Abstract
Study Objective:
Patients with obstructive sleep apnea (OSA) frequently exhibit higher rates of dyslipidemia, a risk factor for cardiovascular and cerebrovascular disorders. Treatment for OSA by CPAP may improve cholesterol metabolism. This meta-regression analysis (MA) estimates the effect of CPAP treatment on dyslipidemia.
Methods:
PubMed and Cochrane libraries were searched by utilizing different combinations of keywords: CPAP, obstructive sleep apnea, serum lipids, dyslipidemia, cholesterol, total cholesterol (TC), low density lipoprotein, LDL, high density lipoprotein, HDL, triglyceride, and TG. Inclusion criteria were: (1) English articles and (2) studies with an adult population with the diagnosis of OSA who were treated with CPAP. The OSA group must have cholesterol profile including TC, LDLc, HDLc, and TG, without and with CPAP treatment. Fifty-four studies were reviewed, while 29 studies pooled for MA.
Results:
Thirty-four datasets from 29 studies with 1,958 subjects pooled. Treatment duration range was from 2 days to 1 year. TC standardized mean differences (SMD) ranged from -41.5 to -0.077, pooled mean difference (PMD) was -5.660 (LL -6.715 to UL -4.606, p < 0.001). SMD in LDL ranged from -3.7 to 0; PMD was -0.488 (LL -0.715 to UL -0.261, p < 0.001). HDL SMD ranged from -0.498 to 1.94. The PMD was 0.207 (LL 0.05 to UL 0.364, p < 0.01). TG SMD ranged from -9.327 to 1.98; PMD was -0.054 (LL -0.124 to UL 0.016, p < 0.129).
Conclusions:
CPAP treatment for OSA seems to improve dyslipidemia (decrease in total cholesterol and LDL, and increase in HDL). It does not appear to affect TG levels.
Citation:
Nadeem R, Singh M, Nida M, Kwon S, Sajid H, Witkowski J, Pahomov E, Shah K, Park W, Champeau D. Effect of CPAP treatment for obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med 2014;10(12):1295-1302.
Keywords: obstructive sleep apnea, dyslipidemia, CPAP, cholesterol, sleep disordered breathing
Obstructive sleep apnea (OSA) is a common disorder that is often asymptomatic; the prevalence of patients with OSA who do not present clinical syndrome, might be as high as 20% to 30% in the middle-aged.1 OSA is a significant source of morbidity and mortality, as OSA has been increasingly linked to cardiovascular and cerebrovascular disease, and many studies have shown that OSA is associated with increased cardiovascular and cerebrovascular morbidity.2–9 OSA is associated with obesity and metabolic syndrome, most likely from associated endocrine abnormalities, especially reduced androgens.10 Subjects with OSA manifest increased dyslipidemia.11 CPAP—the main modality of treatment—serves as a pneumatic splint device and reduces upper airway collapse, which results in reduction of arousals and improvement in oxyhemoglobin saturation. This may result in improvement in hormonal regulation and metabolic abnormalities: blood glucose levels, inflammation, and dyslipidemia. Multiple studies document this effect of OSA treatment by CPAP on dyslipidemia. We performed meta-analysis (MA) and meta-regression (MR) to specifically detect treatment effect by CPAP on dyslipidemia.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Patients with obstructive sleep apnea (OSA) frequently exhibit higher rates of dyslipidemia, a risk factors for cardiovascular and cerebrovascular disorders. Multiple studies with small sample size documented the improvement in dyslipidemia by treatment for OSA by CPAP therapy, hence the meta-regression analysis aim to estimates the effect of CPAP treatment on dyslipidemia.
Study Impact: CPAP treatment for OSA seems to improve dyslipidemia (decrease in total cholesterol and LDL, and increase in HDL). Study suggests that improvement in dyslipidemia may be the mechanism for improvement of cardiovascular and cerebrovascular disorders in patients, treated for sleep apnea by pressure therapy.
METHODS
We performed this review in accordance with PRISMA guidelines for performing meta-analysis. A protocol was prospectively developed, detailing the objectives, criteria for study selection, and approach to assessing the study quality, primary outcome, and methodology.
Data Source and Study Selection
Studies for review were found searching the PubMed, Cochrane, and EMBASE databases from January 1, 1960, to December 31, 2013. Unpublished data from scientific meetings were not searched, since most abstracts do not provide detail data needed for meta-analysis. Searches were conducted using the keywords: sleep apnea, obstructive sleep apnea, CPAP, APAP, AutoPAP, pressure therapy, serum lipids, dyslipidemia, cholesterol, total cholesterol, low density lipoprotein, high density lipoprotein, and triglyceride. Each target outcome was also searched in its abbreviated forms (Chol T, HDL, LDL, TG) to ensure that no relevant source is left out. Additionally, each target and its abbreviated forms were searched in combination with obstructive sleep apnea and CPAP. Multiple authors individually searched for and scored manuscripts for inclusion. If a manuscript was scored differently by 2 authors, then it was reviewed by third author to finalize its inclusion.
Studies and Endpoint Definitions
Lipid profile includes total cholesterol (TC), low density lipoprotein cholesterol (LDL), high density lipoprotein cholesterol HDL, and triglyceride (TG). Inclusion criteria defined for subsequent study selection were as follows: (1) the study must be in English, (2) studies with adult population only, (3) full text manuscripts had to be available, (4) the study must have reported values for at least one of the outcomes of interest, (5) OSA was diagnosed by sleep study and OSA defined as AHI of ≥ 5/h, (6) the study must have included individuals with obstructive sleep apnea treated with PAP device, and (7) the study must have reported values in mean and standard deviation or median with range.
Data Extraction
Study selection, data extraction, and statistical analysis were all done in accordance to previously published methodology for meta-analyses. Studies identified for inclusion then underwent data extraction. Data was extracted at a study level by a single author and then reviewed by a second author to ensure no errors were made. Levels of serum lipids were extracted from studies as mean with standard deviation. Measurement unit of lipid profile was mmol/L. If any of these values were in mg/dl they were converted into mmol/L by dividing them by their molar weight. For studies with data reported in median and range, mean and standard deviation were calculated utilizing methods outlined by Hozo et al.12 Target variables (TC, HDL, LDL, TG) were recorded, as well as reported demographics (age, gender, BMI), confounding factors (AHI, blood glucose, insulin level), and Epworth Sleepiness Scale (ESS) score (when available) to evaluate the effect of these parameters on the target by employing subgroup analysis or meta-regression.
Statistical Analysis
The statistical analysis was performed by the Comprehensive Meta-Analysis software package (version CM 2.2, Biostat, Englewood, NJ). Heterogeneity analysis by the Cochran's Q statistics for individual end points across all studies was performed. An I2 of 25% to 49% was considered to represent a low level of heterogeneity, 50% to 74% a moderate level, and 75% to 100% a high level. A 2-sided α error of less than 0.05 was considered to be statistically significant.
RESULTS
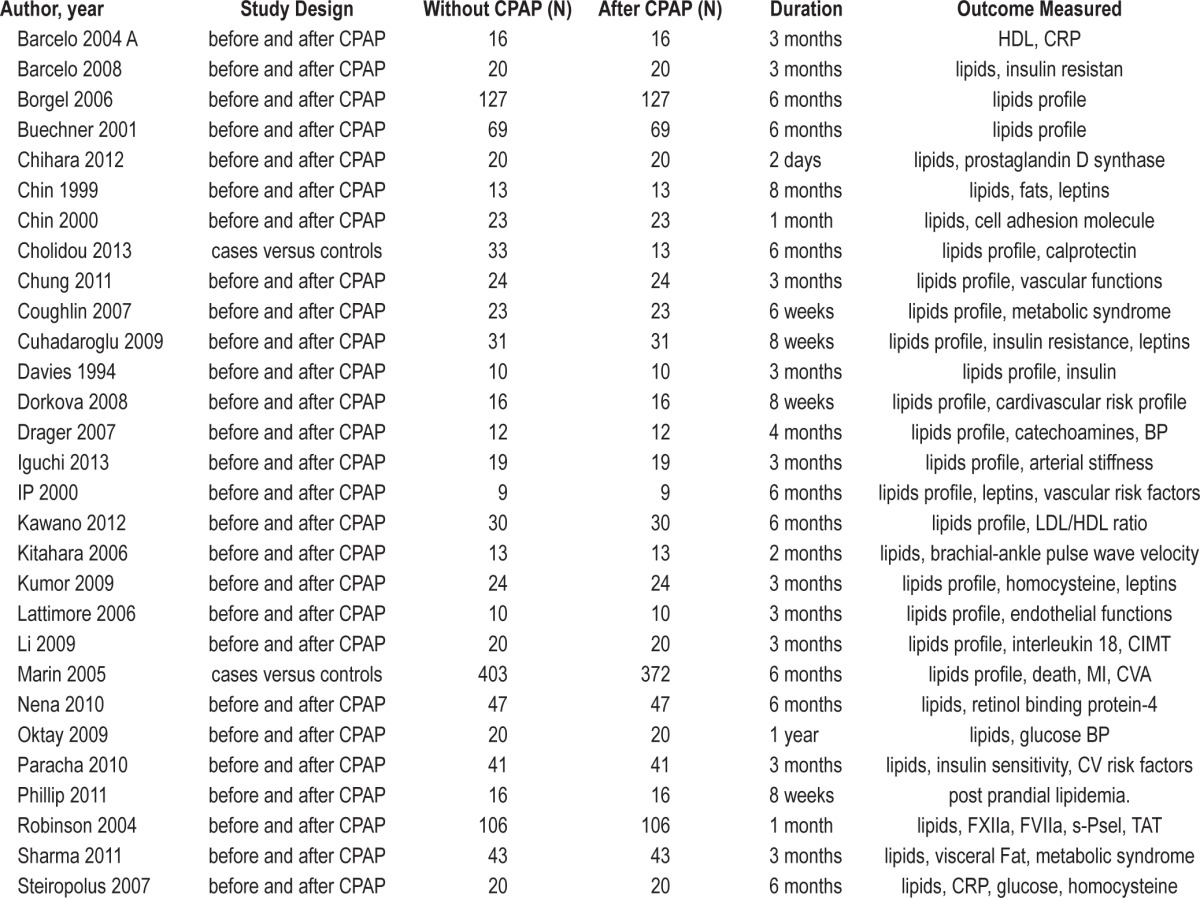
The literature was ranked according to the hierarchy of evidence of Sackett et al.13 Total citations identified from electronic search by using key words without duplication were N = 367; sleep apnea and cholesterol N = 287; sleep apnea and dyslipidemia N = 334; CPAP and lipids N = 59; additional studies found by other combinations N = 57. A total of 313 citations were excluded after screening titles and abstracts; therefore, 54 articles were retrieved for detailed evaluation. Twenty-five studies were excluded for different reasons (full text not available N = 4, non-English manuscript N = 4, inappropriate population N = 4, data not extractable N = 4, missing relevant information N = 2, and data not reported N = 7). Therefore, 29 studies (with 34 datasets) (Table 1) met inclusion criteria and were pooled for meta-analysis including total 1,958 subjects (without CPAP [N = 1,554] and with CPAP treatment [N = 1,260]). Few studies (Borgel 2006, Coughlin 2007, Marin 2005, and Sharma 2011) compare the lipid profile between untreated (N = 698) and treated OSA (N = 404) patients, while majority of studies compared lipid profile on subjects (N = 856) before CPAP usage and after CPAP usage for a specified amount of time. CPAP treatment duration ranged from 2 days to 1 year. Data from all of studies were pooled for analysis. Data regarding compliance was not reported by all studies. Available compliance data was variable across included studies. Another meta-analysis can be performed by including only studies with compliant subjects (when enough studies are available) to address the issue of effect of optimal pressure therapy on lipid profile, although it will not be applicable as compliance rates are not very high in general.
Table 1.
Characteristics of included studies.
Total Cholesterol
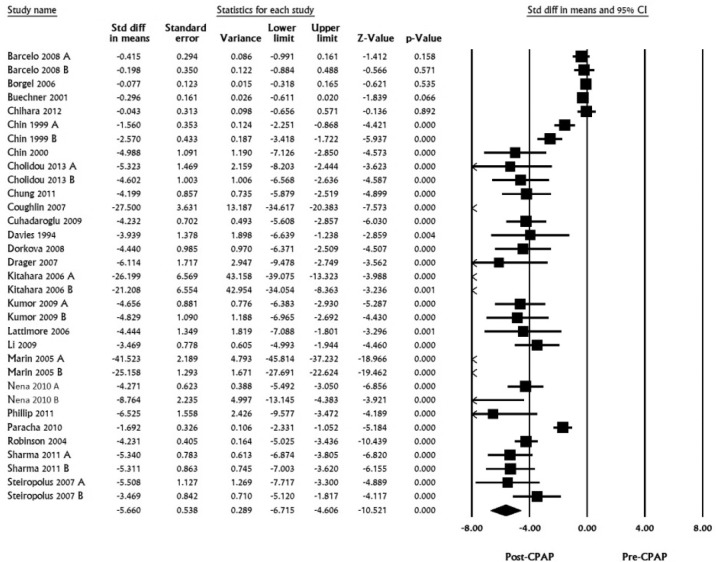
A total of 24 studies with 33 datasets including 1,929 subjects were pooled for TC. Standardized mean differences ranged from -41.5 to -0.077, pooled mean difference was calculated to be -5.660 (LL -6.715 to UL -4.606, I2 for this analysis was 97.37, p < 0.001; Figure 1).
Figure 1. Total cholesterol, standard difference in means, before treatment versus after CPAP treatment.
LDL Cholesterol
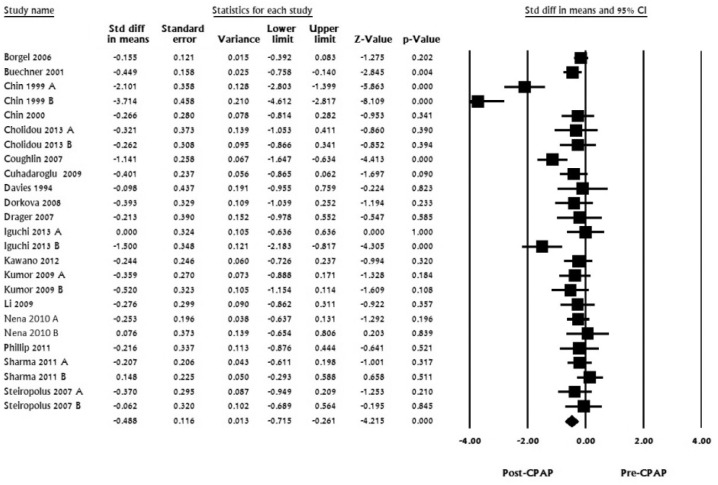
For LDL, 18 studies with 25 datasets including 676 subjects were pooled. Standardized mean difference in LDL ranged from -3.7 to 0; pooled mean difference was calculated to be -0.488 (LL -0.715 to UL -0.26, I2 for this analysis was 78.37, p < 0.001; Figure 2)
Figure 2. LDL, standard difference in means, before treatment versus after CPAP treatment.
HDL Cholesterol
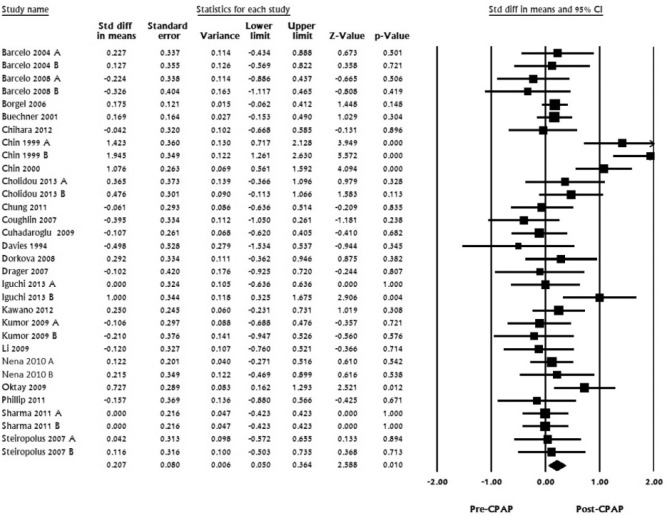
For HDL, 23 studies with 32 datasets including 806 subjects were pooled. Standardized mean difference ranged from -0.498 to 1.94. The pooled mean difference was calculated to be 0.207 (LL 0.05 to UL 0.364, I2 for this analysis was 59.11, p < 0.01; Figure 3).
Figure 3. HDL, standard difference in means, before treatment versus after CPAP treatment.
Triglyceride
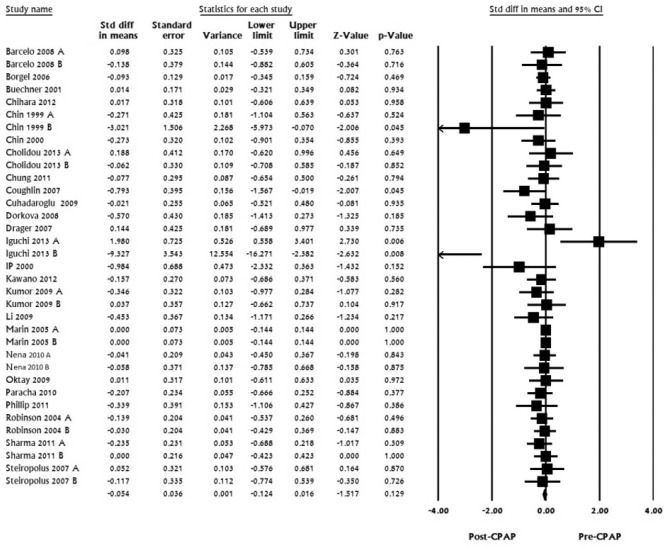
For TG, 25 studies with 35 datasets including 1,926 subjects were pooled and analyzed. Standardized mean difference ranged from -9.327 to 1.98; pooled mean difference was calculated to be -0.054 (LL -0.124 to UL 0.016, I2 for this analysis was 0, p < 0.129; Figure 4).
Figure 4. Triglycerides, standard difference in means, before treatment versus after CPAP treatment.
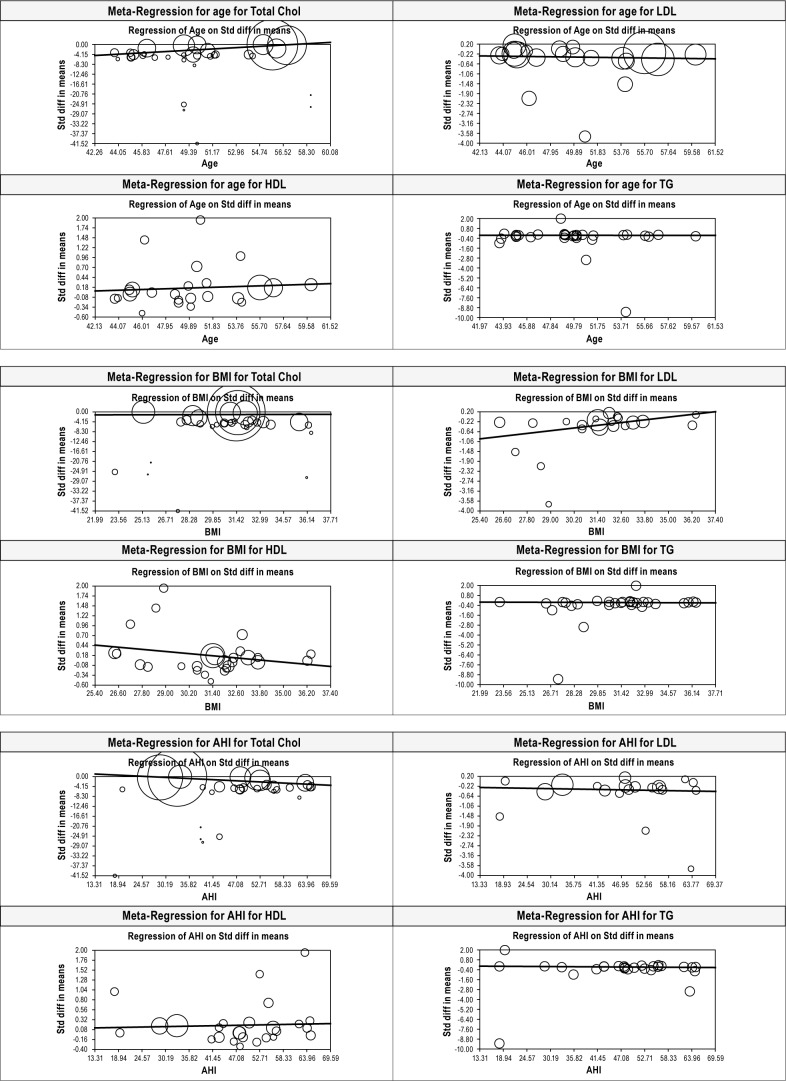
Meta-Regression to Evaluate the Effect of Age, BMI, and AHI on Lipid Levels after CPAP Treatment
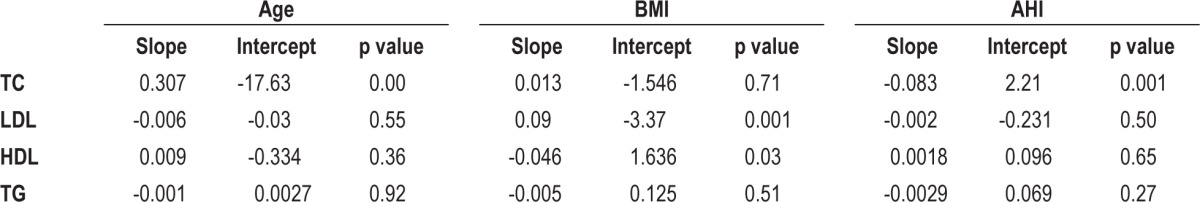
Only age was found to have modest but significant effect on improvement of total cholesterol levels after treatment with CPAP (Beta 0.307, p = 0.0001). There was no effect for age on LDL (p = 0.55), HDL (p = 0.36), or TG (p = 0.92). BMI was found to have modest but significant effect on LDL (Beta 0.09, p = 0.001) and HDL (Beta -0.046, p = 0.03) while there was no significant effect on TC (p = 0.71) or TG (p = 0.511). AHI was found to be a significant but modest confounder for effect on only TC after CPAP treatment (Beta -0.083, p = 0.001). AHI had no effect on LDL (p = 0.50), HDL (p = 0.65), or TG (p = 0.27) (Figure 5, Table 2).
Figure 5. All MR plots for age, BMI, and AHI for TC, LDL, HDL, and TG before versus after CPAP treatment.
Size of the bubbles is proportional to the study sample size.
Table 2.
Meta-regression statistics.
DISCUSSION
There is strong positive association between LDL and the risk of coronary heart disease.14,15 Randomized trials have demonstrated that lowering LDL cholesterol with medications reduces the risk of cardiac death, nonfatal myocardial infarction, ischemic stroke, and the need for revascularization procedures.16–18
The present meta-regression analysis (MR) showed that there is an improvement in the levels of cholesterol in subjects with OSA treated with pressure therapy (decrease in total cholesterol and low density lipoprotein, and an increase in high density lipoprotein) although it did not show any improvement in triglyceride.
This finding of heightened dyslipidemia in patients with obstructive sleep apnea suggest that treatment of sleep apnea may improve this risk factors for heart disease directly or indirectly by affecting other confounding factors—obesity, hypertension, diabetes mellitus, and metabolic syndrome—as CPAP treatment for sleep apnea has been shown to positively impact management of these confounding variables.
The mechanism for lipid metabolism modulation by CPAP is unknown. Chronic intermittent hypoxia (CIH) associated with OSA may adversely affect dyslipidemia, as suggested by animal studies that CIH up-regulates lipoprotein secretion,19 increases free fatty acid flux to the liver,20 may induce sympathetic activity,21 which could induce lipolysis.22 CPAP may improve dyslipidemia by improving hypoxia.23 Another mechanism may be from improvement in insulin resistance, which may increase total cholesterol and LDL cholesterol by decreasing the catabolism of LDL, by down-regulation of LDL receptors.24 CPAP may improve lipids by affecting inflammatory markers.23,25 Another possibility is reduction of hypersomnolence during the day with increase in activity.26 Alternatively, exercise may increase the desire for foods that are high in carbohydrates and reduce the desire for foods that are high in fat.27
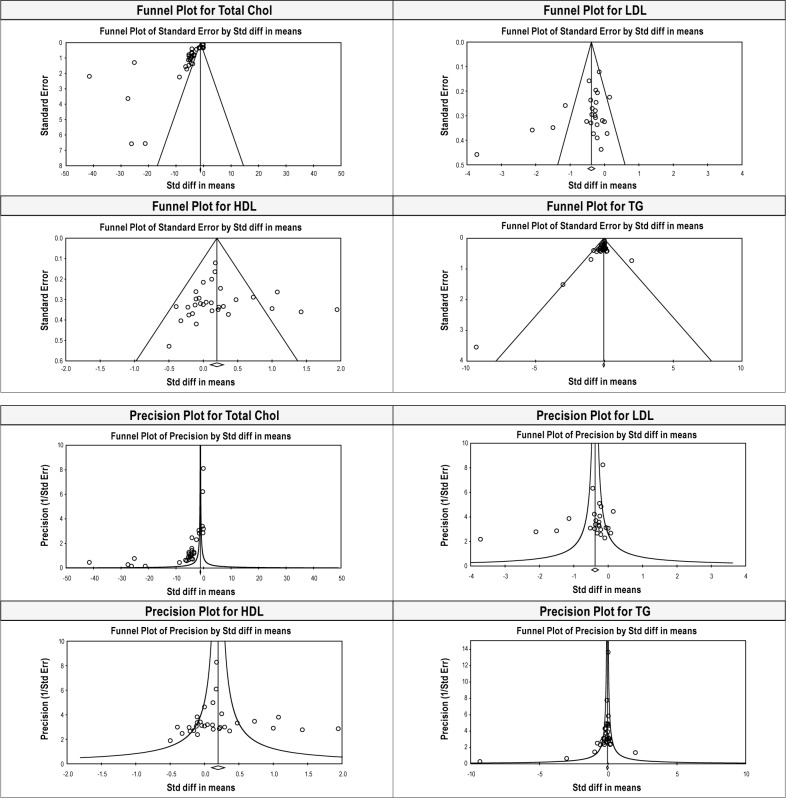
Several limitations of this meta-analysis should be emphasized. Available literature is largely low level evidence. Few of the relevant studies regarding the improvement with CPAP therapy were cross-sectional in nature, so the temporal relationships between these two factors were unclear. There was significant heterogeneity among studies, and funnel plots and precision plots also suggest publication bias in TC, LDL, and HDL analysis (Figure 6). We could not perform the meta-regression for other confounding factors—sleepiness, presence of hypertension or hyperglycemia, or insulin levels—since we have data on these variables only in few studies. These factors have been found to be associated with elevated dyslipidemia.
Figure 6. Funnel plots and precision plot for TC, LDL, HDL, and TG levels before and after CPAP treatment.
Another weakness of our meta-analysis is that all papers written in languages other than English were excluded, and we used only published studies, raising the possibility of publication bias.
In summary, there appears to be some evidence indicating improvement in degree of hypercholesterolemia in patients with OSA treated by pressure therapy. These findings suggest that mechanism of improvement in cardiovascular disorders in patients treated for OSA may be by reducing atherosclerosis by improving cholesterol profile. Prospective studies are needed to asses if this improvement is related to compliance with pressure therapy and if such improvement is only specific to pressure therapy or if it holds true for other modalities, such as oral devices and surgical treatment, as well.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. The study was performed at Rosalind Franklin University of Medicine and Science, North Chicago, IL.
REFERENCES
- 1.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 2.Lavie P, Lavie L. Cardiovascular morbidity and mortality in obstructive sleep apnea. Curr Pharm Des. 2008;14:3466–73. doi: 10.2174/138161208786549317. [DOI] [PubMed] [Google Scholar]
- 3.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 5.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 6.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 7.Imadojemu VA, Gleeson K, Gray KS, Sinoway LI, Leuenberger UA. Obstructive sleep apnea during sleep is associated with peripheral vasoconstriction. Am J Respir Crit Care Med. 2002;165:61–6. doi: 10.1164/ajrccm.165.1.2009062. [DOI] [PubMed] [Google Scholar]
- 8.Sajkov D, Wang T, Saunders NA, Bune AJ, McEvoy RD. Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:152–8. doi: 10.1164/ajrccm.165.2.2010092. [DOI] [PubMed] [Google Scholar]
- 9.Amin RS, Kimball TR, Bean JA, et al. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1395–9. doi: 10.1164/rccm.2105118. [DOI] [PubMed] [Google Scholar]
- 10.Rebuffè-Scrive M, Mårin P, Björntorp P. Effect of testosterone on abdominal adipose tissue in men. Int J Obes. 1991;15:791–5. [PubMed] [Google Scholar]
- 11.Nadeem R, Singh M, Nida M, Waheed I, Khan A, Ahmed S, Naseem J, Champeau D. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a metaregression analysis. J Clin Sleep Med. 2014;10:475–89. doi: 10.5664/jcsm.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sackett DL, Straus SE, Richardson WS, Rosenberg W, Haynes RB. 2nd ed. Oxford: Churchill Livingstone; 2000. Evidence-Based Medicine: How To Practice and Teach EBM; pp. 173–5. [Google Scholar]
- 14.Prospective Studies Collaboration. Lewington S, Whitlock G. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–39. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 15.Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomized trials. Lancet. 2010;376:1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emberson JR, Ng LL, Armitage J, Bowman L, Parish S, Collins R. N-terminal pro-B-type natriuretic peptide, vascular disease risk, and cholesterol reduction among 20,536 patients in the MRC/BHF heart protection study. J Am Coll Cardiol. 2007;49:311–9. doi: 10.1016/j.jacc.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 18.Cleland JGF, McMurray JJV, Kjekshus J, et al. Plasma concentration of amino-terminal pro-brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin: a report from CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure) J Am Coll Cardiol. 2009;54:1850–9. doi: 10.1016/j.jacc.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Savransky V, Nanayakkara A, Smith PL, O'Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol. 2007;102:557–63. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 20.Jun J, Reinke C, Bedja D, et al. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209:381–6. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedner J, Darpo B, Ejnell H, Carlson J, Caidahl K. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. Eur Respir J. 1995;8:222–9. doi: 10.1183/09031936.95.08020222. [DOI] [PubMed] [Google Scholar]
- 22.Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, Sul HS. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1–4. doi: 10.1152/ajpgi.00554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cholidou KG, Kostakis ID, Manali ED, et al. Calprotectin: a protein related to cardiovascular risk in adult patients with obstructive sleep apnea. Cytokine. 2013;61:917–23. doi: 10.1016/j.cyto.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Buechner NJ, Zidek W, Eer M, Haske M, Sanner BM. Obstructive sleep apnea syndrome. Effects of therapy on dyslipidemia. Somnologie. 2001;5:97–102. [Google Scholar]
- 25.Baessler A, Nadeem R, Harvey M, et al. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers - a meta-analysis. J Inflamm (Lond) 2013;22 doi: 10.1186/1476-9255-10-13. 10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–5. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 27.Tremblay A, Buemann B. Exercise-training, macronutrient balance and body weight control. Int J Obes Relat Metab Disord. 1995;19:79–86. [PubMed] [Google Scholar]