Abstract
The results of a high-throughput screening assay using the dengue virus-2 replicon showed that the imidazole 4,5-dicarboxamide (I45DC) derivative (15a) has a high dengue virus inhibitory activity. Based on 15a as a lead compound, a novel class of both disubstituted I45DCs and the resembling pyrazine 2,3-dicarboxamides (P23DCs) were synthesized. Here, we report on their in vitro inhibitory activity against dengue virus (DENV) and yellow fever virus (YFV). Some of these first generation compounds have shown activity against both viruses in the micromolar range. Within this series, compound 15b was observed to display the highest antiviral potency against YFV with an EC50 = 1.85 μM. In addition, compounds 20a and 20b both potently inhibited replication of DENV (EC50 = 0.93 μM) in Vero cells.
Keywords: Flavivirus inhibitors, Dengue virus, Yellow fever virus, Imidazole dicarboxylic acid, Pyrazine dicarboxylic acid
Graphical abstract
Highlights
-
•
Two new series of heterocycles were evaluated for Flavivirus inhibition.
-
•
Activities at micromolar levels were noted for inhibition of dengue virus.
-
•
Remarkable selective inhibitory properties for yellow fever virus were recorded.
-
•
Imidazole-4,5-dicarboxylic amides provide an interesting scaffold for antivirals.
-
•
Pyrazine-2,3-dicarboxylic amides likewise are endowed with anti-flavivirus activities.
1. Introduction
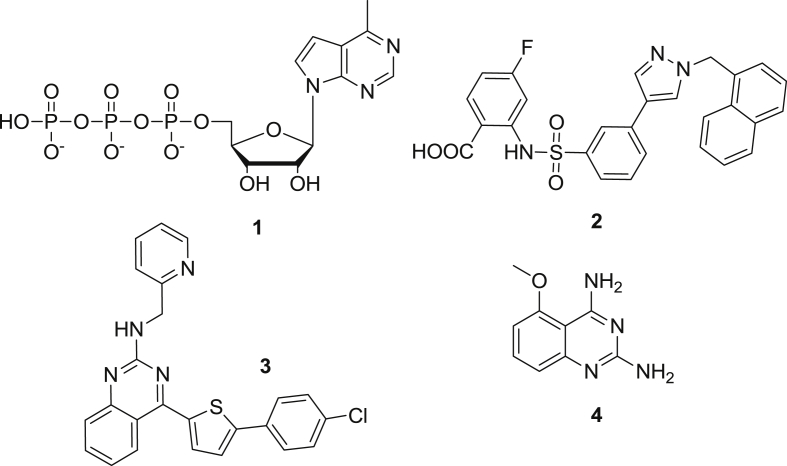
Dengue is the most common arthropod-borne viral infection in the world and is estimated to transmit 390 million new infections every year [3]. Dengue virus (DENV) belongs to the Flavivirus genus of the Flaviviridae. The genome of DENV is comprised of a 10.7 kb, single, positive-stranded RNA with at least four different circulating serotypes (DENV-1 to DENV-4) [4]. Reportedly, a fifth serotype now complicates vaccine development [4]. With increased levels of population growth, urbanization, and international travel, DENV illness has increased 30-fold in the last 50 years [5]. Most of the current research on dengue infections is focused on the treatment of symptoms, which often can be a tedious and intensive process. As there is neither a drug nor any vaccine available to combat DENV infections till date [6], it is highly important to explore and uncover small molecules that have potent anti-dengue activity. In the past half-decade, a number of antiviral agents with such inhibitory properties have been discovered. These include an adenosine analog 1 [7], a N-sulfonylanthranilic acid derivate 2 [8], the chlorophenyl-thiophene derivate 3 [9], and most recently some 2,4-diaminoquinazoline derivatives 4 [10] (see Fig. 1). Unfortunately, none of these substances have entered into clinical trials. Hence, further development of new chemical entities endowed with dengue inhibitory properties is warranted. Our previous efforts herein, focused on tritylated and alkylated nucleoside analogs, resulting in compounds endowed with strong anti-flavivirus inhibitory properties. However, no clear SAR could be determined [1], [2]. Hence we now turned our attention to another lead molecule.
Fig. 1.
Structures of some recently described antiviral compounds being inhibitory for dengue virus.
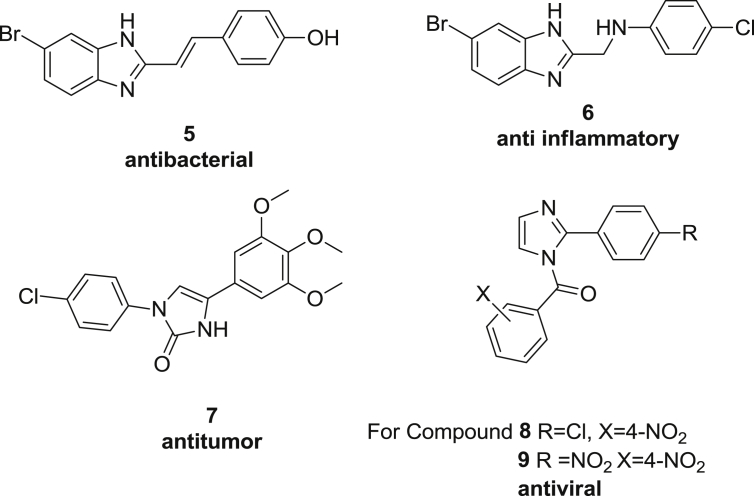
The five-membered imidazole ring is a structural unit found in many biologically active compounds. The strong therapeutic properties of imidazole containing drugs have encouraged medicinal chemists to synthesize a large number of novel chemotherapeutic agents comprising this entity. Amongst others, imidazole core structures are found in different carboxypeptidase, hemeoxygenase and lactamase inhibitors, as well as among anti-inflammatory, anticancer, antibacterial, antifungal, antitubercular, antidiabetic and antiviral products, further highlighted with some examples. Ramya et al. synthesized a series of novel 5-(nitro/bromo)-styryl-2-benzimidazole derivatives (5) and tested them for their antibacterial activity against Staphylococcus aureus, Escherichia coli and likewise evaluated the antifungal activity against Candida albicans and Aspergillus fumigates [11]. Biological activities proved comparable to ciprofloxacin. Kavitha C.S. et al. synthesized a series of 2-methylaminobenzimidazole derivatives in which compound 6 showed analgesic and anti-inflammatory activity comparable with the standard drug nimesulide [12]. Cenzo et al. synthesized a series of 1, 4-diarylimidazole-2(3H)-one derivatives 7 and their 2-thione analogs and found antitumor activity [13]. Finally, Sharma et al. synthesized imidazole derivatives for antiviral screening and different [2-(substituted phenyl)-imidazol-1-yl]-benzamides like 8 and 9 were selected as the most promising antiviral agents [14] (see Fig. 2).
Fig. 2.
Some examples of imidazole containing structures endowed with pronounced biological activity.
Imidazole-4,5-dicarboxylic acid (I45DC) and its derivatives such as those bearing primary or secondary amides have previously been reported to be active against HIV-1 protease [15]. Likewise, some derivatives of this scaffold were uncovered as potential kinase inhibitors with antiproliferative activity against HL-60 cells [16]. In addition, inhibition of protein–protein interaction between Hepatitis C glycoprotein E2 and CD81 has been reported previously [17]. The I45DCs are known as well to be a structural component of some antibiotics [18] and also to affect memory [19]. Substitution on the imidazole moiety herein has been reported, both in solution phase as well as in a combinatorial fashion on solid support.
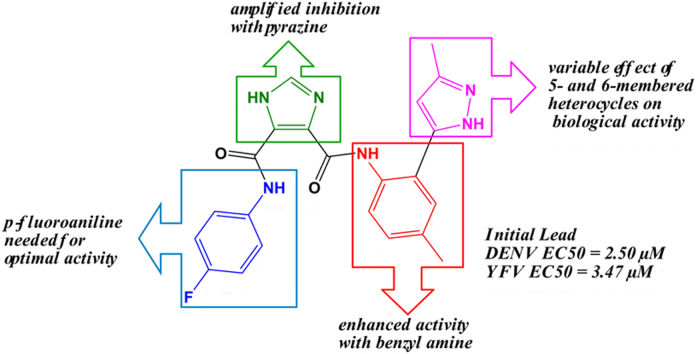
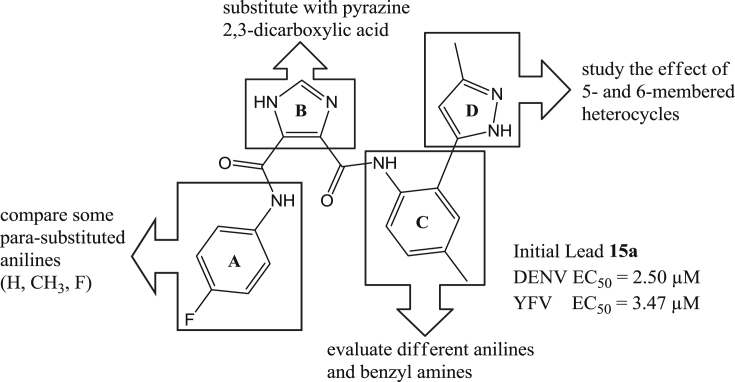
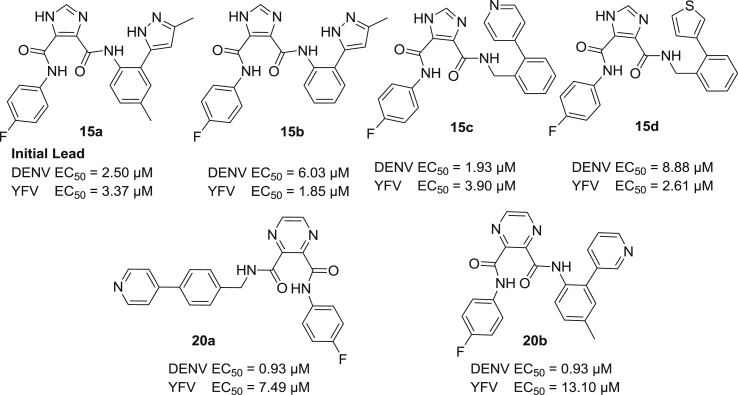
High throughput screening of a compound library led to the identification of N5-(4-fluorophenyl)-N4-(4-methyl-2-(3-methyl-1H-pyrazol-5-yl)phenyl)-1H-imidazole-4,5-dicarboxamide 15a (see Fig. 3) with an EC50 of 2.50 μM and 3.47 μM against DENV and YFV, respectively. In search for new compounds with potential for clinical use as antiviral agents, a series of compounds based on this I45DC scaffold were synthesized with regiospecific attachment of the substituents and these analogs were investigated for their inhibitory properties against DENV and YFV. In addition, the imidazole core was substituted for a pyrazine ring leading to a series of pyrazine-2,3-dicarboxylic acids (P23DC). The intended substitution of the central imidazole ring would result in slightly different orientation of the attached amide groups, and in a change of the hydrogen bonding pattern exchanging the combination of a hydrogen donating and a hydrogen accepting nitrogen into two hydrogen accepting nitrogen atoms. In this communication, we report on the synthesis and biological activity of both new series of dissymmetric I45DCs and P23DCs, which we studied for their inhibitory properties against YFV and DENV.
Fig. 3.
Lead compound 15a and envisaged modifications of different parts of the antiviral lead for optimization of the structure.
2. Results and discussion
2.1. Synthetic aspects
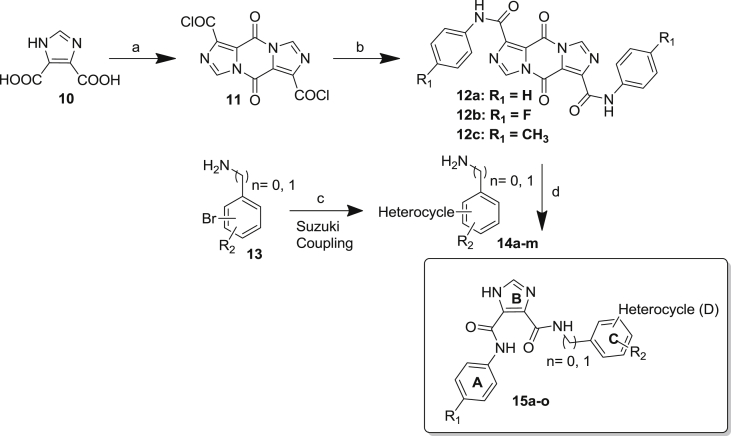
The general procedure employed for the synthesis of dissymmetrically disubstituted I45DCs is shown in Scheme 1. Imidazole-4,5-dicarboxylic acid 10 was allowed to reflux with SOCl2 in toluene with catalytic DMF to afford pyrazinedione diacid dichloride 11 in 92% yield [20]. The literature route for amide formation suggests hydrolysis of the acid chlorides to carboxylic acids by the addition of water. Amines are then added to open both acyl imidazole bonds affording two identical imidazole analogs. Baures et al., replaced the water with phenol, thereby modulating the reactivity of the acid chloride in comparison with an acyl imidazole bond for the subsequent addition of two different amines [20]. The difficulty in preparing such analogs is that their synthesis is highly dependent upon the substituents to be introduced. Primary benzyl amines, for example, are too reactive to provide selectivity in the reaction, whereas anilines can be added in a proper stoichiometric ratio in order to provide a pyrazine dione intermediate 12 which can often be purified by crystallization. In the case of aniline derivatives, the resulting pyrazine intermediates are often insoluble in the reaction solvent and can be isolated simply by vacuum filtration. In parallel, different bromoanilines or benzylamines were subsequently coupled with commercially available heterocyclic boronic acids through palladium catalyzed Suzuki reaction to give the desired second aniline in 60–75% yield. Addition of this second aniline to the pyrazine intermediates 12a–c resulted in opening of the acyl imidazole bond to obtain the expected product. Based on this approach, a series of novel molecules was prepared within this I45DC family containing various substituted phenyl rings. For some of the final products 15, the reaction sequence was changed with prior attachment of the aniline carrying a heterocycle.
Scheme 1.
General reaction scheme for synthesis of the I45DC analogs. Conditions: a) SOCl2, cat. DMF, Toluene, 85 °C; b) DIPEA, substituted aniline, DCM, −10 °C; c) acetonitrile:water (1:1), K2CO3, PdCl2TPP2, boronic acid; d) DIPEA, substituted aniline or benzyl amine, DCM, room temperature (rt).
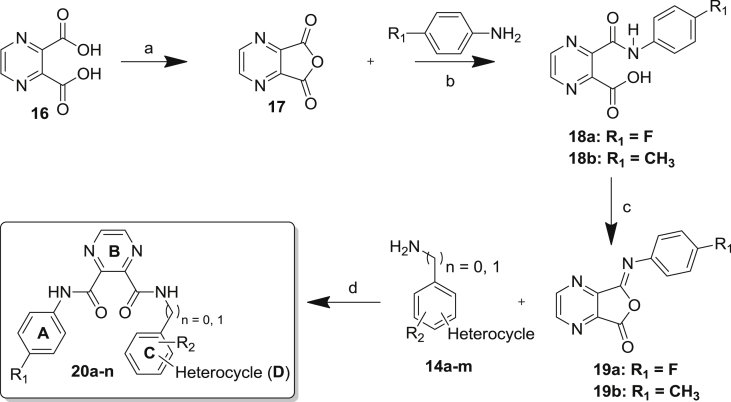
Pyrazines are important pharmacophores present in a number of biologically active compounds such as antimycobacterial, antibacterial, antidiabetic, and hypnotic agents. To further explore previous structure–activity relationship, we planned to substitute the five-membered imidazole ring with a pyrazine ring. Hereto, regioselective functionalization of the pyrazine started from commercial pyrazine-2,3-dicarboxylic acid (P23DA) and a four-step synthesis of the target compounds has been described in Scheme 2. P23DA was converted to its corresponding anhydride 17 by treatment with acetic anhydride at room temperature.
Scheme 2.
General scheme for assembly of different P23DC analogs. Conditions: a) acetic anhydride; b) acetonitrile:water (1:1), substituted aniline, dodecyl hydrogen sulfate sodium salt, rt; c) trifluoroacetic anhydride, TEA, THF; d) substituted aniline or benzyl amine, THF, rt.
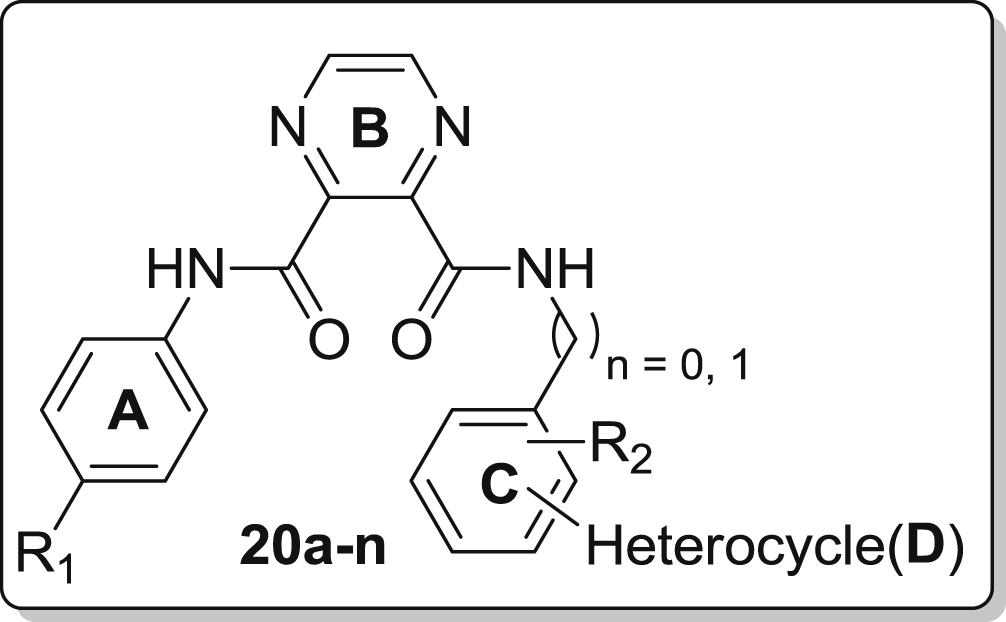
Reaction with p-fluoroaniline or p-toluidine resulted in opening of the five-membered ring with concomitant formation of the first amide bond (18a,b). Reaction with trifluoroacetic anhydride at 0 °C afforded the anhydric activated product 19a or 19b allowing introduction of the second amide. Hereto, the intermediate was reacted with preformed anilines or benzyl amines 14a–m to give the target compounds 20a–n in 60–80% overall yields. All final products were purified by silica gel chromatography and characterized by 1H NMR, 13C NMR and HRMS before evaluation of their inhibitory properties against DENV and YFV.
2.2. Assessment of antiviral activity
As shown in Fig. 3 and Scheme 1, Scheme 2, four main structural domains were chosen for optimization of hit 15a: the aromatic moiety (ring A), the central pyrazine/imidazole core (ring B), the substituted aromatic moiety (ring C) and the heterocyclic group (ring D). These efforts have provided a few compounds that exhibited an improved biological profile with respect to the initial lead compound 15a. Overall, improvement or reduction of the inhibitory activities for either DENV or YFV is mostly running in parallel.
The overall structure of 15a comprises an imidazole core carrying two aniline moieties coupled via an amide bond, with one of the aniline rings substituted with an additional heterocyclic ring (ring D). Therefore, our efforts to understand the structure–activity relationship of the initial lead started with modifying the heterocyclic moiety as shown in Table 1. Using p-fluoroaniline (ring A) and the I45DC core as a fixed fragment, substitution of the five-membered pyrazole moiety (ring D) with a thienyl or furanyl ring mostly led to considerable loss of activity (i.e., compare 15h, 15i, 15j versus 15a). However, activity remarkably was restored upon substitution of a methyl group for the para-fluorine in ring A (15k, 15l). The benzylamine substitution for ring A likewise annihilated the inhibitory properties (15m), while in contrast a p-fluoro-phenylethylamine again displayed nice activity (15n). Exchanging aniline ring C for a benzylamine preserved the activity when the supplementary heterocycle D was attached at the ortho position (15c, 15d), while para substitution led to considerable loss of activity (15e–g) within this context. A single attempt with an aminopyridine ring at site C proved inactive (15o). Overall, the compounds 15b (EC50 = 1.85 μM, YFV), 15c (EC50 = 1.93 μM, DENV), 15d (EC50 = 2.61 μM, YFV) and 15k (EC50 = 2.02 μM, DENV) are endowed with the best inhibitory properties within this imidazole series.
Table 1.
Inhibitory properties for yellow fever virus and dengue virus of the imidazole analogs 15a–15o.
| Sr. No. | n1 | n2 | R1 | R2 | Heterocycle (positiona) | Dengue |
Yellow fever |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (μM) | CC50 (μM) | SI | EC50 (μM) | CC50 (μM) | SI | ||||||
| 15a | 0 | 0 | F | p-Methyl | 3-Methyl-1H-pyrazol-5-yl (ortho) Initial Lead | 2.50 | >120 | 48 | 3.47 | >120 | 34.5 |
| 15b | 0 | 0 | F | H | 3-Methyl-1H-pyrazol-5-yl (ortho) | 6.03 | 21.7 | 3.6 | 1.85 | >121 | 67 |
| 15c | 0 | 1 | F | H | Pyridin-4-yl (ortho) | 1.93 | 50.1 | 26 | 3.90 | >120 | 31 |
| 15d | 0 | 1 | F | H | Thien-3-yl (ortho) | 8.88 | 17.32 | 1.95 | 2.61 | 34.5 | 13.2 |
| 15e | 0 | 1 | F | H | Pyridin-3-yl (para) | 42.10 | >120 | 2.85 | 11.81 | >118 | 10 |
| 15f | 0 | 1 | F | H | Thien-3-yl (para) | 33.69 | 20.21 | 0.60 | 28.77 | >119 | 4.13 |
| 15g | 0 | 1 | F | H | Pyridin-4-yl (para) | >120 | >120 | 1 | >120 | >120 | 1 |
| 15h | 0 | 0 | F | p-Methyl | Thien-3-yl (ortho) | 48.75 | >117 | 2.4 | >119 | >119 | 1 |
| 15i | 0 | 0 | F | p-Methyl | Furan-2-yl (ortho) | >123.6 | >124 | 1 | ND | – | – |
| 15j | 0 | 0 | H | p-Methyl | Thien-3-yl (ortho) | >124 | >124 | 1 | 9.20 | >120 | 13 |
| 15k | 0 | 0 | CH3 | p-Methyl | Thien-3-yl (ortho) | 2.02 | >50 | 24.5 | >120 | >120 | 1 |
| 15l | 0 | 0 | CH3 | p-Methyl | Furan-2-yl (ortho) | 3.47 | >125 | 36.2 | ND | – | – |
| 15m | 1 | 0 | H | p-Methyl | Furan-2-yl (ortho) | >125 | >125 | 1 | ND | – | – |
| 15n | 2 | 0 | F | p-Methyl | Furan-2-yl (ortho) | 3.77 | >116.5 | 30.9 | ND | – | – |
| 15o | 0 | 0 | H | p-Methyl | See structure above | >120 | 26.40 | 0.22 | >120 | >119 | 0.97 |
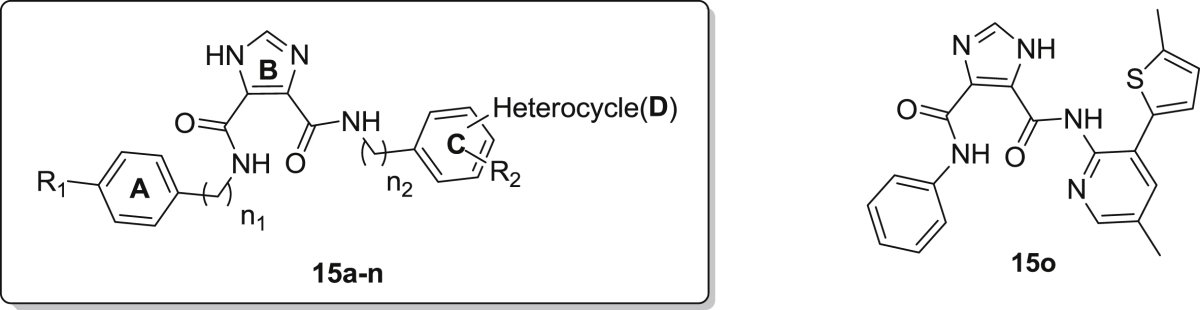
Indicates the attachment point of the heterocycle on the phenyl ring.
With these results in mind, we hoped to further improve the potency via replacement of the central five-membered imidazole (ring B) with a pyrazine scaffold, both rings containing two nitrogen atoms but providing a slightly different orientation for the aniline substituents. Focusing on compound 15c as most potent representative for inhibition of DENV (EC50 = 1.92 μM), we synthesized a series of pyrazine analogs with a pyridine substituent as the heterocyclic moiety D, attached at different positions of ring C. The inhibitory potency of these compounds was likewise examined against DENV and YFV and is summarized in Table 2 (compounds 20a−20n).
Table 2.
Inhibitory properties for yellow fever virus and dengue virus of the pyrazine containing compounds 20a–20n.
| Sr. No. | n | R1 | R2 | Heterocycle (positiona) | Dengue |
Yellow fever |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (μM) | CC50 (μM) | SI | EC50 (μM) | CC50 (μM) | SI | |||||
| 20a | 1 | F | H | Pyridin-4-yl (para) | 0.94 | >117.5 | >125 | 7.49 | >120 | 16 |
| 20b | 0 | F | p-Methyl | Pyridin-3-yl (ortho) | 0.94 | >117.5 | >125 | 13.10 | >118 | 9 |
| 20c | 1 | F | H | Pyridin-3-yl (para) | 2.99 | >115.1 | 38.5 | 8.90 | >116 | 13 |
| 20d | 0 | F | H | Pyridin-4-yl (ortho) | 11.71 | >117.1 | 10.4 | 8.22 | >123.3 | 15 |
| 20e | 0 | F | H | Pyridin-3-yl (ortho) | 23.19 | >120.5 | 5.2 | 47.53 | >119 | 2.5 |
| 20f | 1 | F | H | Pyridin-4-yl (ortho) | 40.70 | >118.0 | 2.9 | 21.29 | >127 | 6 |
| 20g | 0 | F | p-Methyl | Pyridin-4-yl (ortho) | >117 | >117 | 1 | 48.43 | >125 | 2.6 |
| 20h | 1 | CH3 | H | Pyridin-4-yl (para) | 16.60 | 74.7 | 4.5 | 36.83 | >110.5 | 3 |
| 20i | 0 | CH3 | p-Methyl | Pyridin-3-yl (ortho) | 10.55 | 47.5 | 4.5 | 18.41 | 75.5 | 4.1 |
| 20j | 1 | CH3 | H | Pyridin-3-yl (para) | 6.75 | >116.1 | 17.2 | 73.08 | >124 | 1.7 |
| 20k | 0 | CH3 | H | Pyridin-4-yl (ortho) | 4.71 | >122.5 | 26 | 12.46 | >125 | 10 |
| 20l | 0 | CH3 | H | Pyridin-3-yl (ortho) | 14.56 | >128.13 | 8.8 | >122 | >122 | 1 |
| 20m | 1 | CH3 | H | Pyridin-4-yl (ortho) | 57.86 | >118 | 2.04 | 70.61 | >120 | 1.7 |
| 20n | 0 | CH3 | p-Methyl | Pyridin-4-yl (ortho) | 11.90 | >119 | 10 | 30.93 | >117.5 | 3.8 |
Indicates the attachment point of the heterocycle on the phenyl ring.
Unfortunately, the compound 20f corresponding to 15c, exhibited 20-fold decrease in activity against DENV (EC50 = 40.70 μM) and 6-fold against YFV (EC50 = 21.29 μM). Concentrating on anti-DENV activity, we noticed within the imidazole series that the para attachment of the heterocycle to ring C proved deleterious for the biological activity (see 15e–g). In contrast with these results, the para-pyrimidin-4-yl substituent on ring C proved advantageous within the pyrazine series and led to compound 20a displaying a 2-fold increase in anti-DENV potency with EC50 of 0.93 μM. A distinction however can be made having either a benzylamine (n = 1) or an aniline (n = 0) aromatic ring C. Within the benzylamine series, the heterocycle is preferred at the para position with preference for concomitant presence of fluoroaniline at the A-site (20a, 20c) over toluidine (20h, 20j). However, ortho attachment of the heterocycle on the benzylamine ring seriously reduced the activity (20f, 20m). Having an aniline ring at site C the results were less clear with mostly intermediate inhibitory properties (5–25 μM), except for the strongly inhibiting compound 20b and the non-active analog 20e. Within this pyrazine series (20a–n) the YFV inhibitory properties in general were slightly weaker compared to DENV inhibition by these compounds.
3. Conclusion
A series of tetracyclic inhibitors were prepared using I45CD and P23DC scaffolds and which further were part of a structure−activity investigation. The SAR results of both series, I45DCs and P23DCs derivatives, as potent anti-dengue and anti-yellow fever virus agents are described above. Our investigation led to the discovery of 15b, which is the most potent inhibitor of the series (EC50 = 1.85 μM) against YFV, but followed closely in potency by the quite deviating structure 15d. The structural analog 15c on the other hand proved most inhibitory for dengue virus (see Fig. 4). In addition, within the pyrazine series both compounds 20a and 20b were found to have potent DENV inhibitory properties (EC50 = 0.93 μM) uncovered to date. Key features of these inhibitors include the central bis-amide arrangement which is found to be a metal-chelating component in many drugs. In summary, determination of the antiviral activities shows that I45DC and P23DC are attractive candidate scaffolds for further studies and the interesting activities observed so far, warrant an in depth study of their mechanism of action.
Fig. 4.
Overview of all new compounds most inhibitory to either DENV or YFV as reported in this communication.
4. Materials and methods
All other chemicals were provided by Aldrich or ACROS and were of the highest quality. 1H and 13C NMR spectra were determined with a 300 MHz Varian Gemini apparatus with tetramethylsilane as internal standard for the 1H NMR spectra (s = singlet, d = doublet, dd = double doublet, t = triplet, br. s = broad signal, br. d = broad doublet, m = multiplet) and the solvent signal – DMSO-d6 (δ = 39.6 ppm) or CDCl3 (δ = 76.9 ppm) – for the 13C NMR spectra. Exact mass measurements were performed with a quadrupole/orthogonal acceleration time-of-flight tandem mass spectrometer (qTOF2, Micromass, Manchester, UK) fitted with a standard electrospray ionization (ESI) interface. All solvents were carefully dried or bought as such.
4.1. Chemistry
4.1.1. 5,10-Dioxo-5,10-dihydrodiimidazo[1,5-a:1′,5′-d]pyrazine-1,6-dicarbonyl dichloride (11)
To a dry round-bottom flask was added 1.0 g of imidazole-4,5-dicarboxylic acid (10, 1 mmol) and 10 mL of toluene. To this stirred suspension were added 3.0 mL of thionyl chloride (6.5 mmol) and 0.250 mL of DMF (catalytic). The resulting mixture was refluxed for 16 h. After the mixture was cooled in ice bath, the solid product was collected by vacuum filtration, washed with two 20 mL portions of toluene, and dried under vacuum to yield 0.95 g of crude 11 (Yield: 95%) as a yellow solid. This product was used further without characterization.
4.1.2. General procedure for 12a–e
To a suspension of 11 (1.0 mmol) in 10 mL of dichloromethane was added N,N-diethylaniline (2 mmol), and corresponding substituted aniline (1.7 mmol) at −10 °C. The reaction mixture was stirred at room temperature for 3–5 h before collecting highly insoluble colored solid 12a–e by vacuum filtration. The product was washed with portions of 10 mL DCM, cold water, and acetone, respectively. Note: Addition of excess of aniline results in opening of acyl imidazole bonds affording unwanted products.
4.1.2.1. 5,10-Dioxo-N1,N6-diphenyl-5,10-dihydrodiimidazo[1,5-a:1′,5′-d]pyrazine-1,6-dicarboxamide (12a)
Yield: 85%; Orange solid; 1H NMR (300 MHz, CDCl3): δ 10.74 (s, 2H), 9.09 (s, 2H), 7.80 (d, 4H, J = 7.8 Hz), 7.41 (t, 4H, J = 8.1 Hz), 7.17 (t, 2H, J = 7.5 Hz). 13C NMR (75 MHz, CDCl3): δ 162.3, 158.1, 149.2, 145.2, 138.7, 138.2, 129.0, 124.5, 120.1. HRMS calcd for C22H15N6O4 [M+H]+: 427.1149; found: 427.1151.
4.1.2.2. N1,N6-bis(4-fluorophenyl)-5,10-dioxo-5,10-dihydrodiimidazo[1,5-a:1′,5′-d]pyrazine-1,6-dicarboxamide (12b)
Yield: 87%; Yellow Solid; 1H NMR (300 MHz, CDCl3): δ 10.76 (s, 2H), 9.09 (s, 2H), 7.85–7.81 (m, 4H), 7.25 (m, 4H, J = 9.0 Hz). 13C NMR (75 MHz, CDCl3): δ 160.3, 158.1, 157.2, 149.0, 145.0, 138.6, 134.8, 134.8, 122.1, 122.0, 120.7, 115.5. HRMS calcd for C22H15N6O4 [M+H]+: 427.1149; found: 427.1151. HRMS calcd for C22H13F2N6O4 [M+H]+: 463.0961; found: 463.0961.
4.1.2.3. 5,10-Dioxo-N1,N6-di-p-tolyl-5,10-dihydrodiimidazo[1,5-a:1′,5′-d]pyrazine-1,6-dicarboxamide (12c)
Yield: 89%; Red Solid; 1H NMR (600 MHz, DMSO): δ 10.62 (s, 2H), 9.07 (s, 2H), 7.67 (d, 4H, J = 8.4 Hz), 7.20 (d, 4H, J = 7.8 Hz), 2.30 (s, 6H). 13C NMR (125 MHz, DMSO): δ157.8, 149.2, 145.2, 138.6, 135.9, 133.5, 129.4, 120.4, 120.0, 20.6. HRMS calcd for C22H15N6O4 [M+H]+: 427.1149; found: 427.1151. HRMS calcd for C24H19N6O4 [M+H]+: 455.1462; found: 455.1461.
4.1.2.4. N1,N6-bis(4-methyl-2-(thiophen-2-yl)phenyl)-5,10-dioxo-5,10-dihydrodiimidazo [1,5-a:1′,5′-d]pyrazine-1,6-dicarboxamide (12d)
Yield: 74%; Red Solid; 1H NMR (300 MHz, DMSO): 10.01 (s, 2H), 8.97 (s, 2H), 7.79–7.63 (m, 6H), 7.35–7.23 (m, 6H), 2.37 (s, 6H, 2CH3). HRMS calcd for C32H23N6O4S2 [M+H]+: 619.1237; found: 619.1238. In view of insufficient solubility 13C NMR could not be determined.
4.1.2.5. N1,N6-bis(2-(furan-2-yl)-4-methylphenyl)-5,10-dioxo-5,10-dihydrodiimidazo[1,5-a:1′,5′-d]pyrazine-1,6-dicarboxamide (12e)
Yield: 72%; Yellow Solid; 1H NMR (300 MHz, DMSO): 10.46 (s, 2H), 9.07 (s, 2H), 7.81–7.61 (m, 6H), 7.25–6.63 (m, 6H), 2.38 (s, 6H, 2CH3). HRMS calcd for C32H23N6O6 [M+H]+: 587.1673; found: 587.1672. In view of insufficient solubility 13C NMR could not be determined.
4.1.3. General procedure for 14a–m
To a solution of corresponding aniline/benzyl amine (1.0 mmol) in 10 mL dioxane:water (1:1), was added anhydrous K2CO3 (1.5 mmol), aryl boronic acids (1.2 mmol) and Pd(TPP)2Cl2 (0.025 mmol) in a seal tube. The mixture was purged with argon for 30 min at rt and heated at 100 °C for 1 h in microwave. All reactions and manipulations were run under argon atmosphere. After completion, the solvent was evaporated under reduced pressure and the residue was purified by column chromatography on a silica gel to give the desired product.
4.1.3.1. 2-(5-Methyl-1H-pyrazol-3-yl)aniline (14a) [21]
Yield: 52%; White solid; 1H NMR (300 MHz, CDCl3): δ 7.50 (d, 1H, J = 7.2 Hz), 7.13 (t, 1H, J = 7.5 Hz), 6.79–6.75 (m, 2H), 6.39 (s, 1H), 2.37 (s, 3H, CH3).
4.1.3.2. 4-Methyl-2-(pyridin-3-yl) aniline (14b)
Yield: 62%; Yellow Oil; 1H NMR (300 MHz, CDCl3): δ 8.70 (s, 1H), 8.61–8.59 (m, 1H), 7.83–7.80 (m, 1H),7.40–7.35 (m, 1H), 7.05–7.01 (m, 1H), 6.94 (m, 1H), 6.73–6.71 (d, 1H, J = 8.1 Hz), 3.60 (bs, 2H, NH2), 2.30 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 149.2, 147.4, 140.3, 135.7, 130.0, 129.0, 127.3, 122.6, 115.2, 19.5. HRMS calcd for C12H13N2 [M+H]+: 185.1073; found: 185.1078.
4.1.3.3. 4-Methyl-2-(pyridin-4-yl)aniline (14c)
Yield: 59%; White solid; 1H NMR (300 MHz, CDCl3): δ 8.69–8.67 (m, 2H), 7.44–7.42 (m, 2H), 7.06–6.96 (m, 2H), 6.72 (d, 1H, J = 8.1 Hz), 3.69 (bs, 2H, NH), 2.30 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 150.4, 130.6, 130.5, 124.0, 116.4, 20.5. HRMS calcd for C12H13N2 [M+H]+: 185.1073; found: 185.1074.
4.1.3.4. 2-(Pyridin-3-yl)aniline (14d)
Yield: 72%; Yellow solid; 1H NMR (300 MHz, CDCl3): δ 8.73 (s, 1H), 8.62–8.60 (m, 1H), 7.84–7.80 (m, 1H), 7.41–7.36 (m, 1H), 7.24–7.11 (m, 2H), 6.89–6.79 (m, 2H), 3.75 (bs, 2H, NH2). 13C NMR (75 MHz, CDCl3): δ 150.2, 148.6, 143.9, 136.6, 135.4, 130.7, 129.5, 123.6, 119.0, 116.0. HRMS calcd for C11H11N2 [M+H]+: 171.0917; found: 171.0921.
4.1.3.5. 2-(Pyridin-4-yl)aniline (14e)
Yield: 69%; White solid; 1H NMR (300 MHz, CDCl3): δ 8.69–8.67 (m, 2H), 7.44–7.28 (m, 2H), 7.25–7.13 (m, 2H), 6.89–6.78 (m, 2H), 3.82 (bs, 2H, NH2). 13C NMR (75 MHz, CDCl3): δ 149.4, 129.2, 128.8, 123.0, 118.0, 115.2. HRMS calcd for C11H11N2 [M+H]+: 171.0917; found: 171.0925.
4.1.3.6. (4-(Pyridin-3-yl)phenyl)methanamine (14f)
Yield: 55%; White solid; 1H NMR (300 MHz, CDCl3): δ 8.86 (m, 2H), 8.61–8.59 (m, 1H), 7.91–7.87 (m, 1H), 7.60–7.57 (m, 2H), 7.47–7.40 (m, 2H), 7.38–7.36 (m, 1H), 3.96 (s, 2H, CH2), 1.62 (bs, 2H, NH2). 13C NMR (75 MHz, CDCl3): δ 149.9, 127.5, 126.8, 121.1, 45.7. HRMS calcd for C12H13N2 [M+H]+: 185.1073; found: 185.1072.
4.1.3.7. (4-(Pyridin-4-yl)phenyl)methanamine (14g) [22]
Yield: 59%; White solid; 1H NMR (300 MHz, CDCl3): δ 8.64–8.62 (m, 2H), 7.61–7.41 (m, 6H), 3.92 (s, 2H, CH2), 1.77 (bs, 2H, NH2). 13C NMR (75 MHz, CDCl3): δ 149.2, 126.9, 126.2, 120.5, 45.1.
4.1.3.8. (2-(Pyridin-4-yl)phenyl)methanamine (14h) [23]
Yield: 65%; White solid; 1H NMR (300 MHz, CDCl3): δ 8.76–8.74 (m, 1H), 7.56–7.10 (m, 8H), 3.92 (s, 2H, CH2). 13C NMR (75 MHz, CDCl3): δ 149.1, 132.0, 131.3, 131.1, 131.1, 128.2, 127.7, 127.6, 127.5, 126.9, 122.7, 120.5, 46.0.
4.1.3.9. 4-Methyl-2-(thiophen-3-yl)aniline (14i)
Yield: 62%; Off-white solid; 1H NMR (300 MHz, CDCl3): δ 7.47–7.40 (m, 2H), 7.33–7.31 (m, 1H), 7.09 (s, 1H), 7.03–7.00 (m, 1H), 6.75–6.72 (d, 1H, J = 8.1 Hz), 3.73 (bs, 2H, NH2), 2.33 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 141.5, 140.1, 130.7, 129.1, 128.5, 127.8, 126.0, 122.6, 122.5, 116.0, 20.5. HRMS calcd for C11H12N1S1 [M+H]+: 190.0685; found: 190.0686.
4.1.3.10. (4-(Thiophen-3-yl)phenyl)methanamine (14j) [24]
Yield: 58%; Off-white solid; 1H NMR (300 MHz, CDCl3): δ 7.61–7.58 (m, 2H), 7.47–7.28 (m, 6H), 3.92 (s, 2H, CH2). 13C NMR (75 MHz, CDCl3): δ 127.7, 126.8, 126.5, 126.3, 120.2, 46.3.
4.1.3.11. 2-(Thiophen-3-yl)phenyl)methanamine (14k) [25]
Yield: 52%; White solid; 1H NMR (300 MHz, CDCl3): δ 7.58–7.12 (m, 6H), 3.91 (s, 2H, CH2). 13C NMR (150 MHz, CDCl3): δ 162.1, 160.8, 160.5, 142.0, 141.1, 135.8, 132.7, 130.1, 129.8, 129.3, 129.0, 128.8, 128.6, 128.4, 128.2, 127.7, 127.5, 127.4, 127.1, 127.0, 126.8, 125.5, 125.0, 123.4, 122.7, 46.8, 44.2.
4.1.3.12. 2-(Furan-2-yl)-4-methylaniline (14l)
Yield: 63%; White solid; 1H NMR (300 MHz, CDCl3): δ 7.52 (s, 1H), 7.32 (s, 1H), 6.97–6.94 (m, 1H), 6.71–6.52 (m, 3H), 2.30 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 152.5, 141.2, 140.8, 129.5, 127.8, 127.6, 116.9, 116.2, 111.3, 106.3, 20.4. HRMS calcd for C11H12N1O1 [M+H]+: 174.0913; found: 174.0910.
4.1.3.13. 5-Methyl-3-(5-methylthiophen-2-yl)pyridin-2-amine (14m)
Yield: 57%; White solid; 1H NMR (300 MHz, CDCl3): δ 7.84 (s, 1H), 7.28 (s, 1H), 6.99 (d, J = 3.3 Hz, 1H), 6.73–6.72 (m, 1H), 4.96 (bs, 2H, NH2), 2.49 (s, 3H, CH3), 2.18 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 153.6, 146.5, 139.8, 138.5, 137.1, 125.6, 125.5, 122.7, 114.5, 17.0, 14.9.
4.1.4. General procedure for 15b–o
To a stirred solution of 12a–e (1.0 mmol) in 10 mL DCM, was added DIPEA (3.0 mmol), and 14a–m (3.0 mmol) under argon atmosphere at room temperature. The mixture was allowed to reflux for 16–48 h. Two alternative workup procedures were followed depending on whether the product precipitated out of solution or not.
Workup 1 (for soluble products): The DCM was evaporated using a rotavapor. The product was purified by flash chromatography on silica gel.
Workup 2 (for insoluble products): The precipitate was collected by filtration using a Buchner funnel and washed on the frit with DCM (20–25 mL) until the yellow impurities had disappeared. The product obtained from this workup procedure did not need chromatographic purification.
4.1.4.1. N5-(4-fluorophenyl)-N4-(2-(3-methyl-1H-pyrazol-5-yl)phenyl)-1H-imidazole-4,5-dicarboxamide (15b)
Yield: 86%; 1H NMR (500 MHz, DMSO): δ 13.69 (bs, 1H, NH), 13.30 (bs, 1H, NH), 13.05 (bs, 1H, NH), 12.78 (bs, 1H, NH), 8.65 (d, 1H, J = 8.0 Hz), 7.96 (s, 1H), 7.78–7.23 (m, 9H), 6.54 (s, 1H), 2.31 (s, 3H, CH3). 13C NMR (125 MHz, DMSO): δ 163.3, 159.6, 158.0, 156.4, 150.3, 140.2, 137.3, 135.2, 135.0, 133.9, 130.0, 128.7, 124.8, 123.2, 121.9, 121.6, 116.4, 116.2, 103.1, 10.8. HRMS calcd for C21H16F1N6O2 [M−H]+: 403.1324; found: 403.1323.
4.1.4.2. N5-(4-fluorophenyl)-N4-(2-(pyridin-4-yl)benzyl)-1H-imidazole-4,5-dicarboxami-de (15c)
Yield: 83%; 1H NMR (600 MHz, DMSO): δ 8.76 (d, 0.5H, J = 6.6 Hz), 7.96–7.17 (m, 10H), 4.57 (d, 2H, J = 6.0 Hz, CH2). 13C NMR (150 MHz, DMSO): δ 159.5, 157.9, 150.0, 137.4, 127.1, 134.8, 132.7, 132.4, 131.8, 131.7, 129.3, 129.1, 129.0, 128.7, 128.1, 122.4, 122.0, 116.0, 115.8, 43.1. HRMS calcd for C23H18F1N5O2 [M−H]+: 414.1372; found: 414.1372.
4.1.4.3. N5-(4-fluorophenyl)-N4-(2-(thiophen-3-yl)benzyl)-1H-imidazole-4,5-dicarbox amide (15d)
Yield: 86%; 1H NMR (600 MHz, DMSO): δ 13.53 (bs, 0.5H, NH), 13.45 (bs, 0.5H, NH), 9.40 (bs, 0.5H, NH), 9.23 (bs, 0.5H, NH), 7.99–7.18 (m, 12H), 4.58 (d, 2H, J = 6.6 Hz, CH2). 13C NMR (150 MHz, DMSO): δ 164.3, 156.1, 140.5, 137.3, 137.0, 132.7, 132.5, 129.9, 129.0, 128.0, 127.6, 127.1, 126.3, 123.7, 121.1, 115.9, 115.7, 42.9. HRMS calcd for C22H16F1N4O2S1 [M−H]+: 419.0973; found: 419.0967.
4.1.4.4. N5-(4-fluorophenyl)-N4-(4-(pyridin-3-yl)benzyl)-1H-imidazole-4,5-dicarboxamide (15e)
Yield: 76%; 1H NMR (600 MHz, DMSO): δ 13.55 (s, 1H, NH), 13.49 (bs, 1H, NH), 10.49 (s, 1H), 10.06 (s, 1H), 9.47 (s, 1H), 8.85–7.20 (m, 9H), 4.59 (d, 2H, J = 6.6 Hz, CH2). 13C NMR (150 MHz, DMSO): δ 164.2, 156.4, 148.6, 147.7, 139.3, 137.2, 136.0, 135.6, 135.1, 134.4, 133.0, 128.9, 128.4, 128.3, 127.3, 127.2, 124.2, 121.4, 121.4, 116.1, 116.9, 42.3. HRMS calcd for C23H17F1N5O2 [M−H]+: 414.1372; found: 414.1372.
4.1.4.5. N5-(4-fluorophenyl)-N4-(4-(thiophen-3-yl)benzyl)-1H-imidazole-4,5-dicarboxamide (15f)
Yield: 78%; 1H NMR (300 MHz, DMSO): δ 9.84 (bs, 1H, NH), 7.97 (s, 1H), 7.87–7.19 (m, 15H), 4.57 (d, 2H, J = 6.3 Hz, CH2). 13C NMR (75 MHz, DMSO): δ 160.1, 157.0, 141.4, 141.4, 138.0, 137.0, 134.9, 134.0, 128.1, 128.0, 127.6, 127.1, 126.3, 126.0, 121.7, 121.6, 120.9, 120.7, 115.9, 115.6, 42.2. HRMS calcd for C22H16F1N5O2 S1 [M−H]+: 419.0983; found: 419.0993.
4.1.4.6. N5-(4-fluorophenyl)-N4-(4-(pyridin-4-yl)benzyl)-1H-imidazole-4,5-dicarboxamide (15g)
Yield: 81%; 1H NMR (600 MHz, DMSO) δ 13.56 (s, 1H, NH), 13.47 (bs, 1H, NH), 9.50 (s, 1H, NH), 8.61–7.20 (m, 13H), 4.59 (d, 2H, J = 6.0 Hz, CH2). 13C NMR (150 MHz, DMSO): δ 164.2, 156.2, 150.5, 150.3, 146.9, 140.4, 137.1, 135.9, 135.0, 133.0, 128.8, 128.3, 128.2, 127.1, 127.0, 121.3, 121.2, 116.0, 115.9, 42.2. HRMS calcd for C23H17F1N5O2 [M−H]+: 414.1372; found: 414.1369.
4.1.4.7. N5-(4-fluorophenyl)-N4-(4-methyl-2-(thiophen-3-yl)phenyl)-1H-imidazole-4,5-dicarboxamide (15h)
Yield: 89%; 1H NMR (500 MHz, DMSO): δ 13.64 (s, 0.7H, NH), 13.56 (s, 0.3H, NH), 13.22 (s, 0.7H, NH), 12.44 (s, 0.3H, NH), 10.46 (s, 0.3H, NH), 10.12 (s, 0.7H, NH), 7.99–7.15 (m, 13H), 2.36 (s, 3H, CH3). 13C NMR (125 MHz, DMSO): δ 162.5, 156.0, 138.3, 137.1, 136.7, 135.2, 134.9, 132.9, 131.5, 130.7, 130.6, 130.0, 129.2, 128.6, 128.6, 128.4, 128.4, 127.0, 126.2, 124.9, 124.2, 124.0, 122.9, 199.9, 121.2, 121.2, 116.0, 115.8, 115.2, 115.1, 20.6. HRMS calcd for C22H18F1N5O2 S1 [M−H]+: 421.1128; found: 421.1117.
4.1.4.8. N5-(4-fluorophenyl)-N4-(2-(furan-2-yl)-4-methylphenyl)-1H-imidazole-4,5-dicarboxamide (15i)
Yield: 77%; 1H NMR (300 MHz, DMSO): δ 13.69 (bs, 1H, NH), 13.20 (bs, 1H, NH), 10.60 (s, 1H, NH), 8.05–7.55 (m, 6H), 7.27–6.56 (m, 5H), 2.38 (s, 3H, CH3). HRMS calcd for C22H18F1N4O3 [M+H]+: 405.1357; found: 405.1353.
4.1.4.9. N4-(4-methyl-2-(thiophen-3-yl)phenyl)-N5-phenyl-1H-imidazole-4,5-dicarboxamide (15j)
Yield: 81%; 1H NMR (600 MHz, DMSO): δ 13.63 (s, 0.7H, NH), 13.56 (s, 0.3H, NH), 13.18 (s, 0.7H, NH), 12.47 (s, 0.3H, NH), 10.34 (s, 0.3H, NH), 10.12 (s, 0.7H, NH), 7.96–7.12 (m, 12H), 2.36 (s, 3H, CH3). 13C NMR (125 MHz, DMSO): δ 162.4, 156.0, 138.5, 138.3, 137.1, 135.2, 132.8, 131.5, 130.6, 129.9, 129.3, 128.6, 128.4, 127.0, 124.2, 124.0, 120.9, 119.4, 20.6. HRMS calcd for C22H17N4O2 S1 [M−H]+: 401.1078; found: 401.1078.
4.1.4.10. N4-(4-methyl-2-(thiophen-3-yl)phenyl)-N5-(p-tolyl)-1H-imidazole-4,5-dicarboxamide (15k)
Yield: 79%; 1H NMR (600 MHz, DMSO): δ 13.59 (s, 0.7H, NH), 13.54 (s, 0.3H, NH), 13.09 (s, 0.3H, NH), 12.52 (s, 0.7H, NH), 10.26 (s, 0.3H, NH), 10.10 (s, 0.7H, NH), 7.98–7.18 (m, 10H), 2.36 (s, 3H, CH3), 2.28 (s, 3H, CH3). 13C NMR (125 MHz, DMSO): δ 162.4, 155.8, 138.3, 137.0, 136.0, 135.1, 133.1, 132.7, 131.5, 130.6, 129.9, 129.6, 129.4, 129.0, 128.6, 128.5, 128.4, 128.4, 127.0, 124.2, 124.0, 120.9, 119.4, 20.6. HRMS calcd for C23H21N4O2S1 [M+H]+: 417.1380; found: 417.1377.
4.1.4.11. N4-(2-(furan-2-yl)-4-methylphenyl)-N5-(p-tolyl)-1H-imidazole-4,5-dicarboxamide (15l)
Yield: 76%; 13.58 (s, 0.7H, NH), 13.52 (s, 0.3H, NH), 13.07 (s, 0.3H, NH), 12.50 (s, 0.7H, NH), 10.23 (s, 0.3H, NH), 10.08 (s, 0.7H, NH), 7.97–7.93 (m, 2H), 7.71–7.56 (m, 4H), 7.33–7.11 (m, 5H), 2.35 (s, 3H, CH3), 2.28 (s, 3H, CH3). 13C NMR (125 MHz, DMSO): δ 162.4, 155.8, 138.3, 136.9, 136.0, 135.1, 133.1, 132.7, 131.5, 130.6, 129.8, 129.6, 129.4, 129.0, 128.6, 128.5, 128.4, 128.4, 127.0, 124.2, 124.0, 120.9, 119.4, 20.6, 20.6. HRMS calcd for C23H21N4O3 [M+H]+: 401.1608; found: 401.1602.
4.1.4.12. N5-benzyl-N4-(2-(furan-2-yl)-4-methylphenyl)-1H-imidazole-4,5-dicarboxamide (15m)
Yield: 83%; 1H NMR (300 MHz, CDCl3): δ 11.84 (bs, 1H, NH), 11.57 (bs, 1H, NH), 10.32 (bs, 1H, NH), 8.16 (d, 1H, J = 8.4 Hz), 7.61–7.18 (m, 10H), 6.74 (d, 1H, J = 3.3 Hz), 6.57–6.55 (m, 1H), 4.70 (d, 2H, J = 6.0 Hz, CH2), 2.40 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 161.7, 159.0, 142.4, 134.9, 134.8, 130.5, 129.1, 128.6, 127.9, 127.4, 127.2, 123.0, 111.7, 108.5, 43.5, 21.0. HRMS calcd for C23H21N4O3 [M+H]+: 401.1608; found: 401.1590.
4.1.4.13. N5-(4-fluorophenethyl)-N4-(2-(furan-2-yl)-4-methylphenyl)-1H-imidazole-4,5-dicarboxamide (15n)
Yield: 75%; 1H NMR (300 MHz, CDCl3): δ 11.13 (bs, 1H, NH), 10.32 (bs, 1H, NH), 8.18 (d, 1H, J = 8.7 Hz), 7.67–6.55 (m, 10H), 3.73 (m, 2H, CH2), 3.00 (t, 2H, J = 7.5 Hz CH2), 2.42 (s, 3H, CH3). 13C NMR (150 MHz, DMSO): δ 161.5, 157.9, 157.1, 150.6, 150.3, 143.1, 142.8, 142.3, 142.2, 138.4, 137.4, 136.8, 136.5, 135.7, 135.0, 133.3, 132.6, 130.4, 130.2, 129.0, 128.7, 128.3, 127.6, 127.3, 127.1, 125.5, 125.0, 124.5, 123.8, 118.0, 115.7, 115.4, 113.1, 112.8, 112.1, 109.1, 108.9, 77.1, 60.9, 34.81. HRMS calcd for C24H22FN4O3 [M+H]+: 433.1670; found: 433.1668.
4.1.4.14. N5-(5-methyl-3-(5-methylthiophen-2-yl)pyridin-2-yl)-N4-phenyl-1H-imidazole-4,5-dicarboxamide (15o)
Yield: 76%; 1H NMR (600 MHz, CDCl3): δ 13.63 (bs, 1H, NH), 13.08 (bs, 0.7H, NH), 12.92 (bs, 0.3H, NH), 10.70 (bs, 0.7H, NH), 10.54 (bs, 0.3H, NH), 8.31–7.08 (m, 8H), 6.79–6.76 (m, 1H), 2.39 (s, 3H, CH3), 2.36 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3): δ 184.6, 149.5, 147.4, 144.6, 143.7, 141.2, 138.3, 138.2, 137.4, 137.3, 135.6, 132.9, 129.3, 129.2, 129.1, 128.5, 127.1, 126.8, 126.3, 128.5, 17.5, 15.1. HRMS calcd for C22H19N5O2S1 [M−H]+: 416.1187; found: 416.1187.
4.1.5. General procedure for 18a–b
Sodium dodecyl sulfate (SDS, 6 mmol) was added to a stirred heterogeneous suspension of amine (5 mmol) in water (20 mL) until a homogeneous solution was formed, (in case of turbidity, the mixture was warmed to obtain a clear solution). Anhydride 17 (5 mmol) dissolved in acetonitrile (5 mL) was added to this solution in one lot. After stirring for 1 h at room temperature, the acetonitrile was evaporated and the product precipitated from the aqueous layer. To the aqueous solution containing precipitate, solid sodium hydrogen carbonate was added pinch-wise until the effervescence ceased and the pH was 6.0. The remaining precipitated product was filtered, washed with water (20 mL), dried in a vacuum desiccator. In cases where the product did not precipitate, the reaction mixture was extracted with ethyl acetate (2 × 25 mL). The combined organic extracts were dried with anhydrous Na2SO4 and the solvent was removed in a rotary evaporator under reduced pressure to yield the pure product.
4.1.5.1. 3-((4-Fluorophenyl)carbamoyl)pyrazine-2-carboxylic acid (18a) [26]
Yield: 72%; White solid; 1H NMR (300 MHz, DMSO): δ 10.86 (bs, 1H), 8.90 (s, 2H), 7.83–7.78 (m, 2H), 7.22 (t, 2H, J = 9.0 Hz).
4.1.5.2. 3-(p-Tolylcarbamoyl)pyrazine-2-carboxylic acid (18b) [27]
Yield: 65%; White solid; 1H NMR (300 MHz, DMSO): δ 10.68 (bs, 1H), 8.88 (s, 2H), 7.67 (d, J = 8.4 Hz, 2H), 7.17 (t, J = 8.4 Hz, 2H), 2.29 (s, 3H, CH3). 13C NMR (75 MHz, DMSO): δ 166.5, 162.3, 146.5, 145.8, 145.6, 144.6, 136.0, 133.4, 129.3, 120.2, 20.7.
4.1.6. General procedure for 19a–b
Trifluoroacetic anhydride (1.5 equiv) was added dropwise to a stirring solution of acid 18a–b (1 equiv) and Et3N (TEA, 3 equiv) in 1,4-dioxane (20 mL) that was kept at 0 °C with an ice bath. After 15 min the yellow solution was allowed to warm to room temperature and was stirred for 30 min, then it was poured in cold H2O (100 mL). A precipitate formed, which was collected by filtration using a Buchner funnel and washed with H2O. The product was dried overnight under high vacuum.
4.1.6.1. (Z)-7-((4-fluorophenyl)imino)furo[3,4-b]pyrazin-5(7H)-one (19a)
Yield: 70%; Off-white solid; 1H NMR (300 MHz, CDCl3): δ 9.06 (d, 2H, J = 5.1 Hz), 7.73–7.69 (m, 2H), 7.15 (t, J = 8.4 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 150.9, 149.5, 128.7, 128.6, 116.2, 116.0.
4.1.6.2. (Z)-7-(p-tolylimino)furo[3,4-b]pyrazin-5(7H)-one (19b)
Yield: 66%; White solid; 1H NMR (300 MHz, CDCl3): δ 9.07–9.03 (m, 2H), 7.69–7.28 (m, 4H), 2.43 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 150.5, 149.0, 129.5, 126.4, 21.0.
4.1.7. General procedure for 20a–n
Phthalisoimide 19a–b (1 equiv) was added to stirring solution of the aniline/benzylamine 14a–m (1.2 equiv) in THF. The mixture was stirred overnight at room temperature. The THF was evaporated on a rotavapor. The mixture was diluted with EtOAc (triple the volume of THF) and washed three times with 1 M HCl. The organic phase was dried with Na2SO4 and evaporated. The product was purified by flash chromatography on silica gel.
4.1.7.1. N2-(4-fluorophenyl)-N3-(4-(pyridin-4-yl) benzyl) pyrazine-2,3-dicarboxamide (20a)
Yield: 76%; 1H NMR (300 MHz, DMSO): δ 10.65 (bs, 1H, NH), 9.45 (t, J = 6.6 Hz, 1H, NH), 8.88 (q, 2H, J = 2.4 Hz), 8.64–8.61 (m, 2H), 7.78–7.69 (m, 6H), 7.50 (d, J = 8.4 Hz, 2H), 7.21 (t, J = 9.0 Hz, 2H), 2.39 (d, J = 6.3 Hz, 2H, CH2). 13C NMR (75 MHz, DMSO): δ 164.0, 163.7, 160.1, 156.9, 150.3, 148.3, 146.9, 145.4, 145.3, 144.5, 140.5, 135.8, 135.5, 128.3, 126.9, 121.7, 121.6, 121.2, 115.7, 115.4, 42.2. HRMS calcd for C24H19F1N5O2 [M+H]+: 428.1517; found: 428.1520.
4.1.7.2. N2-(4-fluorophenyl)-N3-(4-methyl-2-(pyridin-3-yl) phenyl) pyrazine-2,3-dicarboxamide (20b)
Yield: 74%; 1H NMR (300 MHz, CDCl3): δ 9.02 (bs, 1H, NH), 8.69–8.54 (m, 5H), 8.27 (d, J = 8.4 Hz, 1H), 7.84 (d, J = 8.1 Hz, 1H), 7.70–7.66 (m, 2H), 7.36–7.26 (m, 2H), 7.11–7.03 (m, 3H), 2.39 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 161.8, 161.5, 150.1, 149.1, 144.3, 137.3, 135.7, 134.2, 131.8, 131.0, 130.1, 123.7, 123.2, 122.2, 122.1, 116.1, 115.8, 21.1. HRMS calcd for C24H19F1N5O2 [M+H]+: 428.1517; found: 428.1514.
4.1.7.3. N2-(4-fluorophenyl)-N3-(4-(pyridin-3-yl) benzyl) pyrazine-2,3-dicarboxamide (20c)
Yield: 72%; 1H NMR (300 MHz, CDCl3): δ 9.33 (bs, 1H, NH), 8.76 (bs, 1H, NH), 8.63 (s, 2H), 8.54–8.52 (d, J = 4.8 Hz, 1H), 7.86–7.27 (m, 9H), 7.05 (m, J = 8.7 Hz, 2H), 4.76 (d, J = 6.0 Hz, 2H, CH2). 13C NMR (75 MHz, CDCl3): δ164.9, 161.6, 161.5, 148.6, 148.3, 147.6, 146.1, 144.6, 144.0, 137.8, 137.3, 136.3, 134.4, 133.6, 128.8, 127.6, 123.7, 122.1, 122.0, 116.0, 115.7, 43.7. HRMS calcd for C24H19F1N5O2 [M+H]+: 428.1517; found: 428.1517.
4.1.7.4. N2-(4-fluorophenyl)-N3-(2-(pyridin-4-yl) phenyl) pyrazine-2,3-dicarboxamide (20d)
Yield: 66%; 1H NMR (300 MHz, CDCl3): δ 10.72 (bs, 1H, NH), 10.27 (bs, 1H, NH), 8.90–8.86 (m, 2H), 8.58–8.56 (m, 2H), 7.85–7.76 (m, 3H), 7.55–7.37 (m, 5H), 7.22 (t, J = 9.0 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 163.2, 163.1, 149.8, 147.0, 146.2, 145.1, 144.9, 135.2, 134.4, 133.6, 130.3, 129.3, 126.6, 126.2, 124.0, 121.9, 121.8, 115.7, 115.4, 79.3. HRMS calcd for C23H17F1N5O2 [M+H]+: 414.1361; found: 414.1363.
4.1.7.5. N2-(4-fluorophenyl)-N3-(2-(pyridin-3-yl) phenyl) pyrazine-2,3-dicarboxamide (20e)
Yield: 76%; 1H NMR (300 MHz, CDCl3): δ 9.04 (bs, 1H, NH), 8.78 (bs, 1H, NH), 8.70–8.56 (m, 4H), 8.45 (d, J = 8.1 Hz, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.72–7.69 (m, 2H), 7.52–7.7.28 (m, 4H), 7.07 (t, J = 8.7 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 161.7, 161.5, 150.1, 149.2, 144.3, 137.3, 134.4, 134.0, 133.5, 130.5, 129.9, 129.5, 125.8, 123.7, 123.1, 122.2, 122.1, 116.1, 115.8. HRMS calcd for C23H17F1N5O2 [M+H]+: 414.1361; found: 414.1365.
4.1.7.6. N2-(4-fluorophenyl)-N3-(2-(pyridin-4-yl) benzyl) pyrazine-2,3-dicarboxamide (20f)
Yield: 68%; 1H NMR (600 MHz, DMSO): δ 10.67 (bs, 1H, NH), 9.41 (t, J = 6.0 Hz, 1H), 8.91–8.89 (m, 2H), 7.75–7.19 (m, 8H), 4.51 (d, J = 6.0 Hz, 2H). 13C NMR (125 MHz, DMSO): δ 164.3, 163.6, 159.2, 157.6, 150.6, 149.0, 148.1, 145.3, 144.5, 137.4, 135.3, 132.4, 129.0, 128.5, 127.8, 122.1, 121.5, 115.5, 115.4, 42.9. HRMS calcd for C24H19F1N5O2 [M+H]+: 428.1517; found: 428.1520.
4.1.7.7. N2-(4-fluorophenyl)-N3-(4-methyl-2-(pyridin-4-yl)phenyl)pyrazine-2,3-dicarbox amide (20g)
Yield: 76%; 1H NMR (300 MHz, CDCl3): δ 8.95 (bs, 1H, NH), 8.66–8.58 (m, 5H), 8.32 (d, J = 8.4 Hz, 1H), 7.72–7.67 (m, 2H), 7.43 (d, J = 8.4 Hz, 2H), 7.30 (d, J = 8.4 Hz, 2H), 7.13–7.7.04 (m, 3H), 2.40 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 150.0, 145.9, 144.0, 143.8, 130.9, 130.0, 129.9, 124.0, 122.6, 121.7, 121.6, 115.6, 115.3, 20.6. HRMS calcd for C24H19F1N5O2 [M+H]+: 428.1517; found: 428.1518.
4.1.7.8. N2-(4-(pyridin-4-yl) benzyl)-N3-(p-tolyl) pyrazine-2,3-dicarboxamide (20h)
Yield: 78%; 1H NMR (300 MHz, CDCl3): δ 9.19 (bs, 1H, NH), 8.66–8.62 (m, 4H), 7.64–7.47 (m, 8H), 7.17 (d, J = 8.1 Hz, 3H), 4.78 (d, J = 6.0 Hz, 2H, CH2), 2.36 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 165.1, 161.2, 150.4, 148.1, 148.0, 145.8, 144.8, 143.8, 139.0, 137.6, 134.9, 134.8, 129.7, 128.8, 127.5, 121.7, 120.3, 43.7, 21.1. HRMS calcd for C25H22N5O2 [M+H]+: 424.1768; found: 424.1765.
4.1.7.9. N2-(4-methyl-2-(pyridin-3-yl) phenyl)-N3-(p-tolyl) pyrazine-2,3-dicarboxamide (20i)
Yield: 76%; 1H NMR (300 MHz, CDCl3): δ 8.95 (bs, 1H, NH), 8.72–8.56 (m, 5H), 8.33 (d, J = 8.1 Hz, 1H), 7.89 (d, J = 7.5 Hz, 1H), 7.62 (d, J = 8.1 Hz, 2H), 7.38–7.14 (m, 4H), 2.42 (s, 3H, CH3), 2.37 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 150.2, 149.2, 144.5, 144.1, 137.3, 135.5, 134.8, 134.2, 131.9, 131.0, 130.1, 123.6, 123.3, 120.3, 21.1. HRMS calcd for C25H22N5O2 [M+H]+: 424.1768; found: 424.1767.
4.1.7.10. N2-(4-(pyridin-3-yl) benzyl)-N3-(p-tolyl) pyrazine-2,3-dicarboxamide (20j)
Yield: 71%; 1H NMR (300 MHz, CDCl3): δ 9.24 (bs, 1H, NH), 8.78–8.54 (m, 4H), 7.88–7.84 (m, 1H), 7.63 (d, J = 8.4 Hz, 2H), 7.56–7.17 (m, 8H), 4.79 (d, J = 6.0 Hz, 2H, CH2), 2.36 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 165.2, 161.2, 148.6, 148.3, 148.1, 145.7, 144.6, 143.8, 137.9, 137.2, 136.4, 134.9, 134.7, 134.4, 129.7, 128.9, 127.6, 123.7, 120.3, 43.7, 21.1. HRMS calcd for C25H22N5O2 [M+H]+: 424.1768; found: 424.1769.
4.1.7.11. N2-(2-(pyridin-4-yl) phenyl)-N3-(p-tolyl) pyrazine-2,3-dicarboxamide (20k)
Yield: 72%; 1H NMR (300 MHz, CDCl3): δ 8.95 (bs, 1H, NH), 8.68–8.51 (m, 6H), 7.63 (d, J = 8.4 Hz, 2H), 7.50–7.19 (m, 7H), 2.37 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 150.5, 146.2, 144.6, 144.1, 134.9, 134.1, 129.9, 129.8, 125.7, 124.5, 123.0, 120.3, 21.1. HRMS calcd for C24H20N5O2 [M+H]+: 410.1611; found: 410.1608.
4.1.7.12. N2-(2-(pyridin-3-yl) phenyl)-N3-(p-tolyl) pyrazine-2,3-dicarboxamide (20l)
Yield: 68%; 1H NMR (300 MHz, CDCl3): δ 8.70 (bs, 1H, NH), 8.70–8.47 (m, 6H), 7.90 (d, J = 7.8 Hz, 2H), 7.63–7.18 (m, 8H), 2.36 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 162.0, 160.1, 149.6, 148.5, 144.0, 143.7, 137.0, 123.2, 134.0, 133.6, 120.0, 129.2, 129.1, 125.2, 123.2, 122.8, 119.8, 20.6. HRMS calcd for C24H20N5O2 [M+H]+: 410.1611; found: 410.1606.
4.1.7.13. N2-(4-(pyridin-4-yl) benzyl)-N3-(p-tolyl) pyrazine-2,3-dicarboxamide (20m)
1H NMR (300 MHz, CDCl3): δ 9.13 (bs, 1H, NH), 8.77–8.66 (m, 3H), 7.71–7.16 (m, 11H), 4.82 (s, 2H, CH2), 2.36 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 161.3, 150.8, 144.6, 143.9, 136.9, 132.9, 130.5, 129.7, 129.4, 128.7, 128.6, 128.0, 121.6, 120.3, 44.3, 21.1. HRMS calcd for C25H22N5O2 [M+H]+: 424.1768; found: 424.1768.
4.1.7.14. N2-(4-methyl-2-(pyridin-4-yl) phenyl)-N3-(p-tolyl) pyrazine-2,3-dicarboxamide (20n)
Yield: 73%; 1H NMR (300 MHz, CDCl3): δ 8.94 (bs, 1H, NH), 8.68–8.62 (m, 4H), 8.49 (bs, 1H, NH), 8.37 (d, J = 8.4 Hz, 1H), 7.63 (d, J = 8.4 Hz, 2H), 7.48–7.14 (m, 6H), 2.42 (s, 3H, CH3), 2.37 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3): δ 161.3, 150.5, 146.4, 144.5, 144.1, 134.8, 131.5, 130.4, 129.8, 124.5, 123.2, 120.3, 21.1. HRMS calcd for C25H22N5O2 [M+H]+: 424.1768; found: 424.1770.
4.2. Antiviral activity determination for DENV and YFV
Green monkey kidney cells [Vero-B cells as obtained from the European Collection of Cell Cultures (ECACC)] were grown in minimum essential medium (MEM; Gibco, Merelbeke, Belgium) supplemented with 10% fetal calf serum (FCS), 1% l-glutamine and 1% sodium bicarbonate. Vero-B cells were seeded at a density 7 × 103 cells/well in 100 μL assay medium and allowed to adhere overnight. Antiviral assays were performed in medium supplemented with 2% FCS, 1% l-glutamine and 1% sodium bicarbonate. After washing cells twice with 2% FCS medium, serial compound dilutions (1:2) were added to each well (starting concentration 100 μg/mL), following by adding 100 μL of 2% of phosphate buffered saline (PBS) culture medium containing 100 μL 50% cell culture infectious doses (i.e., CCID50) of virus. After 7 days of incubation, 2% FCS culture medium was discarded and cells were fixed with ethanol and stained with 1% methylene blue, and EC50 and CC50 were determinate visually. The 50% effective concentration (EC50), which is defined as the compound concentration that is required to inhibit the virus-induced cytopathogenic effect (CPE) by 50%, and 50% cytotoxic concentration (CC50), which is defined as the compound concentration that is required to inhibit the cell growth by 50%.
Acknowledgments
Milind Saudi holds a scholarship of the Erasmus Mundus Cooperation Window. Mass spectrometry was made possible by the support of the Hercules Foundation of the Flemish Government (grant 20100225–7). The antiviral work was supported by the Wellcome Trust (grant 089328), EU FP7 project SILVER (contract no HEALTH-F3-2010-260644) and the Marie Curie Initial Training Network “EUVIRNA”, while the chemistry part was supported by the Rega Foundation. We are indebted to C. Biernaux for final typesetting.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejmech.2014.09.062.These data include MOL files and InChiKeys of the most important compounds described in this article.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
MOL files
The following ZIP file contains the MOL files of the most important compound referred to in this article.
ZIP file containing the MOL files of the most important compound in this article.
References
- 1.Saudi M., Zmurko J., Kaptein S., Rozenski J., Neyts J., Van Aerschot A. In search of Flavivirus inhibitors part 2: tritylated, diphenylmethylated and other alkylated nucleoside analogues. Eur. J. Med. Chem. 2014;76:98–109. doi: 10.1016/j.ejmech.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Chatelain G., Debing Y., De Burghgraeve T., Zmurko J., Saudi M., Rozenski J., Neyts J., Van Aerschot A. In search of flavivirus inhibitors: evaluation of different tritylated nucleoside analogues. Eur. J. Med. Chem. 2013;65:249–255. doi: 10.1016/j.ejmech.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., Myers M.F., George D.B., Jaenisch T., Wint G.R., Simmons C.P., Scott T.W., Farrar J.J., Hay S.I. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Normile D. Surprising new dengue virus throws a spanner in disease control efforts. Science. 2013;342:415. doi: 10.1126/science.342.6157.415. [DOI] [PubMed] [Google Scholar]
- 5.Stevens A.J., Gahan M.E., Mahalingam S., Keller P.A. The medicinal chemistry of dengue fever. J. Med. Chem. 2009;52:7911–7926. doi: 10.1021/jm900652e. [DOI] [PubMed] [Google Scholar]
- 6.Mehlhorn H., Wu Z., Ye B. Springer-Verlag; Berlin Heidelberg: 2014. Treatment of Human Parasitosis in Traditional Chinese Medicine. [Google Scholar]
- 7.Wu R., Smidansky E.D., Oh H.S., Takhampunya R., Padmanabhan R., Cameron C.E., Peterson B.R. Synthesis of a 6-methyl-7-deaza analogue of adenosine that potently inhibits replication of polio and dengue viruses. J. Med. Chem. 2010;53:7958–7966. doi: 10.1021/jm100593s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Z., Chen Y.-L., Kondreddi R.R., Chan W.L., Wang G., Ng R.H., Lim J.Y., Lee W.Y., Jeyaraj D.A., Niyomrattanakit P. N-sulfonylanthranilic acid derivatives as allosteric inhibitors of dengue viral RNA-dependent RNA polymerase. J. Med. Chem. 2009;52:7934–7937. doi: 10.1021/jm901044z. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q.-Y., Patel S.J., Vangrevelinghe E., Xu H.Y., Rao R., Jaber D., Schul W., Gu F., Heudi O., Ma N.L. A small-molecule dengue virus entry inhibitor. Antimicrob. Agents Chemother. 2009;53:1823–1831. doi: 10.1128/AAC.01148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao B., Tong X.-K., Tang W., Li D.-W., He P.-L., Garcia J.-M., Zeng L.-M., Gao A.-H., Yang L., Li J. Discovery and optimization of 2, 4-diaminoquinazoline derivatives as a new class of potent dengue virus inhibitors. J. Med. Chem. 2012;55:3135–3143. doi: 10.1021/jm2015952. [DOI] [PubMed] [Google Scholar]
- 11.Shingalapur R.V., Hosamani K.M., Keri R.S. Synthesis and evaluation of in vitro anti-microbial and anti-tubercular activity of 2-styryl benzimidazoles. Eur. J. Med. Chem. 2009;44:4244–4248. doi: 10.1016/j.ejmech.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Achar K., Hosamani K.M., Seetharamareddy H.R. In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur. J. Med. Chem. 2010;45:2048–2054. doi: 10.1016/j.ejmech.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Congiu C., Cocco M.T., Onnis V. Design, synthesis, and in vitro antitumor activity of new 1,4-diarylimidazole-2-ones and their 2-thione analogues. Bioorg. Med. Chem. Lett. 2008;18:989–993. doi: 10.1016/j.bmcl.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Sharma D., Narasimhan B., Kumar P., Judge V., Narang R., De Clercq E., Balzarini J. Synthesis, antimicrobial and antiviral evaluation of substituted imidazole derivatives. Eur. J. Med. Chem. 2009;44:2347–2353. doi: 10.1016/j.ejmech.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Baures P.W. Heterocyclic HIV-1 protease inhibitors. Org. Lett. 1999;1:249–252. doi: 10.1021/ol990586y. [DOI] [PubMed] [Google Scholar]
- 16.Perchellet E.M., Perchellet J.P., Baures P.W. Imidazole-4,5-dicarboxamide derivatives with antiproliferative activity against HL-60 cells. J. Med. Chem. 2005;48:5955–5965. doi: 10.1021/jm050160r. [DOI] [PubMed] [Google Scholar]
- 17.VanCompernolle S.E., Wiznycia A.V., Rush J.R., Dhanasekaran M., Baures P.W., Todd S.C. Small molecule inhibition of hepatitis C virus E2 binding to CD81. Virology. 2003;314:371–380. doi: 10.1016/s0042-6822(03)00406-9. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda N., Iwagami H., Nakanishi E., Nakamiya T., Sasaki Y., Murata T. Synthesis and antibacterial activity of 6- and 7-[2-(5-carboxyimidazole-4-carboxamido)phenylacetamido]-penicillins and cephalosporins. J. Antibiot. Tokyo. 1983;36:242–249. doi: 10.7164/antibiotics.36.242. [DOI] [PubMed] [Google Scholar]
- 19.Kulikova O., Belyavtseva L., Reikhardt B., Borisova G.Y., Aleksandrova I.Y., Borodkin Y.S. Participation of RNA synthesis in the process of the development and maintenance of conditioned reflexes (an investigation using antitheines) Neurosci. Behav. Physiol. 1993;23:404–408. doi: 10.1007/BF01183000. [DOI] [PubMed] [Google Scholar]
- 20.Wiznycia A.V., Baures P.W. An improved method for the synthesis of dissymmetric N,N′-disubstituted imidazole-4,5-dicarboxamides. J. Org. Chem. 2002;67:7151–7154. doi: 10.1021/jo025536c. [DOI] [PubMed] [Google Scholar]
- 21.Asproni B., Murineddu G., Pau A., Pinna G.A., Langgård M., Christoffersen C.T., Nielsen J., Kehler J. Synthesis and SAR study of new phenylimidazole-pyrazolo [1, 5-c] quinazolines as potent phosphodiesterase 10A inhibitors. Bioorg. Med. Chem. 2011;19:642–649. doi: 10.1016/j.bmc.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 22.Z. No, J. Kim, S.-J. Han, J.H. Kim, Y.S. Park, S. Lee, K. Nam, J. Kim, J. Lee, S. Kang, M.J. Seo, S. Lee, G. Choi, Anti-inflammation compounds as therapeutic agents, PCT Int. Appl., 2012143796 (2012).
- 23.E.M. Bunnelle, W.A. Carroll, A.S. Florjancic, G.C. Hirst, B. Li, D.W. Nelson, S. Peddi, A. Perez-Medrano, Amino-tetrazoles analogues and methods of use, PCT Int. Appl., 2005111003 (2005).
- 24.L. Meijer, H. Galons, B. Joseph, F. Popowycz, N. Oumata, Pyrazolo[1,5-a]-1,3,5-triazine derivatives, preparation thereof, and therapeutic use thereof (2010).
- 25.Finch H., Reece D.H., Sharp J.T. An efficient general route to furo-, pyrido-and thieno-[d][2] benzazepines via Pd0 catalysed cross coupling reactions and nitrile ylide cyclisations. J. Chem. Soc. Perkin Trans. 1994;1:1193–1203. [Google Scholar]
- 26.Serrao E., Xu Z.-L., Debnath B., Christ F., Debyser Z., Long Y.-Q., Neamati N. Discovery of a novel 5-carbonyl-1 H-imidazole-4-carboxamide class of inhibitors of the HIV-1 integrase–LEDGF/p75 interaction. Bioorg. Med. Chem. 2013;21:5963–5972. doi: 10.1016/j.bmc.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 27.D.S. Goldfarb, Method for altering the lifespan of eukaryotic organisms, U.S. 20090163545 A1 20090625 (2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ZIP file containing the MOL files of the most important compound in this article.