methods of carcinogenesis (Shen et al. 1994; Towner et al. 2002; Lee et al. 2005; Theumer et al. 2010; Ravinayagam et al. 2012). Thus, the significant increase in the levels of lipid peroxidation products (conjugated dienes, lipid hydroperoxides, and malondialdehydes) (Table 3) shows indiscriminate oxidative assaults on the cellular lipids. These increases (most especially conjugated dienes) could result to mutation (Das et al. 2010). The capability of the beverages to reverse AFB1-mediated increases in conjugated dienes, lipid hydroperoxides, and malondialdehyde may be due to the ROS scavenging activity of the beverages. It may also be due to the capability of polyphenolic components of S. bicolor to promote ROS detoxification through the induction of antioxidant enzymes (Ajiboye et al. 2013b), which could cause the peroxidation of polyunsaturated fatty acids of plasma membrane.
Protein carbonyl is an indicator of irreversible damage to cellular proteins and may have lasting detrimental effects on cells and tissues (Dalle-donne et al. 2003). Thus, the significant increase in protein carbonyl in AFB1-treated microsomes (Table 3) could have resulted from the oxidation of protein by free radicals and ROS generated during AFB1 metabolism. The attenuation of AFB1-mediated increase in the level of protein carbonyl by Obiolor and Pito further shows possible ROS scavenging and capability to halt oxidative onslaught on protein, possibly through ROS detoxification. Similar attenuation of AFB1-mediated increase in protein carbonyl level following the administration of Tridham has been reported (Ravinayagam et al. 2012).
Conclusion
It can be deduced from this study that the sorghum-based beverages (Obiolor and Pito) can serve as functional foods, as evident from the gross energy contents, antioxidant activities, and capabilities to prevent the AFB1-mediated oxidation of lipids and proteins. Thus, the consumption of these beverages is encouraged as functional foods.
Conflict of Interest
None declared.
References
- Achi OK. Microbiology of ‘obiolor’: a Nigerian fermented non-alcoholic beverage. J. Appl. Biotechnol. 1990;69:321–325. doi: 10.1111/j.1365-2672.1990.tb01522.x. [DOI] [PubMed] [Google Scholar]
- Ajiboye TO, Komolafe YO, Oloyede HOB, Ogunbode SM, Adeoye MD, Abdussalami IO, et al. Polyphenolic rich extract from Sorghum bicolor's grains enhance reactive oxygen species detoxification in N-nitrosodiethylamine-treated rats. Food Sci. Hum. Wellness. 2013a;2:39–45. [Google Scholar]
- Ajiboye TO, Komolafe YO, Oyelola H, Oloyede B, Yakubu MT, Adeoye MD, et al. Diethylnitrosamine-induced redox imbalance in rat microsomes: protective role of polyphenolic- rich extract from Sorghum bicolor grains. J. Basic Clin. Physiol. Pharmacol. 2013b;24:41–49. doi: 10.1515/jbcpp-2012-0031. doi: 10.1515/jbcpp-2012-0031. [DOI] [PubMed] [Google Scholar]
- Ajiboye TO, Yakubu MT, Oladiji AT. Electrophilic and reactive oxygen species detoxification potentials of chalcone dimers is mediated by Nrf-2. J. Biochem. Mol. Toxicol. 2014;28:11–22. doi: 10.1002/jbt.21517. [DOI] [PubMed] [Google Scholar]
- American Association of Cereal Chemists (AACC) Approved Methods of the AACC. 10th ed. St. Paul, MN: AACC; 1983. [Google Scholar]
- American Association of Cereal Chemists (AACC) Approved Methods of the AACC. 10th ed. St. Paul, MN: AACC; 1999. [Google Scholar]
- Awika JM, Rooney LW, Wu X, Prior RL, Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agricult.l Food Chem. 2003;51:6657–6662. doi: 10.1021/jf034790i. [DOI] [PubMed] [Google Scholar]
- Bus JS, Costa LG, Hodgson E, Lawrence DA, Reed DJ. Current protocols in toxicology. Hoboken, NJ: John Wiley & Sons, Inc; 2001. doi: 10.1002/0471140856. [Google Scholar]
- Dalle-donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Das AK, Bag S, Sahu R, Dua TK, Sinha MK, Gangopadhyay M, et al. Protective effect of Corchorus olitorius leaves on sodium arsenite-induced toxicity in experimental rats. Food Chem. Toxicol. 2010;48:326–335. doi: 10.1016/j.fct.2009.10.020. doi: 10.1016/j.fct.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Dykes L, Seitz LM, Rooney WL, Rooney LW. Flavonoid composition of red sorghum genotypes. Food Chem. 2009;116:313–317. doi: 10.1016/j.foodchem.2011.03.020. doi: 10.1016/j.foodchem.2009.02.052. [DOI] [PubMed] [Google Scholar]
- Ekundayo JA. The production of pito, a Nigerian fermented beverage. J. Food Technol. 1969;4:217–225. [Google Scholar]
- FAO/WHO/UNU. Energy and protein requirements. Technical reports series 724. Geneva: WHO; 1981. pp. 133–138. [Google Scholar]
- Gornall AC, Bardawill CJ, David MM. Determination of serum protein by means of biuret reaction. J. Biol. Chem. 1949;177:751–756. [PubMed] [Google Scholar]
- Hasler CM. Functional foods: their role in disease prevention and health promotion. Food Tech. 1998;52:57–62. [Google Scholar]
- Kondraganti SR, Muthiah K, Jiang W, Barrios R, Moorthy B. Effects of 3-methylcholanthrene on gene expression profiling in the rat using cDNA microarray analyses. Chem. Res. Toxicol. 2005;18:1634–1641. doi: 10.1021/tx050085n. [DOI] [PubMed] [Google Scholar]
- Lee J, Hye E, Lee K, Sook H. Alleviation of aflatoxin B1-induced oxidative stress in HepG2 cells by volatile extract from Allii Fistulosi Bulbus. Science. 2005;77:2896–2910. doi: 10.1016/j.lfs.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Clement I. Determination of carbonyl content in oxidatively modified proteins. Meth. Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Matz SA. The chemistry and technology of cereals as food and feed. 2nd ed. McAllen, TX: Pan-Tech International INC; 1991. [Google Scholar]
- Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radical Biol. Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Odunfa SA. African fermented foods. In: Woods JB, Oyewole OB, editors. Microbiology of fermented foods. 713–752. London: Blackie Academic and Professional; 1998. [Google Scholar]
- Oloyede OB. All for the love of Nutrients. 78th Inaugural Lecture, University of Ilorin, Ilorin, Nigeria. Ilorin: Univ. of Ilorin Press; 2005. [Google Scholar]
- Oloyede HOB, Ajiboye TO, Komolafe YO, Salau AK. Polyphenolic extract of Blighia sapida arilli prevents N-nitrosodiethylamine-mediated oxidative onslaught on microsomal protein, lipid and DNA. Food Biosci. 2013;1:48–56. [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: antioxidative activities of product of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986;44:307–315. [Google Scholar]
- Pang G, Xie J, Chen Q, Hu Z. How functional foods play critical roles in human health. Food Sci Hum Wellness. 2012;1:26–60. doi: 10.1016/j.fshw.2012.10.001. [Google Scholar]
- Ravinayagam V, Jaganathan R, Panchanadham S, Palanivelu S. Potential antioxidant role of tridham in managing oxidative stress against aflatoxin-B1 -induced experimental hepatocellular carcinoma. Int. J. Hepatol. 2012;2012:1–9. doi: 10.1155/2012/428373. doi: 10.1155/2012/428373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Salinas I, Pro A, Salinas Y, Sosa E, Becerril CM, Cuca M, Cervantes M, Gallegos J. Compositional variation amongst sorghum hybrids: effect of kafirin concentration on metabolizable energy. J. Cereal Sci. 2006;44:342–346. [Google Scholar]
- Shen HM, Shi CY, Lee HP, Ong CN. Aflatoxin B1-induced lipid peroxidation in rat liver. Toxicol. Appl. Pharmacol. 1994;127:145–150. doi: 10.1006/taap.1994.1148. doi: 10.1006/taap.1994.1148. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Cumbes QJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. doi: 10.1016/0031-9422(89)80182-7. [Google Scholar]
- Spanos GA, Wrolstad RE. Influence of processing and storage on the phenolic composition of Thompson seedless grape juice. J. Agricult. Food Chem. 1991;38:1565–1571. [Google Scholar]
- Tamang JP. Dietary cultures and antiquity of fermented foods and beverages. In: Hui YH, Özgül Evranuz E, Samuel D, editors. Fermented foods and beverages of the world. Boca Raton, FL: CRC Press, Taylor and Francis Group; 2010. pp. 1–40. [Google Scholar]
- Theumer MG, Cánepa MC, López AG, Mary VS, Dambolena JS, Rubinstein HR. Subchronic mycotoxicoses in Wistar rats: assessment of the in vivo and in vitro genotoxicity induced by fumonisins and aflatoxin B1, and oxidative stress biomarkers status. Toxicology. 2010;268:104–110. doi: 10.1016/j.tox.2009.12.007. 10.1016/j.tox.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Towner RA, Mason RP, Reinke LA. In vivo detection of aflatoxin-induced lipid free radicals in rat bile. Biochim. Biophys. Acta. 2002;1573:55–62. doi: 10.1016/s0304-4165(02)00326-4. [DOI] [PubMed] [Google Scholar]
- Turkoglu A, Duru ME, Mercan N, Kivrak I, Gezer K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. 2007;101:267–273. doi: 10.1016/j.foodchem.2006.01.025. [Google Scholar]
- WHO/FAO Report. Energy and protein requirements. WHO Technical Report Series No.724. Geneva: WHO; 1985. [PubMed] [Google Scholar]
- Yen G-C, Chen H-Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995;43:27–32. doi: 10.1021/jf00049a007. [Google Scholar]
- Yen GC, Der Duh P. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994;42:629–632. doi: 10.1021/jf00039a005. [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]