Abstract
Vesicomyidae clams harbor sulfide-oxidizing endosymbionts and are typical members of cold seep communities where active venting of fluids and gases takes place. We investigated the central biogeochemical processes that supported a vesicomyid clam colony as part of a locally restricted seep community in the Japan Trench at 5346 m water depth, one of the deepest seep settings studied to date. An integrated approach of biogeochemical and molecular ecological techniques was used combining in situ and ex situ measurements. In sediment of the clam colony, low sulfate reduction rates (maximum 128 nmol mL−1 day−1) were coupled to the anaerobic oxidation of methane. They were observed over a depth range of 15 cm, caused by active transport of sulfate due to bioturbation of the vesicomyid clams. A distinct separation between the seep and the surrounding seafloor was shown by steep horizontal geochemical gradients and pronounced microbial community shifts. The sediment below the clam colony was dominated by anaerobic methanotrophic archaea (ANME-2c) and sulfate-reducing Desulfobulbaceae (SEEP-SRB-3, SEEP-SRB-4). Aerobic methanotrophic bacteria were not detected in the sediment, and the oxidation of sulfide seemed to be carried out chemolithoautotrophically by Sulfurovum species. Thus, major redox processes were mediated by distinct subgroups of seep-related microorganisms that might have been selected by this specific abyssal seep environment. Fluid flow and microbial activity were low but sufficient to support the clam community over decades and to build up high biomasses. Hence, the clams and their microbial communities adapted successfully to a low-energy regime and may represent widespread chemosynthetic communities in the Japan Trench. In this regard, they contributed to the restricted deep-sea trench biodiversity as well as to the organic carbon availability, also for non-seep organisms, in such oligotrophic benthic environment of the dark deep ocean.
Introduction
Cold seep communities establish where tectonic or gravitational forces push free gas, methane-rich pore water, and/or mud upward into sulfate-penetrated surface sediments (Boetius & Wenzhöfer, 2013). High energy availability at and near the sediment surface thereby supports enormous biomasses of chemosynthetic organisms such as siboglinid tubeworms, mytilid and vesicomyid bivalves, and giant sulfide-oxidizing bacteria (Sibuet & Olu, 1998; Levin, 2005; Grünke et al.,2012). These organisms are well adapted to access and use reduced compounds in seep sediments. For instance, most vesicomyid clams have a reduced gut system and thus rely almost entirely on their autotrophic sulfide-oxidizing endosymbionts for nutrient and energy supply (Childress et al., 1993; Goffredi & Barry, 2002, and references therein). To access the sulfide, they dig with their foot several centimeters into the sediment (Dubilier et al., 2008), take the sulfide up, and transport it with their blood to the endosymbionts (Childress et al., 1993). Some vesicomyid species are able to accumulate amounts of sulfide in their body that exceed ambient concentrations more than 60-fold (Childress et al., 1993; Barry & Kochevar, 1998) and are thus found in habitats with a wide range of sulfide concentrations (0.6–20 mm; Barry et al., 1997; Decker et al., 2012; Pop Ristova et al., 2012). Bioturbation by the clams enhances the sulfate transport from the water column into the sediment, resulting in sulfate reduction (SR) at sediment depths that otherwise would be sulfate-limited (Wallmann et al., 1997; Levin et al., 2003; Treude et al., 2003). Hence, vesicomyid clams are able to populate seep sites of low geological activity, where sulfide is not found close to the sediment surface (Fischer et al., 2012).
In methane-enriched seep sediments, sulfide is a product of bacterial SR that is often coupled to the anaerobic oxidation of methane (AOM) mediated by consortia of anaerobic methanotrophic archaea (ANME) and sulfate-reducing bacteria (SRB; Boetius et al., 2000). High densities of these microbial consortia have been described in seep sediments of all continental margins from shallow waters to the deep sea (Knittel & Boetius, 2009, and references therein). The occurrence, distribution, and activity of the microbes involved in AOM have been intensively studied using different molecular ecological tools and biogeochemical measurements (Boetius et al., 2009; Knittel & Boetius, 2009). So far, there are three main ANME clades ANME-1, ANME-2, and ANME-3 (Hinrichs et al., 1999; Niemann et al., 2006b), which contain several sub-clades, such as thermophilic ANME-1 (Holler et al., 2011), ANME-2a-c (Orphan et al., 2001), and the recently described Methanoperedenaceae (Haroon et al., 2013). The involved SRB are close relatives of either Desulfosarcina/Desulfococcus or Desulfobulbus (Knittel et al., 2003; Schreiber et al., 2010; Kleindienst et al., 2012). The different ANME clades can be distinguished using methods based on nucleic acids (Orphan et al., 2001; Knittel et al., 2005; Pernthaler et al., 2008) and membrane lipids (Hinrichs et al., 1999; Elvert et al., 2003; Rossel et al., 2011).
In the last decade, the improvement in deep-sea technologies such as remotely operated vehicles or submersibles enabled the scientific community to explore seep ecosystems in detail by performing focused sampling and in situ measurements. These in situ investigations have significantly increased our knowledge of the small-scale variability of biodiversity and of biogeochemical activities within and between seep ecosystems (Jørgensen & Boetius, 2007; Boetius & Wenzhöfer, 2013, and references therein). However, only a few studies exist in water depths >4000 m because it is a technological challenge to access these remote abyssal habitats for sampling and in situ measurements (Boetius & Wenzhöfer, 2013). It is known from the Nankai Trough or the Japan Trench that cold seeps occur frequently even down to water depths of at least 7500 m (Kobayashi, 2002; Arakawa et al., 2005, and reference therein). This tectonically active area hosts numerous seeps and the deepest known vesicomyid clam colonies at 6437 m (Sibuet et al., 1988; Ogawa et al., 1996; Fujikura et al., 1999). Japan Trench seeps offer a unique opportunity to study microbial community structure and biogeochemical processes at abyssal seep ecosystems as most seep studies have been conducted at shallower sites (Sibuet et al., 1988; Boetius & Wenzhöfer, 2013).
Although chemosynthetic clam colonies in the Japan Trench are known, detailed insights into the underlying biogeochemical processes and predominant microbial communities fueling these remote and high-biomass seep communities are sparse. Here, we combined analyses of sediment pore water chemistry, sediment–water interface exchange processes, as well as methane and sulfate turnover rate measurements with community analyses based on 16S rRNA genes and intact polar lipids (IPLs) to thoroughly investigate the biogeochemistry and microbial community. To our knowledge, this is the first and most comprehensive study on the functioning of an abyssal seep ecosystem using in situ activity measurements in the Japan Trench to date. Our main hypotheses were (i) the key biogeochemical processes in the sediment that fuel the spatially restricted clam colony are similar to those found at shallow seeps and (ii) the microbial community composition of this ecosystem differs from that of shallow seeps.
Materials and Methods
Seafloor observations and sampling
During the cruise YK06-05 in 2006 with the RV Yokosuka to the Japan Trench, we investigated a clam colony inhabited by Abyssogena phaseoliformis (former known as Calyptogena phaseoliformis) and Isorropodon fossajaponicum (former known as Calyptogena fossajaponica) at 5346 m water depth. The names of both species were adapted according to the most recent taxonomic studies of the family Vesicomyidae (Krylova & Sahling, 2010, and references therein) and the accepted nomenclature in the World Register of Marine Species (http://www.marinespecies.org/). The targeted sampling and precise positioning of the in situ instruments were achieved with the manned research submersible Shinkai 6500 (JAMSTEC, Nankoku, Kochi, Japan). Besides the well-defined vesicomyid clam colonies present in this area of the Japan Trench, no other chemosynthetic communities, such as sulfide-oxidizing bacterial mats, were observed. The colonies, however, were associated with different groups of benthic organisms including actiniaria, holothurians, and tube-dwelling polychaetes. Typically, the clam patches were round with diameters ranging from a few decimeters to 2 m (Fig. 1B). Distances between the widespread colonies were a few tens of meters, and we observed several trails of moving clams during the dives.
Figure 1.
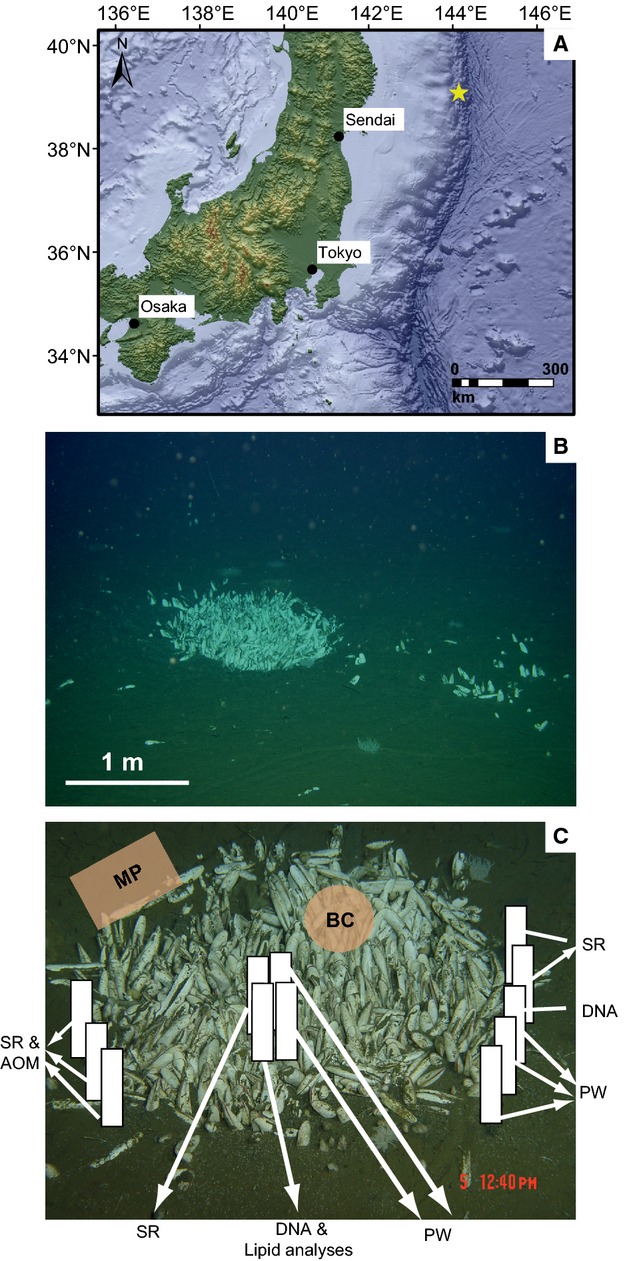
Sampling and in situ measurements were performed at a vesicomyid clam colony in the Japan Trench (A) at a water depth of 5346 m (A,B). The map was generated using the esri arcgis software and the General Bathymetric Chart of the Oceans (GEBCO_09 Grid, version 20091120, http://www.gebco.net). (C) Relative positions of in situ measurements and pushcore sampling (white bars) at the investigated clam patch. MP, microprofiler; BC, benthic chamber; DNA, 16S rDNA analyses; SR, sulfate reduction; AOM, anaerobic oxidation of methane; PW, pore water chemistry, methane concentration and isotopy; calcium carbonate, pyrite, and total organic carbon content.
One large vesicomyid clam colony (Fig. 1A; 39°6.3560′N, 143°53.5619′E) was studied in detail with microbiological and biogeochemical methods. In the following text, this particular Japan Trench clam colony is termed JTC colony. Sampling was first performed close to the rim of the JTC colony and then at the center (Fig. 1C). Immediately after sample recovery onboard, the sediment core was sub-sampled for ex situ rate measurements or preserved for later analyses.
Geochemistry
Ex situ pore water concentrations of sulfate and dissolved inorganic carbon (DIC) were measured, along with the concentrations and isotopic compositions of dissolved methane, total organic carbon (TOC) content, and turnover rates of sulfate as well as methane. In addition, in situ benthic oxygen uptake rates were determined with a microprofiler and a benthic chamber module. The data are available online via the Data Publisher for Earth & Environmental Science PANGAEA (doi: 10.1594/PANGAEA.826602).
Ex situ measurements
To measure the concentrations of pore water constituents, push cores were sub-sampled in 1 cm intervals and pore water was extracted via sediment squeezing (Reeburgh, 1967; 0.45 μm Durapore Filter; Millipore, Bedford, MA, USA). For each sample depth, we obtained 1–5 mL pore water that was immediately preserved and stored at 4 °C until the measurements were taken in the home laboratory. To determine sulfate concentrations, 0.5–1 mL pore water was fixed in 1 mL 2% zinc acetate (ZnAc) solution. Samples were diluted and filtered before concentrations were determined by non-suppressed anion exchange chromatography (Waters IC-Pak anion exchange column, Waters 430 conductivity detector; Waters, Milford, MA, USA). For measuring DIC concentrations, the pore water was preserved with 20 μL saturated mercuric chloride (HgCl2) solution and stored headspace free. DIC content of the samples was measured by the flow injection method (detector VWR scientific model 1054) according to Hall & Aller (1992). Dissolved methane concentrations and isotopic compositions were determined with the headspace method according to Kvenvolden & McDonald (1986) and Ertefai et al. (2010) using gas chromatography and isotope ratio mass spectrometry, respectively. Carbon isotope ratios are reported in the δ-notation as per mil (‰) deviation from Vienna Pee Dee Belemnite standard. Standard deviations of δ13C values were obtained from repeated measurements and were usually less than ±1.0‰.
Pyrite and carbonate content of the sediment was measured by X-ray refraction analysis as previously described (Ertefai et al., 2010). TOC contents were measured from dry and homogenized sediment samples using a Leco CS 200 analyzer (LECO, St. Joseph, MI, USA). Prior to the TOC analysis, the samples were treated with 12.5% hydrogen chloride (HCl) solution to remove any inorganic carbon.
Sulfate reduction and AOM were measured ex situ by the whole core injection method (Jørgensen, 1978). We incubated the samples at in situ temperature (1.5 °C) for 48 h with either 14CH4 (dissolved in water, 2.5 kBq) or carrier-free 35SO4 (dissolved in water, 50 kBq). Sediment was fixed in 25 mL sodium hydroxide (NaOH) solution (2.5%, w/v) or 20 mL ZnAc solution (20%, w/v) for AOM or SR, respectively. Turnover rates were measured as previously described (Treude et al., 2003; Kallmeyer et al., 2004).
In situ measurements
Total oxygen uptake (TOU) and diffusive oxygen uptake (DOU) were measured at the center and the rim of the JTC colony, respectively. The difference between TOU and DOU is commonly dedicated to faunal-mediated consumption, including bioirrigation and bioturbation as well as the animal respiration itself (Glud, 2008 and references therein). TOU of the JTC colony center was determined with a small cylindrical benthic chamber module, which enclosed a sediment area of 284 cm2 (radius = 9.5 cm) together with 15 cm of overlying bottom water (equivalent to approximately 5 L). Two Clark-type minielectrodes continuously recorded the oxygen concentration of the enclosed water body during the incubation (Treude et al., 2009). Sensors were calibrated against bottom water oxygen concentration (determined from Winkler titration) and a zero reading recorded at in situ temperature on board. TOU (mmol m−2 day−1) was calculated from the initial linear change in oxygen concentration vs. time (for more details see Wenzhöfer & Glud, 2002).
Oxygen penetration depth and DOU at the rim of the clam colony were measured with a small deep-sea microprofiler module (Treude et al., 2009), carrying three oxygen Clark-type microelectrodes (Revsbech et al., 1983) and one temperature sensor (Pt100; UST Umweltsensorentechnik GmbH, Geschwenda, Germany). High-resolution microprofiles across the sediment-water interface were measured with a vertical resolution of 100 μm on a total length of 15 cm. Oxygen electrodes had a linear response to the oxygen concentration in seawater and were calibrated in situ using constant readings in the bottom water (oxygen concentration determined by Winkler titration) and the anoxic parts of the sediment (Wenzhöfer et al., 2000; De Beer et al., 2006). DOU (mmol m−2 day−1) was calculated from the measured microprofiles and Fick's first law of diffusion with DOU = D0 × (dC/dz), where D0 (1.26 × 10−9 m−2 s−1) is the molecular diffusion coefficient in water corrected for temperature and salinity (Li & Gregory, 1974), C (μm) is the solute concentration, and z (m) is the depth within the diffusive boundary layer (Rasmussen & Jørgensen, 1992).
Microbial community analysis
IPL analyses
Before intact and free cell membrane constituents were analyzed by liquid and gas chromatography, freeze dried sediment was spiked with internal standards and lipids were extracted using a modified Bligh and Dyer method (Sturt et al., 2004). The total lipid extract was separated chromatographically on a glass column using 3 g of silica gel (60 mesh) into three fractions: a non-polar fraction (dichloromethane), a glycolipid fraction (acetone), and a phospholipid fraction (methanol). The phospholipid fractions were analyzed for IPLs, which were analyzed by high performance liquid chromatography/electrospray ionization-multiple stage-mass spectrometry (HPLC/ESI-MSn) as previously described (Sturt et al., 2004). The non-polar fractions were further separated for gas chromatography analyses, following standard protocols for separation, derivatization, and transesterification (Elvert et al., 2000, 2003) described in detail by Ertefai et al. (2008).
16S rRNA gene analyses
To analyze the microbial community composition, we constructed archaeal and bacterial 16S rRNA gene libraries of sediments from the center and the rim of the JTC colony. On board, sediment cores were sectioned into 1–5 cm intervals and frozen at −20 °C. Total community DNA was retrieved from 5 g of sediment (pooled from the 0 to 10 cm depth horizon) by chloroform extraction as described by Zhou et al. (1996) and purified using the Wizard DNA clean-up system (Promega, Madison, WI, USA). PCRs for 16S rRNA gene libraries were carried out using the Master Taq polymerase (Eppendorf, Hamburg, Germany), 26–30 cycles and the bacterial primers GM3/GM4 (Muyzer et al., 1995) or archaeal primers Arch20F/Uni1392R (Lane et al., 1985; Massana et al., 1997). Purification of PCR products, cloning reactions, and the sequencing of inserts were performed as previously described (Niemann et al., 2006a), and chimeric sequences were removed using Mallard (Ashelford et al., 2006). The 16S rRNA gene sequences were aligned with SILVA INcremental Aligner (SINA; Prüsse et al., 2007) and manually optimized according to the secondary structure. Phylogenetic classification was carried out using the arb software package (Ludwig et al., 2004) based on the SILVA small subunit 16S rRNA reference sequence database (ssuref v111; Quast et al., 2013). Phylogenetic trees were calculated with the maximum likelihood algorithm phyml (100 bootstraps) and a positional variability filter as described before (Ruff et al., 2013). Operational taxonomic units at 98% 16S rRNA gene identity (OTU0.02) and Chao1 richness estimates were calculated using the software mothur v1.24 (Schloss et al., 2009). The nucleotide sequences reported in this paper have been archived in the EMBL, GenBank, and DDBJ nucleotide sequence databases under the accession numbers HG425384–HG425704.
Results
Sediment solid phase
The recovered sediment cores were visually differentiated into upper (0–10 cm below seafloor – cmbsf), middle (10–25 cmbsf), and lower (>25 cmbsf) sections (Fig. 2A). The upper 10 cm showed a light brown color and were characterized by living vesicomyid clams being partly buried into the slightly sandy sediment. The middle section of the core was black with broken shells, and a sulfidic smell was noticed during subsampling. Below 25 cm depth, the sediment was of uniform gray color. The differentiation of the sediment into different horizons was also reflected in the pyrite, carbonate, and TOC contents of the sediment (Fig. 2B). In the upper sediment horizon (0–10 cm), pyrite (FeS2) was absent and carbonate was low (5–7 wt-%). In the middle section, the amount of pyrite and carbonate increased to up to 8 and 32 wt-%, respectively. The carbonate content declined again in the lower section in contrast to pyrite, which reached values of up to 12 wt-%. TOC content in the sediment was constant in the upper 15 cm (approximately 1.7 wt-%), decreased in the middle section of the core (14–18 cmbsf), and remained constant again in the lower section (Fig. 2B).
Figure 2.
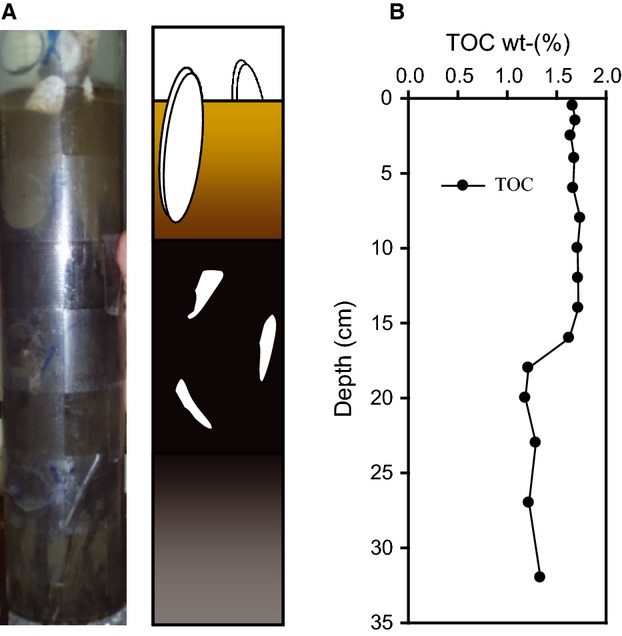
Stratigraphy and mineralogy of the sediment sampled in the center of the JTC colony. Core image and sketch (A) and total organic carbon = TOC (B).
Sediment geochemistry
Pore water geochemistry
In the center and at the rim of the JTC colony, sulfate, DIC, and dissolved methane concentrations as well as the methane isotopic composition were determined. In the center of the clam patch, sulfate concentration decreased to <1 mm at 12 cmbsf (Fig. 3A). The DIC concentration profile showed an opposite behavior to the sulfate profile, as it first increased with depth and then stayed nearly constant at more than 100 mm below 10 cmbsf. In contrast, at the JTC colony rim, sulfate penetrated deeper into the sediment (18 cmbsf) as compared to the center (Fig. 3), and the maximum DIC concentration (approximately 100 mm) was found at 31 cmbsf. Dissolved methane was analyzed in all three lithostratigraphic horizons (Fig. 3). Concentrations and isotopic compositions varied with sediment depth and differed between sampling spots. The center revealed higher dissolved methane concentrations than the rim, with a maximum between 25 and 33 cmbsf (Fig. 3A). At the center, values of dissolved methane ranged from −84 to −79‰. The highest δ13C values were found in the middle section of the core at 15–20 cmbsf. At the rim, the dissolved methane was less depleted in 13C (δ13C values of −72 to −66‰; Fig. 3B).
Figure 3.
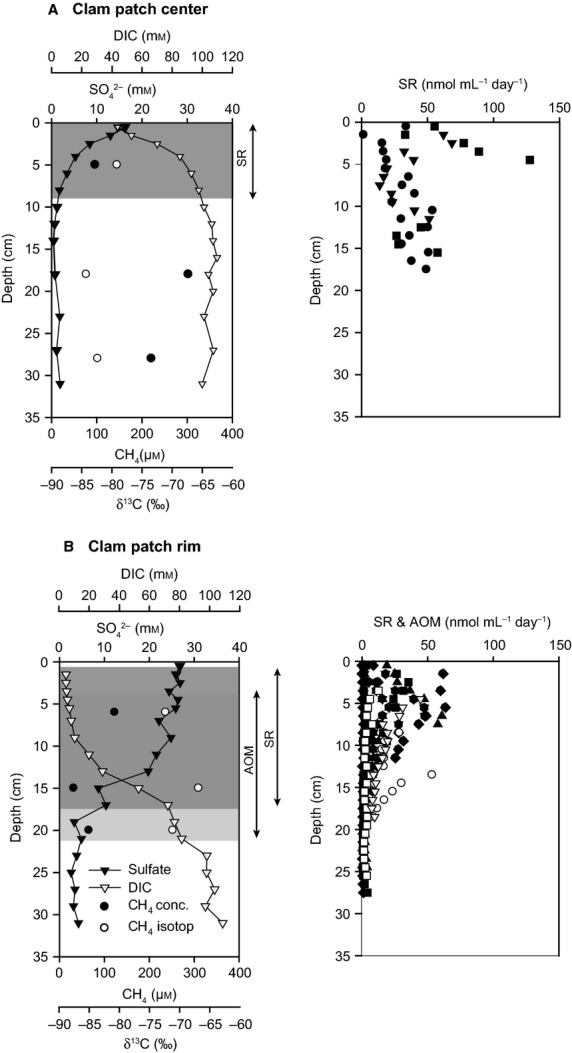
Left panel: Sulfate (black triangles) and DIC (white triangles) concentrations were measured in the pore water from the center (A) and the rim (B) of the JTC colony. Potential SR and AOM horizons according to pore water concentrations are highlighted. Furthermore, methane concentration (black dots) and isotopic composition (white dots) were determined at both locations. Right panel: SR rates (in black) and AOM rates (in white) from the center and the rim of the JTC colony. The different symbols represent replicates of turnover rate measurements.
Methane oxidation and SR rates
Sulfate consumption was measured at the rim and the center of the JTC colony, whereas methane turnover could only be quantified in the rim sediment (Fig. 3). At the center, SR values were scattered over the investigated depth horizon and ranged from 16 to 128 nmolmL−1 day−1. The averaged depth integrated SR rate (0–16 cm) was 6.3 mmol m−2 day−1. At the colony rim, sulfate turnover was lower (1.4–64 nmol mL−1 day−1) with a maximum at about 5 cm below seafloor and decreased with increasing sediment depth. Horizontal distribution of methane consumption at the rim was similar to SR rates with values ranging from 2 to 52 nmol mL−1 day−1. The average depth (0–16 cm below seafloor)-integrated turnover rates of methane and sulfate at the rim were in the same range with 2.4 (n = 3) and 2.1 (n = 3) mmol m−2 day−1, respectively.
In situ oxygen uptake measurements
The microprofiler module was placed at the sediment next to the JTC colony, because a direct placement of the fragile glass sensors in the JTC colony was not possible. Approximately 20 cm beside the colony rim, the average oxygen penetration depth was 1.64 cm (n = 3) with an average DOU of 1.9 mmolm−2 day−1 (Fig. 4). Temperature remained constant at about 1.3 °C for the entire profiling length, and thus no heat flow was observed. In contrast to the microprofiler, the benthic chamber was placed directly on the clams, enclosing about 20 clams. A TOU of 21 mmol m−2 day−1 was measured, which is one order of magnitude higher than the DOU outside the colony. Assuming that the DOU represents the benthic oxygen consumption of the sediment in the Japan Trench at 5346 m, we calculated the oxygen consumption related to the benthic chemosynthetic community (CCOU) by subtracting DOU from TOU (CCOU = TOU − DOU). For our investigated JTC colony, this resulted in a community consumption of 19 mmol O2 m−2 day−1. The chamber enclosed a sediment area of 0.0284 m2, populated by approximately 20 clams, which resulted in a clam density of approximately 700 clams m−2. Thus, one clam consumed about 27 μmol oxygen per day.
Figure 4.
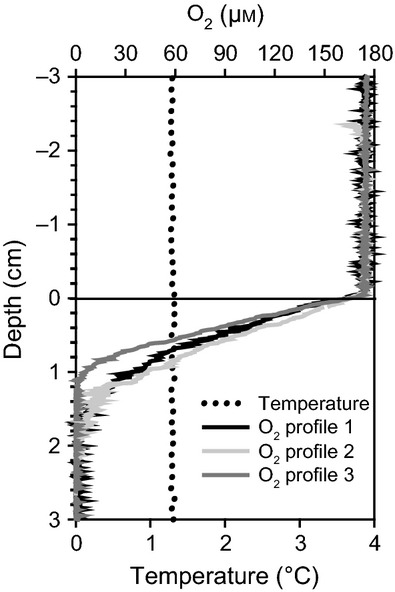
High resolution microsensor measurements outside of the colony showed an oxygen penetration depth of >1 cm and constant temperature throughout the entire sediment layer.
Microbial community
Biomarker analyses
Analyses of microbial lipids as both intact polar membrane lipids and free lipids were performed using sediment from below the clams in the center of the JTC colony (Fig. 5). The HPLC-MSn analysis revealed phosphate-based IPLs in the form of hydroxyarchaeol (OH-Ar) with phosphatidylglycerol (PG) and phosphatidylserine (PS) as polar headgroups. Both IPL types increased with sediment depth from 0.1 to 2.8 μg g−1 dry sediment and were most abundant in the sediment horizon between 12 and 18 cm (2.8 μg g−1) before their concentration declined to 0.5 μg g−1 with sediment depth (Fig. 5). Bacterial dietherglycerolipids (DEG), occurring as phosphatidylethanolamine (PE) and PS, were absent in the upper 6 cm, but increased with sediment depth and peaked in the sediment horizon at 12–18 cm (0.6 μg g−1) and then declined to 0.1 μg g−1 dry sediment. The free (not intact) lipids included OH-Ar and monoalkyl glycerol ethers (MAGE). The δ13C values of free lipids varied with sediment depth, and the most strongly 13C-depleted lipids were present at 12–18 cm below seafloor (Fig. 5).
Figure 5.
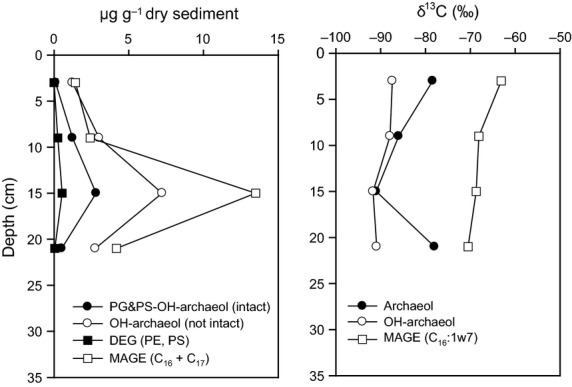
Microbial lipid profiles in the center of the JTC colony. Concentrations of archaeal and bacterial lipids are shown on the left and the isotopic compositions are on the right panel; abbreviations: PG&PS-OH-archaeol (intact): hydroxyarchaeol (OH-Ar) with phosphatidylglycerol (PG) and phosphati-dylserine (PS) as polar headgroups; OH-Ar (OH-Ar without polar headgroup); DEG (PE, PS): dialkyletherglycerolipid as phosphati-dylethanolamine and PS; MAGE (C16 + C17): sum of C16 and C17 monoalkyl glycerol ethers (MAGE), includes C16 and C17 saturated MAGE and three monounsaturated C16-MAGE.
Phylogenetic diversity
Sequencing of selected clones from 16S rRNA gene libraries resulted in a total of 147 archaeal and 173 bacterial sequences (Fig. 6) from the surface sediment (0–10 cm) of the JTC colony center and the rim.
Figure 6.
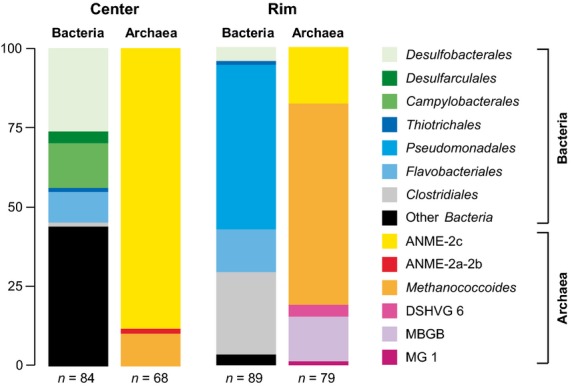
Relative 16S rRNA clone frequencies. Archaeal and bacterial diversity in the center and at the rim of the JTC colony. The scale bar represents relative clone frequencies in percent. The total number of clones per gene library is indicated below the respective column. DSHVG, Deep Sea Hydrothermal Vent Group, MBGB, Marine Benthic Group B, MG1, Marine Group 1.
The archaeal community of the center seemed to be extremely low in diversity (Fig. 7A), because we only obtained four OTU0.02, too few to reasonably estimate Chao1 richness. The OTU0.02 belonged to the ANME-2c clade (88% of all clones), the ANME-2a clade (1%), and the genus Methanococcoides (11%). The bacterial gene library of the center was more diverse (Chao1 = 41 OTU0.02) and was dominated by deltaproteobacterial SRB of the orders Desulfobacterales (26%) and Desulfarculales (4%). The diversity of sulfate reducers was high, including members of the genera Desulfarculaceae, Desulfobacula, Desulforhopalus, and Desulfobacterium (Fig. 7C). Interestingly, we did not detect sequences of the SEEP-SRB-1 clade, which is common at seep ecosystems, instead the SRB community seemed to be dominated by Desulfobulbaceae (20%), such as SEEP-SRB-3 and SEEP-SRB-4. The sulfur-oxidizing community seemed to be dominated by chemolithoautotrophic Sulfurovum species (14%) within the Epsilonproteobacteria, because sequences of Thiotrichales (approximately 1%) were rare. Additional clades included Planctomycetes and the candidate divisions JS1, OD1, and Hyd24-12 (Fig. S2).
Figure 7.
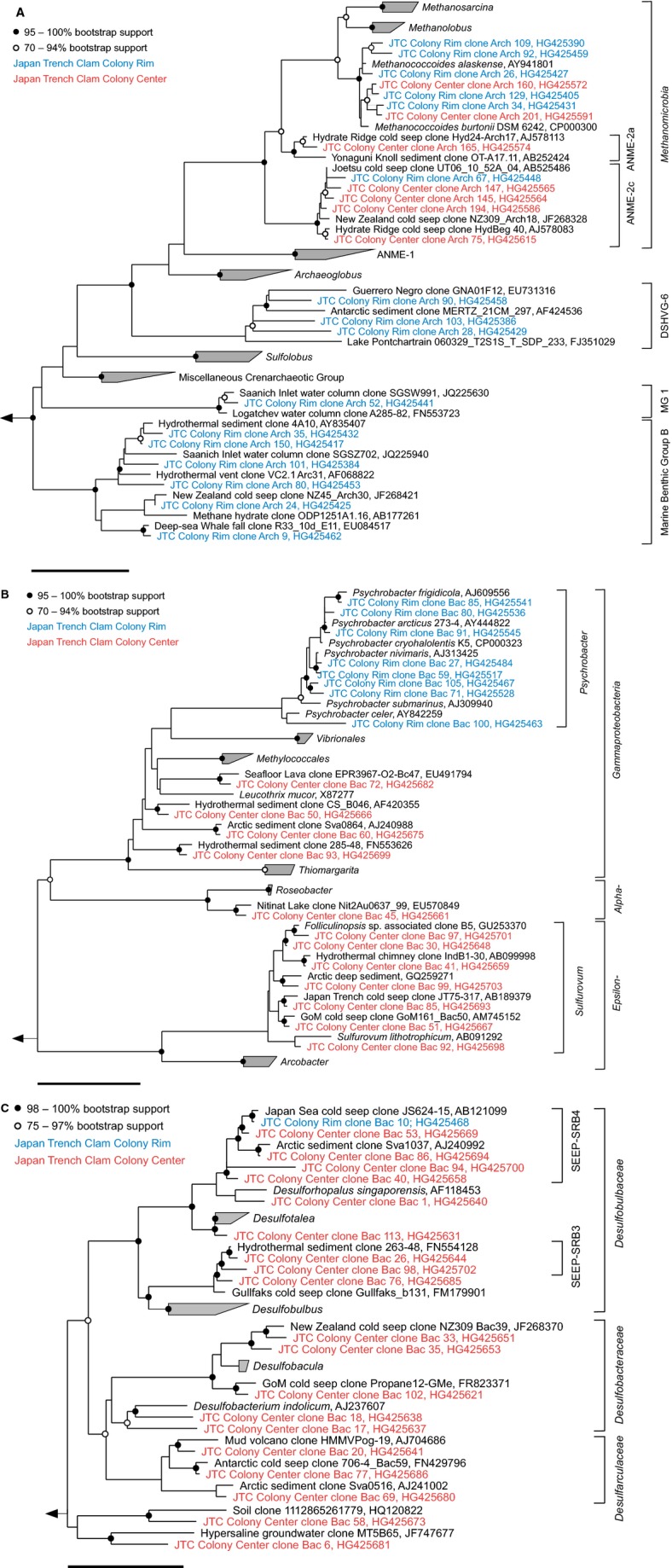
Phylogenetic affiliation of Archaea (A), Alpha-, Gamma-, and Epsilonproteobacteria (B) and Deltaproteobacteria (C) of the JTC colony center (red) and colony rim (blue) sediments based on 16S rRNA gene sequences. The scale bars represent 10% estimated sequence divergence.
The archaeal community of the JTC colony rim appeared to be more diverse than that of the center (Chao1 = 12 OTU0.02). Most sequences belonged to the genus Methanococcoides (63%), followed by ANME-2c archaea (18%). In addition, we found members of Marine Benthic Group B (14%), Deep-Sea Hydrothermal Vent Group 6 (4%), and Marine Group 1 (approximately 1%). The bacterial gene library of the rim sediment (Chao1 = 25 OTU0.02) was greatly dominated by Psychrobacter (51%) of the order Pseudomonadales, showing a high microdiversity (Fig. 7B) and also included many Clostridiales (Fig. S2) and several sequences of a SEEP-SRB-4 organism. The bacterial libraries of the center and rim contained distinct populations of Flavobacteriales and other clades (Figs S1 and S2). Remarkably, the seep sediments seemed to lack clades that are common to many seep sites worldwide, such as SEEP-SRB-1, Methylococcales, ANME-1, and Thermoplasmatales.
Discussion
Seepage intensity at a Japan Trench clam colony
The distribution of clams at seeps is strongly controlled by the biogeochemical processes in underlying sediments, which are influenced by the supply of methane-rich fluids from the subsurface. Upward flow of hydrocarbon-rich fluids through thrust faults (Kobayashi, 2002) at fracture zones of the Japan Trench has been described at sites, where vesicomyid clam colonies with sharp boundaries have been detected (Juniper & Sibuet, 1987; Sibuet et al., 1988; Ogawa et al., 1996). Oxygen and temperature profiles obtained in this study point also to a locally focused release of seep fluids at the investigated JTC colony. The measured oxygen penetration depth of 1.6 cm (Fig. 4) and the corresponding low benthic oxygen consumption rate indicated that sediments a few centimeters away from the colony rim were similar to non-seep influenced sediments (Wenzhöfer & Glud, 2002; De Beer et al., 2006). At typical cold seep habitats, oxygen penetration into the sediment is usually limited to the top few millimeters (Lichtschlag et al., 2010; Felden et al., 2013), and is reduced even at vesicomyid clam sites that exhibit bioturbating activity (Levin et al., 2003). Moreover, a straight temperature profile (Fig. 4) indicated that fluid flow from the deep subsurface was not detectable next to the colony rim. At active seep sediments, upward fluid flow is indicated by increasing temperatures with increasing depths (Feseker et al., 2008). Such temperature gradients have been recorded for numerous clam colonies, for example at the Nankai Trough (Kobayashi, 2002) and at the Peruvian margin (Olu et al., 1996). Unfortunately, we could not measure sediment temperature profiles directly below the clam patch. However, the concave-shaped sulfate concentration profile and the methane concentrations measured at the colony center (Fig. 3) indicated a low seepage activity nourishing the clam community. Methane δ13C values of <−80‰ at the JTC colony center (Fig. 3) suggested biogenic methane formation from CO2 and H2 rather than a deep subsurface thermogenic origin (Whiticar, 1999).
Alternatively, the JTC colony could have also developed, because methane-rich and sulfate-free deep sediment layers were suddenly exposed to oxygenated and sulfate-rich bottom waters as proposed for the Monterey Canyon seep ecosystems (Paull et al., 2005). There, chemosynthetic benthic communities are most common on steep slopes where seafloor erosion occurs and tectonically driven fluid flow is lacking (Paull et al., 2005). Erosion of the sediment in the Japan Trench might have happened when the upper sediment layer was removed during one of the regularly occurring earthquakes (Kobayashi, 2002; Kawagucci et al., 2012), however, our data and dive observations do not indicate such sediment instabilities for the investigated site.
Biogeochemical processes in sediments of the Japan Trench clam colony
To investigate whether there are also similarities to shallow seeps concerning the underlying biogeochemical processes, we analyzed methane and sulfate consumption rates. Fluid flow and associated methane availability at the JTC colony were rather low, but sufficient to maintain a dense seep community of living clams. The depth integrated rates of AOM and SR have a ratio close to one, indicating a close coupling of methane consumption and sulfide production (Treude et al., 2003; Niemann et al., 2006b), which constantly nourished the clams and their chemosynthetic symbionts.
Sulfate reduction rates of clam patches at different seep ecosystems cover a wide range of turnover rates (Treude et al., 2003; Boetius & Suess, 2004; Pop Ristova et al., 2012), and seem to correlate with the methane availability in the sediment. Rates measured at the JTC colony (maximum 64 nmol mL−1 day−1) are in the same range as those of a clam colony at the REGAB pockmarks (maximum 154 nmol mL−1 day−1; Pop Ristova et al., 2012). But these values are nearly two orders of magnitude lower compared to Hydrate Ridge off Oregon, where SR rates of up to 3000 nmol mL−1 day−1 in combination with methane concentration of up to 10 mm have been found (Torres et al., 2002; Boetius & Suess, 2004). In fact, even lower methane concentrations sustain clam habitats (Barry et al., 1997; Wallmann et al., 1997; Cambon-Bonavita et al., 2009), which underlines the capability of Vesicomyidea clams to adapt to different environmental conditions. Low methane concentrations are not only an indicator for low seepage rates, they also result in lower sulfide availability within the sediment. Vesicomyidea clams are able to inhabit sites with low sulfide concentrations simply due to their ability to enrich sulfide in their body fluids above ambient concentrations (Childress et al., 1993; Barry et al., 1997; Barry & Kochevar, 1998). It also has been proposed that a continuous supply of sulfide is more important for these animals than the absolute concentration (Dubilier et al., 2008). Furthermore, clams could influence benthic biogeochemical processes similar to vestimentiferan tubeworms, which supply the microbial community close to their roots with sulfate and thus enhance locally the microbial sulfide production (Cordes et al., 2005). Furthermore, clams can move to sediments with higher sulfide concentrations as soon as sulfide is depleted at one location (Sibuet et al., 1988; Olu et al., 1996; Levin, 2005). In fact, such single moving clams were observed during our exploration. However, because the majority of clams were associated in patches (Fig. 1B), methane seepage and subsequent sulfide availability seemed to be sufficient to maintain the colonies.
At the JTC colony, the low methane concentrations in the surface sediments might not only be due to low seepage activity but could have also resulted from efficient methane consumption by the benthic filter. Indeed, we measured SR rates of 6.3 mmol m−2 day−1 in the sediment below the clam colony. If we assume that oxygen was used as the terminal electron acceptor for sulfide oxidation, which in turn is mainly produced by SR coupled to AOM, then TOU can be used to estimate the in situ methane consumption within the sediment. An oxygen uptake of 21 mmol m−2 day−1 would correspond to a methane consumption rate of 10.5 mmol m−2 day−1 based on the stoichiometric ratios of methane to sulfide (1:1) and of sulfide to oxygen (1:2). Methane efflux measurements at other clam habitats indicated that the uprising methane is completely oxidized in the sediment (Sommer et al., 2006; Pop Ristova et al., 2012). Therefore, the methane flux from the deep subsurface for the entire JTC colony (diameter 1.8 m2) would have been 19 mmol day−1, which corroborated that seepage was relatively low compared to other clam habitats (Torres et al., 2002; Boetius & Suess, 2004; Sommer et al., 2006; Pop Ristova et al., 2012). This could be either a temporal effect because fluid flow may slightly vary over time at seeps (Olu et al., 1996) or the seep community of the JTC colony is well adapted to efficiently use a low, but constant methane supply to build up the observed high biomasses.
Chemosynthetic seep community at the Japan Trench clam colony
Clams as bioengineers
Vesicomyid clams rely on the biogeochemical processes in the sediment for their sulfide supply and at the same time strongly influence the benthic biogeochemical regime by bioirrigation and bioturbation (Wallmann et al., 1997; Fischer et al., 2012). Geochemical gradients (DIC, pyrite, calcium carbonate content) and turnover rates at the JTC colony showed an active community performing SR and AOM in the upper 10–15 cm of the sediment. However, we did not find a distinct production zone at the JTC colony, which is usually present at seep habitats of other associated organisms (Treude et al., 2003; Felden et al., 2010, 2013). The activity was rather spread throughout the sediment, within a depth range that was affected by the clams, which had an average body length of 15–17 cm and were buried up to four-fifths in the sediment. Clams and other seep-associated fauna, such as polychaete tubeworms, are known to enhance the availability of electron acceptors in deeper sediment horizons by bioirrigation, which results in a lowering of the sulfate methane transition zone (Wallmann et al., 1997; Levin et al., 2003; Treude et al., 2003; Fischer et al., 2012; Ruff et al., 2013). By this mechanism, competing chemosynthetic surface organisms are separated from their energy source over time (Fischer et al., 2012; Pop Ristova et al., 2012). At the JTC colony, the clams seemed to have successfully altered the sulfide availability within the sediment and thus other common members of cold seeps such as thiotrophic bacterial mats were not observed (Treude et al., 2003; Felden et al., 2010, 2013; Lichtschlag et al., 2010).
Using average growth rates of other vesicomyid clam species (Barry & Kochevar, 1998) and the measured shell sizes, we estimated an average age of 10–15 years for the living clams at the JTC colony. The mixture of living clams and empty shells suggested that the clam colony existed for more than 15 years; consequently, methane seepage has likely influenced this site at least for several decades. The reduced faunal diversity at the JTC colony dominated by only two clam species indicated relatively stable spatial and temporal environmental conditions (e.g., fluid flow), because it was proposed that the reduction in ecological niches and thus diversity results from habitat stability (Sibuet & Olu-Le Roy, 2002). The comparison of shallow and deep-sea seep ecosystems indicated a positive correlation between faunal diversity, biomass, and fluid flow rate independent of water depth (Sibuet & Olu-Le Roy, 2002; Cordes et al., 2010). Thus, the reduced JTC colony diversity as compared to other seep ecosystems is likely due to the low fluid flow and not an effect of water depth. Contrastingly, the faunal abundance and diversity in non-seep sediments decrease with depth (Rex et al., 2006; Wei et al., 2010) as they rely on the organic carbon input from the photic zone (Smith et al., 2008). Deep cold seep communities (e.g., this study, Kobayashi, 2002; Arakawa et al., 2005) might therefore even influence and nourish the surrounding benthic communities at hadal depth, maintaining an active and biomass-rich benthos such as that found at continental slopes (Boetius & Wenzhöfer, 2013, and references therein).
Benthic microbial community in a low seepage, abyssal clam habitat
We demonstrated the presence of an active microbial community at the JTC colony that couples SR to AOM by biogeochemical measurements, lipid analyses, and 16S rRNA gene analyses. In the sediment below the clams, the lipid analyses revealed diagnostic biomarkers for AOM-specific archaeal and bacterial groups. The depth trend of IPL concentrations indicated an increase of prokaryotic cell abundances at the depth of the geochemical reaction zone, where sulfate and methane were metabolized (Fig. 5). Archaeol-based IPLs such as PI- and PG-OH-AR and high ratios of OH-Ar vs. Ar lipids strongly suggested a predominance of ANME-2 archaea in the center of the JTC colonly (Niemann & Elvert, 2008; Rossel et al., 2008, 2011). The gene library results indicated that the dominant clade for anaerobic methane oxidation was ANME-2c, which is supported by previous findings (Vossmeyer et al., 2012). ANME-2c seems to preferentially occur in sediments bioirrigated by clams, for example at Hydrate Ridge in the Northeast Pacific and the REGAB pockmark in the Kongo Basin (Elvert et al., 2005; Knittel et al., 2005; Pop Ristova et al., 2012), and in low fluid flux regimes (Elvert et al., 2005; Wegener et al., 2008). The ANME-2c organisms that we found were closely related to those of other seeps worldwide, indicating a global distribution. Hence, the environmental niche of ANME-2c seemed to be determined by bioturbation and low methane seepage, rather than water depth and geographic location.
In contrast to other studies (Knittel et al., 2003; Cambon-Bonavita et al., 2009), we did not detect ANME-1 or ANME-3 in sediments below vesicomyids (Fig. 7A). The absence of ANME-1 at the JTC colony was previously observed (Vossmeyer et al., 2012) and might be due to the environmental requirements of this organism. The upper sediment at the JTC colony was light brown (Fig. 2) and pyrite was absent, suggesting oxygenation due to faunal activity. ANME-1 seemed to be oxygen sensitive, because they were absent at bioirrigated seeps of Hikurangi margin (Ruff et al., 2013) and increased with sediment depth and decreasing sediment irrigation at clam colonies (Knittel et al., 2005; Rossel et al., 2011). Additionally, ANME-1 appeared to be more sensitive to cold temperatures than ANME-2 (Rossel et al., 2011). In contrast to our findings, the presence of ANME-3 in the JTC sediments was reported previously based on T-RFLP using Hha1 (Vossmeyer et al., 2012). However, ANME-3 and Methanococcoides spp. can not be distinguished with this method because they are closely related and include organisms that have the same restriction site for this enzyme (not shown).
The presence of sulfate-reducing Deltaproteobacteria, which include the partner SRB of ANME, was shown by the 13C-depleted IPL-derived bacterial lipids and the high amounts of MAGE (Hinrichs et al., 2000; Teske et al., 2002; Niemann & Elvert, 2008) in the center of the JTC colony. Remarkably, we did not detect sequences of the SEEP-SRB1 or SEEP-SRB-2 clades (Fig. 7C), which are typically the syntrophic partner SRB of ANME-2 archaea (Schreiber et al., 2010; Kleindienst et al., 2012). Instead, we found many sequences of the clades SEEP-SRB-3 and SEEP-SRB-4 within the Desulfobulbaceae (Fig. 7C). These clades occured as single cells in surface sediments of bacterial mat- or clam-covered seeps (Knittel et al., 2003; Kleindienst et al., 2012) and decreased with increasing sediment depth (Knittel et al., 2003). SEEP-SRB-3 were also found at a clam colony in the Nankai trough (Li et al., 1999a) and both clades occurred in bioirrigated seeps at Hikurangi margin (Ruff et al., 2013). Hence, SEEP-SRB-3 and SEEP-SRB-4 might have an advantage over other SRB clades in bioturbated and thus oxygenated sediments. The occurrence of ANME-2c without their partner SRB indicated that ANME-2c organisms were either associated to other SRBs, or occurred as aggregates or single cells without direct contact to SRBs (Knittel & Boetius, 2009), or performed AOM without a partner SRB (Milucka et al., 2012).
Unexpectedly, aerobic methylotrophic bacteria were not detected in the sediment of the JTC colony, although they are widespread in methane-rich ecosystems, especially in disturbed or bioirrigated cold seep sediments (Inagaki et al., 2004b; Lösekann et al., 2007; Tavormina et al., 2008; Ruff et al., 2013) and contribute significantly to the benthic methane and oxygen consumption (Felden et al., 2010, 2013; Boetius & Wenzhöfer, 2013). Benthic oxygen consumption at the JTC colony was in the same range as sulfide production, which also indicated that aerobic methane oxidation was low or absent. In contrast, at the REGAB clam colonies benthic oxygen consumption was up to three orders of magnitude higher than sulfide production rates (Decker et al., 2012; Pop Ristova et al., 2012), which was assigned to aerobic methanotrophy (Pop Ristova et al., 2012).
Sulfide oxidation at the JTC colony seemed to be performed not only by the chemosynthetic vesicomyids, but also by Sulfurovum spp., which are sulfur oxidizers that were first isolated from hydrothermal vent sediments of the mid-Okinawa Trough (Inagaki et al., 2004a). Although we cannot exclude that elemental sulfur was present in the JTC colony sediment, their occurrence indicated that Sulfurovum organisms are also able to oxidize sulfide (Li et al., 1999a,b; Inagaki et al., 2002; Fang et al., 2006). Thiotrichales and Arcobacter spp., which are sulfur-oxidizing bacteria commonly detected at cold seeps (Omoregie et al., 2008; Grünke et al., 2011, 2012), did not seem to be important at the JTC colony.
The microbial community at the rim of the JTC colony differed greatly from the one at the center, despite a distance of only 30 cm and mirrored the sharp biogeochemical gradients and defined ecosystem boundaries. The sediment at the rim of the colony was dominated by psychrophilic Gammaproteobacteria and comprised clades that are common to deep-sea sediments, such as Thaumarchaeota (Durbin & Teske, 2011). However, sequ-ences of the Marine Benthic Group B, Desulfobacterales, ANME-2c, and Methanococcoides, which occur in methane-rich subsurface sediments (Biddle et al., 2006; Inagaki et al., 2006), were also found indicating that methane was at least occasionally present, Nevertheless, there was little community overlap between the sediments on species-level (98% 16S rRNA gene identity; Fig. 7A–C; Figs S1 and S2), corroborating distinct differences between these seafloor habitats.
Conclusion
In contrast to non-seep systems, a correlation of biodiversity and biomass with water depth was, so far, not found for methane seeps. However, piezophilic adaptations of the methane-oxidizing microbial community remain speculative, as only a few studies have been conducted below 5000 m water depth. Our investigation includes the first in-depth analysis of the microbial community structure and activity at an abyssal seep site. We could show that an abyssal clam colony in the Japan Trench was similar to the ones found at shallower depths, concerning the predominant biogeochemical processes, such as AOM, SR, and benthic oxygen consumption. The tight coupling of AOM and SR rates indicated that abyssal benthic methane filters are as efficient as those of shallow seeps. Our findings suggested that the environmental niche of the dominant ANME archaea and sulfate reducers may be determined by bioturbation of the clams and low methane seepage rather than by pressure or geographic location. However, other key functional populations, such as thiotrophs, differed from those found at shallow clam seeps, or seemed to be absent, indicating environmental filtering due to the extreme environment or dispersal limitation. We show that advances in deep-sea technology (Boetius & Wenzhöfer, 2013 and references therein) finally enable us to improve our limited knowledge about the biogeochemistry and microbiology of abyssal methane seeps, which occur frequently along deep-sea trenches and faults with active fluid flow (Sibuet & Olu, 1998; Tyler et al., 2002; Judd, 2003) and could be significant for the marine methane budget.
Acknowledgments
We thank the members of the shipboard crew of the RV Yokosuka, the Shinkai 6500 team, and the shipboard scientific party for the excellent support during cruise YK06-05. We are grateful for the technical support from Martina Alisch, Viola Beier, Xavier Prieto Mollar, Gabriele Schüßler, and especially Gabriele Eickert. Special thanks to the ‘SeaTechs’ Axel Nordhausen, Marc Viehweger, Patrick Meyer, and Volker Asendorf for building and maintaining the in situ instruments. We thank Antje Boetius and Katrin Knittel for very helpful discussions during the preparation of the manuscript. This work was performed in the framework of the GEOTECHNOLOGIEN project MUMM II (03G0608C) funded by the German Ministry of Education and Research (BMBF) and German Research Foundation (DFG) as well as the Max Planck Society. The work of S.E. Ruff was supported by the Leibniz program of the DFG to Antje Boetius.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Phylogenetic affiliation of Bacteroidetes in JTC colony center (red) and colony rim (blue) sediments based on 16S rRNA gene sequences. The scale bar represents 10% estimated sequence divergence.
Phylogenetic affiliation of all other bacterial sequences retrieved from JTC colony center (red) and colony rim (blue) sediments based on 16S rRNA gene sequences. The scale bar represents 10% estimated sequence divergence.
References
- Arakawa S, Mori M, Nogi Y, Sato T, Yoshida Y, Usami R, Kato C. Cold-seep microbial communities are more abundant at deeper depths in the Japan Trench land slope. Journal of Japanese Society for Extremophiles. 2005;4:50–55. [Google Scholar]
- Ashelford KE, Chuzhanova NA, Fry JC, Jones AJ, Weightman AJ. New screening software shows that most recent large 16S rRNA gene clone libraries contain chimeras. Applied and Environment Microbiology. 2006;72:5734–5741. doi: 10.1128/AEM.00556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JP, Kochevar RE. A tale of two clams: differing chemosynthetic life styles among vesicomyids in Monterey Bay cold seeps. Cahiers de Biologie Marine. 1998;39:329–331. [Google Scholar]
- Barry JP, Kochevar RE, Baxter CH. The influence of pore-water chemistry and physiology on the distribution of vesicomyid clams at cold seeps in Monterey Bay: implications for patterns of chemosynthetic community organization. Limnology and Oceanography. 1997;42:318–328. [Google Scholar]
- Biddle JF, Lipp JS, Lever MA, Lloyd KG, Sorensen KB, Anderson R, Fredricks HF, Elvert M, Kelly TJ, Schrag DP, Sogin ML, Brenchley JE, Teske A, House CH, Hinrichs KU. Heterotrophic Archaea dominate sedimentary subsurface ecosystems off Peru. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3846–3851. doi: 10.1073/pnas.0600035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boetius A, Suess E. Hydrate Ridge: a natural laboratory for the study of microbial life fueled by methane from near-surface gas hydrates. Chemical Geology. 2004;205:291–310. [Google Scholar]
- Boetius A, Wenzhöfer F. Seafloor oxygen consumption fuelled by methane from cold seeps. Nature Geoscience. 2013;6:725–734. [Google Scholar]
- Boetius A, Ravenschlag K, Schubert CJ, Rickert D, Widdel F, Gieseke A, Amann R, Jørgensen BB, Witte U, Pfannkuche O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature. 2000;407:623–626. doi: 10.1038/35036572. [DOI] [PubMed] [Google Scholar]
- Boetius A, Holler T, Knittel K, Felden J, Wenzhöfer F. The seabed as natural laboratory: lessons from uncultivated methanotrophs. In: Epstein S, editor. Uncultivated Microorganisms. Berlin/Heidelberg: Springer; 2009. pp. 59–82. [Google Scholar]
- Cambon-Bonavita MA, Nadalig T, Roussel E, Delage E, Duperron S, Caprais JC, Boetius A, Sibuet M. Diversity and distribution of methane-oxidizing microbial communities associated with different faunal assemblages inagiant pockmark of the Gabon continental margin. Deep-Sea Research Part II. 2009;56:2248–2258. [Google Scholar]
- Childress JJ, Fisher CR, Favuzzi JA, Arp AJ, Oros DR. The role of a zinc-based, serum-borne sulfide-binding component in the uptake and transport of dissolved sulfide by the chemoautotrophic symbiont-containing clam Calyptogena Clongata. Journal of Experimental Biology. 1993;179:131–158. [Google Scholar]
- Cordes EE, Arthur MA, Shea K, Arvidson RS, Fisher CR. Modeling the mutualistic interactions between tubeworms and microbial consortia. PLoS Biology. 2005;3:497–506. doi: 10.1371/journal.pbio.0030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes EE, Cunha MR, Galeron J, Mora C, Olu-Le Roy K, Sibuet M, Van Gaever S, Vanreusel A, Levin LA. The influence of geological, geochemical, and biogenic habitat heterogeneity on seep biodiversity. Marine Ecology. 2010;31:51–65. [Google Scholar]
- De Beer D, Sauter E, Niemann H, Kaul N, Foucher JP, Witte U, Schlüter M, Boetius A. In situ fluxes and zonation of microbial activity in surface sediments of the Håkon Mosby mud volcano. Limnology and Oceanography. 2006;51:1315–1331. [Google Scholar]
- Decker C, Caprais JC, Khripounoff A, Olu K. First respiration estimates of cold-seep vesicomyid bivalves from in situ total oxygen uptake measurements. Comptes Rendus Biologies. 2012;335:261–270. doi: 10.1016/j.crvi.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Dubilier N, Bergin C, Lott C. Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nature Reviews Microbiology. 2008;6:725–740. doi: 10.1038/nrmicro1992. [DOI] [PubMed] [Google Scholar]
- Durbin AM, Teske A. Microbial diversity and stratification of South Pacific abyssal marine sediments. Environmental Microbiology. 2011;13:3219–3234. doi: 10.1111/j.1462-2920.2011.02544.x. [DOI] [PubMed] [Google Scholar]
- Elvert M, Suess E, Greinert J, Whiticar MJ. Archaea mediating anaerobic methane oxidation in deep-sea sediments at cold seeps of the eastern Aleutian subduction zone. Organic Geochemistry. 2000;31:1175–1187. [Google Scholar]
- Elvert M, Boetius A, Knittel K, Jørgensen BB. Characterization of specific membrane fatty acids as chemotaxonomic markers for sulfate-reducing bacteria involved in anaerobic oxidation of methane. Geomicrobiology Journal. 2003;20:403–419. [Google Scholar]
- Elvert M, Hopmans EC, Treude T, Boetius A, Suess E. Spatial variations of methanotrophic consortia at cold methane seeps: implications from a high-resolution molecular and isotopic approach. Geobiology. 2005;3:195–209. [Google Scholar]
- Ertefai TF, Fisher MC, Fredricks HF, Lipp JS, Pearson A, Birgel D, Udert KM, Cavanaugh CM, Gschwend PM, Hinrichs KU. Vertical distribution of microbial lipids and functional genes in chemically distinct layers of a highly polluted meromictic lake. Organic Geochemistry. 2008;39:1572–1588. [Google Scholar]
- Ertefai TF, Heuer VB, Prieto-Mollar X, Vogt C, Sylva SP, Seewald J, Hinrichs KU. The biogeochemistry of sorbed methane in marine sediments. Geochimica et Cosmochimica Acta. 2010;74:6033–6048. [Google Scholar]
- Fang JS, Shizuka A, Kato C, Schouten S. Microbial diversity of cold-seep sediments in Sagami Bay, Japan, as determined by 16S rRNA gene and lipid analyses. FEMS Microbiology Ecology. 2006;57:429–441. doi: 10.1111/j.1574-6941.2006.00126.x. [DOI] [PubMed] [Google Scholar]
- Felden J, Wenzhöfer F, Feseker T, Boetius A. Transport and consumption of oxygen and methane in different habitats of the Håkon Mosby Mud Volcano (HMMV) Limnology and Oceanography. 2010;55:2366–2380. [Google Scholar]
- Felden J, Lichtschlag A, Wenzhofer F, De Beer D, Feseker T, Ristova PP, De Lange G, Boetius A. Limitations of microbial hydrocarbon degradation at the Amon mud volcano (Nile deep-sea fan) Biogeosciences. 2013;10:3269–3283. [Google Scholar]
- Feseker T, Foucher JP, Harmegnies F. Fluid flow or mud eruptions? Sediment temperature distributions on Håkon Mosby mud volcano, SW Barents Sea slope. Marine Geology. 2008;247:194–207. [Google Scholar]
- Fischer D, Sahling H, Nothen K, Bohrmann G, Zabel M, Kasten S. Interaction between hydrocarbon seepage, chemosynthetic communities, and bottom water redox at cold seeps of the Makran accretionary prism: insights from habitat-specific pore water sampling and modeling. Biogeosciences. 2012;9:2013–2031. [Google Scholar]
- Fujikura K, Kojima S, Tamaki K, Maki Y, Hunt J, Okutani T. The deepest chemosynthesis-based community yet discovered from the hadal zone, 7326 m deep, in the Japan Trench. Marine Ecology Progress Series. 1999;190:17–26. [Google Scholar]
- Glud RN. Oxygen dynamics of marine sediments. Marine Biology Research. 2008;4:243–289. [Google Scholar]
- Goffredi SK, Barry JP. Species-specific variation in sulfide physiology between closely related Vesicomyid clams. Marine Ecology Progress Series. 2002;225:227–238. [Google Scholar]
- Grünke S, Felden J, Lichtschlag A, Girnth AC, De Beer D, Wenzhöfer F, Boetius A. Niche differentiation among mat-forming, sulfide-oxidizing bacteria at cold seeps of the Nile Deep Sea Fan (Eastern Mediterranean Sea) Geobiology. 2011;9:330–348. doi: 10.1111/j.1472-4669.2011.00281.x. [DOI] [PubMed] [Google Scholar]
- Grünke S, Lichtschlag A, De Beer D, Felden J, Salman V, Ramette A, Schulz-Vogt HN, Boetius A. Mats of psychrophilic thiotrophic bacteria associated with cold seeps of the Barents Sea. Biogeosciences. 2012;9:2947–2960. [Google Scholar]
- Hall PO, Aller RC. Rapid, small-volume, flow-Injection analysis for Sigma-CO2 and NH4+ in marine and fresh-waters. Limnology and Oceanography. 1992;37:1113–1119. [Google Scholar]
- Haroon MF, Hu SH, Shi Y, Imelfort M, Keller J, Hugenholtz P, Yuan ZG, Tyson GW. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature. 2013;500:567–570. doi: 10.1038/nature12375. [DOI] [PubMed] [Google Scholar]
- Hinrichs KU, Hayes JM, Sylva SP, Brewer PG, Delong EF. Methane-consuming archaebacteria in marine sediments. Nature. 1999;398:802–805. doi: 10.1038/19751. [DOI] [PubMed] [Google Scholar]
- Hinrichs KU, Summons RE, Orphan V, Sylva SP, Hayes JM. Molecular and isotopic analysis of anaerobic methane-oxidizing communities in marine sediments. Organic Geochemistry. 2000;31:1685–1701. [Google Scholar]
- Holler T, Widdel F, Knittel K, Amann R, Kellermann MY, Hinrichs K-U, Teske A, Boetius A, Wegner G. Thermophilic anaerobic oxidation of methane by marine microbial consortian. ISME Journal. 2011;5:1946–1956. doi: 10.1038/ismej.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki F, Sakihama Y, Inoue A, Kato C, Horikoshi K. Molecular phylogenetic analyses of reverse-transcribed bacterial rRNA obtained from deep-sea cold seep sediments. Environmental Microbiology. 2002;4:277–286. doi: 10.1046/j.1462-2920.2002.00294.x. [DOI] [PubMed] [Google Scholar]
- Inagaki F, Takai K, Nealson KH, Horikoshi K. Sulfurovum lithotrophicum gen. nov., sp nov., a novel sulfur-oxidizing chemolithoautotroph within the epsilon-Proteobacteria isolated from Okinawa Trough hydrothermal sediments. International Journal of Systematic and Evolutionary Microbiology. 2004a;54:1477–1482. doi: 10.1099/ijs.0.03042-0. [DOI] [PubMed] [Google Scholar]
- Inagaki F, Tsunogai U, Suzuki M, Kosaka A, Machiyama H, Takai K, Nunoura T, Nealson KH, Horikoshi K. Characterization of C1-metabolizing prokaryotic communities in methane seep habitats at the Kuroshima Knoll, Southern Ryukyu Arc, by analyzing pmoA mmoX mxaF mcrA, and 16S rRNA genes. Applied and Environment Microbiology. 2004b;70:7445–7455. doi: 10.1128/AEM.70.12.7445-7455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki F, Nunoura T, Nakagawa S, Teske A, Lever M, Lauer A, Suzuki M, Takai K, Delwiche M, Colwell FS, Nealson KH, Horikoshi K, D'hondt S, Jørgensen BB. Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments, on the Pacific Ocean Margin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2815–2820. doi: 10.1073/pnas.0511033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen BB. A comparison of methods for the quantification of bacterial sulfate reductin in coastal marine sediments I. Measurements with radiotracer techniques. Geomicrobiology Journal. 1978;1:11–27. [Google Scholar]
- Jørgensen BB, Boetius A. Feast and famine – microbial life in the deep-sea bed. Nature Reviews Microbiology. 2007;5:770–781. doi: 10.1038/nrmicro1745. [DOI] [PubMed] [Google Scholar]
- Judd AG. The global importance and context of methane escape from the seabed. Geo-Marine Letters. 2003;23:147–154. [Google Scholar]
- Juniper SK, Sibuet M. Cold seep benthic communities in Japan subduction zones – spatial-organization, trophic strategies and evidence for temporal evolution. Marine Ecology Progress Series. 1987;40:115–126. [Google Scholar]
- Kallmeyer J, Ferdelman TG, Weber A, Fossing H, Jørgensen BB. A cold chromium distillation procedure for radiolabeled sulfide applied to sulfate reduction measurements. Limnology and Oceanography, Methods. 2004;2:171–180. [Google Scholar]
- Kawagucci S, Yoshida YT, Noguchi T, Honda MC, Uchida H, Ishibashi H, Nakagawa F, Tsunogai U, Okamura K, Takaki Y, Nunoura T, Miyazaki J, Hirai M, Lin WR, Kitazato H, Takai K. Disturbance of deep-sea environments induced by the M9.0 Tohoku Earthquake. Scientific Reports. 2012;2:1–6. doi: 10.1038/srep00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst S, Ramette A, Amann R, Knittel K. Distribution and in situ abundance of sulfate-reducing bacteria in diverse marine hydrocarbon seep sediments. Environmental Microbiology. 2012;14:2689–2710. doi: 10.1111/j.1462-2920.2012.02832.x. [DOI] [PubMed] [Google Scholar]
- Knittel K, Boetius A. Anaerobic oxidation of methane: progress with an unknown process. Annual Review of Microbiology. 2009;63:311–314. doi: 10.1146/annurev.micro.61.080706.093130. [DOI] [PubMed] [Google Scholar]
- Knittel K, Boetius A, Lemke A, Eilers H, Lochte K, Pfannkuche O, Linke P, Amann R. Activity, distribution, and diversity of sulfate reducers and other bacteria in sediments above gas hydrate (Cascadia margin, Oregon) Geomicrobiology Journal. 2003;20:269–294. [Google Scholar]
- Knittel K, Lösekann T, Boetius A, Kort R, Amann R. Diversity and distribution of methanotrophic archaea at cold seeps. Applied and Environment Microbiology. 2005;71:467–479. doi: 10.1128/AEM.71.1.467-479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K. Tectonic significance of the cold seepage zones in the eastern Nankai accretionary wedge – an outcome of the 15 years’ KAIKO projects. Marine Geology. 2002;187:3–30. [Google Scholar]
- Krylova EM, Sahling H. Vesicomyidae (Bivalvia): current taxonomy and distribution. PLoS One. 2010;5:107–132. doi: 10.1371/journal.pone.0009957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvenvolden KA, McDonald TJ. Organic geochemistry on the JOIDES Resolution – an essay 6. ODP Technology Note. 1986.
- Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR. Rapid-determination of 16S ribosomal-RNA sequences for phylogenetic analyses. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin LA. Ecology of cold seep sediments: interactions of fauna with flow, chemistry and microbes. In: RN Gibson, RJA Atkinson, JDM Gordon., editors. Oceanography and Marine Biology – An Annual Review. London, UK: Taylor & Francis Group; 2005. pp. 1–46. [Google Scholar]
- Levin LA, Ziebis W, Mendoza GF, Growney VA, Tryon MD, Brown KM, Mahn C, Gieskes JM, Rathburn AE. Spatial heterogeneity of macrofauna at northern California methane seeps: influence of sulfide concentration and fluid flow. Marine Ecology Progress Series. 2003;265:123–139. [Google Scholar]
- Li YH, Gregory S. Diffusion of ions in sea-water and in deep-sea sediments. Geochimica et Cosmochimica Acta. 1974;38:703–714. [Google Scholar]
- Li L, Guenzennec J, Nichols P, Henry P, Yanagibayashi M, Kato C. Microbial Diversity in Nankai Trough Sediments at a Depth of 3,843 m. Journal of Oceanography. 1999a;55:635–642. [Google Scholar]
- Li L, Kato C, Horikoshi K. Microbial diversity in sediments collected from the deepest cold-seep area, the Japan Trench. Marine Biotechnology. 1999b;1:391–400. doi: 10.1007/pl00011793. [DOI] [PubMed] [Google Scholar]
- Lichtschlag A, Felden J, Brüchert V, Boetius A, De Beer D. Geochemical processes and chemosynthetic primary production in different thiotrophic mats of the Håkon Mosby Mud Volcano (Barents Sea) Limnology and Oceanography. 2010;55:931–949. [Google Scholar]
- Lösekann T, Knittel K, Nadalig T, Fuchs B, Niemann H, Boetius A, Amann R. Diversity and abundance of aerobic and anaerobic methane oxidizers at the Håkon Mosby mud volcano, Barents Sea. Applied and Environment Microbiology. 2007;73:3348–3362. doi: 10.1128/AEM.00016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar Buchner A, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH. ARB: a software environment for sequence data. Nucleic Acids Research. 2004;32:1363–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massana R, Murray AE, Preston CM, Delong EF. Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara Channel. Applied and Environment Microbiology. 1997;63:50–56. doi: 10.1128/aem.63.1.50-56.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milucka J, Ferdelman TG, Polerecky L, Franzke D, Wegener G, Schmid M, Lieberwirth I, Wagner M, Widdel F, Kuypers MMM. Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature. 2012;491:541–546. doi: 10.1038/nature11656. [DOI] [PubMed] [Google Scholar]
- Muyzer G, Teske A, Wirsen CO, Jannasch HW. Phylogenetic-relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel-electrophoresis of 16S rDNA fragments. Archives of Microbiology. 1995;164:165–172. doi: 10.1007/BF02529967. [DOI] [PubMed] [Google Scholar]
- Niemann H, Elvert M. Diagnostic lipid biomarker and stable carbon isotope signatures of microbial communities mediating the anaerobic oxidation of methane with sulphate. Organic Geochemistry. 2008;39:1668–1677. [Google Scholar]
- Niemann H, Duarte J, Hensen C, Omoregie E, Magalhaes VH, Elvert M, Pinheiro LM, Kopf A, Boetius A. Microbial methane turnover at mud volcanoes of the Gulf of Cadiz. Geochimica et Cosmochimica Acta. 2006a;70:5336–5355. [Google Scholar]
- Niemann H, Lösekann T, De Beer D, Elvert M, Nadalig T, Knittel K, Amann R, Sauter EJ, Schluter M, Klages M, Foucher JP, Boetius A. Novel microbial communities of the Håkon Mosby mud volcano and their role as a methane sink. Nature. 2006b;443:854–858. doi: 10.1038/nature05227. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Fujioka K, Fujikura K, Iwabuchi Y. En echelon patterns of Calyptogena colonies in the Japan trench. Geology. 1996;24:807–810. [Google Scholar]
- Olu K, Duperret A, Sibuet M, Foucher JP, Fialamedioni A. Structure and distribution of cold seep communities along the Peruvian active margin: relationship to geological and fluid patterns. Marine Ecology Progress Series. 1996;132:109–125. [Google Scholar]
- Omoregie EO, Mastalerz V, De Lange G, Straub KL, Kappler A, Roy H, Stadnitskaia A, Foucher JP, Boetius A. Biogeochemistry and community composition of iron- and sulfur-precipitating microbial mats at the Chefren mud volcano (Nile Deep Sea fan, Eastern Mediterranean) Applied and Environment Microbiology. 2008;74:3198–3215. doi: 10.1128/AEM.01751-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orphan VJ, Hinrichs KU, Ussler W, Paull CK, Taylor LT, Sylva SP, Hayes JM, Delong EF. Comparative analysis of methane-oxidizing archaea and sulfate-reducing bacteria in anoxic marine sediments. Applied and Environment Microbiology. 2001;67:1922–1934. doi: 10.1128/AEM.67.4.1922-1934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull CK, Schlining B, Ussler W, Paduan JB, Caress D, Greene HG. Distribution of chemosynthetic biological communities in Monterey Bay, California. Geology. 2005;33:85–88. [Google Scholar]
- Pernthaler A, Dekas AE, Brown CT, Goffredi SK, Embaye T, Orphan VJ. Diverse syntrophic partnerships from deep-sea methane vents revealed by direct cell capture and metagenomics. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7052–7057. doi: 10.1073/pnas.0711303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop Ristova P, Wenzhöfer F, Ramette A, Zabel M, Fischer D, Kasten S, Boetius A. Bacterial diversity and biogeochemistry of different chemosynthetic habitats of the REGAB cold seep (West African margin, 3160 m water depth) Biogeosciences. 2012;9:5031–5048. [Google Scholar]
- Prüsse E, Quast C, Knittel K, Fuchs BM, Ludwig WG, Peplies J, Glöckner FO. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Research. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Research. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H, Jørgensen BB. Microelectrode studies of seasonal oxygen-uptake in a coastal sediment – role of molecular-diffusion. Marine Ecology Progress Series. 1992;81:289–303. [Google Scholar]
- Reeburgh WS. An improved interstitial water sampler. Limnology and Oceanography. 1967;12:163–165. [Google Scholar]
- Revsbech NP, Jørgensen BB, Blackburn TH, Cohen Y. Microelectrode studies of the photosynthesis and O2, H2S, and pH profiles of a microbial mat. Limnology and Oceanography. 1983;28:1062–1074. [Google Scholar]
- Rex MA, Etter RJ, Morris JS, Crouse J, Mcclain CR, Johnson NA, Stuart CT, Deming JW, Thies R, Avery R. Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Marine Ecology Progress Series. 2006;317:1–8. [Google Scholar]
- Rossel PE, Lipp JS, Fredricks HF, Arnds J, Boetius A, Elvert M, Hinrichs KU. Intact polar lipids of anaerobic methanotrophic archaea and associated bacteria. Organic Geochemistry. 2008;39:992–999. [Google Scholar]
- Rossel PE, Elvert M, Ramette A, Boetius A, Hinrichs KU. Factors controlling the distribution of anaerobic methanotrophic communities in marine environments: evidence from intact polar membrane lipids. Geochimica et Cosmochimica Acta. 2011;75:164–184. [Google Scholar]
- Ruff SE, Arnds J, Knittel K, Amann R, Wegener G, Ramette A, Boetius A. Microbial communities of deep-sea methane seeps at Hikurangi continental margin (New Zealand) PLoS One. 2013;8:e72627. doi: 10.1371/journal.pone.0072627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environment Microbiology. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L, Holler T, Knittel K, Meyerdierks A, Amann R. Identification of the dominant sulfate-reducing bacterial partner of anaerobic methanotrophs of the ANME-2 clade. Environmental Microbiology. 2010;12:2327–2340. doi: 10.1111/j.1462-2920.2010.02275.x. [DOI] [PubMed] [Google Scholar]
- Sibuet M, Olu K. Biogeography, biodiversity and fluid dependence of deep-sea cold-seep communities at active and passive margins. Deep-Sea Research Part II. 1998;45:517–567. [Google Scholar]
- Sibuet M, Olu-Le Roy K. Cold seep communities on continental margins: structure and quantitative distribution relative to geological and fluid venting patterns. In: Wefer G, Billett D, Hebbeln D, Jørgensen BB, Schlüter M, Van Weering TCE, editors. Ocean Margin System. Berlin-Heidelberg: Springer-Verlag; 2002. pp. 235–251. [Google Scholar]
- Sibuet M, Juniper SK, Pautot G. Cold-seep benthic communities in the Japan subduction zones – geological control of community-development. Journal of Marine Research. 1988;46:333–348. [Google Scholar]
- Smith CR, De Leo FC, Bernardino AF, Sweetman AK, Arbizu PM. Abyssal food limitation, ecosystem structure and climate change. Trends in Ecology & Evolution. 2008;23:518–528. doi: 10.1016/j.tree.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Sommer S, Pfannkuche O, Linke P, Luff R, Greinert J, Drews M, Gubsch S, Pieper M, Poser M, Viergutz T. Efficiency of the benthic filter: biological control of the emission of dissolved methane from sediments containing shallow gas hydrates at Hydrate Ridge. Global Biogeochemical Cycles. 2006;20:GB2019. [Google Scholar]
- Sturt HF, Summons RE, Smith K, Elvert M, Hinrichs K-U. Intact polar membrane lipids in prokaryotes and sediments deciphered by high-performance liquid chromatography/electrospray ionization multistage mass spectrometry—new biomarkers for biogeochemistry and microbial ecology. Rapid Communications in Mass Spectrometry. 2004;18:617–628. doi: 10.1002/rcm.1378. [DOI] [PubMed] [Google Scholar]
- Tavormina PL, Ussler W, Orphan VJ. Planktonic and sediment-associated aerobic methanotrophs in two seep systems along the North American margin. Applied and Environment Microbiology. 2008;74:3985–3995. doi: 10.1128/AEM.00069-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske A, Hinrichs KU, Edgcomb V, Gomez AD, Kysela D, Sylva SP, Sogin ML, Jannasch HW. Microbial diversity of hydrothermal sediments in the Guaymas Basin: evidence for anaerobic methanotrophic communities. Applied and Environment Microbiology. 2002;68:1994–2007. doi: 10.1128/AEM.68.4.1994-2007.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres ME, Mcmanus J, Hammond DE, De Angelis MA, Heeschen KU, Colbert SL, Tryon MD, Brown KM, Suess E. Fluid and chemical fluxes in and out of sediments hosting methane hydrate deposits on Hydrate Ridge, OR, I: hydrological provinces. Earth and Planetary Science Letters. 2002;201:525–540. [Google Scholar]
- Treude T, Boetius A, Knittel K, Wallmann K, Jørgensen BB. Anaerobic oxidation of methane above gas hydrates at Hydrate Ridge, NE Pacific Ocean. Marine Ecology Progress Series. 2003;264:1–14. [Google Scholar]
- Treude T, Smith CR, Wenzhöfer F, Carney E, Bernardino AF, Hannides AK, Krüger M, Boetius A. Biogeochemistry of a deep-sea whale fall: sulfate reduction, sulfide efflux and methanogenesis. Marine Ecology Progress Series. 2009;382:1–21. [Google Scholar]
- Tyler PA, German CR, Ramirez-Llodra E, Van Dover CL. Understanding the biogeography of chemosynthetic ecosystems. Oceanologica Acta. 2002;25:227–241. [Google Scholar]
- Vossmeyer A, Deusner C, Kato C, Inagaki F, Ferdelman T. Substrate-specific pressure dependence of microbial sulfate reduction in deep-sea cold seep sediments of the Japan Trench. Frontiers in Microbiology. 2012;3:1–12. doi: 10.3389/fmicb.2012.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallmann K, Linke P, Suess E, Bohrmann G, Sahling H, Schlüter M, Dahlmann A, Lammers S, Greinert J, Von Mirbach N. Quantifying fluid flow, solute mixing, and biogeochemical turnover at cold vents of the eastern Aleutian subduction zone. Geochimica et Cosmochimica Acta. 1997;61:5209–5219. [Google Scholar]
- Wegener G, Niemann H, Elvert M, Hinrichs K-U, Boetius A. Assimilation of methane and inorganic carbon by microbial communities mediating the anaerobic oxidation of methane. Environmental Microbiology. 2008;10:2287–2298. doi: 10.1111/j.1462-2920.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- Wei CL, Rowe GT, Escobar-Briones E, Boetius A, Soltwedel T, Caley MJ, Soliman Y, Huettmann F, Qu FY, Yu ZS, Pitcher CR, Haedrich RL, Wicksten MK, Rex MA, Baguley JG, Sharma J, Danovaro R, Macdonald IR, Nunnally CC, Deming JW, Montagna P, Levesque M, Weslawski JM, Wlodarska-Kowalczuk M, Ingole BS, Bett BJ, Billett DSM, Yool A, Bluhm BA, Iken K, Narayanaswamy BE. Global patterns and predictions of seafloor biomass using random forests. PLoS One. 2010;5:e15323. doi: 10.1371/journal.pone.0015323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzhöfer F, Glud RN. Benthic carbon mineralization in the Atlantic: a synthesis based on in situ data from the last decade. Deep-Sea Research Part I. 2002;49:1255–1279. [Google Scholar]
- Wenzhöfer F, Holby O, Glud RN, Nielsen HK, Gundersen JK. In situ microsensor studies of a shallow water hydrothermal vent at Milos, Greece. Marine Chemistry. 2000;69:43–54. [Google Scholar]
- Whiticar MJ. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chemical Geology. 1999;161:291–314. [Google Scholar]
- Zhou JZ, Bruns MA, Tiedje JM. DNA recovery from soils of diverse composition. Applied and Environment Microbiology. 1996;62:316–322. doi: 10.1128/aem.62.2.316-322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic affiliation of Bacteroidetes in JTC colony center (red) and colony rim (blue) sediments based on 16S rRNA gene sequences. The scale bar represents 10% estimated sequence divergence.
Phylogenetic affiliation of all other bacterial sequences retrieved from JTC colony center (red) and colony rim (blue) sediments based on 16S rRNA gene sequences. The scale bar represents 10% estimated sequence divergence.


