Abstract
Study Objectives:
To characterize the nature and impact of sleep disturbances on quality of life (QOL) in women with interstitial cystitis/bladder pain syndrome (IC/BPS).
Methods:
Participants were 3,397 women from a telephone probability survey who met IC/BPS symptom criteria. Sleep quality, duration, and IC/BPS nocturnal symptoms (i.e., trouble sleeping due to bladder pain, urgency, or needing to use the bathroom), general QOL (mental and physical health and sexual functioning), and IC/BPS QOL impairment were assessed via self-report during telephone interview.
Results:
Over half of the sample reported poor sleep quality, sleep duration ≤ 6 hours, or trouble sleeping due to IC/BPS symptoms. After covariate adjustment, short sleep duration was significantly associated with greater IC/BPS QOL impairment (β = -0.04; p < 0.001) and poorer self-reported physical health (β = 1.86; p < 0.001). Poor sleep quality was significantly associated with greater IC/BPS QOL impairment (β = 0.06; p < 0.001), poorer self-reported physical health (β = -2.86; p < 0.001), and greater sexual dysfunction (β = -0.04; p < 0.05). IC/BPS nocturnal symptoms were significantly associated with greater IC/BPS impairment (β = 0.14; p < 0.001), poorer physical health (β = -2.76; p < 0.001) and mental health (β = 0.52; p < 0.01), and greater sexual dysfunction (β = -0.10; p < 0.001), after covariate adjustment. After further adjustment for IC/BPS nocturnal symptoms, we found that poor sleep quality and short sleep duration were independent correlates of poor self-reported physical health.
Conclusions:
Poor sleep quality and short sleep duration, as well as disorder-specific sleep disturbances, are highly prevalent in women with IC/BPS and are associated with poorer disease-specific and general QOL.
Citation:
Troxel WM, Booth M, Buysse DJ, Elliott MN, Suskind AM, Clemens JQ, Berry SH. Sleep disturbances and nocturnal symptoms: relationships with quality of life in a population-based sample of women with interstitial cystitis/bladder pain syndrome. J Clin Sleep Med 2014;10(12):1331-1337.
Keywords: pain, bladder symptoms, sleep quality, sleep duration, interstitial cystitis, women's health
Interstitial cystitis (IC)/ bladder pain syndrome (BPS) is a chronic pain syndrome which disproportionately affects women and is characterized by symptoms of urinary urgency and frequency, pelvic pain, and nocturia.1 The etiology and pathophysiology of IC/BPS are currently unknown; however, prevailing theories implicate chronic or subclinical infection, autoimmunity, neurogenic inflammation, or bladder urothelial defects (i.e., epithelial lining of the urinary tract) as potential mechanisms.2
In the absence of known or measurable pathophysiological biomarkers, the clinical diagnosis of IC/BPS is based on patient-reported symptoms, and many cases remain undiag-nosed. For instance, in the RAND Interstitial Cystitis Epidemiology (RICE) Study (from which the current data are drawn), a nationally representative population survey, up to 6.5% of US women reported experiencing IC/BPS symptoms according to established case criteria, but less than 10% of these women had an IC/BPS diagnosis.3 Adding to the diagnostic uncertainty is the considerable symptom overlap and comorbidity between IC/BPS and other chronic pain conditions, such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome (IBS).4
BRIEF SUMMARY
Current Knowledge/Study Rationale: Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic pain syndrome that disproportionately affects women and is characterized by a constellation of bladder symptoms, including bladder/pelvic pain and nocturia. Although there has been a great deal of research on musculoskeletal pain conditions and sleep disturbances, there has been very little research on the nature or impact of sleep disturbances in populations with organ-specific pain conditions, such as IC/BPS.
Study Impact: In this population-based sample of women with IC/BPS symptoms, sleep problems, including short sleep duration, poor sleep quality, and disorder-specific nocturnal symptoms, were highly prevalent and were associated with greater impairment on multiple quality of life indicators. Findings suggest that augmenting IC/BPS treatment strategies with sleep-focused treatments may improve quality of life in IC/BPS patients.
Notably, sleep disturbances are known to covary with all of these conditions.5 Evidence from the broader chronic pain literature suggests that sleep disturbances can exacerbate pain and vice versa, that pain can initiate or exacerbate sleep disturbances.6–8 This vicious cycle, in turn, can have profound negative influences on quality of life (QOL).5 Moreover, such bidirectional relationships highlight the importance of evaluating sleep disturbances in the context of chronic pain. Importantly, however, much of the research on sleep and pain conditions has focused on musculoskeletal pain, rather than organ-specific pain, such as IC/BPS symptoms.
Despite the fact that several of the most prevalent IC/BPS symptoms are nocturnal, and that sleep disturbances are commonly comorbid with IC/BPS symptoms or diagnoses,4,9 there have been few studies of the nature or impact of sleep disturbances on QOL in IC/BPS populations. For instance, in a web-based sample of 407 women with IC who were recruited from the website of the Interstitial Cystitis Association (ICA), Panzera and colleagues found that 100% of the sample exceeded threshold criteria for clinically significant sleep disturbances on the Pittsburgh Sleep Quality Index (PSQI).10,11 Examination of IC/BPS-specific symptoms revealed that nocturia, pain, and urgency contributed to 21% of the variance in overall sleep quality scores. Thus, poor sleep quality appears ubiquitous in the IC/BPS population, and IC/BPS-specific symptoms explain some, but not all of the variance in poor sleep quality. Importantly, however, given the recruitment method in Panzera's study (i.e., a website providing support and treatment options for diagnosed IC/BPS patients), the sample may have been biased towards a more severe population and/or those with better access to healthcare in order to receive an accurate diagnosis. In a clinical trial of multidose pentosan polysulfate sodium for patients with IC/BPS diagnoses, sleep problems at baseline were associated with poorer mental and physical health QOL, and improvements in IC/BPS symptoms were associated with improvements in sleep.12 Together these findings suggest the prominent role of sleep disturbances in the clinical presentation and impact of IC/BPS symptoms; however, no study to date has examined sleep disturbances in a national prevalence sample using established case definitions of IC/BPS. Moreover, given robust and prospective associations between poor sleep quality and short sleep duration and diverse indicators of QOL, morbidity, and mortality, the important question remains whether sleep quality and duration contributes to QOL over and above IC/BPS-specific nocturnal symptoms.
The RICE study provides a unique opportunity to characterize the nature and impact of sleep disturbances in a large and representative population of US women with IC/BPS symptoms. Specifically, aims of the current study were three-fold: (1) to characterize sleep quality, duration, and IC/BPS-specific nocturnal symptoms in women with IC/BPS symptoms; (2) to examine the extent to which sleep quality, duration, or IC/ BPS nocturnal symptoms contributes to QOL, after adjusting for factors which are known to covary with QOL and/or sleep problems, including socidemographics (age, sex, race/ethnicity, marital status), menstrual status, and depressive symptoms; and (3) to examine the unique contribution of poor sleep quality and short sleep duration to QOL, over and above the contribution of IC/BPS-specific nocturnal symptoms and other covariates. To provide a more comprehensive assessment of the degree to which sleep problems are associated with QOL in the IC/BPS population, we included diverse indicators of QOL domains, which have been identified in previous research as being particularly germane to women with IC/BPS, including general, non-condition specific physical and mental health and sexual functioning, and IC/BPS-specific QOL (described below).
METHODS
Participants
Data were from the RICE Study, a telephone probability survey of 146,231 US households the only national study of women with IC/BPS symptoms. Detailed descriptions of the RICE Study methods and design are provided elsewhere.3 To reduce screening costs for a rare population, screening was conducted in 2 stages from 2007-2009. To identify households with an adult female (age 18 or older) who had current symptoms of IC/BPS and/or had previously been diagnosed with IC/BPS for the first stage of population screening, 2 BPS/IC screening items were added to a random digit dial national probability sample weekly omnibus survey that uses standard statistical methods for selecting the sample and weighting for nonresponse. We used these weights at the first stage of our weighting procedure. An examination of these weights showed that the adjustments were small, indicating a close fit between the demographic characteristics of the screening sample and the population as a whole.
Among the 131,691 households with an adult female, 32,474 households (24.7%) had a woman aged 18 years or older with bladder symptoms and/or an IC/BPS diagnosis and were asked to undergo a second-stage telephone screening for study eligibility. Among those eligible for second-stage screening, 39.3% (12,752) agreed to further screening, and 82.1% (10,474) of those completed screening. Among women screened, 32.4% (3,397) met the following validated high-sensitivity RICE symptom criteria (81% sensitivity and 54% specificity) to establish caseness: (1) pain, pressure, or discomfort in pelvic area; (2) urinary frequency 10+ times/day or urgency due to pain, pressure, or discomfort (not fear of wetting, which is associated with overactive bladder);13 (3) pain that worsens as bladder fills; (4) no resolution of bladder symptoms after antibiotic treatment; and (5) never treated with hormone injections for endometriosis.14
Consistent with IC/BPS diagnostic exclusion criteria, women were excluded if they were: ever diagnosed with genital herpes, bladder cancer, diverticulum of the urethra, a spinal cord injury, stroke, Parkinson disease, multiple sclerosis, spina bifida, tuberculosis affecting the bladder, or cancer of the uterus, ovaries, vagina, or urethra; ever had cyclophosphamide (Cytoxan) therapy, or radiation therapy to pelvic area; or were currently or possibly pregnant. We conducted multivariate modeling of screening participation (including sociodemographic, household composition, and clinical characteristics) to construct population and nonresponse weights accounting for the second phase of screening, which were used in the analyses.
All procedures were approved by the RAND Human Subjects Protection Committee (#b4822-04-01). All participants provided informed consent.
Measures
Participants provided information on sociodemographics (age, race/ethnicity marital status, and highest level of education), menopausal status (“did you have a period in the past 12 months?”), sleep, depression, and quality of life measures during a telephone interview. Covariates, including age, marital status, education, menstrual status, and depressive symptoms that have previously been shown to correlate with QOL outcomes,15,16 and were associated with sleep and/ or outcomes in bivariate correlations in the current sample (analyses not shown) were included in the multivariate regression models.
Sleep
Participants' self-reported sleep duration and sleep quality were assessed using single-items modified from the PSQI.11 The sleep duration item was recorded in hours of sleep per night. The sleep quality item was rated on a scale of 1 (“very good”) to 4 (“very bad”). Sleep duration and sleep quality were analyzed separately. Three items assessed the degree to which participants' experienced “trouble sleeping” as a result of: (1) having to go to the bathroom; (2) feelings of urgency or needing to urinate; and (3) bladder or pelvic pain; on a scale ranging from “not during the past month” to “every night or nearly every night.” We created an IC/BPS-specific nocturnal symptoms score by summing the 3 IC/BPS-specific nocturnal symptoms, which yielded adequate internal reliability (Cronbach α = 0.69). Consistent with the PSQI scoring, higher scores on the sleep quality item and each of the individual IC/ BPS-specific nocturnal symptoms represent higher levels of sleep disturbance.
General Health-Related QOL
Participants completed the MOS Short Form (SF-36) Health Survey, which is a well-established QOL instrument, with well-documented internal reliability (α ranging from 0.80-0.90 in previously published literature) and validity based on normed data, that assesses 7 domains of QOL including: physical functioning, role functioning, bodily pain, general health, vitality, social functioning, and mental health.17,18 Aggregate scores of mental health and physical health based on the 7 domains were utilized in the current analyses and reported as T-scores (M = 50; SD = 10), with higher scores indicating better functioning.
Sexual Function
Women were asked the extent to which they experienced 5 general sexual dysfunction symptoms in the past 4 weeks on a scale ranging from “not a problem” to “very much a problem.” Items for the sexual dysfunction scale were drawn from a population-based survey19 and included items such as “lack of sexual interest,” “difficulty becoming aroused,” or “difficulty having orgasm.” The items were averaged and yielded very good internal consistency (Cronbach α = 0.88). Higher scores indicate greater sexual dysfunction.
IC/BPS Specific Impairment
The RICE Bladder Symptom Impact Scale (RICE BSI-6) was developed and validated to provide a more comprehensive assessment of the extent to which bladder symptoms affect key domains of QOL that have been identified in prior IC/ BPS research as being most salient to this population. Specifically, the scale includes 6 items that assess the degree to which bladder symptoms affect emotional functioning (e.g., mood, feelings of self-worth), social functioning (“social life”), and role responsibilities (“ability to carry out your home responsibilities”), with higher scores indicating higher levels of impairment in IC/BPS-specific QOL. The RICE BSI-6 has been previously validated and has demonstrated excellent internal consistency (Cronbach α = 0.92), strong convergent validity, and good face validity.20
Depression
The Patient Health Questionnaire-8 items (PHQ-8)21 was administered to assess depressive symptoms. Scores on the PHQ-8 range from 0-24, with higher scores indicating greater depression symptoms. The scale has a sensitivity of 73% and a specificity of 98% for the diagnosis of major depression, and internal reliability of 0.86.21 For the current analyses, PHQ-8 scores were included as a covariate for all models except for mental health QOL (to avoid redundancy with this outcome).
Statistical Analysis
Descriptive statistics regarding the sample characteristics for all primary study variables and covariates were computed. Pearson correlations were computed to examine the correlations among sleep duration, sleep quality, and IC/BPS-specific nocturnal symptoms. The primary analyses employed multivariate linear regression analyses and used survey weights to account for sample design and nonresponse rates. Separate regression models were used to evaluate the relationship between sleep problems and QOL outcomes, after adjusting for age, race/ ethnicity (dummy coded), education, menopausal status, and depressive symptoms. Depressive symptoms were excluded from the mental health models to avoid redundancy with the outcome. Finally, to examine the unique contribution of sleep duration or sleep quality to QOL, follow-up regression models additionally adjusted for IC/BPS-specific nocturnal symptoms (summary score).
RESULTS
Sample Characteristics
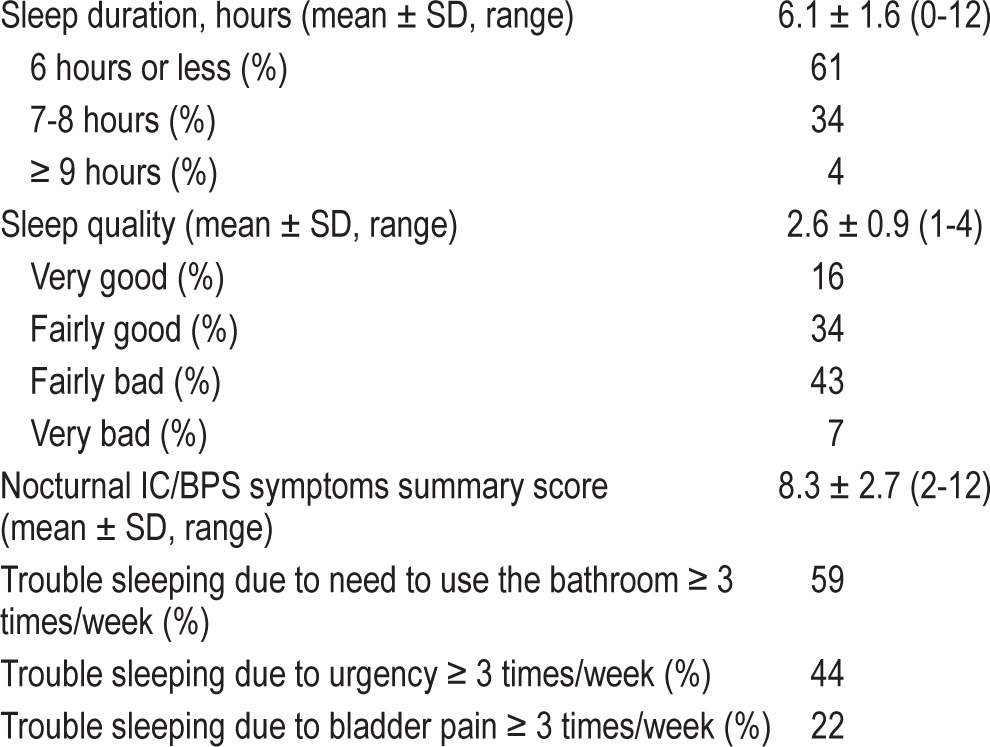
As shown in Table 1, 74% of the sample was white, 58% were married, and 36% had a high school education or less. Average sleep quality for the sample was 2.6 (SD 0.9; range: 1-4). Mean sleep duration was 6.1 h (SD 1.6; range 0-12). To provide a more qualitative description of the sleep characteristics of the sample, Table 2 shows the mean (SD) for each continuous sleep variable as well as the frequency for each response category of sleep quality, sleep duration (≤ 6 h, 7-8 h, or ≥ 9 h), and the percentage of respondents who endorsed each of the nocturnal IC symptoms at a frequency of ≥ 3 times per week. However, primary analyses used the continuous sleep measures. As shown, 50% of the sample reported “fairly bad” or “very bad” sleep quality, and 61% of the sample reported sleep duration ≤ 6 h. Of the IC/BPS-specific nocturnal symptoms, the most prevalent symptom was trouble sleeping “due to need to use the bathroom” (59%), with 44% of sample reporting trouble sleeping due to urgency, and 22% reporting trouble sleeping due to bladder pain.
Table 1.
Sample characteristics of women with IC/BPS symptoms (N = 3,397).

Table 2.
Sleep characteristics of the sample (N = 3,397).
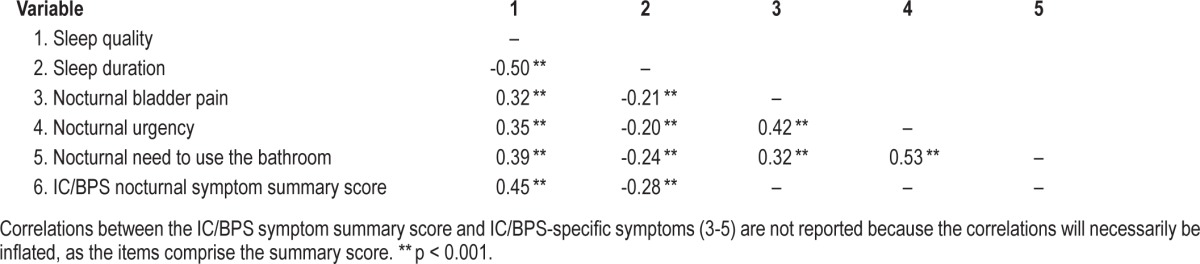
Correlations Among Sleep Problems
As expected, there were significant correlations across all of the sleep variables (as shown in Table 3). Sleep quality and sleep duration were significantly and inversely related (Pearson r = -0.50, p < 0.001), indicating that poorer sleep quality is associated with shorter sleep duration.
Table 3.
Correlations among sleep quality, sleep duration, and IC/BPS nocturnal symptoms.
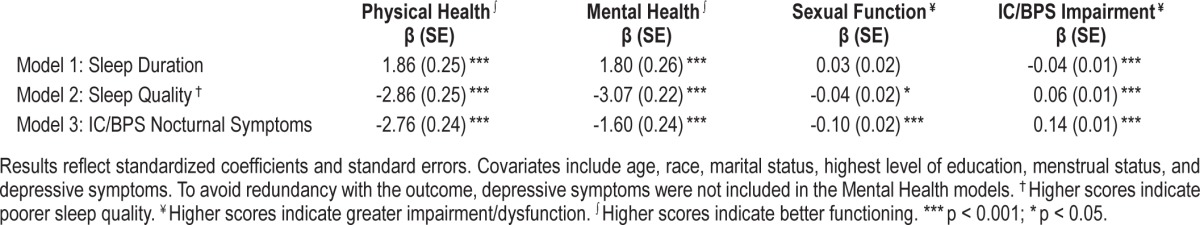
Multivariate Linear Regressions
Table 4 depicts the results of multivariate linear regression models that regressed each of the sleep problems (sleep duration, sleep quality, and IC/BPS-specific nocturnal symptoms) in separate models on QOL indicators. After covariate adjustment, short sleep duration was significantly associated with poorer self-reported physical health (β = 1.86; p < 0.001) and greater IC/BPS impairment (β = -0.04; p < 0.001). Poor sleep quality was significantly associated with poorer self-reported physical health (β = -2.86; p < 0.001), greater sexual dysfunction (β = -0.04; p < 0.05), and greater IC/BPS impairment (β = 0.06; p < 0.001), even after adjusting for covariates. IC/BPS nocturnal symptoms were significantly associated with poorer physical (β = -2.76; p < 0.001) and mental health (β = 0.52; p < 0.01), greater sexual dysfunction (β = -0.10; p < 0.001), and greater IC/BPS impairment (β = 0.14; p < 0.001), after covariate adjustment.
Table 4.
Results of multivariate linear regression models predicting general QOL and disorder-specific QOL according to sleep duration, sleep quality, or IC/BPS nocturnal symptoms.
Finally, to examine the unique contribution of sleep quality and duration on QOL, over and above the influence of disorder-specific nocturnal symptoms, we conducted follow-up regression models that additionally adjusted for the IC/BPS nocturnal symptoms. These models (Table 5) revealed that sleep duration and sleep quality remained a significant, independent correlate of self-reported physical health (p < 0.001). Associations between sleep and IC/BPS impairment and sexual function were not significant in these models.
Table 5.
Results of multivariate linear regression models predicting general QOL and disorder-specific QOL according to sleep duration or sleep quality, after adjustment for covariates.

DISCUSSION
In this well-characterized, population-based sample of women with IC/BPS symptoms, analyses confirmed a high prevalence of sleep problems, including short sleep duration, poor sleep quality, and nocturnal symptoms that are specifically related to the disorder. In fact, 61% of the current sample reported sleeping 6 or fewer hours per night, and only about one-third of the current sample (34%) reported the optimal 7-8 hours of sleep, as recommended by the Centers for Disease Control22 and others. Such high rates of insufficient sleep duration are of particular concern given increasing reports of prospective associations between short sleep duration and increased risk of adverse health outcomes, including cardiovascular diseases, diabetes, and mortality.23–25 Roughly half of the population reported “fairly bad or very bad” sleep quality in the past month, suggesting that poor sleep quality is a highly salient problem in this population. Notably, poor sleep quality and insomnia-related symptoms have also been prospectively linked with adverse cardiometabolic outcomes in community-based population studies.26,27
The most commonly reported IC/BPS nocturnal symptoms reported were nocturnal urgency and frequent need to use the bathroom, whereas bladder pain was somewhat less frequently endorsed (22% of sample). Our analyses further confirmed that sleep problems are independent contributors to QOL indicators in this population, even after controlling for sociodemographic characteristics and depressive symptoms (except in models with mental health as the outcome). Finally, this study afforded the opportunity to examine the extent to which short sleep duration and poor sleep quality contribute to QOL, over and above nocturnal symptoms of the disorder, including urinary urgency, pain, and need to use the bathroom. Multivariate models found that short sleep duration and poor sleep quality were associated with poorer self-reported physical health, even after further adjustment for IC/BPS specific nocturnal symptoms. However, other relationships with QOL indicators were not significant after accounting for IC/BPS specific nocturnal symptoms. Comparing the magnitude of the associations and the consistency of results across QOL outcomes, it is evident that IC/BPS nocturnal symptoms are the most consistent correlates of a range of QOL outcomes, and generally the magnitude of the association was greater than that for sleep quality and sleep duration. However, these findings may reflect greater overlap between the independent and dependent variables when examining IC/ BPS symptoms.
Taken together, these findings have important clinical implications as they highlight the multidimensional nature of sleep, the high prevalence of a variety of nocturnal symptoms in IC/ BPS populations, and the unique prognostic importance of different types of sleep disturbances, in contributing to QOL in IC/BPS populations. Clinically, providers may perceive sleep problems as typical symptoms of IC/BPS, rather than specific targets of intervention. Given that behavioral and pharmacologic treatments for sleep problems are efficacious,28 and that emerging evidence suggests that treating sleep problems can have downstream effects on co-occurring conditions (e.g., pain, depression),29–31 whereas left untreated, sleep problems can portend poorer depression treatment response,32 an important area for future research is to evaluate the impact of sleep problems on IC/BPS treatment outcomes. In fact, recent research has found that in older adults with concurrent insomnia and nocturia, patients who received a brief behavioral treatment for insomnia (BBTI) showed improvements in sleep as well as self-reported insomnia.33 Furthermore, patients with concurrent insomnia and nocturia had poorer treatment response to BBTI than those with insomnia only.34 Thus, sleep clinicians and researchers should also consider the impact of nocturnal symptoms related to IC/BPS or other causes on the course and treatment response for sleep disorders, particularly in populations at higher risk for nocturnal bladder symptoms and insomnia, such as older adults and women.
There are several limitations of the current analyses. First, the cross-sectional nature of the data precludes determination of causality. However, we did statistically adjust for many potential confounders of the relationship, including sociodemo-graphic characteristics, depressive symptoms, and menopausal status, which are known to covary with both sleep and QOL. In addition, although the inclusion of several domains of sleep was a strength of the study, we used specific items modified from the PSQI, rather than the full, validated PSQI scale, which may have limited validity and reliability. Regarding the sample, diagnosis of IC/BPS requires a clinical evaluation, which was not performed in this population-based study. Although participants endorsed bladder symptoms consistent with an IC/BPS diagnosis, it is possible that some participants would not meet diagnostic criteria in a clinical evaluation. However, a recent paper by our group has shown that the RICE sample reports similar severity of IC/BPS symptoms as a clinical population.35 Finally, the telephone survey techniques used to screen this large population yielded low response rates, which may have introduced bias in the sample. However, recent reviews of the survey methodology literature suggest that among probability samples conducted with a standardized process that adheres to typical survey methodology standards (as in the present study), response rates are only weakly associated with nonresponse bias and may not be a strong indicator of survey data quality.36–39
These limitations notwithstanding, the current analyses fill several key gaps in the literature. First, ours is the first community-based probability sample study to examine the relationship between several domains of sleep and key indicators of QOL in a large, representative sample of women with IC/BPS symptoms. In addition, our study provides some evidence to disentangle the association between general sleep characteristics (duration and quality) and QOL, independent of IC/BPS nocturnal symptoms. Given that sleep complaints are common comorbid symptoms of IC/BPS, these findings have clinical relevance inasmuch as they suggest that some of the sleep disturbances experienced by IC/BPS patients are more general in nature, rather than specific symptoms of the disorder. Thus, these findings suggest potential avenues for augmenting IC/ BPS treatment strategies with sleep-focused treatments. Finally, our findings add to the broader literature on sleep and pain, which has tended to focus primarily on musculoskeletal pain,40 and extends the research to organ-specific pain syndromes. In conclusion, findings demonstrate high prevalence of poor sleep quality and short sleep duration, as well as nocturnal symptoms related to IC/BPS. Given the links between sleep disturbances and QOL, interventions to improve sleep in IC/BPS populations may be an important next step to improve QOL in this population.
DISCLOSURE STATEMENT
This research was supported by U01 DK 070234 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH). Support for the first author (WMT) was provided by an Early Career Award from the National Heart, Lung and Blood Institute (NHLBI; HL093220). The National Institutes of Health, NIDDK, and NHLBI specifically disclaim responsibility for any analyses, interpretations, or conclusions. Work was performed at the RAND Corporation. Dr. Troxel has served on the advisory board of Hypno Core Ltd. Dr. Buysse has consulted for General Sleep Corp., Merck, Purdue Pharma, and Philips Respironics. Dr. Clemens has received royalties from UpToDate. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Bogart LM, Berry SH, Clemens JQ. Symptoms of interstitial cystitis, painful bladder syndrome and similar diseases in women: a systematic review. J Urol. 2007;177:450–6. doi: 10.1016/j.juro.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 2.Hanno PM. Interstitial cystitis-epidemiology, diagnostic criteria, clinical markers. Rev Urol. 2002;4(Suppl 1):S3–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540–4. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chelimsky G, Heller E, Buffington CA, Rackley R, Zhang D, Chelimsky T. Comorbidities of interstitial cystitis. Front Neurosci. 2012;6:114. doi: 10.3389/fnins.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–32. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 6.Brand S, Gerber M, Puhse U, Holsboer-Trachsler E. The relation between sleep and pain among a non-clinical sample of young adults. Eur Arch Psychiatry Clin Neurosci. 2010;260:543–51. doi: 10.1007/s00406-010-0113-2. [DOI] [PubMed] [Google Scholar]
- 7.Onen SH, Onen F, Courpron P, Dubray C. How pain and analgesics disturb sleep. Clin J Pain. 2005;21:422–31. doi: 10.1097/01.ajp.0000129757.31856.f7. [DOI] [PubMed] [Google Scholar]
- 8.Roehrs T, Roth T. Sleep and pain: interaction of two vital functions. Semin Neurol. 2005;25:106–16. doi: 10.1055/s-2005-867079. [DOI] [PubMed] [Google Scholar]
- 9.Tsai CF, Ouyang WC, Tsai SJ, Hong CJ, Lin TL. Risk factors for poor sleep quality among patients with interstitial cystitis in Taiwan. Neurourol Urodyn. 2010;29:568–72. doi: 10.1002/nau.20799. [DOI] [PubMed] [Google Scholar]
- 10.Panzera AK, Reishtein J, Shewokis P. Sleep disruption and interstitial cystitis symptoms in women. Urol Nurs. 2011;31:159–65. [PubMed] [Google Scholar]
- 11.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 12.Nickel JC, Payne CK, Forrest J, Parsons CL, Wan GJ, Xiao X. The relationship among symptoms, sleep disturbances and quality of life in patients with interstitial cystitis. J Urol. 2009;181:2555–61. doi: 10.1016/j.juro.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Dell JR, Mokrzycki ML, Jayne CJ. Differentiating interstitial cystitis from similar conditions commonly seen in gynecologic practice. Eur J Obstet Gynecol Reprod Biol. 2009;144:105–09. doi: 10.1016/j.ejogrb.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 14.Berry SH, Bogart LM, Pham C, et al. Development, validation and testing of an epidemiological case definition of interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1848–52. doi: 10.1016/j.juro.2009.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogart LM, Suttorp MJ, Elliott MN, Clemens JQ, Berry SH. Validation of a quality-of-life scale for women with bladder pain syndrome/interstitial cystitis. Qual Life Res. 2012;21:1665–70. doi: 10.1007/s11136-011-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franco OH, Wong YL, Kandala NB, et al. Cross-cultural comparison of correlates of quality of life and health status: the Whitehall II Study (UK) and the Western New York Health Study (US) Eur J Epidemiol. 2012;27:255–65. doi: 10.1007/s10654-012-9664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware JE, Kosinski M, Dewey JE, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Quality Metric Inc. 2000 [Google Scholar]
- 18.McHorney CA, Ware JE, Jr., Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–44. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 20.Bogart LM, Suttorp MJ, Elliott MN, Clemens JQ, Berry SH. Prevalence and correlates of sexual dysfunction among women with bladder pain syndrome/ interstitial cystitis. Urology. 2011;77:576–80. doi: 10.1016/j.urology.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Centers of Disease Control and Prevention [Internet] Atlanta, GA: Office of the Associate Director for Communication, Digital Media Branch, Division of Public Affairs; c2014. Are you getting enough sleep?; April 14, 2014 [July 24, 2014]; [about 2 screens]. Available from: http://www.cdc.gov/features/sleep/ [Google Scholar]
- 23.Xi B, He D, Zhang M, Xue J, Zhou D. Short sleep duration predicts risk of metabolic syndrome: a systematic review and meta-analysis. Sleep Med Rev. 2014;18:293–7. doi: 10.1016/j.smrv.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Holliday EG, Magee CA, Kritharides L, Banks E, Attia J. Short sleep duration is associated with risk of future diabetes but not cardiovascular disease: a prospective study and meta-analysis. PLoS One. 2013;8:e82305. doi: 10.1371/journal.pone.0082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojima M, Wakai K, Kawamura T, et al. Sleep patterns and total mortality: a 12-year follow-up study in Japan. J Epidemiol. 2000;10:87–93. doi: 10.2188/jea.10.87. [DOI] [PubMed] [Google Scholar]
- 26.Troxel WM, Buysse DJ, Matthews KA, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33:1633–40. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grandner MA, Jackson NJ, Pak VM, Gehrman PR. Sleep disturbance is associated with cardiovascular and metabolic disorders. J Sleep Res. 2012;21:427–33. doi: 10.1111/j.1365-2869.2011.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 29.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. J Clin Oncol. 2005;23:6083–96. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 30.Roehrs TA. Does effective management of sleep disorders improve pain symptoms? Drugs. 2009;69(Suppl 2):5–11. doi: 10.2165/11531260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Riemann D. Does effective management of sleep disorders reduce depressive symptoms and the risk of depression? Drugs. 2009;69(Suppl 2):43–64. doi: 10.2165/11531130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Troxel WM, Kupfer DJ, Reynolds CF, 3rd, et al. Insomnia and objectively measured sleep disturbances predict treatment outcome in depressed patients treated with psychotherapy or psychotherapy-pharmacotherapy combinations. J Clin Psychiatry. 2012;73:478–85. doi: 10.4088/JCP.11m07184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyagi S, Resnick NM, Perera S, Monk TH, Hall MH, Buysse DJ. Behavioral treatment of insomnia: also effective for nocturia. J Am Geriatr Soc. 2014;62:54–60. doi: 10.1111/jgs.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyagi S, Resnick NM, Perera S, Monk TH, Hall MH, Buysse DJ. Behavioral treatment of chronic insomnia in older adults: does nocturia matter? Sleep. 2014;37:681–87. doi: 10.5665/sleep.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konkle KS, Berry SH, Elliott MN, et al. Comparison of an Interstitial cystitis/ bladder pain syndrome clinical cohort with symptomatic community women from the RAND Interstitial Cystitis Epidemiology Study. J Urol. 2012;187:508–12. doi: 10.1016/j.juro.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davern M. Nonresponse rates are a problematic indicator of nonresponse bias in survey research. Health Serv Res. 2013;48:905–12. doi: 10.1111/1475-6773.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groves RM, Fowler FJ, Couper MP, Lepkowski JM, Singer E, Tourangeau R. Survey Methodology. Hoboken, NJ: Wiley; 2009. [Google Scholar]
- 38.Halbesleben JR, Whitman MV. Evaluating survey quality in health services research: a decision framework for assessing nonresponse bias. Health Serv Res. 2013;48:913–30. doi: 10.1111/1475-6773.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson TP, Wislar JS. Response rates and nonresponse errors in surveys. JAMA. 2012;307:1805–6. doi: 10.1001/jama.2012.3532. [DOI] [PubMed] [Google Scholar]
- 40.Doufas AG, Panagiotou OA, Ioannidis JP. Concordance of sleep and pain outcomes of diverse interventions: an umbrella review. PLoS One. 2012;7:e40891. doi: 10.1371/journal.pone.0040891. [DOI] [PMC free article] [PubMed] [Google Scholar]


