Abstract
Aims
Glucagon-like peptide-1 (GLP-1) receptor agonists improve blood glucose control by enhancing glucose-sensitive insulin release, delaying gastric emptying and reducing postprandial glucagon secretion. The studies reported here investigated the insulin response to an intravenous (iv) glucose challenge after injection of lixisenatide (LIXI) 20 µg or placebo.
Methods
Two single-centre, double-blind, randomized, placebo-controlled, single-dose, crossover studies were performed in healthy subjects (HS) and people with type 2 diabetes mellitus (T2DM). Participants received subcutaneous LIXI or placebo 2 h before an iv glucose challenge. Study endpoints included first- and second-phase insulin response, insulin concentration (INS), glucagon response and glucose disposal rate (Kglucose). LIXI exposure was measured over 12 h.
Results
LIXI 20 µg reached maximum concentration after 2 h and resensitized first-phase insulin secretion by 2.8-fold in T2DM to rates comparable with those in HS on placebo, and raised second-phase insulin secretion by 1.6-fold in T2DM. INS rose correspondingly and glucose disposal was accelerated by 1.8-fold in T2DM. First-phase insulin secretion and glucose disposal were also augmented by LIXI in HS, whereas second-phase insulin secretion reduced blood glucose concentrations to below fasting levels and then ceased, accompanied by a rapid, short-lasting rise in glucagon. Otherwise, suppression of glucagon release subsequent to augmentation of insulin release was unaffected in T2DM and in HS.
Conclusions
LIXI resensitized the insulin response to an iv glucose challenge in people with T2DM, thereby accelerating glucose disposal to nearly physiological intensity, and did not impair counter-regulation to low glucose levels by glucagon.
Keywords: healthy subjects, insulin response, lixisenatide, pharmacodynamics, pharmacokinetics, type 2 diabetes mellitus
Introduction
Glucagon-like peptide-1 (GLP-1) receptor agonists are an established therapeutic option for people with type 2 diabetes mellitus (T2DM), and both short- and long-acting agents are available 1. GLP-1 receptor agonists enhance insulin secretion in a glucose-dependent manner, which differentiates them from insulin therapy and insulin secretagogues, such as sulphonylureas and glinides, and results in a much lower risk of hypoglycaemia 1,2. Even though they share the same basic mechanism of action, long- and short-acting GLP-1 receptor agonists have different impacts on prandial and fasting plasma glucose (FPG), owing to differences in their pharmacokinetic and pharmacodynamic properties 1. Short-acting GLP-1 receptor agonists act predominantly on postprandial plasma glucose (PPG), attributable to intermittent activation of the GLP-1 receptor and slowed gastric emptying 1. Long-acting GLP-1 receptor agonists have a greater impact on FPG due to their sustained activation of the GLP-1 receptor, and lesser impact on gastric emptying owing to tachyphylaxis 1,3–6.
In healthy individuals, insulin secretion following a meal occurs in two distinct phases: a first phase that occurs during the first 10 min following a sudden rise in plasma glucose concentration, which reduces basal glucagon secretion and hepatic glucose production; and a second phase that is sustained until normoglycaemia is restored 7,8. Early-phase insulin secretion is critical for the maintenance of glucose homeostasis, with studies suggesting that an early insulin rise restrains excessive glucose excursions after nutrient ingestion 9,10. The first-phase insulin response is characteristically absent or severely blunted in people with T2DM, owing to impaired β-cell function 7,9–11. The GLP-1 receptor agonists, exenatide and liraglutide, both augment first- and second-phase insulin secretion in response to an intravenous (iv) glucose bolus 12,13.
Lixisenatide (LIXI) is a once-daily, short-acting prandial GLP-1 receptor agonist that has been demonstrated to significantly improve glycaemic control, with a pronounced effect on PPG and a low incidence of hypoglycaemia 14–20. In order to understand its effect on insulin secretion, the two complementary phase I studies reported here were undertaken, investigating the impact of LIXI on first- and second-phase insulin responses after a standardized iv glucose load in both healthy subjects (HS) and in people with T2DM.
Patients and Methods
The two individual, parallel, single-centre, double-blind, randomized, placebo-controlled, single-dose, two-period, two-treatment, two-sequence, crossover studies compared the insulin response to an iv glucose challenge after injection of LIXI 20 µg with the response after placebo injection. One study was performed in HS and the other study was performed in people with T2DM. These studies were carried out at the PROFIL Institut für Stoffwechselforschung GmbH, Neuss, Germany. The studies were approved by the institutional review boards or ethics committees and were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All participants gave written informed consent to participate in the studies.
Study Design
In both studies, participants were randomized 1 : 1 to one of two treatment sequences (LIXI 20 µg in study period 1 and placebo in study period 2 or vice versa). The two study periods were separated by a washout period of between 1 and 7 days. During each study period, participants fasted overnight (at least 10 h for HS and 13 h for people with T2DM) to adjust blood glucose to euglycaemic levels [about 5.5 mmol/l (100 mg/dl)]. In the study in people with T2DM, an iv infusion of insulin [soluble human insulin (Actrapid®), NovoNordisk, Mainz, Germany] could be given to people who did not reach euglycaemia. Participants in both studies received a subcutaneous injection of LIXI 20 µg or placebo in the left or right abdominal wall. Two hours after LIXI or placebo injection, participants received an iv bolus of glucose (0.3 g/kg body weight; 50% aqueous solution) over a period of 30 s.
Study Participants
Both studies combined included a total of 42 participants: 20 (14 male, 6 female) in the healthy study and 22 (13 male, 9 female) in the T2DM study. In the study in HS, the mean age, weight and body mass index (BMI) were 35.6 years, 75.7 kg and 24.5 kg/m2, respectively. Screening included FPG and glycated haemoglobin (HbA1c). In the T2DM study, all subjects were confirmed as having T2DM by HbA1c determination and being treated with metformin and/or lifestyle modifications. In the study in people with T2DM, the mean age, weight and BMI were 54.6 years, 88.7 kg and 29.9 kg/m2, respectively. In this study, baseline FPG ranged from 4.9 to 7.7 mmol/l (88139 mg/dl), with a mean value of 6.3 ± 0.5 mmol/l (114 ± 11 mg/dl), and baseline HbA1c ranged from 44 to 64 mmol/mol (6.2–8.0%), with a mean value of 52 ± 1 mmol/mol (6.9 ± 0.5%). HbA1c of people with T2DM treated with lifestyle modifications only was 49–55 mmol/mol (6.2–7.2%).
Study Assessments
In both studies the first- and second-phase insulin secretion response was assessed as the area under the insulin secretion rate (ISR) curve after iv glucose challenge (ISR-AUC0–10 min, ISR-AUC10–120 min). The ISR was calculated by deconvolution analysis based on a two-compartment model of C-peptide (C-PEP) elimination and was calculated from the C-PEP concentrations using the insulin secretion (ISEC) computer program to calculate insulin secretion 21. First- and second-phase insulin concentrations (INS) as well as C-PEP and glucagon concentrations (and associated AUCs), and glucose disposal according to the glucose disappearance constant (Kglucose), were also evaluated.
Kglucose was calculated as 100× the slope of the regression line fitted to the natural logarithm of glucose concentrations from 15 to 120 min after the glucose bolus in people with T2DM and from 10 to 30 min after the glucose bolus in HS. LIXI plasma concentrations were determined using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) technique (lower limit of quantification, 12 pg/ml) from blood samples frequently collected over 12 h after LIXI injection. Adverse event (AE) data were collected and summarized for all participants who received at least one administration of investigational product, including those who were withdrawn prematurely.
In healthy volunteers, blood samples were collected for 300 min (for 60 min before injection of LIXI or placebo and for 240 min after injection). In people with T2DM, blood samples were collected 30 min before and 240 min after injection of LIXI or placebo, that is for 120 min after iv glucose challenge. INS, C-PEP and glucagon concentrations were determined by peptide-specific radioimmunoassay, and glucose concentration was determined using a photometric hexokinase assay. In both the studies, blood samples for the determination of LIXI plasma concentration were collected from LIXI injection until 12 h thereafter.
Statistical Analyses
The primary analysis population comprised all subjects with completed profiles on both study days and with both treatments. Per protocol, INS-AUC0–10 min served as primary endpoint in the study in T2DM and ISR-AUC0–10 min in the subsequent study in HS.
A sample size analysis determined that at least 18 volunteers were required in the T2DM study to detect a threefold increase in INS-AUC0–10 min after subcutaneous injection of LIXI relative to placebo with a statistical power of 90% and a type 1 error of 5%, assuming a within-subject standard deviation of 0.9. Twenty subjects were enroled into the study to allow for dropouts. These assumptions were based on data reported by Fehse et al. for INS-AUC0–10 min observed after iv administration of exenatide relative to placebo 12. For the study in HS, the sample size analysis determined that at least 16 volunteers were required to detect a 1.5-fold increase in ISR-AUC0–10 min after subcutaneous injection of LIXI relative to placebo with a statistical power of 90% and a type 1 error of 5%, assuming a within-subject standard deviation of 0.3. These assumptions were based upon the study in people with T2DM. Eighteen subjects were enroled into the study to allow for dropouts. Naturally log-transformed ISR-AUC0–10 min, ISR-AUC10–120 min, INS-AUC0–10 min, INS-AUC10–120 min, C-PEP-AUC0–10 min, C-PEP-AUC10–120 min and Kglucose were analysed using linear mixed models with fixed terms for sequence, period, sex and treatment, and with an unstructured R matrix of treatment variances and covariances for subject within sequence blocks (using SAS PROC MIXED). Estimates and 90% confidence intervals (CIs) for the ratios of geometric means of LIXI versus placebo were calculated within the linear mixed-effect model framework. The pharmacokinetic parameters of maximum plasma concentration (Cmax), tmax (time to Cmax), half-life (t1/2z), area under the curve at the last quantifiable time-point (LIXI-AUClast) and LIXI-AUC were calculated using non-compartmental methods from plasma concentration data of LIXI, and were summarized by descriptive statistics. All data are presented as mean ± standard deviation unless otherwise stated.
Results
In the healthy study, all 20 participating subjects completed the study, with no study discontinuations; however, only 18 were included in the pharmacodynamic analysis as two subjects had incomplete pharmacodynamic profiles. In the T2DM study, 22 subjects were enroled and 20 subjects completed the planned study period. The two remaining subjects discontinued due to AEs, neither of which was considered to be related to study treatment.
Three participants who completed the T2DM study required intermediate low-dose iv infusions of insulin to stabilize glycaemic levels on the first study day that were discontinued 30 min prior to glucose challenge. Two of these participants randomized to the placebo–LIXI sequence also required stabilization on the second study day and therefore received insulin infusions matching day one to enhance comparability. These insulin infusions did not interfere with the response pattern as verified by strong individual differences in insulin responses to iv glucose following placebo and LIXI treatments.
Pharmacodynamics – Fasting State
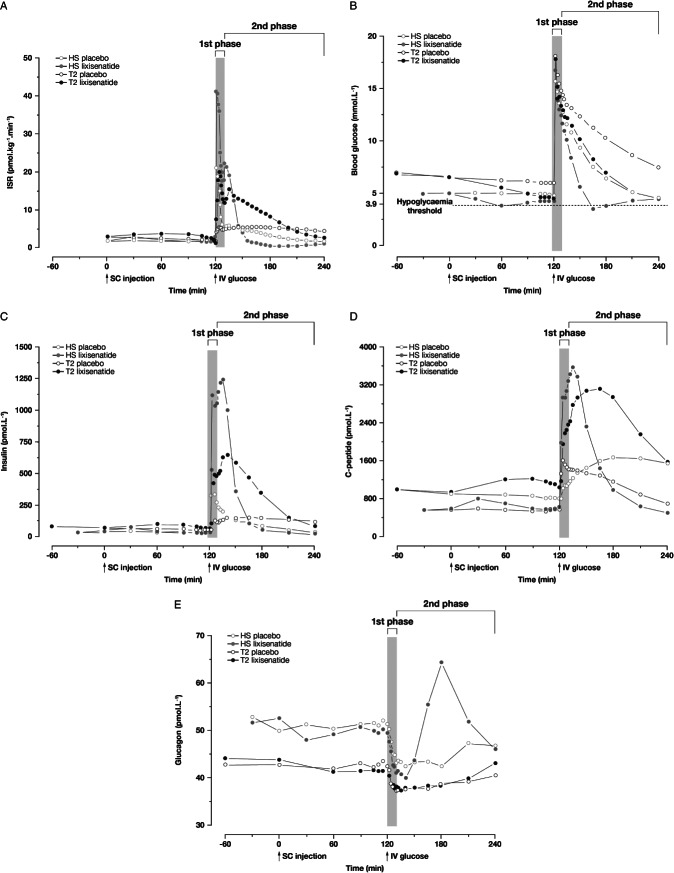
People with T2DM presented with elevated FPG 180 min prior to LIXI or placebo administration – mean (s.d.) 7.51 (1.11) and 7.49 (0.92) mmol/l [135 (20) and 135 (17) mg/dl), respectively – which gradually decreased to 6.60 (0.69) and 6.65 (0.76) mmol/l [119 (12) and 120 (14) mg/l] immediately prior to injection of medication. LIXI administration was associated with a small increase in fasting basal insulin secretion and INS, and a sizeable decrease in basal blood glucose levels in both HS and people with T2DM (Figure 1B, C). Both effects were short-lived in HS but were more sustained in T2DM, establishing euglycaemia prior to the iv glucose challenge. The resulting baseline FPG were 6.09 (0.68) mmol/l [110 (12) mg/dl] 120 min after placebo and 4.56 (0.97) mmol/l [82 (17) mg/dl] 120 min after LIXI in people with T2DM, and 4.97 (0.27) and 4.36 (0.25) mmol/l [90 (5) and 78 (5) mg/dl] in HS.
Figure 1.
Plots of mean values for (A) insulin secretion rate, (B) blood glucose concentration, (C) insulin concentration, (D) C-peptide concentration and (E) glucagon concentration, all during iv glucose challenge following injection of lixisenatide 20 µg or placebo. ISR, insulin secretion rate; HS, healthy subjects; T2, type 2 diabetes mellitus; SC, subcutaneous; IV, intravenous.
Pharmacodynamics – iv Glucose Bolus
Pharmacodynamic outcomes from both studies are shown in Table1. Treatment with LIXI significantly increased first-phase insulin secretion (ISR-AUC0–10 min) by 2.4-fold (90% CI: 2.1–2.6) in HS and 2.8-fold (90% CI: 2.5–3.1) in people with T2DM (p < 0.001 for both; Figure 1A). LIXI also increased second-phase insulin secretion (ISR-AUC10–120 min) by 1.6-fold (90% CI: 1.4–1.7) in people with T2DM (p < 0.001), but did not change ISR-AUC10–120 min in HS compared with placebo (0.9-fold; 90% CI: 0.8–1.0). In HS, blood glucose levels did not decrease further due to rapid reduction of the ISR when blood glucose fell below normal baseline levels, approximately 20 min after the glucose challenge, owing to normal feedback regulation. ISR-AUC10–120 min was higher in people with T2DM compared with HS following treatment with LIXI.
Table 1.
Pharmacodynamic comparisons for lixisenatide versus placebo
| People with T2DM (n = 20) | Healthy subjects (n = 18) | |||||
|---|---|---|---|---|---|---|
| Placebo | Lixisenatide 20 µg | Geometric mean ratio LIXI/PBO (90% CI) | Placebo | Lixisenatide 20 µg | Geometric mean ratio LIXI/PBO (90% CI) | |
| Insulin response | ||||||
| ISR-AUC0–10 min, pmol/kg | 48 (19) | 133 (49) | 2.8 (2.5, 3.1) | 112 (30) | 268 (78) | 2.4 (2.1, 2.6) |
| ISR-AUC10–120 min,pmol/kg | 593 (158) | 925 (210) | 1.6 (1.4, 1.7) | 370 (111) | 341 (118) | 0.9 (0.8, 1.0) |
| INS-AUC0–10 min,pmol min/l | 503 (385) | 2835 (1778) | 6.6 (5.0, 8.7) | 2620 (1253) | 8269 (3758) | 3.2 (2.7, 3.8) |
| INS-AUC10–120 min,pmol min/l | 10 402 (5158) | 31 602 (17 307) | 3.0 (2.7, 3.3) | 6371 (2327) | 21 885 (8841) | 3.4 (2.7, 4.2) |
| C-peptide response | ||||||
| C-PEP-AUC0–10 min,pmol min/l | 1809 (1432) | 7796 (3990) | 6.1 (4.2, 8.8) | 8661 (3406) | 19 726 (6685) | 2.3 (2.0, 2.7) |
| C-PEP-AUC10–120 min,pmol min/l | 81 555 (25 986) | 167 725 (42 743) | 2.1 (1.9, 2.3) | 65 029 (20 726) | 97 208 (24 858) | 1.5 (1.3, 1.7) |
| Glucose disposal | ||||||
| Glucose disposal, Kglucose,% per min | 0.57 (0.11) | 0.98 (0.11) | 1.8 (1.6, 1.9) | 1.6 (0.8) | 3.8 (1.8) | 2.3 (1.9, 3.0) |
All data are reported as arithmetic mean ± s.d. (standard deviation) unless otherwise stated.
AUC, area under the curve; CI, confidence interval; ISR, insulin secretion rate; ISR-AUC0–10 min, first-phase insulin secretion; ISR-AUC10–120 min, second-phase insulin secretion; INS-AUC0–10 min, first-phase insulin concentration; INS-AUC10–120 min, second-phase insulin concentration; Kglucose, glucose disposal constant; LIXI, lixisenatide; PBO, placebo; T2DM, type 2 diabetes mellitus.
The increase in insulin secretion was accompanied by an increase in glucose disposal rate (Kglucose) in both HS and in people with T2DM receiving LIXI (Figure 1B). A 2.3-fold (90% CI: 1.9–3.0) increase in Kglucosewas seen in HS, and a 1.8-fold (90% CI: 1.6–1.9) increase was seen in people with T2DM treated with LIXI compared with placebo.
As a result of the increase in insulin secretion, INS-AUC0–10 minincreased in both HS and in people with T2DM (Figure 1C). A 3.2-fold (90% CI: 2.7–3.8) increase was seen in HS compared with placebo and a 6.6-fold (90% CI: 5.0–8.7) increase was observed in people with T2DM compared with placebo. INS-AUC10–120 minwas also significantly (p < 0.001) increased in both HS [3.4-fold increase (90% CI: 2.7–4.2); p < 0.001] and people with T2DM [3.0-fold increase (90% CI: 2.7–3.3); p < 0.001].
In the study in people with T2DM, 15 completers were receiving metformin and five were treated with lifestyle modification alone, prior to the study. Of the 15 participants treated with metformin, seven were strong responders to LIXI (INS-AUC0–10 min > 3800 pmol min/l), while only one participant who had previously been treated with lifestyle modification alone was identified as a strong responder. The baseline HbA1c of the metformin-treated strong responders was 44–53 mmol/mol (6.2–7.0%), and the baseline HbA1c of the lifestyle-treated strong responder was 48 mmol/mol (6.5%). The baseline HbA1c levels for the participants identified as weaker responders to LIXI (INS-AUC0–10 min < 2000 pmol/min) were 48–64 mmol/mol (6.5–8.0%).
The increase in insulin secretion was reflected in the C-PEP levels, with LIXI increasing first- and second-phase C-PEP levels in both HS and people with T2DM compared with placebo (Figure 1D).
In HS, the suppression of glucagon release was augmented following LIXI administration but returned to normal levels more rapidly than after placebo (Figure 1E). Glucagon levels are affected by both INS and blood glucose levels, and the drop in glucagon levels observed in HS is the result of a drop in blood glucose levels. Glucagon release recovered faster in the few participants with blood glucose levels <3.9 mmol/l (<70 mg/dl) attributable to normal feedback pathways. In the study in people with T2DM, glucagon suppression was not affected by LIXI.
Pharmacokinetics
The mean LIXI plasma concentration profile over time, following a single subcutaneous dose of LIXI 20 µg in HS and people with T2DM, is shown in Figure 2and Table2. Maximum LIXI concentrations (Cmax) were achieved 2 h after a single 20 µg LIXI injection in both people with T2DM and HS, and then declined with a similar mean (s.d.) half-life of 2.6 (0.7) and 2.1 (0.4) h, respectively. However, a greater peak plasma concentration was recorded in HS [LIXI-Cmax; mean (s.d.) 145 (63.6) pg/ml] compared with people with T2DM [LIXI-Cmax; mean (s.d.) 83.9 (21.3) pg/ml].
Figure 2.
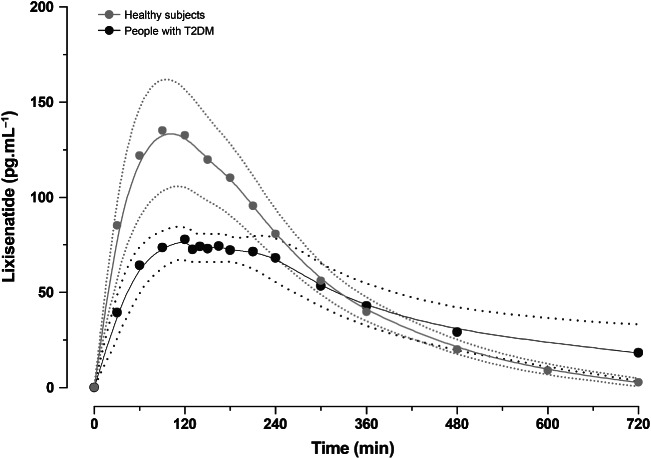
Mean plasma concentrations of lixisenatide over time following a single subcutaneous 20 µg dose. Dotted line indicates the 25th and 75th percentiles. T2DM, type 2 diabetes mellitus.
Table 2.
Pharmacokinetic characteristics of lixisenatide
| Parameter mean (s.d.) | People with T2DM* | Healthy subjects |
|---|---|---|
| LIXI-Cmax,pg/ml | 83.9 (21.3) | 145 (63.6) |
| tmax,h† | 2.0 (1.50–2.25) | 2.0 (1.98–2.75) |
| t½z,h | 2.6 (0.7) | 2.1 (0.4) |
| LIXI-AUClast,pg h/ml | 449 (149) | 611 (216) |
| LIXI-AUC, pg h/ml | 529 (165) | 661 (216) |
LIXI-AUC, area under the curve extrapolated to infinity; LIXI-AUClast, area under the curve at the last quantifiable time point; LIXI-Cmax, maximum plasma concentration; s.d., standard deviation; T2DM, type 2 diabetes mellitus; tmax, time to LIXI-Cmax; t½z, terminal plasma half-life (i.e. time from LIXI-Cmaxto half LIXI-Cmax).
Data are from 21 participants (one participant was excluded from AUC calculation owing to extrapolation >30%).
Median (interquartile range).
Safety and Tolerability
In the study in HS, 20 treatment-emergent adverse events (TEAEs) were reported by eight participants, seven (35%) while receiving LIXI and four (20%) while receiving placebo, with some participants experiencing TEAEs in both study arms; all were mild-to-moderate in intensity. The most frequent TEAEs with LIXI treatment were gastrointestinal, which were reported by five participants [nausea (n = 5), vomiting (n = 3), diarrhoea (n = 1])], followed by headache (n = 2).
In the study in people with T2DM, four participants reported a total of 13 AEs, all of which were mild-to-moderate in intensity. Ten of these events were documented as treatment-emergent and a further three as posttreatment events. Three participants experienced AEs during LIXI exposure and one during placebo exposure. The AEs with LIXI were mostly gastrointestinal disturbances [nausea (n = 2), vomiting (n = 2)] and headache (n = 2).
Discussion
In this study, conducted under experimental conditions, a single dose of LIXI enhanced first-phase insulin secretion by 2.8-fold and second-phase insulin secretion by 1.6-fold in response to an iv glucose challenge in people with T2DM. This enhanced insulin secretion was seen to result in 6.6-fold elevated INS and an acceleration of glucose disposal comparable with that seen in HS on placebo, with Kglucoseincreasing by 1.8-fold. The first-phase insulin secretion response in people with T2DM was comparable with that seen in HS receiving placebo. In HS, LIXI also enhanced first-phase insulin secretion by 2.4-fold; first-phase INS was also higher by threefold and acceleration of glucose disposal was higher by 2.3-fold. However, the rise in second-phase insulin secretion was short lived and (in total) not increased, as insulin release rapidly ceased when blood glucose fell below baseline. Overall, these effects on insulin response are what would be predicted for a prandial GLP-1 receptor agonist based on previous studies with native GLP-1 or exenatide 1,12,22–30. It is important to note, however, that this was an acute study and the effect of LIXI on insulin secretion following longer-term use is not known.
The ability of LIXI to resensitize glucose-dependent insulin release was observed in all people with T2DM. However, there was a trend observed for LIXI to be more effective in those with modestly elevated HbA1c levels, indicative of the early stages of T2DM, compared with those with higher HbA1c levels. This is probably related to the greater insulin secretory capacity remaining in earlier disease stages.
Glucagon suppression was not affected in either patient population, with glucagon levels returning to normal more rapidly in HS after LIXI than after placebo. It is likely that physiological counter-regulatory pathways are not affected by LIXI, enabling the body to combat hypoglycaemia. The effect of LIXI on glucagon suppression is consistent with observations from a previous 13-week study that showed enhanced suppression with LIXI during the 4 h following a standardized meal test 31.
The iv glucose bolus coincided well with the maximum plasma concentrations of LIXI, which occurred 2 h after subcutaneous injection of a 20 µg dose, and the terminal plasma half-life was similar between people with T2DM (2.6 h) and HS (2.1 h), which is consistent with a previous study using repeated dosing of LIXI 32. Higher maximum plasma concentrations of LIXI were seen in HS compared with people with T2DM. This resulted in higher overall exposure to LIXI in HS than in people with T2DM – reflecting the greater dose per body weight of 0.26 µg/kg in HS compared with 0.23 µg/kg in people with T2DM – yet indicates otherwise similar exposure profiles in people with T2DM and in HS.
The mechanism of action underlying the observed acute effects of LIXI on insulin secretion probably involves direct stimulation of GLP-1 receptors located in the pancreatic islet β-cells, resensitizing the glucose concentration-dependent exocytotic release from insulin-secretory vesicles via a cyclic AMP-dependent signalling pathway 3,4,33. Although the overall impact of GLP-1 receptor agonists on glycaemic control is likely to involve a multifactorial mechanism of action, with retardation of gastric emptying being predominant to the PPG-lowering efficacy of LIXI, the acute effects on the insulin-secretory profile are likely to be highly relevant as they mark restoration of glucose-sensitive stimulation of insulin secretion 3,4,34–36.
At least in its early stages, T2DM is characterized by fasting hyperglycaemia despite hyperinsulinaemia and relative hyperglucagonaemia, all of which were present in people with T2DM. The sustained lowering of blood glucose from hyperglycaemic levels to those of HS after injection of LIXI, and prior to the iv glucose challenge, which is not seen in subjects treated with placebo, is indicative of resensitizing glucose-sensitive insulin release and readjusting glucohomeostasis. This effect on fasting hyperglycaemia is effective on its own and adds to the accelerated PPG disposal. The corresponding effect in HS was short lived, which could be explained by intact glucohomeostatic regulation.
In conclusion, LIXI resensitized the insulin response to an iv glucose challenge in people with T2DM, thereby accelerating glucose disposal to nearly physiological intensities, and did not impair counter-regulation to low glucose by glucagon. The first-phase insulin secretion response in people with T2DM was comparable with that seen in HS receiving placebo.
Acknowledgments
We would like to thank all of the study staff and participating subjects at PROFIL. We would like to thank Peter Ruus and Yan-Hong Liu for initial study design support. Editorial support for this manuscript was provided by Alexander Jones of Medicus International (London, UK) and funded by Sanofi.
Conflict of Interest
R. H. A. B. initiated the investigation, supervised the clinical part in subjects with type 2 diabetes, pooled the information, contributed to the discussion, wrote the manuscript, and reviewed and edited the manuscript. R. H. A. B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. J. S. joint supervised the investigation, supervised the clinical part in HS, contributed to the discussion, and reviewed and edited the manuscript. J. M. researched and deconvoluted the data, conducted the statistical evaluations and contributed to the discussion. C. K. headed the clinical part and reviewed and edited the manuscript.
R. H. A. B., J. S. and J. M. are employees of Sanofi. C. K. is managing director and co-owner of Profil and has received honoraria from Sanofi.
The study was funded by Sanofi, the manufacturer of LIXI. The investigators and representatives from Sanofi were responsible for the study design, protocol, statistical analysis plans, analysis and reporting of the results. Final responsibility for the decision to submit the manuscript for publication was made jointly by all authors.
References
- 1.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 2.Meloni AR, DeYoung MB, Lowe C, Parkes DG. GLP-1 receptor activated insulin secretion from pancreatic beta-cells: mechanism and glucose dependence. Diabetes Obes Metab. 2013;15:15–27. doi: 10.1111/j.1463-1326.2012.01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 4.Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007;113:546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 6.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60:1561–1565. doi: 10.2337/db10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Prato S, Marchetti P, Bonadonna RC. Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes. 2002;51(Suppl. 1):S109–116. doi: 10.2337/diabetes.51.2007.s109. [DOI] [PubMed] [Google Scholar]
- 8.Polonsky KS, Given BD, Hirsch LJ, et al. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med. 1988;318:1231–1239. doi: 10.1056/NEJM198805123181903. [DOI] [PubMed] [Google Scholar]
- 9.Del Prato S. Loss of early insulin secretion leads to postprandial hyperglycaemia. Diabetologia. 2003;46(Suppl. 1):M2–8. doi: 10.1007/s00125-002-0930-6. [DOI] [PubMed] [Google Scholar]
- 10.Del Prato S, Tiengo A. The importance of first-phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2001;17:164–174. doi: 10.1002/dmrr.198. [DOI] [PubMed] [Google Scholar]
- 11.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D., Jr Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984;74:1318–1328. doi: 10.1172/JCI111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:5991–5997. doi: 10.1210/jc.2005-1093. [DOI] [PubMed] [Google Scholar]
- 13.Chang AM, Jakobsen G, Sturis J, et al. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes. 2003;52:1786–1791. doi: 10.2337/diabetes.52.7.1786. [DOI] [PubMed] [Google Scholar]
- 14.Ahren B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M) Diabetes Care. 2013;36:2543–2550. doi: 10.2337/dc12-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonseca VA, Alvarado-Ruiz R, Raccah D, et al. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono) Diabetes Care. 2012;35:1225–1231. doi: 10.2337/dc11-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinget M, Goldenberg R, Niemoeller E, Muehlen-Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P) Diabetes Obes Metab. 2013;15:1000–1007. doi: 10.1111/dom.12121. [DOI] [PubMed] [Google Scholar]
- 17.Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L) Diabetes Care. 2013;36:2489–2496. doi: 10.2337/dc12-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riddle MC, Forst T, Aronson R, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GETGOAL-DUO-1) Diabetes Care. 2013;36:2497–2503. doi: 10.2337/dc12-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenstock J, Raccah D, Koranyi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X) Diabetes Care. 2013;36:2945–2951. doi: 10.2337/dc12-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seino Y, Min KW, Niemoeller E, Takami A, Investigators EG-LAS. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia) Diabetes Obes Metab. 2012;14:910–917. doi: 10.1111/j.1463-1326.2012.01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hovorka R, Soons PA, Young MA. ISEC: a program to calculate insulin secretion. Comput Methods Programs Biomed. 1996;50:253–264. doi: 10.1016/0169-2607(96)01755-5. [DOI] [PubMed] [Google Scholar]
- 22.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 23.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 24.Gutniak M, Orskov C, Holst JJ, Ahrén B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7–36) amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 25.Rachman J, Gribble FM, Barrow BA, Levy JC, Buchanan KD, Turner RC. Normalization of insulin responses to glucose by overnight infusion of glucagon-like peptide 1 (7–36) amide in patients with NIDDM. Diabetes. 1996;45:1524–1530. doi: 10.2337/diab.45.11.1524. [DOI] [PubMed] [Google Scholar]
- 26.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 27.Nauck MA, Niedereich-Holz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273:E981–988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 28.Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab. 2001;281:E155–161. doi: 10.1152/ajpendo.2001.281.1.E155. [DOI] [PubMed] [Google Scholar]
- 29.Egan JM, Clocquet AR, Elahi D. The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab. 2002;87:1282–1290. doi: 10.1210/jcem.87.3.8337. [DOI] [PubMed] [Google Scholar]
- 30.Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3082–3089. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- 31.Ratner RE, Rosenstock J, Boka G, Silvestre L. Post-meal pharmacodynamic profile of AVE0010, a once-daily GLP-1 receptor agonist, in patients with type 2 diabetes inadequately controlled on metformin (Abstract) Diabetologia. 2009;52:S60. [Google Scholar]
- 32.Distiller LA, Ruus P. on behalf of the ACT6011 Study Group. Pharmacokinetics and pharmacodynamics of a new GLP-1 agonist AVE0010 in type 2 diabetes patients [Abstract] Diabetes. 2008;57:A154. [Google Scholar]
- 33.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenz M, Pfeiffer C, Steinsträsser A, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes – relationship to postprandial glycemia. Regul Pept. 2013;185:1–8. doi: 10.1016/j.regpep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept. 2008;151:123–129. doi: 10.1016/j.regpep.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Holst JJ, Christensen M, Lund A, et al. Regulation of glucagon secretion by incretins. Diabetes Obes Metab. 2011;13(Suppl. 1):89–94. doi: 10.1111/j.1463-1326.2011.01452.x. [DOI] [PubMed] [Google Scholar]