Abstract
Aim
The efficacy and safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, was evaluated in patients with type 2 diabetes mellitus (T2DM) inadequately controlled with metformin and pioglitazone.
Methods
In this randomized, double-blind, phase 3 study, patients (N = 342) received canagliflozin 100 or 300 mg during a 26-week, placebo-controlled, core period and a 26-week, active-controlled extension in which placebo-treated patients were switched to sitagliptin 100 mg. Efficacy comparisons for canagliflozin versus placebo at week 26 are reported, with no comparisons versus sitagliptin at week 52 (sitagliptin used to maintain double-blind and control for safety). Safety data are reported for canagliflozin and placebo/sitagliptin.
Results
Canagliflozin 100 and 300 mg significantly lowered haemoglobin A1c (HbA1c) compared with placebo at week 26 (−0.89%, −1.03% and −0.26%; p < 0.001); reductions with canagliflozin 100 and 300 mg were maintained at week 52 (−0.92% and −1.03%). Relative to placebo, both canagliflozin doses significantly reduced body weight (−2.5 and −3.5 kg), fasting plasma glucose and systolic blood pressure (BP) at week 26 (p < 0.05 for all), with reductions maintained at week 52. Overall adverse event (AE) incidence over 52 weeks was 69.9, 76.3 and 76.5% with canagliflozin 100 and 300 mg and placebo/sitagliptin; AE-related discontinuation and serious AE rates were low. Incidences of genital mycotic infections and AEs related to osmotic diuresis and volume depletion were higher with canagliflozin than placebo/sitagliptin.
Conclusion
Canagliflozin improved glycaemic control, reduced body weight and systolic BP, and was generally well tolerated in patients with T2DM on metformin and pioglitazone over 52 weeks.
Keywords: metformin, phase 3 study, SGLT2 inhibitor, thiazolidinediones, type 2 diabetes
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease that often necessitates combination therapies to achieve and maintain effective glycaemic control 1,2. Metformin is the recommended first-line pharmacologic therapy for the management of T2DM 2. For patients who do not achieve and/or maintain glycaemic control on metformin, thiazolidinediones can be added as second agents 1,2. Although thiazolidinediones have been reported to have good durability, these agents can lead to weight gain, fluid retention and increased risk of congestive heart failure; in addition, many patients do not achieve or maintain glycaemic goals with two agents and require additional therapies 2. There remains a need for new, oral antihyperglycaemic agents (AHAs) that can improve glycaemic control as third agent in patients with T2DM with a favourable safety and tolerability profile, in addition to having a positive effect on body weight, an effect that may be particularly desirable when used in combination with a thiazolidinedione.
Canagliflozin is a sodium glucose co-transporter 2 (SGLT2) inhibitor developed for the treatment of adult patients with T2DM 3–10. Canagliflozin decreases blood glucose by lowering the renal threshold for glucose (RTG) and increasing urinary glucose excretion (UGE) 3,11–13, which results in a mild osmotic diuresis and a net caloric loss. The insulin-independent mechanism of action of canagliflozin may allow for additive glucose-lowering effects and promote weight loss when it is combined with other oral AHAs. Across phase 3 studies of adult patients with T2DM on a variety of background AHAs, canagliflozin showed improvement in glycaemic control and reduced body weight and systolic blood pressure (BP), and was generally well tolerated with a low risk of hypoglycaemia 5–10.
This 52-week, phase 3 study evaluated the efficacy and safety of canagliflozin as an add-on therapy in patients with T2DM with inadequate glycaemic control with metformin and pioglitazone. The study consisted of a 26-week, placebo-controlled, core treatment period and a 26-week, active-controlled, extension period, during which patients who received placebo were switched to sitagliptin 100 mg; the blinded switch from placebo to sitagliptin was performed to maintain the study's double-blinding, but not to support an efficacy comparison at week 52 as treatments were not concurrently initiated. Therefore, only descriptive efficacy results are reported for the placebo/sitagliptin group at week 52. Safety findings are reported for both canagliflozin groups and the placebo/sitagliptin group over 52 weeks.
Patients and Methods
Study Design
This randomized, double-blind, placebo- and active-controlled, phase 3 study (ClinicalTrials.gov identifier: NCT01106690) was conducted at 74 centres in 11 countries. The study consisted of a 2-week, single-blind, placebo run-in period; a 26-week, placebo-controlled, double-blind core treatment period followed by a 26-week, active-controlled, double-blind extension period; and a 4-week follow-up period.
Eligible patients were men and women aged ≥18 and ≤80 years with T2DM, fasting plasma glucose (FPG) <15 mmol/l (270 mg/dl) at week −2, and fasting fingerstick glucose ≥6.1 mmol/l (110 mg/dl) and <15 mmol/l (270 mg/dl) on day 1. Patients on protocol-specified doses of metformin [≥2000 mg/day (or 1500 mg/day if unable to tolerate higher dose)] and pioglitazone (30 or 45 mg/day) with haemoglobin A1c (HbA1c) ≥7.0% to ≤10.5% at screening directly entered the placebo run-in period. Patients on other background therapies entered a metformin/pioglitazone dose-titration/dose-stable period of up to 12 weeks; patients with HbA1c ≥7.0% to ≤10.5% on metformin and pioglitazone (at the doses described above) after the dose-titration/dose-stable period then entered the placebo run-in period.
Exclusion criteria included repeated FPG and/or fasting self-monitored blood glucose (SMBG) ≥15.0 mmol/l (270 mg/dl) during the pretreatment phase; history of type 1 diabetes, cardiovascular disease (including myocardial infarction, unstable angina, revascularization procedure or cerebrovascular accident) within 3 months prior to screening, or uncontrolled hypertension; ongoing eating disorder or 5% change in body weight within 12 weeks; and estimated glomerular filtration rate (eGFR) <55 ml/min/1.73 m2 (or <60 ml/min/1.73 m2 if based upon restriction of metformin use in local label) or serum creatinine ≥124 µmol/l for men and ≥115 µmol/l for women.
At the week −2 visit, patients received standard counselling on diet and exercise. Patients were also provided with a glucose meter, testing supplies and testing instructions, and were expected to perform fasting SMBG at least three times per week and record testing results in a protocol-specific diary, which was reviewed by study research staff at each visit.
The study was conducted in accordance with ethical principles that comply with the Declaration of Helsinki, and are consistent with Good Clinical Practices and applicable regulatory requirements. The study protocol and amendments were approved by institutional review boards at participating institutions. All patients provided written informed consent prior to participation.
Randomization and Treatments
During the placebo run-in period, patients received single-blind placebo capsules matching study drug once daily. Patients were randomized via an Interactive Voice Response System/Interactive Web Response System to receive canagliflozin 100 or 300 mg or placebo (1 : 1 : 1) once daily during the 26-week core treatment period. Randomization was balanced using permuted blocks of six patients per block and stratified according to: (i) whether a patient entered the AHA adjustment period and (ii) dose of pioglitazone at randomization. After randomization, HbA1c and FPG values were masked to the study centres unless they met pre-specified glycaemic rescue criteria. After completion of the core treatment period, the database was locked and the study was unblinded by the sponsor for regulatory filing. Patients and study centre and local sponsor personnel remained blinded throughout the extension period.
For patients who entered the extension period, those randomized to canagliflozin 100 or 300 mg continued on these treatments while those randomized to placebo switched to sitagliptin 100 mg in a blinded fashion. During the entire 52-week, double-blind treatment period, glycaemic rescue therapy with glimepiride was initiated if FPG >15.0 mmol/l (270 mg/dl) after day 1 to week 6, >13.3 mmol/l (240 mg/dl) after weeks 6 to 12, and >11.1 mmol/l (200 mg/dl) after weeks 12 to 26, and if HbA1c >8.0% after week 26.
Endpoints and Assessments
The pre-specified primary efficacy endpoint was change from baseline in HbA1c at week 26; change from baseline in HbA1c at week 52 was a secondary efficacy endpoint. Other pre-specified secondary endpoints assessed at weeks 26 and 52 included the proportion of patients reaching HbA1c <7.0%; change from baseline in FPG, systolic BP and the fasting index of β-cell function, Homeostasis Model Assessment (HOMA2-%B); and percent change from baseline in body weight, high-density lipoprotein cholesterol (HDL-C) and triglycerides.
Safety and tolerability over the 52-week treatment period were evaluated based on adverse event (AE) reports, safety laboratory tests, vital sign measurements, physical examinations, SMBG and 12-lead electrocardiograms. AEs pre-specified for additional data collection included urinary tract infections (UTIs) and genital mycotic infections; specific analyses were performed for AEs related to osmotic diuresis and volume depletion. Documented hypoglycaemia episodes included biochemically confirmed episodes [concurrent fingerstick or plasma glucose ≤3.9 mmol/l (70 mg/dl)] with or without symptoms and severe hypoglycaemia episodes (i.e. those for which patients required assistance from another person or resulting in seizure or loss of consciousness).
Statistical Analyses
Sample size determination was based on showing the superiority of canagliflozin to placebo in reducing HbA1c from baseline to week 26. An estimated 86 patients per treatment group were required to achieve 90% power, assuming a between-group difference of 0.5%, a common standard deviation (s.d.) of 1.0%, and using a two-sample, two-sided t-test with a type I error rate of 0.05. Sample size was expanded to 120 patients per group to enhance the safety and tolerability assessment of canagliflozin in patients on metformin plus pioglitazone. No hypothesis testing was conducted for week 52 assessments.
Efficacy endpoints at week 26 were assessed using the modified intent-to-treat (mITT) population (all randomized patients who received ≥1 dose of double-blind study drug). Efficacy endpoints at week 52 were assessed in both the mITT analysis set and the extension mITT analysis set (all patients in the mITT population who entered the extension treatment period, took ≥1 dose of extension double-blind study drug, and did not receive rescue therapy prior to entering the extension period). Efficacy data reported at week 52 in this manuscript are for the mITT analysis set unless otherwise indicated.
Efficacy data were analysed according to randomized treatment assignment using the last observation carried forward (LOCF) approach to impute missing data; for patients who received glycaemic rescue therapy, the last post-baseline value prior to initiation of rescue was used for analysis. An analysis of covariance (ancova) model with treatment and stratification factors as fixed effects and the corresponding baseline value for each endpoint as a covariate was used to assess primary and continuous secondary endpoints. At week 26, least squares (LS) mean differences between groups and two-sided 95% confidence intervals (CIs) were estimated. The categorical secondary endpoint (proportion of patients reaching HbA1c <7.0%) was analysed with a logistic model with treatment and stratification factors as fixed effects and baseline HbA1c as a covariate.
Comparisons were performed for canagliflozin versus placebo at week 26 based on pre-specified hierarchical testing sequences implemented to strongly control overall type I error because of multiplicity. Two-sided statistical tests were conducted at the 5% significance level for all endpoints except systolic BP, HDL-C, triglycerides and HOMA2-%B, which were grouped into two separate families for testing canagliflozin 100 and 300 mg versus placebo, respectively. Each subfamily was assessed using the Hochberg procedure at the 2.5% significance level. p Values were calculated by comparing the LS means and are reported for pre-specified comparisons at week 26 only. At week 52, LS means for the change from baseline in efficacy parameters and two-sided 95% CIs were estimated for canagliflozin 100 and 300 mg and are reported for descriptive purposes. Since the purpose of the use of sitagliptin in the extension period was to maintain blinding and support appropriate safety assessments, and not to provide an efficacy comparison group, only descriptive efficacy results for the sitagliptin arm are reported.
Safety analyses included all reported AEs, regardless of rescue therapy, and laboratory results including data up to within 2 days after the last dose of study drug. The safety analysis set for the 52-week, double-blind treatment period was composed of the same patients as those in the mITT analysis set. An analysis of safety during the 26-week extension period consisted of the extension safety analysis set (all patients who entered the extension period, regardless of rescue therapy and received ≥1 dose of extension double-blind study drug).
Results
Patient Disposition and Baseline Characteristics
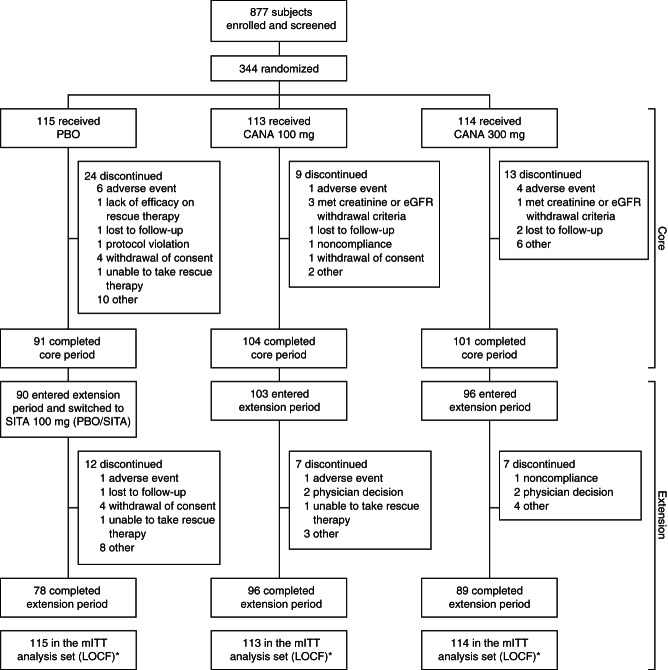
A total of 342 patients were randomized into the core treatment period and received ≥1 dose of study drug, comprising the mITT analysis set (Figure 1). Of the 342 patients, 296 (86.5%) completed the core period; of these, 289 entered the extension period and 263 completed 52 weeks of treatment. Rates of study discontinuation among the 342 mITT patients were 15.0, 21.9 and 32.2% over 52 weeks in the canagliflozin 100 and 300 mg and placebo/sitagliptin groups, respectively (8.0, 11.4 and 20.9%, respectively, during the core period). The most common reasons for discontinuation were ‘other,’ ‘AEs’ and ‘physician decision.’ The most common reason in the category of ‘other’ was withdrawal from dosing but agreed to allow follow-up contact. Among patients who discontinued due to AEs, few (<2%) discontinued due to any individual specific AE term. Over 52 weeks, 4.3, 2.6 and 16.5% of patients in the canagliflozin 100 and 300 mg and placebo/sitagliptin groups, respectively, received glycaemic rescue therapy (0.9, 0 and 12.2%, respectively, during the core period).
Figure 1.
Study flow diagram. PBO, placebo; CANA, canagliflozin; eGFR, estimated glomerular filtration rate; SITA, sitagliptin; mITT, modified intent-to-treat; LOCF, last observation carried forward. *All randomized patients who received ≥1 dose of double-blind study drug.
Baseline patient demographic and disease characteristics were generally similar across treatment groups, with the exception of the canagliflozin 300 mg group where the proportion of females was modestly higher and the proportion of Asian patients was modestly lower (Table 1). Overall, 90% of patients were on ≥2000 mg/day of metformin, and the majority (98%) of patients across treatment groups maintained a stable dose of metformin during the 52-week, double-blind treatment period (mean metformin dose of 2065 mg/day). For pioglitazone, 68% of patients were on pioglitazone 30 mg/day (32% on 45 mg/day); nearly all patients (99%) maintained stable doses of pioglitazone over 52 weeks. At baseline, 71% of patients were on lipid-modifying agents, including 66, 71 and 61% receiving statin therapy in the placebo/sitagliptin and canagliflozin 100 and 300 mg groups, respectively; 5.2, 6.2 and 4.4% of patients, respectively, initiated or modified statin therapy during the study.
Table 1.
Baseline demographics and disease characteristics.
| Characteristic | PBO/SITA (n = 115) | CANA 100 mg (n = 113) | CANA 300 mg (n = 114) | Total (N = 342) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 76 (66.1) | 77 (68.1) | 63 (55.3) | 216 (63.2) |
| Female | 39 (33.9) | 36 (31.9) | 51 (44.7) | 126 (36.8) |
| Age, years | 58.3 ± 9.6 | 56.7 ± 10.4 | 57.0 ± 10.2 | 57.4 ± 10.0 |
| Race, n (%) | ||||
| White | 79 (68.7) | 83 (73.5) | 90 (78.9) | 252 (73.7) |
| Black or African American | 6 (5.2) | 4 (3.5) | 10 (8.8) | 20 (5.8) |
| Asian | 21 (18.3) | 23 (20.4) | 11 (9.6) | 55 (16.1) |
| Other | 9 (7.8) | 3 (2.7) | 3 (2.6) | 15 (4.4) |
| HbA1c, % | 8.0 ± 1.0 | 8.0 ± 0.9 | 7.9 ± 0.9 | 7.9 ± 1.0 |
| FPG, mmol/l (mg/dl) | 9.1 ± 2.2 (164.0 ± 39.6) | 9.4 ± 2.2 (169.4 ± 39.6) | 9.1 ± 2.3 (164.0 ± 41.4) | 9.2 ± 2.2 (165.8 ± 39.6) |
| Body weight, kg | 93.8 ± 22.4 | 94.2 ± 22.2 | 94.4 ± 25.9 | 94.1 ± 23.5 |
| BMI, kg/m2 | 32.5 ± 6.4 | 32.3 ± 6.2 | 32.8 ± 7.7 | 32.5 ± 6.8 |
| eGFR, ml/min/1.73 m2 | 87.2 ± 18.8 | 84.6 ± 17.5 | 87.4 ± 19.5 | 86.4 ± 18.6 |
| Duration of T2DM, years | 10.1 ± 6.6 | 10.5 ± 6.6 | 11.0 ± 7.6 | 10.5 ± 7.0 |
PBO, placebo; SITA, sitagliptin; CANA, canagliflozin; FPG, fasting plasma glucose; BMI, body mass index; eGFR, estimated glomerular filtration rate; T2DM, type 2 diabetes mellitus; s.d., standard deviation.
Data are mean ± s.d. unless otherwise indicated.
Percentages may not total 100.0% due to rounding.
Includes multiple and other.
Efficacy
Glycaemic Efficacy Endpoints
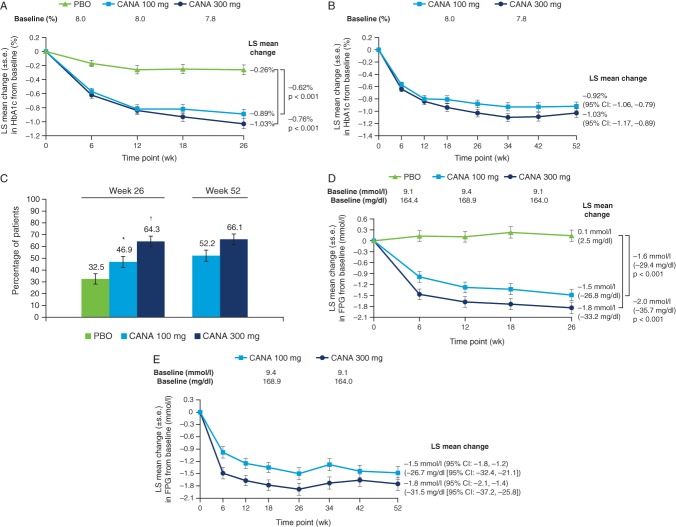
At week 26 (the primary efficacy analysis time point), canagliflozin 100 and 300 mg significantly reduced HbA1c from baseline relative to placebo (LS mean changes of −0.89, −1.03 and −0.26%, respectively; Figure 2A). Placebo-subtracted differences in LS mean changes were −0.62 and −0.76% with canagliflozin 100 and 300 mg, respectively (p < 0.001 for both). Reductions in HbA1c with canagliflozin 100 and 300 mg were sustained over 52 weeks of treatment (Figure 2B), with LS mean changes (95% CI) from baseline of −0.92% (−1.06, −0.79) and −1.03% (−1.17, −0.89), respectively, at week 52. Among patients who switched from placebo to sitagliptin during the extension period, mean HbA1c levels decreased from 7.72% at week 26 to 7.35% at week 52. A greater proportion of patients treated with canagliflozin 100 and 300 mg versus placebo achieved HbA1c <7.0% at week 26 (p < 0.01 and p < 0.001, respectively), with additional modest increases observed at week 52 (Figure 2C).
Figure 2.
Changes in glycaemic parameters (LOCF). (A) Change in HbA1c at week 26, (B) change in HbA1c at week 52, (C) proportion of patients achieving HbA1c <7.0% at weeks 26 and 52, (D) change in FPG at week 26 and (E) change in FPG at week 52. LOCF, last observation carried forward; FPG, fasting plasma glucose; PBO, placebo; CANA, canagliflozin; LS, least squares; s.e., standard error; CI, confidence interval. *p < 0.01 vs. PBO. †p < 0.001 vs. PBO.
Significant improvements from baseline in FPG were observed at week 26 with canagliflozin 100 and 300 mg compared with placebo; differences in the LS mean change versus placebo were −1.6 mmol/l (−29.4 mg/dl) and −2.0 mmol/l (−35.7 mg/dl), respectively (p < 0.001 for both; Figure 2D). LS mean changes (95% CI) from baseline in FPG at week 52 were −1.5 mmol/l (−1.8, –1.2) and −1.8 mmol/l (−2.1, −1.4) [−26.7 mg/dl (−32.4, −21.1) and −31.5 mg/dl (−37.2, −25.8)] with canagliflozin 100 and 300 mg, respectively (Figure 2E). Mean FPG decreased from 9.3 mmol/l at week 26 to 8.6 mmol/l at week 52 in the placebo/sitagliptin group.
Similar changes in glycaemic efficacy parameters were observed with canagliflozin 100 and 300 mg in the extension mITT analysis set (Table S1, Supporting Information).
Body Weight, BP and Lipids
At week 26, significant dose-related reductions from baseline in body weight were observed with canagliflozin 100 and 300 mg compared with placebo; LS mean percent changes relative to placebo were −2.7% (−2.5 kg) and −3.7% (−3.5 kg), respectively (p < 0.001 for both; Figure 3A). LS mean percent changes (95% CI) from baseline in body weight at week 52 were −2.7% (−3.6, –1.9) and −3.7% (−4.6, −2.9) with canagliflozin 100 and 300 mg, respectively; mean absolute changes (95% CI) were −2.5 kg (−3.3, −1.7) and −3.6 kg (−4.4, −2.7) (Figure 3B). During the extension period from weeks 26 to 52, sitagliptin treatment was associated with a minimal change in body weight from 93.8 to 94.1 kg. At week 26, 28.3, 38.6 and 6.1% of patients achieved ≥5% body weight reduction with canagliflozin 100 and 300 mg and placebo, respectively; 25.7 and 36.0% of patients achieved ≥5% body weight reduction with canagliflozin 100 and 300 mg, respectively, at week 52.
Figure 3.
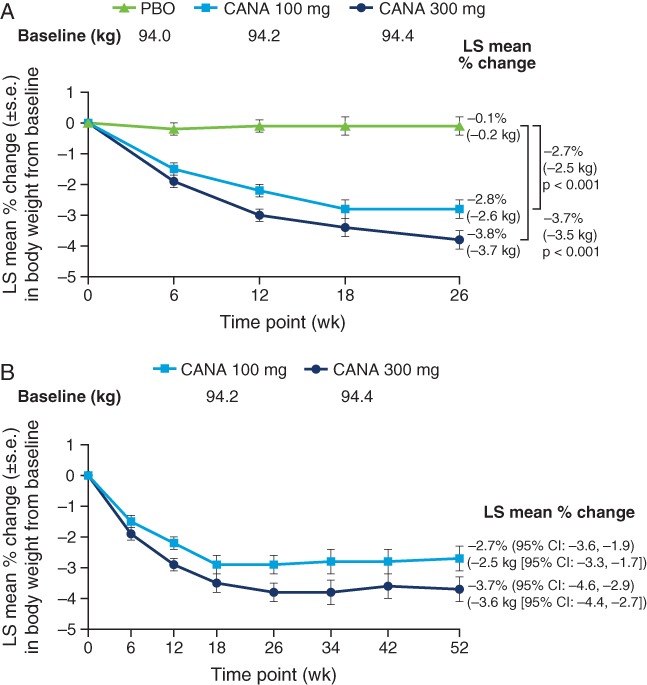
Percent change in body weight (LOCF) at (A) week 26 and (B) week 52. LOCF, last observation carried forward; PBO, placebo; CANA, canagliflozin; LS, least squares; s.e., standard error CI, confidence interval.
At week 26, canagliflozin 100 and 300 mg were associated with significant reductions from baseline in systolic BP compared with placebo (−5.3, −4.7 and −1.2 mmHg, respectively; p < 0.01 for canagliflozin 100 mg and p < 0.025 for canagliflozin 300 mg vs. placebo; Table 2); reductions from baseline in diastolic BP compared with placebo were also observed. LS mean changes (95% CI) from baseline in systolic BP at week 52 were −3.4 mmHg (−5.5, −1.4) and −3.7 mmHg (−5.8, −1.6) with canagliflozin 100 and 300 mg, respectively (Table 2); reductions in diastolic BP were also seen. Minimal changes in systolic BP (from 126.5–126.2 mmHg) and diastolic BP (from 75.9–75.5 mmHg) were seen between weeks 26 and 52 with sitagliptin treatment. No notable mean changes in pulse rate were observed with canagliflozin 100 or 300 mg or placebo at week 26 (−0.3, −1.3 and −0.5 beats/min, respectively) or with canagliflozin 100 or 300 mg at week 52 (0.5 and −1.0 beats/min, respectively).
Table 2.
Summary of changes from baseline in blood pressure and fasting plasma lipids at weeks 26 and 52 (LOCF)
| Week 26 | Week 52 | ||||
|---|---|---|---|---|---|
| Parameter | PBO (n = 115) | CANA 100 mg (n = 113) | CANA 300 mg (n = 114) | CANA 100 mg (n = 113) | CANA 300 mg (n = 114) |
| Systolic BP, n | 114 | 113 | 112 | 113 | 112 |
| Mean ± s.d. baseline, mmHg | 128.2 ± 12.3 | 126.4 ± 12.3 | 126.7 ± 12.0 | 126.4 ± 12.3 | 126.7 ± 12.0 |
| LS mean ± s.e. change | −1.2 ± 1.0 | −5.3 ± 1.0 | −4.7 ± 1.0 | −3.4 ± 1.1 | −3.7 ± 1.1 |
| Difference vs. PBO (95% CI) | −4.1 (−6.9, −1.3) | −3.5 (−6.3, −0.6) | |||
| Diastolic BP, n | 114 | 113 | 112 | 113 | 112 |
| Mean ± s.d. baseline, mmHg | 77.1 ± 8.2 | 75.6 ± 7.8 | 76.6 ± 8.3 | 75.6 ± 7.8 | 76.6 ± 8.3 |
| LS mean ± s.e. change | −0.9 ± 0.7 | −3.3 ± 0.7 | −3.5 ± 0.7 | −2.5 ± 0.7 | −2.7 ± 0.7 |
| Difference vs. PBO (95% CI) | −2.4 (−4.2, −0.5) | −2.6 (−4.4, −0.7) | |||
| Triglycerides, n | 105 | 108 | 109 | 110 | 110 |
| Mean ± s.d. baseline, mmol/l(mg/dl) | 1.6 ± 1.0 (143.9 ± 86.0) | 1.7 ± 1.1 (146.7 ± 97.6) | 1.6 ± 1.1 (143.8 ± 100.1) | 1.7 ± 1.1 (145.7 ± 97.0) | 1.7 ± 1.2 (146.6 ± 103.9) |
| LS mean ± s.e. change, mmol/l(mg/dl) | 0.10 ± 0.08 (8.6 ± 7.0) | −0.06 ± 0.08 (−5.3 ± 6.9) | −0.16 ± 0.08 (−13.9 ± 6.9) | −0.06 ± 0.11 (−5.4 ± 9.5) | −0.05 ± 0.11 (−4.6 ± 9.5) |
| Median (IQR) percent change | 6.3 (−19.2, 37.8) | −2.4 (−23.5, 27.3) | −3.1 (−23.4, 10.9) | −1.6 (−21.3, 28.7) | −8.3 (−29.4, 16.3) |
| LS mean ± s.e. percent change | 15.3 ± 4.1 | 3.1 ± 4.1 | −1.7 ± 4.1 | 4.7 ± 4.0 | −0.6 ± 4.0 |
| Difference vs. PBO (95% CI) | −12.1 (−23.3, −1.0) | −17.0 (−28.1, −5.8) | |||
| LDL-C, n | 105 | 107 | 109 | 110 | 110 |
| Mean ± s.d. baseline, mmol/l(mg/dl) | 2.5 ± 0.9 (96.9 ± 34.5) | 2.4 ± 0.9 (92.1 ± 33.5) | 2.3 ± 0.8 (89.0 ± 31.1) | 2.4 ± 0.9 (92.7 ± 33.4) | 2.3 ± 0.9 (90.2 ± 33.5) |
| LS mean ± s.e. change, mmol/l(mg/dl) | −0.10 ± 0.06 (−3.9 ± 2.5) | 0.08 ± 0.06 (3.3 ± 2.5) | 0.19 ± 0.06 (7.2 ± 2.5) | 0.16 ± 0.07 (6.1 ± 2.8) | 0.20 ± 0.07 (7.8 ± 2.9) |
| Median (IQR) percent change | −3.0 (−18.5, 14.1) | 2.7 (−7.6, 20.6) | 6.8 (−7.5, 23.5) | 4.7 (−9.1, 22.7) | 5.4 (−7.4, 29.5) |
| LS mean ± s.e. percent change | −0.4 ± 2.6 | 7.1 ± 2.5 | 11.3 ± 2.5 | 10.9 ± 3.2 | 14.3 ± 3.2 |
| Difference vs. PBO (95% CI) | 7.5 (0.6, 14.4) | 11.7 (4.8, 18.6) | |||
| HDL-C, n | 105 | 107 | 109 | 110 | 110 |
| Mean ± s.d. baseline, mmol/l(mg/dl) | 1.3 ± 0.3 (49.1 ± 11.9) | 1.3 ± 0.3 (49.2 ± 12.9) | 1.4 ± 0.3 (52.1 ± 12.4) | 1.3 ± 0.3 (49.3 ± 12.7) | 1.3 ± 0.3 (52.0 ± 12.4) |
| LS mean ± s.e. change, mmol/l(mg/dl) | 0.02 ± 0.02 (0.7 ± 0.7) | 0.08 ± 0.02 (3.2 ± 0.7) | 0.10 ± 0.02 (3.8 ± 0.7) | 0.08 ± 0.02 (3.0 ± 0.8) | 0.13 ± 0.02 (5.1 ± 0.8) |
| Median (IQR) percent change | 1.3 (−5.9, 11.0) | 5.1 (−2.3, 17.8) | 8.0 (−1.8, 17.3) | 6.8 (−3.2, 16.3) | 9.3 (−1.7, 20.7) |
| LS mean ± s.e. percent change | 2.4 ± 1.4 | 7.2 ± 1.4 | 8.9 ± 1.3 | 7.0 ± 1.6 | 11.4 ± 1.6 |
| Difference vs. PBO (95% CI) | 4.8 (1.1, 8.5) | 6.5 (2.8, 10.2) | |||
| LDL-C/HDL-C, n | 105 | 107 | 109 | 110 | 110 |
| Mean ± s.d. baseline, mol/mol | 2.1 ± 0.9 | 2.0 ± 0.8 | 1.8 ± 0.7 | 2.0 ± 0.8 | 1.8 ± 0.8 |
| LS mean ± s.e. change | −0.11 ± 0.06 | −0.02 ± 0.06 | 0.00 ± 0.06 | 0.05 ± 0.06 | −0.03 ± 0.06 |
| Median (IQR) percent change | −4.4 (−21.5, 15.6) | −3.6 (−16.0, 8.1) | 1.2 (−13.3, 16.7) | −0.4 (−17.7, 20.4) | −1.4 (−15.5, 18.3) |
| LS mean ± s.e. percent change | −0.7 ± 2.8 | 1.8 ± 2.8 | 3.5 ± 2.8 | 5.5 ± 3.2 | 4.4 ± 3.3 |
| Difference vs. PBO (95% CI) | 2.4 (−5.2, 10.1) | 4.1 (−3.6, 11.8) | |||
| Non–HDL-C, n | 105 | 107 | 109 | 110 | 110 |
| Mean ± s.d. baseline, mmol/l(mg/dl) | 3.2 ± 1.0 (125.2 ± 39.8) | 3.2 ± 1.0 (121.7 ± 40.4) | 3.0 ± 1.0 (117.2 ± 37.3) | 3.2 ± 1.0 (122.3 ± 40.5) | 3.1 ± 1.0 (118.6 ± 39.9) |
| LS mean ± s.e. change, mmol/l(mg/dl) | −0.05 ± 0.08 (−1.9 ± 3.0) | 0.05 ± 0.08 (1.8 ± 3.0) | 0.13 ± 0.08 (5.2 ± 3.0) | 0.14 ± 0.08 (5.3 ± 3.1) | 0.24 ± 0.08 (9.1 ± 3.2) |
| Median (IQR) percent change | 0.3 (−14.3, 12.3) | 1.2 (−8.0, 11.6) | 3.4 (−7.6, 16.1) | 2.6 (−8.3, 17.9) | 3.8 (−7.6, 21.9) |
| LS mean ± s.e. percent change | 1.4 ± 2.4 | 3.3 ± 2.3 | 6.2 ± 2.3 | 6.8 ± 2.6 | 9.0 ± 2.6 |
| Difference vs. PBO (95% CI) | 1.9 (−4.6, 8.3) | 4.8 (−1.6, 11.2) |
LOCF, last observation carried forward; PBO, placebo; CANA, canagliflozin; BP, blood pressure, s.d., standard deviation; LS, least squares; s.e., standard error; CI, confidence interval; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; NS, not significant.
p < 0.01 versus PBO.
p < 0.025 versus PBO.
Statistical comparison versus PBO not performed (not pre-specified).
p = NS versus PBO based on Hochberg's testing approach.
p < 0.001 versus PBO.
Relative to placebo, dose-related increases in HDL-C were observed with canagliflozin 100 mg (p < 0.025) and canagliflozin 300 mg (p < 0.001) at week 26 (Table 2 and Figure S1A, Supporting Information). Increases from baseline in HDL-C seen with both canagliflozin doses at week 52 were generally similar to those seen at week 26, with a slightly larger percent increase observed with canagliflozin 300 mg at week 52. At week 26, triglycerides decreased from baseline with canagliflozin 300 mg (p < 0.01 vs. placebo), whereas increases were seen with canagliflozin 100 mg and placebo; changes were similar with both canagliflozin doses at week 52. Dose-related increases from baseline in low-density lipoprotein cholesterol (LDL-C) were seen with canagliflozin relative to placebo at week 26, with small further increases observed at week 52 (Table 2 and Figure S1B). Increases in non–HDL-C that were smaller than observed increases in LDL-C and small increases in the LDL-C/HDL-C ratio were seen with both doses of canagliflozin relative to placebo at week 26 (Table 2); small further increases in these parameters were seen at week 52. Mean fasting plasma lipid levels remained generally unchanged between weeks 26 and 52 with sitagliptin treatment (HDL-C, 1.29–1.28 mmol/l; LDL-C, 2.39–2.40 mmol/l; triglycerides, 1.73–1.61 mmol/l; LDL-C/HDL-C ratio, 1.97–1.96; non–HDL-C, 3.19–3.15 mmol/l).
Changes from baseline at week 52 in body weight, BP and fasting plasma lipids with canagliflozin 100 and 300 mg in the extension mITT analysis set (Table S1) were generally similar to those in the mITT analysis set.
β-Cell Function
At week 26, canagliflozin 100 and 300 mg showed a significant increase in HOMA2-%B, an indirect measure of β-cell function, compared with placebo (LS mean changes from baseline of 15.2, 18.1 and 0.9, respectively; p < 0.001 for both canagliflozin doses vs. placebo). Improvements in HOMA2-%B with canagliflozin 100 and 300 mg were sustained over 52 weeks of treatment, with LS mean changes (95% CI) from baseline of 16.3 (12.4, 20.2) and 18.2 (14.3, 22.0), respectively.
Safety and Tolerability
The overall incidence of AEs was 69.9, 76.3 and 76.5% with canagliflozin 100 and 300 mg and placebo/sitagliptin, respectively (Table 3). Incidences of AEs leading to discontinuation and serious AEs were low and similar across groups. Incidence of AEs during the extension period (weeks 26–52) are reported in Table S2.
Table 3.
Summary of overall safety and selected AEs over 52 weeks.
| Patients, n (%) | PBO/SITA(n = 115) | CANA 100 mg(n = 113) | CANA 300 mg(n = 114) |
|---|---|---|---|
| Any AE | 88 (76.5) | 79 (69.9) | 87 (76.3) |
| AEs leading to discontinuation | 7 (6.1) | 2 (1.8) | 5 (4.4) |
| AEs related to study drug† | 27 (23.5) | 22 (19.5) | 33 (28.9) |
| Serious AEs | 6 (5.2) | 8 (7.1) | 7 (6.1) |
| Deaths | 0 | 0 | 0 |
| Selected AEs | |||
| UTI | 9 (7.8) | 6 (5.3) | 9 (7.9) |
| Genital mycotic infection | |||
| Maleठ| 0 | 3 (3.9) | 3 (4.8) |
| Female‖,¶ | 3 (7.7) | 6 (16.7) | 11 (21.6) |
| Osmotic diuresis-relatedAEs** | 1 (0.9) | 11 (9.7) | 11 (9.6) |
| Volume depletion AEs†† | 4 (3.5) | 9 (8.0) | 5 (4.4) |
AE, adverse event; PBO, placebo; SITA, sitagliptin; CANA, canagliflozin; UTI, urinary tract infection.
All AEs are reported for regardless of rescue medication.
Possibly, probably or very likely related to study drug, as assessed by investigators.
PBO/SITA, n = 76; CANA 100 mg, n = 77; CANA 300 mg, n = 63.
Including balanitis, balanitis candida, balanoposthitis, balanoposthitis infective and genital infection fungal.
PBO/SITA, n = 39; CANA 100 mg, n = 36; CANA 300 mg, n = 51.
Including genital infection fungal, vaginal infection, vulvitis, vulvovaginal candidiasis, vulvovaginal mycotic infection and vulvovaginitis.
Including dry mouth, micturition urgency, nocturia, pollakiuria, polyuria and thirst.
Including dehydration, dizziness postural, hypotension, orthostatic hypotension and syncope.
Over 52 weeks, canagliflozin 100 and 300 mg were associated with higher incidences of genital mycotic infections in males and females compared with placebo/sitagliptin (Table 3). All these events were considered by the investigator to be mild to moderate in intensity and none led to study discontinuation. Most genital mycotic infection AEs were reported during the first 26 weeks of treatment and were generally treated with antifungal agents (topical and/or oral) that were self-initiated or given at the discretion of the treating physician. Incidences of UTIs over 52 weeks were 5.3, 7.9 and 7.8% with canagliflozin 100 and 300 mg and placebo/sitagliptin, respectively, with no upper UTIs reported.
Canagliflozin 100 and 300 mg were associated with higher rates of AEs related to osmotic diuresis [e.g. pollakiuria (increased urine frequency), polyuria (increased urine volume)] over 52 weeks (Table 3). Incidences of volume depletion AEs (e.g. postural dizziness, orthostatic hypotension) were low across groups but numerically higher with canagliflozin 100 mg compared with canagliflozin 300 mg and placebo/sitagliptin over 52 weeks (Table 3). AEs related to osmotic diuresis and volume depletion were generally considered by the investigator to be mild to moderate in intensity and infrequently led to study discontinuation; the majority of events occurred during the first 26 weeks of treatment.
The proportion of patients with documented hypoglycaemia episodes over 52 weeks was 4.4, 6.1 and 6.1% with canagliflozin 100 and 300 mg and placebo/sitagliptin, respectively; 2.7, 3.5 and 3.5% had >1 documented hypoglycaemia episode. There were no reports of severe hypoglycaemia.
In general, only small differences were seen in mean percent changes in safety laboratory parameters with canagliflozin compared with placebo/sitagliptin over 52 weeks (Table 4). Decreases in alanine aminotransferase and gamma glutamyl transferase were seen with canagliflozin versus placebo/sitagliptin. Small decreases in eGFR were seen with both canagliflozin groups at week 6 and subsequently trended towards baseline with canagliflozin 100 mg and remained generally stable with canagliflozin 300 mg, with values in the canagliflozin 300 mg and placebo/sitagliptin groups generally similar at week 52. Increases from baseline in serum creatinine were observed at week 6 across treatment groups, followed by stable or diminishing increases over 52 weeks. Increases in blood urea nitrogen were seen with canagliflozin, whereas a decrease was seen with placebo/sitagliptin. Decreases in serum urate were seen with canagliflozin, whereas an increase was seen with placebo/sitagliptin. No meaningful changes from baseline were observed in serum electrolytes, including potassium and sodium, across treatment groups. Both canagliflozin doses were associated with increases in magnesium and phosphate versus placebo/sitagliptin. Increases in haemoglobin and haematocrit were observed with canagliflozin, whereas decreases were seen with placebo/sitagliptin.
Table 4.
Mean percent changes in clinical laboratory parameters from baseline to week 52
| Parameter | PBO/SITA | CANA 100 mg | CANA 300 mg |
|---|---|---|---|
| ALT, n | 78 | 95 | 87 |
| Mean baseline, U/l | 22.5 | 25.9 | 21.9 |
| Mean ± s.d. percentchange | 1.9 ± 32.4 | −3.1 ± 36.6 | −7.0 ± 27.9 |
| BUN, n | 78 | 95 | 88 |
| Mean baseline, mmol/l | 5.9 | 5.6 | 5.7 |
| Mean ± s.d. percentchange | −1.5 ± 20.1 | 13.6 ± 28.9 | 21.3 ± 31.4 |
| Creatinine, n | 78 | 95 | 88 |
| Mean baseline, µmol/l | 75.8 | 77.5 | 75.5 |
| Mean ± s.d. percentchange | 4.3 ± 10.4 | 2.3 ± 11.6 | 5.6 ± 10.6 |
| GGT, n | 78 | 95 | 88 |
| Mean baseline, U/l | 26.0 | 29.9 | 29.3 |
| Mean ± s.d. percentchange | −1.2 ± 38.6 | −7.5 ± 28.7 | −14.0 ± 27.9 |
| eGFR, n | 78 | 95 | 88 |
| Mean baseline,ml/min/1.73 m2 | 87.3 | 85.4 | 88.7 |
| Mean ± s.d. percentchange | −3.9 ± 11.0 | −1.6 ± 12.7 | −5.3 ± 10.7 |
| Magnesium, n | 78 | 95 | 88 |
| Mean baseline, mmol/l | 0.8 | 0.8 | 0.8 |
| Mean ± s.d. percentchange | 0.0 ± 10.1 | 7.6 ± 8.9 | 11.4 ± 9.6 |
| Phosphate, n | 78 | 95 | 88 |
| Mean baseline, mmol/l | 1.2 | 1.2 | 1.1 |
| Mean ± s.d. percentchange | −1.4 ± 12.7 | 4.3 ± 15.6 | 4.2 ± 14.6 |
| Potassium, n | 78 | 95 | 88 |
| Mean baseline, mmol/l | 4.3 | 4.4 | 4.3 |
| Mean ± s.d. percentchange | 0.1 ± 6.7 | −0.1 ± 7.6 | 0.4 ± 8.4 |
| Sodium, n | 78 | 95 | 88 |
| Mean baseline, mmol/l | 139.2 | 139.5 | 139.8 |
| Mean ± s.d. percentchange | 0.9 ± 1.5 | 0.5 ± 1.7 | 0.4 ± 1.8 |
| Urate, n | 78 | 95 | 88 |
| Mean baseline, µmol/L | 321.9 | 318.0 | 315.0 |
| Mean ± s.d. percentchange | 4.2 ± 15.8 | −10.1 ± 17.4 | −8.0 ± 16.1 |
| Haemoglobin, n | 78 | 94 | 87 |
| Mean baseline, g/l | 139.4 | 137.9 | 135.5 |
| Mean ± s.d. percentchange | −1.6 ± 5.5 | 4.9 ± 7.9 | 5.6 ± 7.0 |
| Haematocrit, n | 77 | 93 | 87 |
| Mean baseline, % | 41.6 | 41.1 | 40.4 |
| Mean ± s.d. percentchange | −1.2 ± 6.2 | 5.7 ± 8.9 | 6.4 ± 7.5 |
PBO, placebo; SITA, sitagliptin; CANA, canagliflozin; ALT, alanine aminotransferase; s.d., standard deviation; BUN, blood urea nitrogen; GGT, gamma glutamyl transferase; eGFR, estimated glomerular filtration rate.
Discussion
The patients enrolled in this study reflect a broad range of ages, ethnicities and racial backgrounds, were generally overweight or obese (BMI ≥30 kg/m2), and had a relatively long history of T2DM, consistent with patients with T2DM already on dual AHA combination therapy. In this population of patients with T2DM inadequately controlled with metformin and pioglitazone, canagliflozin 100 and 300 mg significantly reduced HbA1c, FPG, systolic BP and body weight compared with placebo at week 26. Importantly, these improvements were sustained over the entire 52-week treatment period.
Improvements in glycaemic control have been reported with pioglitazone in patients with T2DM inadequately controlled on other AHA therapies (e.g. metformin ± sulphonylurea), but increases in body weight have been observed in clinical studies evaluating pioglitazone compared with placebo or other AHAs 14–17. In this study, both canagliflozin doses provided significant reductions in body weight compared with placebo at week 26 that were sustained over 52 weeks of treatment; in contrast, no change in body weight was observed with sitagliptin during the extension period. In a 48-week study in patients with T2DM on background pioglitazone, a slight decrease in body weight was seen initially with dapagliflozin 5 and 10 mg compared with placebo; body weight then gradually increased over 48 weeks (net weight gains from baseline of 3.0, 1.4 and 0.7 kg, respectively) 18. In a 24-week study of the SGLT2 inhibitor empagliflozin in patients with T2DM on background pioglitazone ± metformin, significant reductions in body weight were seen with empagliflozin 10 and 25 mg compared with placebo 19. The reduction in body weight observed with canagliflozin over 52 weeks of treatment suggests that this agent may provide effective weight control in patients receiving background pioglitazone therapy. While changes in body composition were not assessed in this study, findings from other phase 3 studies showed that approximately two-thirds of body weight loss with canagliflozin was due to loss of fat mass 8,20. Because pioglitazone treatment is associated with fluid retention leading to oedema and increased plasma volume 2, whereas osmotic diuresis, volume depletion and increased haematocrit have been seen with canagliflozin, some of the body weight loss observed in this study may have resulted from reversal of pioglitazone-related fluid retention by canagliflozin treatment.
Canagliflozin 100 and 300 mg were associated with significant increases in HDL-C compared with placebo at week 26, with similar changes in HDL-C seen at week 52 with both canagliflozin doses. Overall, minimal changes in triglycerides were seen with both canagliflozin doses over the 52-week treatment period. Both canagliflozin doses were associated with increases in LDL-C, with smaller increases in non–HDL-C and no meaningful changes in the LDL-C/HDL-C ratio. Most of the changes from baseline in lipid parameters were observed at week 26, with only small further increases through week 52. Effects on lipids parameters were generally similar to those reported in other phase 3 studies with canagliflozin as monotherapy and add-on to metformin or metformin plus sulphonylurea 4,8–10. The mechanism of LDL-C increase with canagliflozin treatment is unknown. The impact of these lipid changes on cardiovascular disease is being assessed in the ongoing CANagliflozin cardioVascular Assessment Study (CANVAS) 21.
Declining β-cell function is a hallmark of T2DM progression 22. In this study, population with a mean duration of T2DM of 10.5 years, canagliflozin was associated with significant improvements in HOMA2-%B, an indirect measure of β-cell function, compared with placebo at week 26, with sustained effects seen over 52 weeks of treatment. These findings are consistent with those observed in previous studies evaluating canagliflozin in patients with T2DM 3–5,10 as well as preclinical effects observed with other SGLT2 inhibitors 23–26. It has been suggested that prevention of glucose toxicity (e.g. hyperglycaemia) via the insulin-independent mechanism of action of SGLT2 inhibitors may help to preserve β-cell function by maintaining β-cell mass and reducing β-cell death 27. Additional studies are needed to more fully evaluate the effect of canagliflozin on β-cell function and determine whether these initially observed improvements could contribute to a delay in disease progression.
Canagliflozin was generally well tolerated over 52 weeks, with a safety profile consistent with previous reports, including increased incidences of genital mycotic infections and AEs related to osmotic diuresis and volume depletion 4–10. These AEs generally occurred during the first 26 weeks of treatment, were considered mild or moderate in intensity, and infrequently led to discontinuation. Incidence of UTIs and documented hypoglycaemia were low and similar across treatment groups.
One limitation of this study is the lack of a control group for efficacy at 52 weeks. To avoid prolonged placebo treatment, patients receiving placebo switched to sitagliptin 100 mg after 26 weeks. As sitagliptin treatment was not concurrently initiated, comparisons at week 52 were not appropriate (and hence not pre-specified). Although the 52-week duration of this study provides some longer duration efficacy results, still longer-term data will be needed to fully assess durability. A strength of the study is a patient population that is reflective of a typical profile of patients with T2DM (e.g. broad range of ages and ethnic/racial backgrounds, and mostly overweight or obese), suggesting that findings may be generalizable to a broad T2DM population. Furthermore, the favourable efficacy and safety profile of canagliflozin in this patient population with a relatively long duration of T2DM on background combination therapy suggests a potential benefit of canagliflozin treatment in patients with advanced disease.
In summary, treatment with canagliflozin significantly improved glycaemic control and reduced systolic BP and body weight compared with placebo over 26 weeks in patients with T2DM inadequately controlled with metformin and pioglitazone; these improvements were sustained over 52 weeks of treatment. Canagliflozin was generally well tolerated in these patients, with a pattern of specific AEs that are consistent with the mechanism of action of canagliflozin and previous reports 4–10. These findings provide further support for the clinical utility of canagliflozin as add-on therapy in patients with T2DM.
Acknowledgments
This study was sponsored by Janssen Research & Development, LLC. The authors thank all investigators, study teams and patients for participating in this study. Editorial support was provided by Katie McClendon, PhD, of MedErgy and was funded by Janssen Global Services, LLC. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation. This study was previously presented, in part, in abstract form at the 4th World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension, Barcelona, Spain, 8–11 November 2012; at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, USA, 21–25 June 2013; and at the 16th Annual Canadian Diabetes Association/Canadian Society of Endocrinology and Metabolism Professional Conference & Annual Meetings, Montreal, Quebec, Canada, 17–20 October 2013.
Conflict of Interest
T.F. has received research support from Böehringer Ingelheim, Eli Lilly, Novartis and Sanofi; served on advisory panels for Böehringer Ingelheim and Eli Lilly; and participated in speaker bureaus for Böehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, Sanofi and MSD. R. Guthrie has received research support from Janssen, and serves as a consultant to Janssen, Eli Lilly and Böehringer Ingelheim. R. Goldenberg has received research support from Abbott, AstraZeneca, Böehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Medtronic, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Takeda; has served on advisory panels for AstraZeneca, Böehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck, Novo Nordisk, Roche and Takeda; has participated in speaker bureaus for Abbott, AstraZeneca, Böehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck and Novo Nordisk; and has served as a consultant for AstraZeneca, Böehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Janssen, Novo Nordisk and Takeda. J.Y., U.V. and G.M. are full-time employees of Janssen Research & Development, LLC. P.S. was a full-time employee of Janssen Research & Development, LLC, at the time the study was conducted.
T.F., R.G., R.G. and J.Y. contributed to the conduct of the study; contributed to the acquisition, analysis and interpretation of data; and reviewed and approved the manuscript. U.V. contributed to the analysis and interpretation of data, and reviewed and approved the manuscript. G.M. and P.S contributed to the design and conduct of the study; contributed to the acquisition, analysis and interpretation of data; and reviewed and approved the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Percent change in (A) HDL-C and (B) LDL-C (LOCF).
Summary of changes from baseline in efficacy endpoints at week 52 (Extension mITT, LOCF).
Summary of overall safety and selected AEs during the 26-week double-blind extension period (weeks 26–52).
References
- 1.American Diabetes Association. Standards of medical care in diabetes–2013. Diabetes Care. 2013;36:S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenstock J, Aggarwal N, Polidori D, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232–1238. doi: 10.2337/dc11-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenlöf K, Cefalu WT, Kim K-A, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week, randomized trial. Diabetes Care. 2013;36:2508–2515. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–473. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bode B, Stenlof K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract. 2013;41:72–84. doi: 10.3810/hp.2013.04.1020. [DOI] [PubMed] [Google Scholar]
- 8.Cefalu WT, Leiter LA, Yoon K-H, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 9.Lavalle-Gonzalez F, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–2592. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilding JP, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267–1282. doi: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devineni D, Morrow L, Hompesch M, et al. Canagliflozin improves glycemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012;14:539–545. doi: 10.1111/j.1463-1326.2012.01558.x. [DOI] [PubMed] [Google Scholar]
- 12.Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13:669–672. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 13.Polidori D, Sha S, Ghosh A, Plum-Morschel L, Heise T, Rothenberg P. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin-treated subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98:E867–E871. doi: 10.1210/jc.2012-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolli G, Dotta F, Colin L, Minic B, Goodman M. Comparison of vildagliptin and pioglitazone in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Obes Metab. 2009;11:589–595. doi: 10.1111/j.1463-1326.2008.01023.x. [DOI] [PubMed] [Google Scholar]
- 15.Charpentier G, Halimi S. Earlier triple therapy with pioglitazone in patients with type 2 diabetes. Diabetes Obes Metab. 2009;11:844–854. doi: 10.1111/j.1463-1326.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 16.Scheen AJ, Tan MH, Betteridge DJ, Birkeland K, Schmitz O, Charbonnel B. Long-term glycaemic effects of pioglitazone compared with placebo as add-on treatment to metformin or sulphonylurea monotherapy in PROactive (PROactive 18) Diabet Med. 2009;26:1242–1249. doi: 10.1111/j.1464-5491.2009.02857.x. [DOI] [PubMed] [Google Scholar]
- 17.Bergenstal RM, Wysham C, MacConell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 18.Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, on hemoglobin A1c, body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473–1478. doi: 10.2337/dc11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs CS, Seshiah V, Swallow R, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16:147–158. doi: 10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- 20.Toubro S, Cefalu WT, Xie J, et al. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces body weight mainly through loss of fat mass in subjects with type 2 diabetes (Abstract 762) Diabetologia. 2012;55(1):S313–S314. [Google Scholar]
- 21.Neal B, Perkovic V, de Zeeuw D, et al. Am Heart J. 2013;166:217–223. doi: 10.1016/j.ahj.2013.05.007. Rationale, design, and baseline characteristics of the canagliflozin cardiovascular assessment study (CANVAS)--a randomized placebo-controlled trial. [DOI] [PubMed] [Google Scholar]
- 22.Kahn SE. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 86:4047–4058. doi: 10.1210/jcem.86.9.7713. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Klein T, Leung P. Effects of combining linagliptin treatment with BI-38335, a novel SGLT2 inhibitor, on pancreatic islet function and inflammation in db/db mice. Curr Mol Med. 2012;12:995–1004. doi: 10.2174/156652412802480970. [DOI] [PubMed] [Google Scholar]
- 24.Kurosaki E, Ogasawara H. Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: Preclinical and clinical data. Pharmacol Ther. 2013;139:51–59. doi: 10.1016/j.pharmthera.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Macdonald FR, Peel JE, Jones HB, et al. The novel sodium glucose transporter 2 inhibitor dapagliflozin sustains pancreatic function and preserves islet morphology in obese, diabetic rats. Diabetes Obes Metab. 2010;12:1004–1012. doi: 10.1111/j.1463-1326.2010.01291.x. [DOI] [PubMed] [Google Scholar]
- 26.Nagata T, Fukuzawa T, Takeda M, et al. Tofogliflozin, a novel sodium-glucose co-transporter 2 inhibitor, improves renal and pancreatic function in db/db mice. Br J Pharmacol. 2013;17:519–531. doi: 10.1111/bph.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurczak MJ, Lee HY, Birkenfeld AL, et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes. 2011;60:890–898. doi: 10.2337/db10-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percent change in (A) HDL-C and (B) LDL-C (LOCF).
Summary of changes from baseline in efficacy endpoints at week 52 (Extension mITT, LOCF).
Summary of overall safety and selected AEs during the 26-week double-blind extension period (weeks 26–52).