Abstract
Aim
The broadly used combination of metformin and sulphonylurea (SU) often fails to bring patients to glycaemic goal. This study assessed the efficacy and safety of vildagliptin as add-on therapy to metformin plus glimepiride combination in patients with type 2 diabetes mellitus (T2DM) who had inadequate glycaemic control.
Methods
A multicentre, double-blind, placebo-controlled study randomized patients to receive treatment with vildagliptin 50 mg bid (n = 158) or placebo (n = 160) for 24 weeks.
Results
After 24 weeks, the adjusted mean change in haemoglobin A1c (HbA1c) was −1.01% with vildagliptin (baseline 8.75%) and −0.25% with placebo (baseline 8.80%), with a between-treatment difference of −0.76% (p < 0.001). Significantly more patients on vildagliptin achieved the HbA1c target <7% (28.3% vs. 5.6%; p < 0.001). The difference in fasting plasma glucose reduction between vildagliptin and placebo was −1.13 mmol/l (p < 0.001). In subgroup of patients with baseline HbA1c ≤8%, vildagliptin reduced HbA1c by 0.74% from baseline 7.82% (between-treatment difference: –0.97%; p < 0.001) with significantly more patients achieving the HbA1c target <7% (38.6% vs. 13.9%; p = 0.014). Vildagliptin was well tolerated with low incidence of hypoglycaemia, slightly higher than with placebo (5.1% vs. 1.9%) and no clinically relevant weight gain.
Conclusions
Vildagliptin significantly improved glycaemic control in patients with T2DM inadequately controlled with metformin plus glimepiride combination. The addition of vildagliptin was well tolerated with low risk of hypoglycaemia and weight gain. This makes vildagliptin an attractive treatment option for patients failing on metformin plus SU particularly in patients with baseline HbA1c ≤8%.
Keywords: DPP-4 inhibitor, glimepiride, metformin, oral antidiabetic drug, type 2 diabetes, vildagliptin
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic progressive disease that often requires combination of antidiabetic drugs with different mechanisms of action to achieve glycaemic targets 1–3. The broadly used combination of metformin and a sulphonylurea (SU) fails to maintain glycaemic control over time 4 and the addition of a third antihyperglycaemic agent is required.
When choosing options for the third agent, physicians should consider improvement of glycaemic control without additional risks such as hypoglycaemia and weight gain 5. While insulin is recommended as a preferred next step by many international and local guidelines in patients failing on dual therapy 1–3, parenteral administration, increased risk of hypoglycaemia and weight gain may limit the use of insulin. Negative attitudes towards initiation of insulin and a preference for oral therapies by many patients should also be taken into account. Hence, a third oral agent that provides sustained glycaemic control and delays the time to permanent use of insulin could benefit patients who are reluctant to start injectable therapy 1,6.
Vildagliptin, a potent and selective inhibitor of dipeptidyl peptidase-4 (DPP-4), improves glycaemic control by increasing the availability of endogenous incretin hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) 7,8. Complementing the pharmacological effect of metformin, vildagliptin enhances glucose-dependent insulin secretion and suppresses glucagon release, thereby improving glycaemic control, and contributing to weight-neutrality and reduced hypoglycaemia 9. Vildagliptin has demonstrated similar efficacy as an add-on to metformin when compared to SU with markedly reduced hypoglycaemia risk and no weight gain 10,11.
This study evaluated the efficacy and safety of vildagliptin 50 mg bid as an add-on therapy in patients with T2DM inadequately controlled with dual therapy of metformin (≥1500 mg) and glimepiride (≥4 mg). Of particular interest was whether and which patient population could achieve glycaemic control with such a triple combination.
Methods
Study Design and Patients
This was a 24-week, multicentre, randomized, double-blind, placebo-controlled study in patients with T2DM. Eligible patients were 18–80 years of age, had body mass index (BMI) ≥22 to ≤45 kg/m2, and were inadequately controlled on a stable dose of oral antidiabetic drugs (OADs) for at least 12 weeks prior to the screening visit. Acceptable background therapy prior to enrollment included metformin ≥1500 mg as monotherapy [haemoglobin A1c (HbA1c) ≥8.5 and ≤11%] or dual combination of metformin ≥1500 mg with SU, thiazolidinedione (TZD) or glinide (HbA1c ≥7.5 and ≤11%). Eligible patients continued their current metformin treatment ≥1500 mg throughout the study. Patients were excluded if they had fasting plasma glucose (FPG) ≥15.0 mmol/l; significant hepatic, renal or cardiovascular medical conditions; significant laboratory abnormalities; and pregnant or lactating females.
The study consisted of a 1–2 week screening period, an up to 12-week titration and/or stabilization period (depending on the type and dose of background OAD at study entry) and a 24-week double-blind treatment period. After screening, eligible patients who were (i) on metformin ≥1500 mg plus glimepiride ≥4 mg for at least 12 weeks proceeded directly to randomization; (ii) on metformin monotherapy ≥1500 mg entered a titration and stabilization period for glimepiride up to 4 mg; (iii) on any other combination with metformin discontinued their SU, TZD or glinide therapy, and entered titration and/or stabilization for glimepiride up to 4 mg. Patients were discontinued from the study if they could not tolerate the prescribed dose of metformin ≥1500 mg or glimepiride ≥4 mg during stabilization period. Eligible patients with HbA1c ≥7.5 and ≤11% at the end of stabilization period were randomized (1 : 1) to receive either vildagliptin 50 mg bid or placebo in addition to their metformin ≥1500 mg plus glimepiride ≥4 mg therapy for 24 weeks.
After randomization, the dose of metformin (≥1500 mg) was kept stable. The dose of glimepiride, however, could be adjusted downward for safety reasons at the investigators’ discretion. Rescue medication (insulin or pioglitazone, per investigator discretion) was prescribed if the patient had FPG <13.3 mmol/l between week 6 and 12, FPG >11.1 mmol/l between week 12 and 24 or symptoms of worsening of hyperglycaemia at any visit.
Study Assessments and Endpoints
The primary endpoint was change in HbA1c from baseline to week 24 or to the final visit. Secondary efficacy assessments included change in FPG from baseline to week 24 endpoint and responder rates achieving HbA1c targets of <7 or ≤6.5%. Safety assessments included recording and monitoring of treatment-emergent adverse events (AEs); biochemistry and haematology laboratory test results; electrocardiogram (ECG) findings and vital signs. Hypoglycaemia was defined by symptoms suggestive of hypoglycaemia and a self-monitored plasma glucose measurement <3.1 mmol/l. Severe hypoglycaemia was defined as an episode that required assistance of another person or hospitalization with or without a plasma glucose measurement <3.1 mmol/l.
Statistical Analysis
A sample size of 246 completed patients (123 per arm) would ensure 90% power with a one-sided significance level of 2.5% to declare superiority of vildagliptin 50 mg bid over placebo in HbA1c reduction (%) from baseline after 24 weeks of treatment, assuming a clinically relevant difference of 0.5 absolute units between treatments and a standard deviation of 1.2%. Assuming a drop-out rate of 15%, about 290 patients (145 patients per arm) were to be randomized with an equal randomization ratio 1 : 1 to the two treatment groups.
The adjusted mean changes in HbA1c and FPG from baseline to week 24 were compared between vildagliptin and placebo using an analysis of covariance model with treatment and pooled centre as a classification factor and baseline HbA1c as a covariate. This comparison was performed on full analysis set (FAS) consisting of all randomized patients who received at least one dose of the study drug and had at least one post-randomization efficacy parameter measurement. In addition, responder rates (percentage of patients achieving endpoint HbA1c <7.0 or ≤6.5%) were compared between treatments using a chi-squared test.
Efficacy data used in the analyses were censored at the start of rescue medication. The last observation carried forward (LOCF) method was used to handle missing data because of early discontinuation or data censoring. Safety data were summarized descriptively by treatment. All data of patients who received at least one dose of study medication were included in analysis for safety assessment.
Ethical Statement
This trial was conducted in accordance with the Declaration of Helsinki. An independent ethics committee or institutional review board at each research site reviewed the study protocol. Each patient gave written informed consent before randomization.
Results
Patient Disposition and Baseline Characteristics
The disposition of patients from screening to study endpoint is depicted in figure 1. Of the 564 patients screened, 318 were randomized to vildagliptin (n = 158) and placebo (n = 160). The most common reason for screen failure was having not met the diagnostic/severity criteria (66.8%) and unacceptable laboratory values (55.7%). The percentage of randomized patients who discontinued the study was overall low and slightly higher in the vildagliptin group (8.9%) than in the placebo group (3.1%) mainly due to a greater percentage of patients who withdrew consent (4.4% vs. 1.3%, respectively).
Figure 1.
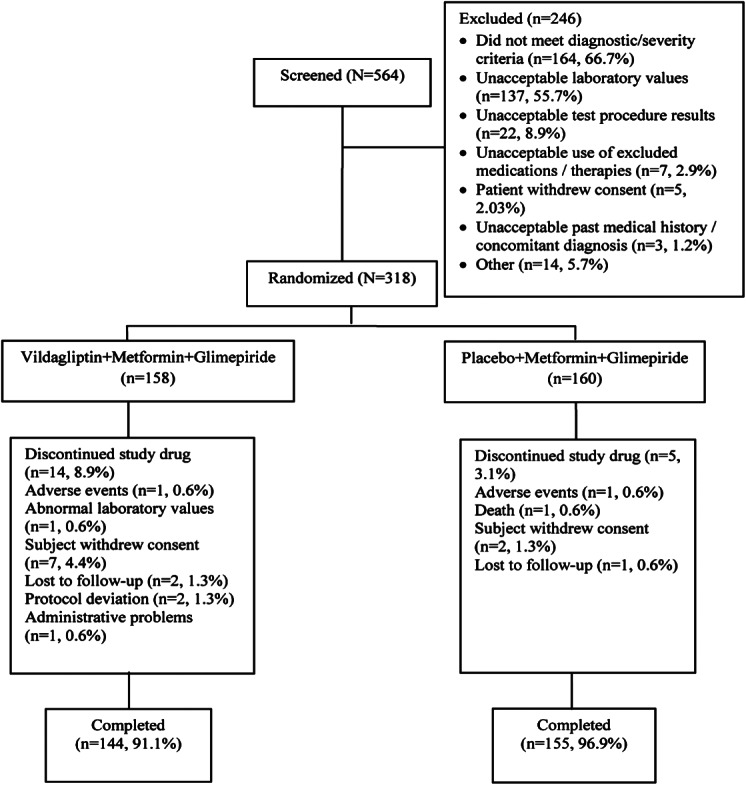
Patient disposition.
The demographic and baseline characteristics of the randomized patients were generally similar between the treatment groups (Table 1). The mean dose of metformin at screening was 1810 mg/day with 43.7% of patients receiving ≥2000 mg daily dose. Approximately 80% of patients (n = 254) were already treated with metformin plus glimepiride with a mean daily glimepiride dose of 4.4 mg; 35 of those patients who were on glimepiride <4 mg were up-titrated to 4 mg. Besides glimepiride, 59 (18.5%) patients were receiving other SUs, 1 (0.3%) patient was on a TZD and 4 (1.2%) patients were on metformin monotherapy at screening; all these patients were switched and up-titrated to glimepiride 4 mg as per protocol.
Table 1.
Patient baseline demographic and background characteristics (randomized set)
| Vildagliptin + Metformin + GlimepirideN = 158 | Placebo + Metformin + Glimepiride N = 160 | Total N = 318 | |
|---|---|---|---|
| Age, years | 55.3 (10.2) | 55.0 (11.1) | 55.1 (10.6) |
| ≥65, n (%) | 29 (18.4) | 38 (23.8) | 67 (21.1) |
| Gender, female, n (%) | 78 (49.4) | 88 (55.0) | 166 (52.2) |
| Race, n (%) | |||
| Asian | 116 (73.4) | 116 (72.5) | 232 (73.0) |
| Indian | 81 (51.3) | 77 (48.1) | 158 (49.7) |
| Chinese | 12 (7.6) | 21 (13.1) | 33 (10.4) |
| Caucasian | 34 (21.5) | 38 (23.8) | 72 (22.6) |
| Other | 8 (5.1) | 6 (3.8) | 14 (4.4) |
| BMI, kg/m2 | 27.9 (4.6) | 28.0 (4.5) | 28.0 (4.5) |
| HbA1c, % | 8.7 (0.9) | 8.8 (0.9) | 8.8 (0.9) |
| ≤8%, n (%) | 48 (30.4) | 36 (22.5) | 84 (26.4) |
| ≤9%, n (%) | 99 (62.7) | 102 (63.8) | 201 (63.2) |
| FPG, mmol/l | 9.3 (2.4) | 9.5 (2.1) | 9.4 (2.3) |
| Duration of T2DM, years | 7.1 (6.2) | 7.5 (6.1) | 7.3 (6.1) |
| GFR (MDRD), ml/min/1.73 m2, n (%) | |||
| Normal, >80 | 99 (62.7) | 104 (65.0) | 203 (63.8) |
| Mild, ≥50 to ≤80 | 55 (34.8) | 50 (31.3) | 105 (33.0) |
| Moderate, ≥30 to <50 | 4 (2.5) | 6 (3.8) | 10 (3.1) |
Values are mean (s.d.) unless indicated otherwise. BMI, body mass index; FPG, fasting plasma glucose; GFR, glomerular filtration rate; HbA1c, haemoglobin A1c; MDRD, modification of diet in renal disease; T2DM, type 2 diabetes mellitus.
Efficacy
Vildagliptin had a sustained glucose-lowering effect during 24 weeks of treatment (figure 2A). The adjusted mean change in HbA1c at study end point in the vildagliptin group of −1.01% (baseline 8.75%) was significantly different from the 0.25% reduction in the placebo group (baseline 8.80%) with a between-treatment difference of −0.76% (p < 0.001) (figure 2B). Changes in HbA1c in subgroup analyses by baseline HbA1c, BMI, age, gender and race were in line with the overall study results.
Figure 2.
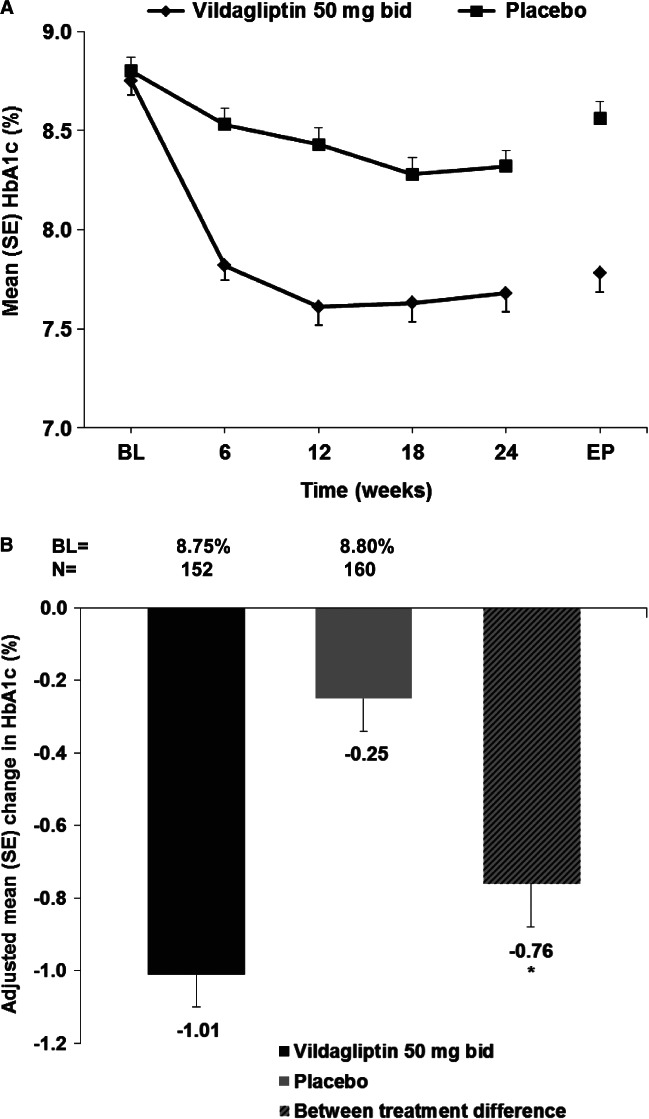
(A) Mean HbA1c (%) by treatment and visit (full analysis set). (B) Adjusted mean change in HbA1c from baseline to endpoint (full analysis set). BL, baseline; EP, endpoint; *p < 0.001.
After 24 weeks of treatment, a significantly higher percentage of patients on vildagliptin achieved HbA1c targets compared with placebo (HbA1c <7%: 28.3% vs. 5.6%, or HbA1c ≤6.5%: 13.2% vs. 1.3%, respectively; p < 0.001).
Vildagliptin demonstrated a clinically relevant reduction in FPG of 1.11 mmol/l (baseline 9.34 mmol/l) compared with nearly no change in the placebo group of +0.02 mmol/l (baseline 9.52 mmol/l). The difference versus placebo of −1.13 mmol/l was clinically and statistically significant (p < 0.001). Rescue medication was used by fewer patients in the vildagliptin group (n = 6/158, 3.8%) compared with the placebo group (n = 22/160, 13.8%).
In a subgroup of patients with baseline HbA1c ≤8% (n = 80, 44 patients on vildagliptin and 36 patients on placebo), vildagliptin provided a significant HbA1c reduction of 0.74% from baseline HbA1c 7.82% compared with an increase of 0.23% in the placebo group from baseline HbA1c 7.67%; the between-treatment difference was −0.97% (p < 0.001). The responder analysis demonstrated that significantly more patients receiving vildagliptin (38.6%) achieved an HbA1c target of <7.0% vs. placebo (13.9%) (p = 0.014) in this subgroup (figure 3).
Figure 3.
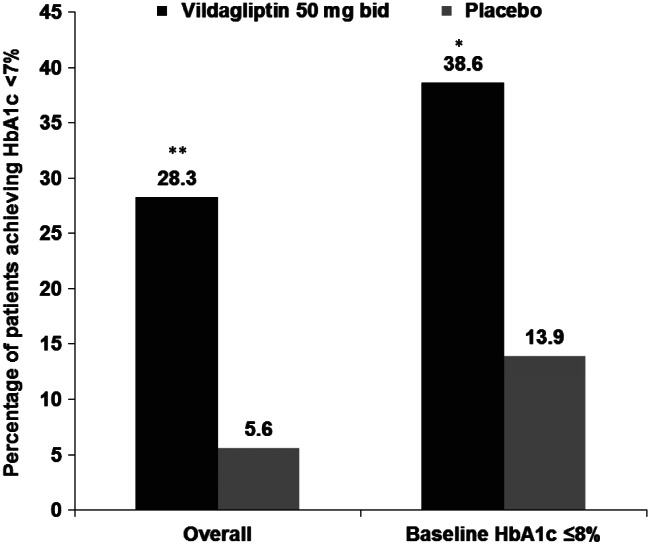
Proportion of patients achieving haemoglobin A1c (HbA1c) <7% with baseline ≥7% in overall population and in subgroup of patients with baseline HbA1c ≤8% (full analysis set). *p = 0.014; **p < 0.001.
Safety
The overall safety and tolerability of vildagliptin was similar to placebo when used in triple combination with metformin and SU (Table 2). The overall incidence of AEs was comparable between treatments, 50.3% vs. 47.5% in the vildagliptin and the placebo groups, respectively. Serious AEs and discontinuations due to AEs were similarly low in both treatment groups. There were no deaths in the vildagliptin group, one placebo-treated patient died due to suicide.
Table 2.
Overall summary of adverse events (AEs) by treatment group (safety set)
| Event | Vildagliptin + Metformin + GlimepirideN = 157, n (%) | Placebo + Metformin + Glimepiride N = 160, n (%) |
|---|---|---|
| AEs | 79 (50.3) | 76 (47.5) |
| SAEs | 3 (1.9) | 2 (1.3) |
| Discontinuation due to AEs | 1 (0.6) | 2 (1.3) |
| Deaths | 0 (0.0) | 1 (0.6) |
| Hypoglycaemic events | 8 (5.1) | 3 (1.9) |
| Severe hypoglycaemia | 1 (0.6) | 0 (0.0) |
| Discontinuation due to hypoglycaemia | 0 (0.0) | 0 (0.0) |
Urinary tract infection was the most frequent AE in both groups (vildagliptin: 6.4% and placebo: 8.1%). Slightly more vildagliptin-treated patients reported dizziness (7.0% vs. 1.9% with placebo) and hyperhidrosis (6.4% vs. 0.6% with placebo), which could be symptoms of hypoglycaemia.
The incidence of hypoglycaemia was overall low with both treatments; however, it was slightly higher in the vildagliptin group (n = 8, 5.1%) than in the placebo group (n = 3, 1.9%). Most of these patients had a single episode of hypoglycaemia; multiple hypoglycaemic events were only reported for one patient in each treatment group. The majority of the hypoglycaemic events were mild and none of them led to the discontinuation from the study. One patient in the vildagliptin group experienced a grade 2 hypoglycaemic event that was not study drug-suspected, but considered due to decreased food intake after a surgery.
No clinically relevant changes in the mean body weight from baseline to week 24 were observed: 73.1 kg at baseline vs. 73.6 kg at study endpoint in the vildagliptin group and 72.4 kg vs. 72.3 kg in the placebo group, respectively. There were no meaningful changes in laboratory values, physical examination results or ECGs between treatment groups. No patients receiving vildagliptin had a treatment emergent alanine aminotransferase or aspartate aminotransferase elevation ≥3x upper limit of normal.
Discussion
In this study, vildagliptin 50 mg bid added as a third OAD to a stable combination of metformin (≥1500 mg) and glimepiride (≥4 mg) provided a robust and clinically relevant reduction in HbA1c of 1.01% from a baseline of 8.75% in patients with T2DM not adequately controlled with dual therapy. The reduction in HbA1c observed in this study is similar to the reductions seen with vildagliptin when used in dual combination with metformin or SU from similar baseline 12,13. About 30% of vildagliptin-treated patients (almost five-fold greater than in the placebo group) achieved HbA1c of <7% demonstrating that a significant proportion of patients who failed on broadly used dual combination could reach treatment target when vildagliptin was added as a third OAD. Other DPP-4 inhibitors have been studied as well in the combination with metformin and glimepiride. Both sitagliptin and linagliptin in combination with metformin and glimepiride decreased HbA1c by 0.6% compared with placebo from a similar baseline HbA1c of 8.3 and 8.2%, respectively 14,15, which appears slightly lower than the 0.8% difference versus placebo seen in our study.
Considerable uncertainty exists regarding optimal treatment for patients in whom glycaemic targets cannot be met with metformin and a SU in combination. Recent meta-analyses conducted to evaluate the benefits of the third antihyperglycaemic agent added to metformin and SU in patients with T2DM inadequately controlled on dual therapy demonstrated statistically significant reductions in HbA1c with different drug classes, including insulin (basal, biphasic and bolus), DPP-4 inhibitors, GLP-1 analogues and TZDs (−0.89 to −1.17%), but not with meglitinides and alpha-glucosidase inhibitors 5,16. In light of relatively minor differences in terms of the glucose lowering efficacy between these drug classes, tolerability and absence of weight gain are key factors in the choice of the drug to be added to existing metformin plus SU therapy.
The addition of a third glucose lowering agent to existing dual therapy with metformin and SU has been associated with an increased risk of hypoglycaemia. This has been demonstrated for TZDs, and GLP-1 analogues. Insulin in triple combination even doubled the incidence of severe hypoglycaemic events 5,16. Although DPP-4 inhibitors typically do not increase the risk of hypoglycaemia; in triple combination with metformin plus SU, sitagliptin and linagliptin reported more hypoglycaemic events than placebo, 16.4% versus 0.9% and 22.7% versus 14.8%, respectively. Thus, a slightly higher rate of hypoglycaemia with vildagliptin (5.1%) compared to placebo (1.9%) in our study is consistent with the experience with other DPP-4 inhibitors 14,15.
The risk of hypoglycaemia seen in this study slightly differs from the data recently reported with vildagliptin in combination with insulin. In patients with advanced T2DM not adequately controlled with metformin plus insulin, the addition of vildagliptin 50 mg bid provided robust reduction in HbA1c with an incidence of hypoglycaemia similar to placebo 17. The generally low risk of hypoglycaemia in triple combinations might be due to vildagliptin’s effect to increase GIP levels between meals and overnight when hypoglycaemia is most likely to occur 18, thereby increasing glucagon levels in hypoglycaemia 19. The slightly greater risk of hypoglycaemia versus placebo with vildagliptin added to metformin plus SU compared to similar incidence of hypoglycaemic events with vildagliptin in combination with metformin and insulin might be due to uncoupling of the glucose-dependent insulinotropic effect of GLP-1 in the presence of SU 20.
Consistent with other findings from the DPP-4 inhibitors 21–23 the glucose-lowering effect of vildagliptin was not associated with an increase in weight. In contrast, TZDs or insulin added to metformin plus SU have been associated with an increase in body weight 24,25.
Ultimately, the clinical objective of treatment intensification is to achieve glycaemic control in as many patients as possible. We were therefore interested which patient group might get closest to this goal and analysed the efficacy and safety in patients having baseline HbA1c ≤8%. The results showed that approximately 40% of patients receiving vildagliptin responded to treatment demonstrating the benefit of triple therapy for this subgroup.
One limitation of our study is its relatively short 24-week duration. Longer study will be required to assess the durability effect of vildagliptin in triple combination as well as its potential in delaying the time to permanent use of insulin. However, durable glycaemic control with vildagliptin as monotherapy has been demonstrated previously 26.
In conclusion, vildagliptin in triple combination with metformin and SU demonstrated robust glucose-lowering efficacy and good safety with low risk of hypoglycaemia and weight gain. This makes vildagliptin an attractive treatment option for patients with T2DM failing on metformin and SU who require a third antihyperglycaemic agent and are not candidates for an insulin therapy, especially in patients with baseline HbA1c ≤8% as a substantial number of patients in this subgroup achieved treatment targets.
Acknowledgments
The authors gratefully acknowledge the support of all the investigators and medical staff at the participating centres. The authors also thank Sanchika Agarwal for editorial assistance. This work was funded by Novartis Pharmaceuticals Corporation.
Investigators
Australia: Dr Greg Fulcher, Prof Joseph Proietto and Dr Richard Simpson. Germany: Dr med. Helmut Anderten, Dr med. Christina Bondke and Dr med. Dietrich Reimer. Hungary: Dr Peter Torzsa and Dr Zsombor Haraszty. India: Dr Manohar Nageshappa, Dr Prasad Gurumallappa, Dr Sharda Ardhanareeswaran, Dr S. Parmesh, Dr S.S. Srikanta, Dr Sanjay Reddy, Dr Usha Rani, Dr Aarti Dharskar, Dr Ashok Dash and Dr Mala Dharmalingam. Italy: Dr Piermarco Piatti and Prof. Stefano Del Prato. Korea: Dr Bongyun Cha, Prof. Dongseop Choi, Dr Seonmee Kang, Prof. Jaehyeon Kim and Prof. Haejin Kim. Mexico: Dr Guillermo González, Dr Marco Morales de Teresa, Dr Enrique Morales and Dra. Guadalupe Morales. Philippines: Dr Grace Delos Santos and Dr Tomas Lazatin. Romania: Dr Miheala Popoviciu, Dr Luiza Demian, Dr Adriana Cif and Dr Diana Barbonta. Taiwan: Prof. Wayne Huey-Herng Sheu, Dr Kai -Jen Tien, Dr Yi-Jen Hung and Dr Shih-Te Tu. UK: Dr Hamish Simpson.
Conflict of Interest
V. L. was critical to designing and conducting the trial, data collection and initial data interpretation. M. A. was responsible for the statistical analysis. W. K. contributed to study design, the initial data interpretation and overall clinical interpretation. S. D. P. represented the study investigators and contributed to the clinical interpretation of the data. All authors were involved in manuscript revisions and are responsible for intellectual content. V. L., M. A. and W. K. are employed by and own shares in Novartis. S. D. P. has served on advisory boards, received honoraria for speaking engagements and received research support from Novartis.
References
- 1.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15:540–559. doi: 10.4158/EP.15.6.540. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook MN, Girman CJ, Stein PP, Alexander CM, Holman RR. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care. 2005;28:995–1000. doi: 10.2337/diacare.28.5.995. [DOI] [PubMed] [Google Scholar]
- 5.Gross JL, Kramer CK, Leitao CB, et al. Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis. Ann Intern Med. 2011;154:672–679. doi: 10.7326/0003-4819-154-10-201105170-00007. [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation. IDF Diabetes Atlas, 5th edn. Brussels, Belgium. 2011. Available from URL: http: // www.idf.org/diabetesatlas. Accessed 20 November 2013
- 7.Mari A, Sallas WM, He YL, et al. Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:4888–4894. doi: 10.1210/jc.2004-2460. [DOI] [PubMed] [Google Scholar]
- 8.Balas B, Baig MR, Watson C, et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab. 2007;92:1249–1255. doi: 10.1210/jc.2006-1882. [DOI] [PubMed] [Google Scholar]
- 9.Ahren B, Foley JE, Bosi E. Clinical evidence and mechanistic basis for vildagliptin’s action when added to metformin. Diabetes Obes Metab. 2011;13:193–203. doi: 10.1111/j.1463-1326.2010.01321.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferrannini E, Fonseca V, Zinman B, et al. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11:157–166. doi: 10.1111/j.1463-1326.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Dejager S, Ahren B, et al. Vildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2-year study. Diabetes Obes Metab. 2010;12:780–789. doi: 10.1111/j.1463-1326.2010.01233.x. [DOI] [PubMed] [Google Scholar]
- 12.Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–895. doi: 10.2337/dc06-1732. [DOI] [PubMed] [Google Scholar]
- 13.Garber AJ, Foley JE, Banerji MA, et al. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10:1047–1056. doi: 10.1111/j.1463-1326.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 14.Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–745. doi: 10.1111/j.1463-1326.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 15.Owens DR, Swallow R, Dugi KA, Woerle HJ. Efficacy and safety of linagliptin in persons with Type2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med. 2011;28:1352–1361. doi: 10.1111/j.1464-5491.2011.03387.x. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh B, Cameron C, Singh SR, Yu C, Dolovich L, Houlden R. Choice of therapy in patients with type 2 diabetes inadequately controlled with metformin and a sulphonylurea: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2012;6:e62–e74. [PMC free article] [PubMed] [Google Scholar]
- 17.Kothny W, Foley J, Kozlovski P, Shao Q, Gallwitz B, Lukashevich V. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:252–257. doi: 10.1111/dom.12020. [DOI] [PubMed] [Google Scholar]
- 18.Ahren B, Schweizer A, Dejager S, Villhauer EB, Dunning BE, Foley JE. Mechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humans. Diabetes Obes Metab. 13:775–783. doi: 10.1111/j.1463-1326.2011.01414.x. [DOI] [PubMed] [Google Scholar]
- 19.Christensen M, Vedtofte L, Holst JJ, Vilsboll T, Knop FK. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60:3103–3109. doi: 10.2337/db11-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Heer J, Holst JJ. Sulfonylurea compounds uncouple the glucose dependence of the insulinotropic effect of glucagon-like peptide 1. Diabetes. 2007;56:438–443. doi: 10.2337/db06-0738. [DOI] [PubMed] [Google Scholar]
- 21.Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–2643. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- 22.Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:376–386. doi: 10.1111/j.1463-1326.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- 23.Foley JE, Jordan J. Weight neutrality with the DPP-4 inhibitor, vildagliptin: mechanistic basis and clinical experience. Vasc Health Risk Manag. 2010;6:541–548. doi: 10.2147/vhrm.s10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charpentier G, Halimi S. Earlier triple therapy with pioglitazone in patients with type 2 diabetes. Diabetes Obes Metab. 2009;11:844–854. doi: 10.1111/j.1463-1326.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 25.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 2009;52:2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Göke B, Hershon K, Kerr D, et al. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naïve patients with type 2 diabetes: comparison with metformin. Horm Metab Res. 2008;40:892–895. doi: 10.1055/s-0028-1082334. [DOI] [PubMed] [Google Scholar]


