Abstract
Aims
To compare the efficacy and safety of two insulin intensification strategies in patients with type 2 diabetes inadequately controlled on basal insulin glargine with metformin and/or pioglitazone.
Methods
A multinational, randomized, open-label trial that compared insulin lispro low mixture (LM25; n = 236) twice daily with a basal–prandial regimen of insulin glargine once daily and insulin lispro once daily (IGL; n = 240) over 24 weeks in patients with HbA1c 7.5–10.5% and fasting plasma glucose ≤6.7 mmol/l. The primary objective was to assess non-inferiority [per-protocol (PP) population], and then superiority [intention-to-treat (ITT) population], of LM25 versus IGL according to change in HbA1c after 24 weeks (non-inferiority margin 0.4%, two-sided significance level 0.05).
Results
Estimated change [least squares (LS) mean (95% CI)] in HbA1c after 24 weeks: −1.30 (−1.44, −1.16)% with LM25 and −1.08 (−1.22, −0.94)% with IGL. Non-inferiority was shown [LS mean (95% CI) HbA1c treatment difference −0.21 (−0.38, −0.04) (PP population)]; gated superiority assessment showed a statistically significant advantage for LM25 (p = 0.010; ITT population). Mean blood glucose, glycaemic variability, overall tolerability and hypoglycaemic episodes per patient-year did not show significant differences between treatments during the study.
Conclusions
In patients with type 2 diabetes inadequately controlled on once-daily basal insulin glargine and metformin and/or pioglitazone, intensification with LM25 was superior to a basal–prandial approach in terms of reduction in HbA1c after 24 weeks and did not increase hypoglycaemia episodes.
Keywords: insulin glargine, insulin intensification, insulin lispro, insulin lispro mixture, type 2 diabetes
Introduction
In patients with type 2 diabetes, glycaemic control typically deteriorates over time due to the progressive nature of the disease, and a significant proportion of patients with type 2 diabetes will require exogenous insulin therapy to reach glycaemic goals 1,2.
There are several options available for starting and progressing with insulin therapy. Once-daily basal insulin is the most convenient strategy for insulin initiation because it is practical and simple to use, but its efficacy is limited since fewer than 40% of patients meet glycaemic targets 3. At this time, intensification of insulin therapy is recommended 2,4 and different approaches can be used 2,5–11. The administration of a rapid-acting analogue before the meal associated with the largest postprandial glycaemic value in addition to basal insulin is considered to be more flexible and is usually recommended. Alternatively, a premixed insulin analogue twice daily (bid) may be used, although this strategy is considered to be less flexible and studied 2. There is a lack of head-to-head studies comparing the efficacy and safety of a premixed insulin analogue bid with that of once-daily basal insulin and once-daily prandial insulin as the second insulin therapy step after the initial basal insulin regimen has failed.
This study was therefore conducted to test the hypothesis that glycaemic control with insulin lispro low mixture (LM25) bid is non-inferior to that achieved with once-daily basal insulin glargine and once-daily prandial insulin lispro (IGL), as measured by the change in HbA1c after 24 weeks of treatment, in patients with type 2 diabetes mellitus who have inadequate glycaemic control on once-daily basal insulin glargine and oral antihyperglycaemic medication (metformin and/or pioglitazone).
Materials and Methods
Study Design and Interventions
This multinational, open-label, randomized, parallel-arm, non-inferiority, phase IV trial compared the efficacy and safety of two insulin intensification strategies in patients with type 2 diabetes who were inadequately controlled on once-daily basal insulin glargine and metformin and/or pioglitazone.
Patients entered a 2-week screening period, and those eligible for the study were randomized in a 1 : 1 ratio, using a computer-generated random sequence, to subcutaneous LM25 (insulin lispro protamine suspension 75% and insulin lispro solution 25%) bid or IGL for 24 weeks. Patients continued taking their stable dose of metformin and/or pioglitazone throughout the study, unless dose modifications were required for safety reasons. Insulin LM25 was administered before breakfast and dinner, whereas basal insulin glargine was administered at bedtime and prandial insulin lispro was administered before the largest meal of the day [defined as the meal with the highest 2-h postprandial blood glucose concentration, as determined from three separate 7-point self-monitoring of blood glucose (SMBG) profiles recorded during the screening period]. The initial total daily dose of insulin LM25 was equivalent to the patient‘s last dose of insulin glargine, although the total daily dose was split into two equally sized doses. Patients in the IGL group continued on the same dose of insulin glargine that they were receiving during the screening period and initiated insulin lispro 4 IU daily.
Randomization was stratified by country and baseline HbA1c concentration (<8.5% or ≥8.5%). All patients provided written informed consent and the study was conducted in accordance with applicable laws and regulations, Good Clinical Practices and the ethical principles who have their origin in the Declaration of Helsinki.
At the screening visit, patients provided a medical history including pre-existing conditions, concomitant medications, previous diabetes treatments and current dosages of antihyperglycaemic medications; they underwent a physical examination; and they provided a fasting blood sample to allow measurement of fasting plasma glucose (FPG) at a central laboratory.
At the beginning, at regular intervals throughout, and at the completion of the 24-week treatment period, patient data were collected including weight and vital signs, blood glucose data and adverse events, including hypoglycaemia. Patients completed 7-point SMBG profiles within the 2 weeks before weeks 4, 12 and 24 of study treatment or before an early discontinuation visit if applicable. Patients recorded medication usage, blood glucose measurements and episodes of hypoglycaemia or any other adverse events throughout the treatment period. For each hypoglycaemia episode, patients recorded details of the signs, symptoms, treatment, associated blood glucose concentrations, if obtained, and recovery.
Clinic or telephone visits were scheduled every 2 weeks during the first 12 weeks of treatment and every 4 weeks thereafter; additional visits were permitted at any time. Investigators reviewed patient diaries and adjusted the patients study insulin doses on an individual basis at each scheduled clinic or telephone visit, if appropriate, according to insulin titration algorithms based on those from previous publications (Table S1, Supporting Information) 6,12,13.
Health outcomes questionnaires [the Insulin Treatment Satisfaction Questionnaire (ITSQ) and Perceptions About Diabetes-Medications 21 (PAM-D21)] were completed at the beginning and completion of the study treatment period.
Patients
Eligible patients were aged between ≥18 and ≤75 years with type 2 diabetes 14 and had HbA1c levels ≥7.5 and ≤10.5% at the screening visit; a FPG concentration ≤6.7 mmol/l at the screening visit, as determined by central laboratory, or >6.7 mmol/l if the investigator considered further titration of basal insulin glargine was not possible for safety reasons; and were receiving stable doses of metformin (≥1500 mg/day for at least 8 weeks) and/or a stable dose of pioglitazone [≥30 mg/day (≥15 mg/day in Korea)] for at least 12 weeks (fixed dose combinations were acceptable) before screening. All patients must have been receiving treatment with once-daily basal insulin glargine for at least 90 days before the first screening visit.
Non-pregnant women who were taking precautions against pregnancy or were unable to become pregnant were eligible. Patients were excluded from the study if they had a body mass index (BMI) >45 kg/m2 at first screening visit; diagnosis of type 1 diabetes; stable dose of pioglitazone greater than the maximum dose approved for use in combination with insulin in their country; history of more than 2 weeks of scheduled prandial insulin use (including mixtures) within 12 weeks of screening; more than one episode of severe hypoglycaemia within 24 weeks before screening; history of concomitant disorders that contraindicated use of study medications; or were currently participating, or had previously participated, in another study within 30 days of screening.
Outcome Measures
The primary efficacy endpoint was the change in HbA1c levels after 24 weeks of treatment. Secondary efficacy endpoints were the change in HbA1c levels from baseline to 12 weeks; the proportion of patients who achieved a target HbA1c of <7.0 or ≤6.5% at 24 weeks; the change in FPG concentration from baseline to 12 and 24 weeks; 7-point SMBG profiles at 12 and 24 weeks; glycaemic variability, defined as the standard deviation in 7-point SMBG profiles at 12 and 24 weeks; daily insulin doses (total, basal and prandial) at 12 and 24 weeks; and the change in weight from baseline at 12 and 24 weeks.
Safety and tolerability were measured by treatment-emergent adverse events (TEAEs) and the incidence, rate and severity of hypoglycaemic episodes (categorized as overall and documented symptomatic ≤3.9 mmol/l, nocturnal and severe).
The patients‘ satisfaction with their insulin treatment was assessed using the ITSQ 15. Patients‘ perceptions about the acceptability and effectiveness of their diabetes medications and perceived emotional and physical adverse events were assessed using the PAM-D21 questionnaire 16.
Statistical Analysis
A total of 382 patients in the per-protocol (PP) population gave 90% power to conclude non-inferiority at the 5% significance level using a non-inferiority margin of 0.4, assuming the two treatment regimens were truly no different and the common standard deviation was 1.2%. The recruitment target was set at 478 patients, assuming that 20% of patients would not qualify for the PP population.
The primary analysis was a gated procedure that tested the two treatment regimens with respect to the change from baseline in HbA1c to 24 weeks for non-inferiority and, if this was met, for superiority. Non-inferiority of LM25 to IGL was concluded if the upper limit of the 95% confidence interval (CI) for the treatment difference (LM25 minus IGL) was entirely below 0.4%. The treatment difference was estimated using a likelihood-based mixed model for repeated measures (MMRM) analysis with baseline HbA1c as a covariate; treatment, country, week of visit and the treatment-by-week interaction as fixed effects; and patient and error as random effects. This analysis was performed on the PP population. For superiority testing, the same model was applied to the intention-to-treat (ITT) population. This hierarchical testing procedure required no adjustment of significance levels to maintain the overall significance level at 5%.
The PP population was defined as all randomized patients except those who did not complete the study or were substantially non-compliant; received study drug different from their randomized study treatment; or violated study inclusion, exclusion or discontinuation criteria. The ITT population was defined as all randomized patients who received at least one dose of study drug.
Changes in FPG, SMBG profile measures and glycaemic variability were analysed using MMRM. The model included the baseline value of the variable being tested treatment, country, baseline HbA1c stratification level, visit and the treatment-by-visit interaction as fixed effects; and patient as random effects.
The number of hypoglycaemic events per patient-year was estimated using a generalized linear model with the assumption of an underlying negative binomial distribution.
ITSQ and PAM-D21 questionnaires were analysed using analysis of covariance (ancova). Last observation carried forward (LOCF) was applied to these analyses, and missing items handled according to the corresponding scoring algorithm.
Results
Patients
Of the 743 patients screened for participating in the study, 476 were randomized to 24 weeks of treatment with LM25 (n = 236) or IGL (n = 240) and received at least one dose of study drug; an additional two patients were randomized to IGL but received no study treatment (Figure 1). Patients were most commonly excluded from the study because they did not meet the participating criteria (n = 255; 96.2% of those excluded). Patients were enrolled at 55 sites across 11 countries (Argentina, Brazil, China, Egypt, India, Mexico, Republic of Korea, Romania, Russia, Spain and Turkey).
Figure 1.
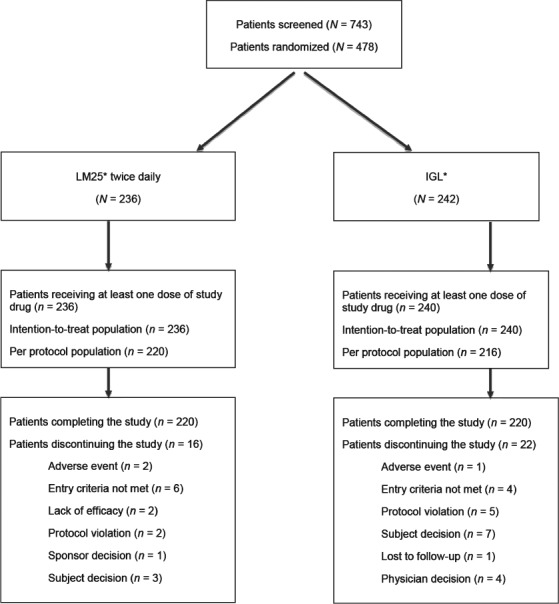
Summary of patient disposition. *Insulin lispro low mixture (insulin lispro protamine suspension 75% and insulin lispro solution 25%) twice daily; basal insulin glargine once daily and prandial insulin lispro once daily.
At baseline, patients had a mean (s.d.) age of 57.5 (9.5) years and mean (s.d.) HbA1c level of 8.62 (0.77)%; 47.1% had baseline HbA1c < 8.5%. Overall, 99.8% of patients were receiving metformin and 5.9% were receiving pioglitazone at baseline (one patient in the IGL group received concomitant pioglitazone as the only oral medication; all other patients receiving pioglitazone also received metformin). Pre-existing conditions were reported by 81.3% of patients, most commonly vascular (62.2%), and metabolic and nutritional (51.3%) disorders, and 81.7% of patients were receiving concomitant medications for conditions other than diabetes. Other baseline characteristics are summarized in Table1. The majority of patients in the IGL group received insulin lispro before lunch (45.8%) or dinner (35.0%).
Baseline patient characteristics for the intention-to-treat population
| Characteristics | LM25 bid* (N = 236) | IGL* (N = 240) | Overall (N = 476) |
|---|---|---|---|
| Sex (male, %) | 49.2 | 40.8 | 45.0 |
| Race (%) | |||
| White | 56.4 | 56.7 | 56.5 |
| Asian | 33.9 | 33.3 | 33.6 |
| Black or African American | 2.1 | 0.4 | 1.3 |
| American Indian or Alaskan Native | 6.4 | 7.1 | 6.7 |
| Age, years [mean (s.d.)] | 57.4 (9.9) | 57.7 (9.1) | 57.5 (9.5) |
| Weight, kg [mean (s.d.)] | 78.7 (15.6) | 78.5 (15.2) | 78.6 (15.4) |
| BMI, kg/m2 [mean (s.d.)] | 29.4 (5.1) | 29.8 (5.1) | 29.6 (5.1) |
| Duration of diabetes, years [mean (s.d.)] | 12.2 (7.7) | 11.3 (6.8) | 11.7 (7.3) |
| HbA1c, % [mean (s.d.)] | 8.7 (0.8) | 8.6 (0.7) | 8.6 (0.8) |
| HbA1c, mmol/mol [mean (s.d.)] | 71 (8.6) | 70 (8.2) | 71 (8.4) |
| FPG, mmol/l [mean (s.d.)] | 6.4 (2.0) | 6.2 (1.8) | 6.3 (1.9) |
| SMBG, mmol/l [mean (s.d.)] | |||
| Before breakfast | 6.7 (1.7) | 6.4 (1.5) | 6.5 (1.6) |
| After breakfast | 10.2 (2.6) | 10.0 (2.4) | 10.1 (2.5) |
| Before lunch | 8.2 (2.1) | 8.3 (2.3) | 8.2 (2.2) |
| After lunch | 10.7 (2.6) | 11.0 (2.5) | 10.9 (2.6) |
| Before dinner | 9.0 (2.4) | 9.5 (2.5) | 9.2 (2.5) |
| After dinner | 11.2 (2.7) | 11.2 (2.8) | 11.2 (2.7) |
| 03:00 hours | 10.1 (2.8) | 10.2 (2.9) | 10.1 (2.9) |
| Daily average | 9.4 (1.9) | 9.5 (1.8) | 9.4 (1.8) |
| Glycaemic variability, mmol/L [mean (s.d.)]† | 2.5 (1.0) | 2.7 (1.0) | 2.6 (1.0) |
| Insulin glargine dose at screening visit, IU | 33.8 (18.7) | 33.5 (17.1) | 33.6 (17.9) |
| Concomitant oral antihyperglycaemic medication | |||
| Metformin, % | 100.0 | 99.6 | 99.8 |
| Daily dose, mg [mean (s.d.)] | 1937.2 (394.9) | 1972.7 (379.1) | 1955.1 (387.0) |
| Pioglitazone, % | 5.5 | 6.3 | 5.9 |
| Daily dose, mg [mean (s.d.)] | 28.8 (4.2) | 30.0 (0.0) | 29.5 (2.8) |
bid, twice daily; BMI, body mass index; FPG, fasting plasma glucose, HbA1c, glycosylated haemoglobin; IGL, insulin glargine once daily and insulin lispro once daily; LM25, lispro low mixture; s.d., standard deviation; SMBG, self-monitoring of blood glucose.
*Insulin lispro low mixture (insulin lispro protamine suspension 75% and insulin lispro solution 25%); basal insulin glargine once daily and prandial insulin lispro once daily.
†Measured using the standard deviation of SMBG results.
Primary Endpoint
The least squares (LS) mean (95% CI) treatment difference at week 24 was −0.21 (−0.38, −0.04), which showed non-inferiority between the two treatment strategies for the PP population. The corresponding LS mean (95% CI) treatment difference for the ITT population was −0.22 (−0.39, −0.05) indicating a statistically significant advantage for LM25 compared with IGL (p = 0.010). The estimated change [LS mean (95% CI)] in HbA1c for the ITT population after 24 weeks was −1.30 (−1.44, −1.16)% with LM25 and −1.08 (−1.22, −0.94)% with IGL.
Secondary Efficacy Endpoints
All secondary analyses were performed using the ITT population. There was no significant between-treatment difference shown in the secondary efficacy end points of change in HbA1c after 12 weeks; change in FPG at 12 or 24 weeks; daily average blood glucose level, measured using SMBG, at 4 (data not shown), 12 and 24 weeks; or glycaemic variability at 4, 12 and 24 weeks (Table S2). The unadjusted changes in HbA1c levels throughout the study are presented in Figure 2a. No difference between treatments was shown for the percentages of patients achieving HbA1c targets of <7.0 or ≤6.5% at 12 or 24 weeks (34.5% of the LM25 group vs. 30.0% of the IGL group achieved HbA1c < 7.0% at week 24; p = 0.359; other data not presented). However, some differences were observed between treatments when SMBG results were compared at each measurement time: at 12 and 24 weeks, SMBG levels were significantly lower in the IGL group before breakfast (p < 0.01), and significantly lower in the LM25 group before lunch (p < 0.01; Table S2). Unadjusted changes in SMBG levels are shown in Figure 2b.
Figure 2.
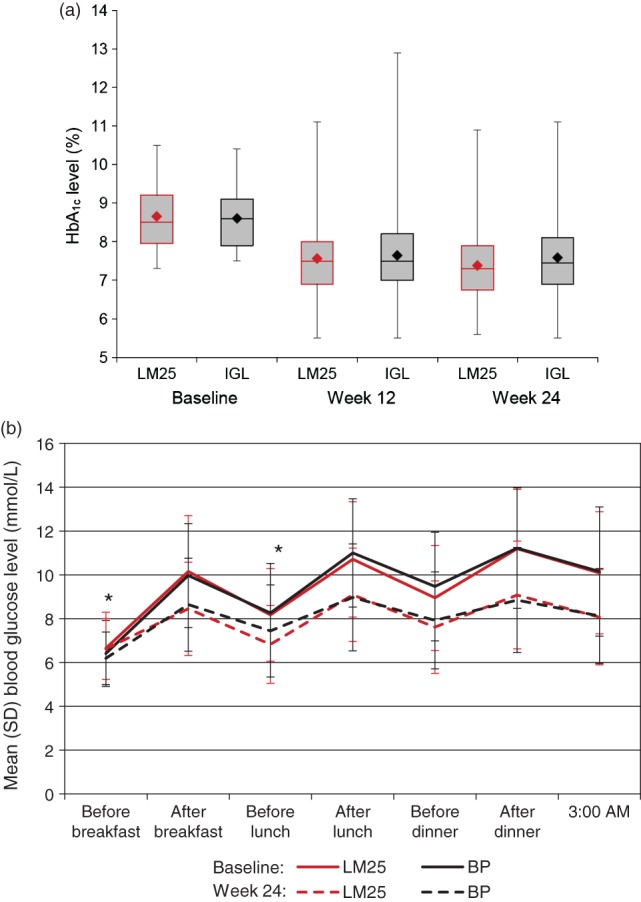
Glycaemic control throughout the study for the intention-to-treat population receiving insulin lispro low mixture (LM25; insulin lispro protamine suspension 75% and insulin lispro solution 25%) twice daily or basal insulin glargine once daily and prandial insulin lispro once daily (IGL). (a) Box plot of unadjusted HbA1c levels, showing median values (horizontal line) and interquartile range (shaded box), with minimum and maximum values (whiskers) and mean values (diamond) at baseline, and 12 and 24 weeks. (b) Mean unadjusted 7-point self-monitoring of blood glucose levels at baseline and 24 weeks. *At 12 and 24 weeks, self-monitoring of blood glucose (SMBG) levels estimated using a likelihood-based mixed model for repeated measure analysis were significantly lower in the IGL group before breakfast (p < 0.01), and significantly lower in the LM25 group before lunch (p < 0.01; estimated values are reported in Table S2).
On the patients‘ final visit, total mean (s.d.) daily dose of LM25 was 53.1 (24.6) IU and total mean (s.d.) dose of IGL was 50.8 (22.0) IU (p = 0.409). Respective doses of basal insulin were 39.8 (18.5) IU and 37.4 (18.8) IU (p = 0.102), and respective doses of prandial insulin were 13.3 (6.2) IU versus 13.5 (6.5) IU (p = 0.488).
Safety and Tolerability
At least one TEAE was reported by 114 patients (48.3%) in the LM25 group and 94 patients in the IGL group (39.2%; p = 0.052), although only 9 (3.8%) and 7 (2.9%) patients, respectively, experienced events that were considered possibly related to the study treatments (p = 0.621). Serious TEAEs were reported by 11 (4.7%) and 8 (3.3%) patients, respectively (p = 0.492). Two patients in the LM25 group and one patient in the IGL group discontinued the study because of adverse events. No patient died during the study.
The percentages of patients experiencing at least one episode of overall, symptomatic, nocturnal or severe hypoglycaemia (see Table2 for definitions) did not differ significantly between treatment groups (p > 0.05 for all assessment periods; Table2). Similarly, using negative binomial models, the number of episodes of symptomatic (p = 0.513), nocturnal (p = 0.810) and severe (p = 0.787) hypoglycaemia per patient-year did not differ significantly between treatment groups.
Reported hypoglycaemia with insulin lispro low mixture (LM25; insulin lispro protamine suspension 75% and insulin lispro solution 25%) twice daily or basal insulin glargine once daily and prandial insulin lispro once daily (IGL) in patients with type 2 diabetes
| Hypoglycaemia | LM25 (N = 236) | IGL (N = 240) | ||
|---|---|---|---|---|
| Patients with ≥1 episode n (%) | No. of episodes per patient-year [mean (s.d.)] | Patients with ≥1 episode n (%) | No. of episodes per patient-year [mean (s.d.)] | |
| Overall (≤3.9 mmol/l) | 144 (61.0) | 13.07 (22.03) | 150 (62.5) | 16.51 (26.44) |
| Documented symptomatic (≤3.9 mmol/l) | 109 (46.2) | 7.21 (14.55) | 110 (45.8)* | 7.72 (15.67) |
| Asymptomatic (≤3.9 mmol/l) | 97 (41.1) | 5.18 (12.62) | 109 (45.4) | 8.34 (18.00) |
| Nocturnal | 50 (21.2) | 1.54 (4.58) | 52 (21.7) | 1.82 (5.25) |
| Severe | 2 (0.8)† | 0.04 (0.45) | 0 | 0 |
Documented symptomatic hypoglycaemia (≤3.9 mmol/l) was defined as any event during which typical symptoms of hypoglycaemia are accompanied by a measured plasma glucose concentration ≤3.9 mmol/l. Asymptomatic hypoglycaemia ≤3.9 mmol/l was defined as any event not accompanied by typical symptoms of hypoglycaemia but with a measured plasma glucose concentration ≤3.9 mmol/l. Nocturnal hypoglycaemia was defined as any hypoglycaemic event that occurred between bedtime and waking. Severe hypoglycaemia was defined as any hypoglycaemic event in which the patient required the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions; the blood glucose concentration may not have been measured during the event, but neurologic recovery that was attributable to the restoration of a normal blood glucose concentration was considered as sufficient evidence that the event was induced by a low-plasma glucose concentration. Between-treatment differences were not significant (p > 0.05).
*One patient discontinued treatment because of hypoglycaemia.
†Neither patient required discontinuation of treatment.
By 24 weeks of treatment, the LS mean (95% CI) body weight increase was 1.13 (0.75, 1.52) kg with LM25 and 0.50 (0.11, 0.89) kg with IGL (p = 0.018).
Health Outcomes
ITSQ domain and total scores, and PAM-D21 questionnaires domain scores at last visit, and changes in these scores from baseline to last visit, showed no statistically significant differences between treatments.
Discussion
To our knowledge, this is the first randomized study comparing these two insulin intensification strategies after basal insulin failure in a clearly defined population during a standard period of time. The published head-to-head data we identified typically involved the use of multiple prandial insulin doses 9,10,17, in contrast with the single prandial dose used in the basal–prandial insulin regimen in our study. However, Riddle et al. studied three strategies to initiate and progress insulin therapy, and compared basal plus one prandial and basal plus up to three prandial injections with premixed insulin bid 11.
In this study, patients had inadequate glycaemic control, defined as baseline HbA1c≥7.5% and a FPG concentration of ≤6.7 mmol/l (or >6.7 mmol/l if further titration of basal insulin glargine was not possible for safety reasons or previous recent measurements confirmed that the screening test results were not consistent with the patient‘s clinical situation). Collectively, these inclusion criteria ensured that enrolled patients had inadequate glycaemic control on their baseline treatment regimen, and required advancing insulin therapy that also targeted postprandial glycaemia. The improvement in HbA1c was achieved without a clinically significant effect on FPG, suggesting that it was mainly the postprandial component of hyperglycaemia that was reduced. These results are consistent with other communications 7,18, probably reflecting the fact that the basal insulin glargine regimen had already been optimized in these patients and the need was for additional prandial coverage. Our study differs from others in that we did not include a run-in period to optimize insulin glargine, although it seems that we correctly identified patients who were already receiving optimized doses of basal insulin, as shown by the FPG concentration ≤6.7 mmol/l. This can be seen as a more pragmatic approach which is applicable in real life clinical practice.
Although data comparing bid insulin regimens are lacking, the non-inferiority of LM25 compared with IGL in this study could be expected. The impact on postprandial glycaemia after breakfast and dinner with LM25 should show at least similar results to the addition of a single injection of insulin lispro with the main meal. Despite the significant decrease in HbA1c in both groups after 24 weeks, the percentages of patients attaining goals of <7.0 or ≤6.5% were low, in agreement with other reports 3, which might indicate the need for further treatment intensification or that patients entered the study with high baseline HbA1c levels. We cannot speculate on the potential results of a long-term follow-up, as our study was designed to compare two insulin intensification strategies as a first step after the initial insulin regimen had failed, with a duration that was similar to that of other studies of this type 8–10,19.
In this study, we did not observe significant differences between the two groups with regard to the total, basal or prandial insulin doses used. Interestingly, no obvious differences seemed to exist in the split (basal/prandial) between the groups, although the prandial dose was divided into two for patients receiving LM25, whereas there was only one prandial injection for the IGL group. Hypoglycaemia has typically been a concern when premixed insulin is considered as a treatment option. In this study, as opposed to that of Riddle et al. 11, we did not find any significant difference in the proportions of patients experiencing overall, symptomatic, asymptomatic or nocturnal hypoglycaemic episodes with LM25 and IGL. There were only two patients in the LM25 group with severe hypoglycaemia, but neither of them had to be discontinued from the study. We consider that the mean observed changes in weight were not clinically relevant, and this may be related to the lack of observed differences in insulin dose at the end of the study.
Several factors require consideration when making the final decision regarding the best therapeutic option for an individual patient, such as patient‘s lifestyle, extent of education on the disease, likely compliance with therapy and the available resources 2. Convenient and effective insulin intensification regimens are important as studies have shown that, despite a knowledge of glycaemic targets, insulin regimens used in clinical practice are often inadequate to achieve or maintain good control 20,21. In this study, the lack of differences observed in responses to the questionnaires suggests that both regimens are likely to be similarly accepted as therapeutic options by patients with type 2 diabetes. However, that the number of daily injections was same in both groups, and that all patients were previously receiving insulin, may have accounted for the lack of differences observed in responses to the questionnaires.
Limitations of this study included the open-label design, dictated by the fact that the two regimens used insulin with different appearances, dosing requirements and injection devices. That the mealtime bolus was given with the same meal throughout the study and no flexibility in dose scheduling was allowed may also have been limitations; however, we believe that this would not have had an impact on study results, as the bolus was given with the largest daily meal (previously defined by glucose values and patients‘ reports), irrespective of the country, diet or time of the day. The study was not powered to assess potential differences between the insulin regimens depending on the timing of the main meal, so any impact of this on glycaemic profile, variability or hypoglycaemia incidence may be further assessed in future studies. The study duration may be seen as a limitation, as long-term effects of these or further intensification strategies were not explored. However, the study was designed to determine patient outcomes after the first insulin intensification step only, and this duration enables comparability with the results of previous studies 8–10,19.
The heterogeneous nature of the patient population included in this study is both a strength and limitation. Between-country differences may exist in clinical practice guidelines, prescribing practices and dietary habits, and the study was not powered to assess the potential differences between treatment arms in each country/region. In contrast, by conducting the study globally in 11 countries, patients of different ethnic origins were included, thereby improving the generalizability of results. Further studies may be needed to elucidate the best regimen for any specific population/country.
Conclusions
In patients with type 2 diabetes inadequately controlled on once-daily basal insulin glargine and metformin and/or pioglitazone, intensification with either LM25 or IGL improved glycaemic control with similar tolerability and acceptability. The reduction in HbA1c after 24 weeks was greater with LM25 than with IGL. The results of our study support current guidelines, and add to the body of evidence supporting the use of both insulin regimens as an option for insulin intensification in patients inadequately controlled with a basal-only insulin regimen plus oral antihyperglycaemic medications.
Acknowledgments
The study was sponsored by Eli Lilly. The authors would like to thank the patients and their families, and all the investigators, nurses and study coordinators who cared for them. The authors also thank the study team for conducting the study, and Tibor Ivanyi and Helene Sapin for critically reviewing the manuscript. In addition, we acknowledge Caroline Spencer and Claire Lavin (Rx Communications, UK) for medical writing assistance with this manuscript. The trial was registered with ClinicalTrials.gov. number NCT01175824.
Conflict of Interest
Angel Rodriguez and Simon Cleall are employees of and shareholders in Eli Lilly. Adriana Onaca has no conflicts of interest. J. L. G. has received grants/research support from Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly and Novo-Nordisk, and served as a consultant or member of a scientific advisory panel/speakers bureau for Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly and Novo-Nordisk. F. J. T. has served on scientific advisory boards and received honoraria or consulting fees or grants/research support from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Hoffmann-La Roche, Merck Serono, MSD, Novartis, Novo-Nordisk and Sanofi. F. J. T. contributed to the study design. F. J. T., J. L. G., A. O., S. C. and A. R. involved in drafting the manuscript and have read and approved the final version. F. J. T., J. L. G. and A. O. also contributed to patient enrollment. S. C. contributed to the statistical analysis. A. R. contributed to the study conception and design.
Supporting Information
Table S1. Dosing algorithm.
Table S2. Glycaemic efficacy variables after treatment with insulin lispro low mixture (LM25; insulin lispro protamine suspension 75% and insulin lispro solution 25%) twice daily or basal insulin glargine once daily and prandial insulin lispro once daily (IGL) in patients with type 2 diabetes; intention-to-treat population.
References
- 1.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 3.Esposito K, Chiodini P, Bellastella G, Maiorino MI, Giugliano D. Proportion of patients at HbA1c target <7% with eight classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78 945 patients. Diabetes Obes Metab. 2012;14:228–233. doi: 10.1111/j.1463-1326.2011.01512.x. [DOI] [PubMed] [Google Scholar]
- 4.Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19:327–336. doi: 10.4158/endp.19.2.a38267720403k242. [DOI] [PubMed] [Google Scholar]
- 5.Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA. Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008;10:1178–1185. doi: 10.1111/j.1463-1326.2008.00967.x. [DOI] [PubMed] [Google Scholar]
- 6.Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept‘ study. Diabetes Obes Metab. 2011;13:1020–1027. doi: 10.1111/j.1463-1326.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson MB, Raskin P, Tanenberg R, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395–403. doi: 10.4158/EP10323.OR. [DOI] [PubMed] [Google Scholar]
- 8.Malone JK, Bai S, Campaigne BN, Reviriego J, Augendre-Ferrante B. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycaemic control in patients with type 2 diabetes. Diabet Med. 2005;22:374–381. doi: 10.1111/j.1464-5491.2005.01511.x. [DOI] [PubMed] [Google Scholar]
- 9.Miser WF, Arakaki R, Jiang H, Scism-Bacon J, Anderson PW, Fahrbach JL. Randomized, open-label, parallel-group evaluations of basal-bolus therapy versus insulin lispro premixed therapy in patients with type 2 diabetes mellitus failing to achieve control with starter insulin treatment and continuing oral antihyperglycemic drugs: a noninferiority intensification substudy of the DURABLE trial. Clin Ther. 32:896–908. doi: 10.1016/j.clinthera.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care. 31:20–25. doi: 10.2337/dc07-1122. [DOI] [PubMed] [Google Scholar]
- 11.Riddle MC, Rosenstock J, Vlajnic A, Gao L. Randomized, 1-year comparison of three ways to initiate and advance insulin for type 2 diabetes: twice-daily premixed insulin versus basal insulin with either basal-plus one prandial insulin or basal-bolus up to three prandial injections. Diabetes Obes Metab. 2010;16:396–402. doi: 10.1111/dom.12225. 2008–2014. [DOI] [PubMed] [Google Scholar]
- 12.Buse JB, Wolffenbuttel BHR, Herman WH, et al. DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24-week results. Diabetes Care. 2009;32:1007–1013. doi: 10.2337/dc08-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R AT.LANTUS study group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes. Comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282–1288. doi: 10.2337/diacare.28.6.1282. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. 1999. . Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. World Health Organization, Department of Noncommunicable Diseases Surveillance, Geneva, Switzerland, . Document WHO/NCD/NCS/99.2.
- 15.Anderson RT, Skovlund SF, Marrero D, et al. Development and validation of the insulin treatment satisfaction questionnaire. Clin Ther. 2004;26:565–578. doi: 10.1016/s0149-2918(04)90059-8. [DOI] [PubMed] [Google Scholar]
- 16.Monahan PO, Lane KA, Hayes RP, McHorney CA, Marrero DG. Reliability and validity of an instrument for assessing patients perceptions about medications for diabetes: the PAM-D. Qual Life Res. 2009;18:941–952. doi: 10.1007/s11136-009-9510-2. [DOI] [PubMed] [Google Scholar]
- 17.Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Ceriello A, Esposito K. Efficacy of insulin analogs in achieving the hemoglobin A1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Care. 2011;34:510–517. doi: 10.2337/dc10-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelman SV, Liu R, Johnson J, Glass LC. AUTONOMY: The first randomized trial comparing two patient-driven approaches to initiate and titrate prandial insulin lispro in type 2 diabetes. Diabetes Care. 2014;37:2132–2140. doi: 10.2337/dc13-2664. [DOI] [PubMed] [Google Scholar]
- 19.Gumprecht J, Benroubi M, Borzi V, et al. IMPROVE study group expert panel. Intensification to biphasic insulin aspart 30/70 (BIAsp 30, NovoMix 30) can improve glycaemic control in patients treated with basal insulins: a subgroup analysis of the IMPROVE observational study. Int J Clin Pract. 2009;63:966–972. doi: 10.1111/j.1742-1241.2009.02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris SB, Kapor J, Lank CN, Willan AR, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Physician. 2010;56:e418–e424. [PMC free article] [PubMed] [Google Scholar]
- 21.Liebl A, Jones S, Goday A, et al. Clinical outcomes after insulin initiation in patients with type 2 diabetes: 24-month results from INSTIGATE. Diabetes Ther. 2012;3:9. doi: 10.1007/s13300-012-0009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Dosing algorithm.
Table S2. Glycaemic efficacy variables after treatment with insulin lispro low mixture (LM25; insulin lispro protamine suspension 75% and insulin lispro solution 25%) twice daily or basal insulin glargine once daily and prandial insulin lispro once daily (IGL) in patients with type 2 diabetes; intention-to-treat population.


