Abstract
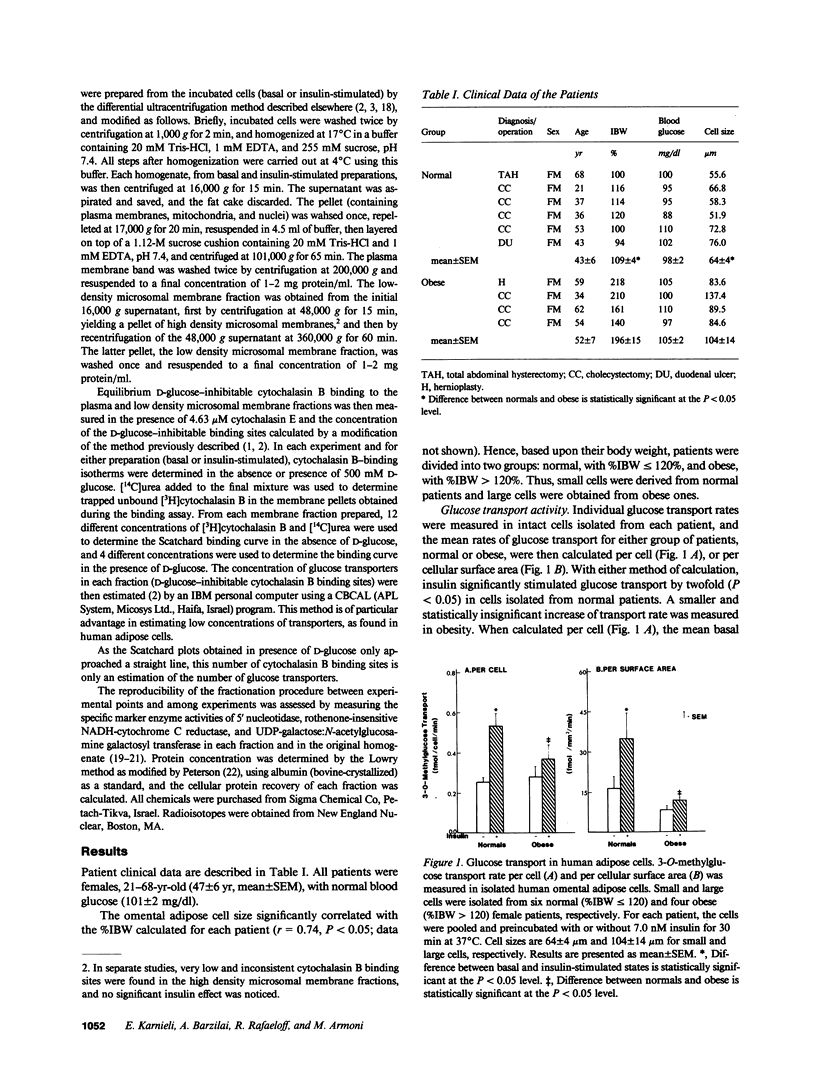
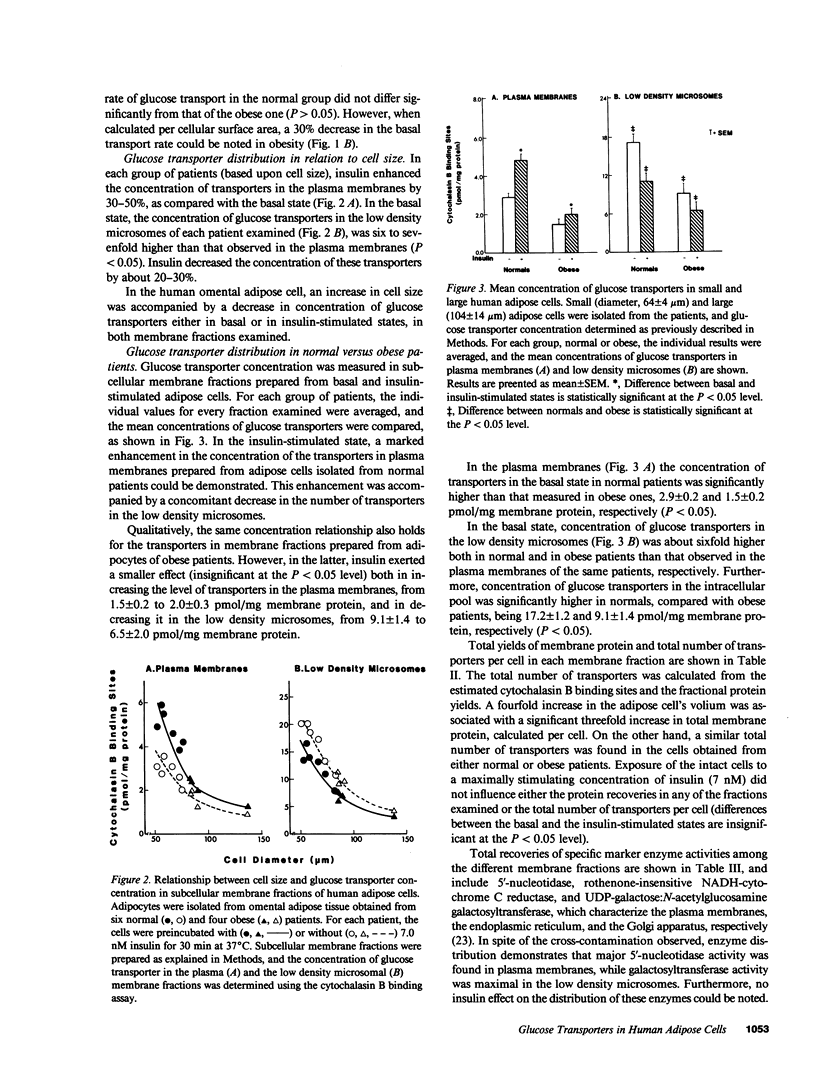
We examined insulin's effects on glucose transport and on subcellular transporter distribution in isolated human omental adipocytes of various sizes. Insulin stimulated 3-O-methylglucose transport by twofold in small cells, while a smaller and insignificant effect was measured in large cells. In the small cells, basal concentrations of glucose transporters were 2.9 and 17.2 pmol/mg membrane protein in the plasma and the low density microsomal membranes, respectively. Increasing cell size was associated with a 50% decrease in the concentration of transporters in each fraction, with no change in their total number per cell. Insulin stimulated the translocation of transporters from the intracellular pool to the plasma membranes, irrespective of cell size. Thus, insulin resistance at the postreceptor level, observed in human obesity, may be associated with a relative depletion of total transporters per cell together with a reduction in their intrinsic activity at the plasma membrane level.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. II. Synthesis of constitutive microsomal enzymes in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):97–117. doi: 10.1083/jcb.30.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki O., Kono T. Effects of temperature on basal and insulin-stimulated glucose transport activities in fat cells. Further support for the translocation hypothesis of insulin action. J Biol Chem. 1982 Dec 10;257(23):14306–14310. [PubMed] [Google Scholar]
- Fleischer B. Isolation and characterization of Golgi apparatus and membranes from rat liver. Methods Enzymol. 1974;31:180–191. doi: 10.1016/0076-6879(74)31020-8. [DOI] [PubMed] [Google Scholar]
- Hissin P. J., Foley J. E., Wardzala L. J., Karnieli E., Simpson I. A., Salans L. B., Cushman S. W. Mechanism of insulin-resistant glucose transport activity in the enlarged adipose cell of the aged, obese rat. J Clin Invest. 1982 Oct;70(4):780–790. doi: 10.1172/JCI110674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissin P. J., Karnieli E., Simpson I. A., Salans L. B., Cushman S. W. A possible mechanism of insulin resistance in the rat adipose cell with high-fat/low-carbohydrate feeding. Depletion of intracellular glucose transport systems. Diabetes. 1982 Jul;31(7):589–592. doi: 10.2337/diab.31.7.589. [DOI] [PubMed] [Google Scholar]
- Horuk R., Rodbell M., Cushman S. W., Wardzala L. J. Proposed mechanism of insulin-resistant glucose transport in the isolated guinea pig adipocyte. Small intracellular pool of glucose transporters. J Biol Chem. 1983 Jun 25;258(12):7425–7429. [PubMed] [Google Scholar]
- Karnieli E., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. A possible mechanism of insulin resistance in the rat adipose cell in streptozotocin-induced diabetes mellitus. Depletion of intracellular glucose transport systems. J Clin Invest. 1981 Sep;68(3):811–814. doi: 10.1172/JCI110318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Kashiwagi A., Verso M. A., Andrews J., Vasquez B., Reaven G., Foley J. E. In vitro insulin resistance of human adipocytes isolated from subjects with noninsulin-dependent diabetes mellitus. J Clin Invest. 1983 Oct;72(4):1246–1254. doi: 10.1172/JCI111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T., Robinson F. W., Blevins T. L., Ezaki O. Evidence that translocation of the glucose transport activity is the major mechanism of insulin action on glucose transport in fat cells. J Biol Chem. 1982 Sep 25;257(18):10942–10947. [PubMed] [Google Scholar]
- Kono T., Suzuki K., Dansey L. E., Robinson F. W., Blevins T. L. Energy-dependent and protein synthesis-independent recycling of the insulin-sensitive glucose transport mechanism in fat cells. J Biol Chem. 1981 Jun 25;256(12):6400–6407. [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O., Gliemann J. Hexose transport in human adipocytes: factors influencing the response to insulin and kinetics of methylglucose and glucose transport. Diabetologia. 1981 Jun;20(6):630–635. doi: 10.1007/BF00257432. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Hjøllund E., Beck-Nielsen H., Lindskov H. O., Sonne O., Gliemann J. Insulin receptor binding and receptor-mediated insulin degradation in human adipocytes. Diabetologia. 1981 Jun;20(6):636–641. [PubMed] [Google Scholar]
- Pedersen O., Hjøllund E., Lindskov H. O. Insulin binding and action on fat cells from young healthy females and males. Am J Physiol. 1982 Aug;243(2):E158–E167. doi: 10.1152/ajpendo.1982.243.2.E158. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Simpson I. A., Yver D. R., Hissin P. J., Wardzala L. J., Karnieli E., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta. 1983 Dec 19;763(4):393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- Smith U., Kuroda M., Simpson I. A. Counter-regulation of insulin-stimulated glucose transport by catecholamines in the isolated rat adipose cell. J Biol Chem. 1984 Jul 25;259(14):8758–8763. [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardzala L. J., Cushman S. W., Salans L. B. Mechanism of insulin action on glucose transport in the isolated rat adipose cell. Enhancement of the number of functional transport systems. J Biol Chem. 1978 Nov 25;253(22):8002–8005. [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Identification of the D-glucose-inhibitable cytochalasin B binding site as the glucose transporter in rat diaphragm plasma and microsomal membranes. Biochim Biophys Acta. 1983 Apr 21;730(1):49–56. doi: 10.1016/0005-2736(83)90315-2. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm. Apparent translocation of intracellular transport units to the plasma membrane. J Biol Chem. 1981 Jul 25;256(14):7090–7093. [PubMed] [Google Scholar]
- Whitesell R. R., Gliemann J. Kinetic parameters of transport of 3-O-methylglucose and glucose in adipocytes. J Biol Chem. 1979 Jun 25;254(12):5276–5283. [PubMed] [Google Scholar]



