Abstract
Parturition does not occur in transgenic mice lacking the prostaglandin F receptor (Ptgfr−/−) because luteolysis is forestalled and progesterone production persists. Ovariectomy of pregnant Ptgfr−/− mice leads to a decline in circulating progesterone and delivery of live pups. The objective of the present study was to test the hypothesis that immigration of macrophages and increased innervation of the cervix of Ptgfr−/− mice was associated with ripening and parturition. The cervix of pregnant Ptgfr−/− mice was studied on Days 15–21 after breeding; additional groups were ovariectomized on Day 19 of pregnancy, and the cervix obtained on Day 20 of pregnancy before birth or the next day at about 24 h after birth. On Days 18–19 of pregnancy, macrophage numbers and nerve fiber density increased more than 3-fold compared with findings in nonpregnant or Day 15 or 21 pregnant Ptgfr−/− mice. The magnitude and time course of these changes were comparable to those found in wild-type controls that delivered on Day 19 after breeding. Thus, the mechanism regulating macrophage immigration, innervation, and cervical remodeling in Ptgfr−/− mice with delayed parturition is similar to wild-type controls that deliver at term. By contrast, ovariectomy forestalled the decrease in cervical macrophages in Ptgfr−/− mice. By Day 21 after breeding, macrophage numbers more than double those after ovariectomy, relative to those found in pregnant Ptgfr−/− mice, whereas nerve fiber density was the same regardless of birth. Density of collagen structure in these mice directly matched macrophage traffic in the cervix. The findings indicate that the prostaglandin F2alpha receptor and progesterone withdrawal are a necessary part of the final common pathway for ripening of the cervix and the process of parturition.
Keywords: cervix, cyclooxygenase, labor, leukocyte, macrophage, neuropeptides, parturition, progesterone, progesterone receptor
INTRODUCTION
Prostaglandins are a critical component of the final common mechanism for parturition. Inhibition of prostaglandin F2alpha (PGF2alpha) production suppresses myometrial contractility and delays delivery in mice and sheep [1, 2]. The process of birth also is blocked in mice that are deficient in Ptgs1 (cyclooxygenase 1), that is, no constitutive PGF2alpha synthesis, or are lacking receptors for PGF2alpha (Ptgfr−/−) [3, 4]. Prolonged pregnancy in Ptgfr−/− mice results from a failure in luteolysis and sustained production of progesterone from the corpus lutea. Ordinarily in rodents, the decline in ovarian production of progesterone begins about 2–3 days before birth [5, 6]. In addition to the systemic decline in progesterone, local withdrawal of progestational support could lead to a loss in uterine quiescence, because upregulation of inhibitory progesterone receptor isoform expression may inhibit transactivation in the human myometrial cells and pregnant mouse uterus at term [7]. Similar evidence for a shift in progesterone isoforms is reported in the primate uterus at term [8, 9] as a mechanism to block the ability of progesterone to sustain pregnancy. In biopsies of cervices from peripartum women, concentrations of progesterone receptor protein decreased at term to 25% of the level found in nonpregnant women [10]. Other recent findings suggest that PGF2alpha promotes the expression of inhibitory progesterone receptor isoforms in myometrium from parturient women [11, 12]. Thus, in rodents, as in primates, actions of PGF2alpha may contribute to a reduced efficacy of progesterone and promote processes that enhance uterine contractility in preparation for parturition.
The process that remodels the cervix begins well before the day of birth [13, 14] and involves actions by prostaglandins [15, 16]. Restructuring of the cervix at the conclusion of pregnancy is associated with degradation of collagen, increases in proinflammatory cytokines, and infiltration of leukocytes [17, 18]. Of importance, the recruitment and activation of macrophages before labor indicate a role for this immune cell in cervical ripening [19–22]. Activated macrophages produce prostaglandins and proinflammatory cytokines that stimulate collagenase production by cervical fibroblasts [23–25]. Indeed, prostaglandins may directly promote restructuring of the extracellular collagen matrix [26–28] as well as act indirectly through vasoactive effects to enhance permeability of blood vessels to leukocytes and fluid [29, 30]. Both actions are consistent with the suggestion by Liggins that ripening of the cervix resembles an inflammatory process [31]. In rodents before parturition, evidence suggests that collagenases and elastases are imported into the cervix by recruitment of leukocytes rather than production by cervical smooth muscle or stromal fibroblasts [32, 33]. These immune cells are present in increasing numbers near blood vessels, in subepithelial stroma, and between smooth muscle bundles during the peripartum period [34–36]. Thus, infiltration of leukocytes could account for increases in local prostaglandin production in the cervix before parturition [37]. Although parturition fails to occur in pregnant Ptgfr−/− mice, the importance of the PGF2alpha receptor for processes associated with ripening of the cervix has not been investigated. Therefore, the present study was designed to test the hypothesis that delayed parturition in pregnant Ptgfr−/− mice is a consequence of failed recruitment of macrophages and lack of restructuring in the cervix.
MATERIALS AND METHODS
Mice lacking the PGF2alpha receptor were generated at Kyoto University as previously described [4, 38]. This mutant strain was back-crossed 10 times to B6 (C57BL/6) mice from Japan SLC [39]. Except for the defect in luteolysis at term pregnancy, no differences were found in other aspects of reproductive function among homozygous Ptgfr−/− mice or wild-type females (see below). Mice were housed in a 12L:12D photoperiod at about 23°C with free access to food and water, and all experiments were conducted under an IACUC-approved protocol in accordance with the National Research Council's publication “Guide for Care and Use of Laboratory Animals.” Adult Ptgfr−/− virgin female mice (9–14 wk of age) were placed overnight with homozygous males, and the presence of a vaginal plug was counted as Day 1 of pregnancy. On Day 15, 18, 19, or 21 of pregnancy, mice were deeply anesthetized, and the cervix, including attached vaginal and uterine tissue, was excised through a midabdominal incision (n = 3 per group). To determine whether immigration of macrophages into the cervix depends upon gonadal steroids, two groups of Ptgfr−/− mice were ovariectomized on the morning of Day 19 after coitus. Based upon evidence that pregnant Ptgfr−/− mice typically give birth about 24 h after removal of the ovaries [40], the cervix was obtained either 20 h after ovariectomy (before birth on Day 20 of pregnancy; Ovx d20) or the next day at about 24 h after birth (Ovx d21). Pups appeared fully formed and vital when dams were killed. The unripened cervix from nonpregnant mice in the luteal phase of the estrous cycle of each strain served as an additional baseline control. Cervices were immersion fixed in 4% paraformaldehyde for about 24 h. Tissue samples were dehydrated in a graded series of alcohol, stored in 100% ethanol, and shipped with cold packs to Loma Linda University. Upon receipt, samples were paraffinized, blocked, sectioned at 10 μm, and processed by immunohistochemistry to identify either macrophages with BM8 antisera [35] or antisera to the intermediate neurofilament protein peripherin [41], as previously described. Sections of cervix that were processed without primary antisera, a negative control, had a pale light brown background, but stained cells were absent. Other sections were stained with picrosirius red to visualize collagen structure [41]. As controls for Ptgfr−/− mice, pregnant B6 (C57BL/ 6NHsd) mice were obtained on Day 12 after breeding from Harlan (Indianapolis, IN). These wild-type mice were killed on Days 15, 18, and 19 of pregnancy (n = 4–5 each); the Day 19 group was about 4 h postpartum. The cervix from each mouse was obtained and processed as described above.
Macrophage Counts
Macrophages were visually identified under bright-field microscopy as morphologically dark brown in or surrounding a hematoxylin-counterstained nucleus. BM8-stained cells were counted in two to three sections of cervix from each mouse. Stained cells were counted in five to nine nonoverlapping vertical and horizontal placements of an eyepiece reticle grid (10 × 10 boxes, 10 110 μm3 per box at 40× objective; 19.8–39.7 3 106 lm3). As in previous studies, to account for hypertrophy and hyperplasia of the reproductive tract tissue during pregnancy, the number macrophages per area of cervix was normalized to cell nuclei density for each individual and relative to the mean of macrophages in nonpregnant cervices. The cell nuclei density per cervix was determined from a digitized image of one grid in each of two hematoxylin-counterstained sections from the cervix of each mouse, equivalent to a total area of 17 538 μm2. In confirmation of previous findings in C3H/HeN mice of cervical hypertrophy with pregnancy [41], the mean 6 SEM cell nuclei density in cervix from nonpregnant wild-type and Ptgfr−/− mice was 10.4 6 ±.15 and 17.2 ± 0.5 ± 104 μm3, respectively. During pregnancy, average cell nuclei numbers in the cervix were 6.3, 6.5, and 8.5 ± 104 μm3 in wild-type mice (Days 15, 18, and 19, respectively; SEM range, 0.24–0.31), and 4.2, 4.6, 6.8, and 8.0 ± 104 μm3 in Ptgfr−/− mice (Days 15, 18, 19, and 21, respectively; SEM range, 0.11–0.78), whereas in Ovx Ptgfr−/− mice, whether pregnant (Ovx d20) or 1 day postpartum (Ovx d21), average cell density was 4.4 ± 0.56 or 5.3 ± 0.39 ± 104 μm3. An average of normalized macrophage counts was taken of all sections for each individual relative to the mean in nonpregnant females of the same strain; group mean and variance then were determined.
Collagen Structure
Collagen-specific birefringence was evaluated in duplicate or triplicate 10-μm sections of cervix from Ptgfr−/− mice after staining with picrosirius red, as previously described [41, 42]. Briefly, nine adjacent but nonoverlapping grids in a 10 ± 10 eyepiece reticle were arbitrarily positioned over a portion of the cervix that did not contain lumen. A black-and-white photograph was taken of the polarized light image using an Apogee microscope camera (Scientific Instrument Co., Temecula, CA). Mean optical density (OD) was assessed with National Institutes of Health ImageJ software (grayscale threshold was calibrated using the Rodbard standard curve) [43]. Darkly stained areas of high collagen density and cross-linking complexity had low OD values, whereas bright regions with reduced collagen content and diffuse structure produced high OD numbers. As detailed previously, OD data were normalized with respect to cell nuclei density for each individual to account for tissue hypertrophy due to reproductive status [41].
Nerve Fiber Density
Nerve fibers were identified morphologically as fine lines that were occasionally interspersed with beaded varicosities or appeared as dark brown spherules, indicative of a cross-section of fiber bundles. Using a 20± objective, at least 21 nonoverlapping vertical and horizontal grid placements in a total of two to three sections from each cervix were evaluated. Boxes that contained peripherin-immunoreactive fibers were counted, and the area of tissue that contained fibers was calculated. Nerve fiber density was normalized for cell nuclei density and relative to the mean in nonpregnant females of the same strain to correct for the hypertrophy of tissue with pregnancy as previously described [41].
Data Analyses
In experiment 1, data for Ptgfr−/− and control were separately evaluated by ANOVA. Levene test for homogeneity of variance on raw or log-transformed data was not statistically significant, and individual comparisons were made with the least significant difference test. Optical density data from picrosirius red-stained sections of cervix from wild-type controls were not normally distributed, and the Kruskal-Wallis test was used. For all endpoints, the Student t-test with Bonferroni correction for multiple comparisons was used to evaluate statistical differences between strains relative to group (nonpregnant or day after breeding). In experiment 2, an ANOVA was used to evaluate the effects of ovariectomy in Ptgfr−/− mice before and after birth compared with intact Ptgfr−/− mice with delayed birth (same day after breeding), as well as compared with wild-type controls before and after birth, Days 18 and 19 after breeding (postpartum), respectively. P < 0.05 was considered significant. All data are presented as mean ± SEM.
RESULTS
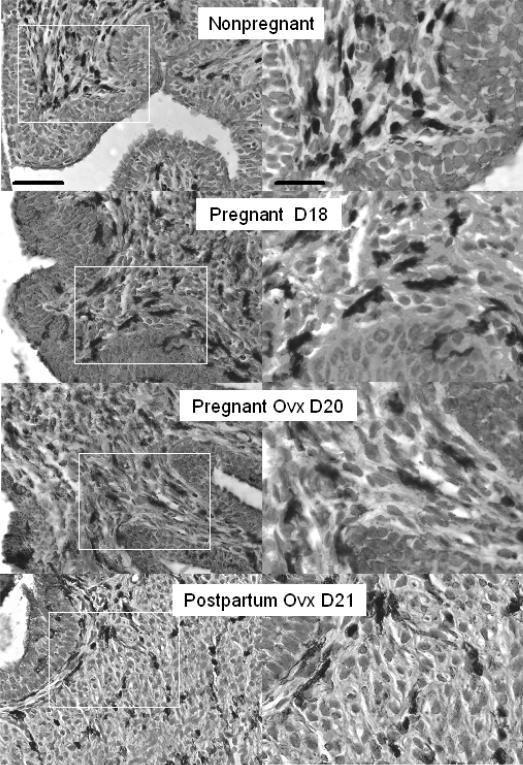
The morphology and anatomical distribution of BM8-stained macrophages was comparable to that in other reports of macrophages in the murine cervix. The cervices of all mice contained BM8-stained cells in the stroma, around blood vessels, in the submucosal epithelium and, to a limited extent, in the epithelium (Fig. 1 photomicrographs shown for Ptgfr −/− mice). Dark cell bodies and processes (dark-brown in actual sections) were associated with counterstained cell nuclei. During pregnancy, hypertrophy was evident in the thickness of the luminal epithelium and greater space surrounding cells and their nuclei in the stroma and smooth muscle cells in the cervix. In addition to reduced density of cell nuclei, an indication of cellular hypertrophy, more macrophages were present in peripartum cervix than in nonpregnant mice or earlier in pregnancy in both Ptgfr−/− mice and wild-type controls.
FIG. 1.
Photomicrographs of BM8 stained macrophages in cervices of Ptgfr−/− mice that were ovary intact (Nonpregnant and Pregnant D18 (day 18 of pregnancy)), ovariectomized, and pregnant (Ovx D20), or postpartum (Ovx D21). Postbreeding day is indicated. The areas within the white boxes in the left panels are magnified and presented in the right panels. Scale bar = 50 μm (left) and 25 μm (right), and applies to photomicrographs in each column. Macrophages with similar morphology and density were present in cervices from wild-type controls (data not shown).
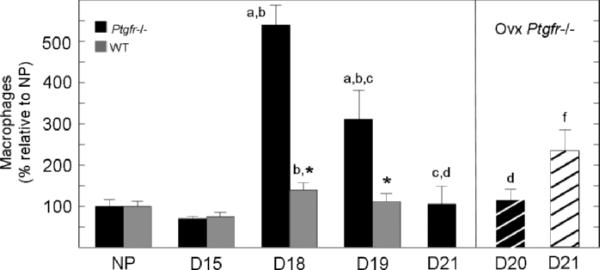
Stereologic analyses of tissue sections indicated that the number of macrophages in the cervix varied with respect to pregnancy. Relatively low numbers of macrophages were evident in the cervix from nonpregnant and Day 15 mice (P < 0.05, df = 10, F = 10.03, ANOVA; Fig. 2). With consideration of actual counts of BM8-stained cells, significantly more macrophages were present in cervices from nonpregnant wild-type versus Ptgfr−/− mice (70 ± 3 and 24 ± 4 per cell nuclei number per μm3 × 10 −3, respectively). By Day 18 of pregnancy, macrophage numbers in Ptgfr−/− increased more than 5-fold relative to those observed in nonpregnant mice or the Day 15 pregnant group. Similarly, in wild-type controls, peak numbers of macrophages were found on Day 18 of pregnancy and did not significantly decline on Day 19 after breeding, some 4 h after birth. The peak in wild-type versus Ptgfr−/− mice on Day 18 of pregnancy did not necessarily reflect a difference in actual cell counts (98.5 ± 11 and 129.4 ± 11 per cell nuclei number per μm3 ± 10−3, respectively; P > 0.05). Rather, macrophages were more prevalent in the cervix of both strains on Day 18 of pregnancy compared with Day 15 or to that in nonpregnant mice (P < 0.05, Ptgfr−/− Day 18 vs. Day 15 or nonpregnant P < 0.05 wild-type Day 18 vs. Day 15; P = 0.08 wild-type Day 18 vs. nonpregnant). By contrast, on Day 19 of pregnancy, macrophage numbers in Ptgfr−/− mice with delayed birth were significantly reduced from the Day 18 peak, although they still were 3-fold higher compared with that 4 days earlier on Day 15 of pregnancy or in nonpregnant Ptgfr−/− mice. By Day 21 of pregnancy, the census of macrophages declined to that found in the cervix of nonpregnant Ptgfr−/− mice.
FIG. 2.
Mean number of macrophages in the cervix of Ptgfr−/− mice and wild-type (±SEM, n = 3–5 per group) adjusted for hypertrophy of pregnancy and normalized to nonpregnant controls in each strain (NP). Postbreeding day is indicated. Wild-type D19 and Ptgfr−/− Ovx D21 groups were postpartum. Statistical symbols indicate P < 0.05 when comparing a specified group to the same strain of NP mice (a) or mice on Days 15 (b), 18 (c), or 19 (d) of pregnancy, or to Ovx Ptgfr−/− mice (f) on Day 20 of pregnancy (ANOVA for Ptgfr−/− mice, F = 41.42, df 4; or for wild-type controls, F = 3.65, df = 3). *A significant difference of P < 0.05 between Ptgfr−/− vs. NP control (t = 3.38, df = 5).
The reduction in macrophages in the cervix of prepartum Ptgfr−/− mice with delayed birth was not blocked by ovariectomy. Fewer macrophages were evident in the cervix of Day 20 pregnant Ptgfr−/− mice 20 h after ovariectomy, compared with numbers observed on Day 18 or 19 of pregnancy. The number of resident macrophages was the same in the cervix of ovariectomized Ptgfr−/− mice on Day 20 of pregnancy as on Day 15 of pregnancy and in nonpregnant Ptgfr−/− mice, as well as wild-type controls on Day 18 of pregnancy (day before birth). On the day after ovariectomy (i.e., 4 h after the cervix was obtained from the pregnant Ovx d20 group), Ptgfr−/− mice gave birth to live pups. The next day, Day 21 after breeding (Ovx d21 group), the number of macrophages in the cervix of postpartum Ptgfr−/− mice increased more than 2-fold compared with the census of immune cells in the cervix of ovary-intact Ptgfr−/− mice on Day 21 of pregnancy.
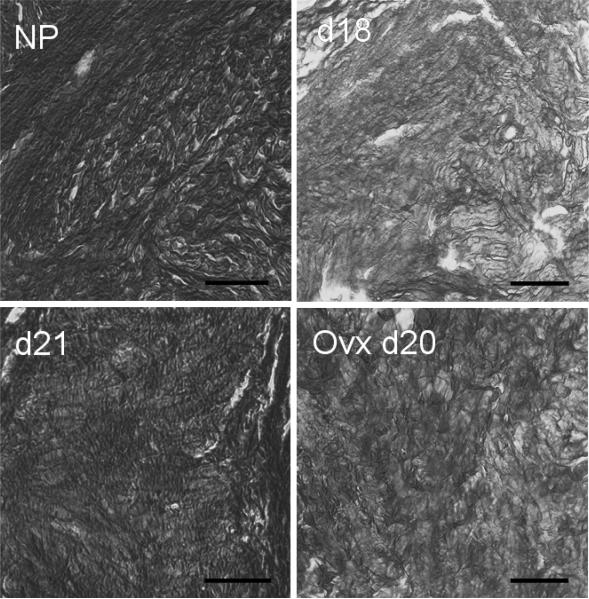
The intensity of picrosirius red-stained collagen varied with respect to pregnancy. In both strains of mice, collagen structure in cervices from nonpregnant mice was densely packed, and fibrils were regularly arranged in the perimetria between luminal epithelium and stroma (Fig. 3 photomicrographs shown for Ptgfr−/− mice). By Day 18 of pregnancy, birefringence of polarized light in sections of the cervix declined. Decreased staining, evidenced by dark areas (pale orange in tissue) and structural disarray, persisted on Day 19 after breeding in both Ptgfr−/− mice with delayed birth and in postpartum wild-type controls. This decline in birefringence indicated a reduced density of collagen structure in cervix on Day 18 of pregnancy and Day 19 after breeding. With continued delay in birth, the collagen staining was increased in the cervix of Ptgfr−/− mice by Day 21 after breeding. By contrast, ovariectomized Ptgfr−/− mice on Day 20 of pregnancy, 20 h after removal of the ovary, maintained a level of staining that was similar to that in sections from prepartum ovary-intact mice on Day 18 after breeding and greater compared with that on Day 21 of pregnancy.
FIG. 3.
Photomicrographs of cervices from Ptgfr−/− mice at different stages of pregnancy stained with picrosirius red to visualize collagen. Coronal sections of cervices were obtained from Ptgfr−/− mice that were nonpregnant (NP), pregnant on Day 18 or 21 of postbreeding (d18 or d21), or ovariectomized on Day 20 (Ovx d20). Using polarized light, birefringence was evident as intense orange on a light yellow background before conversion to grayscale. After conversion to a grayscale image, regions of dense, highly structured collagen were dark (i.e., high birefringence with low light transmittance), whereas bright areas represent reduced collagen structure and scattered fibrils. Picrosirius red stained collagen structure to a similar extent in cervices from wild-type controls (data not shown) as to those photomicrographs shown here for Ptgfr −/− mice. Bar = 50 μm.
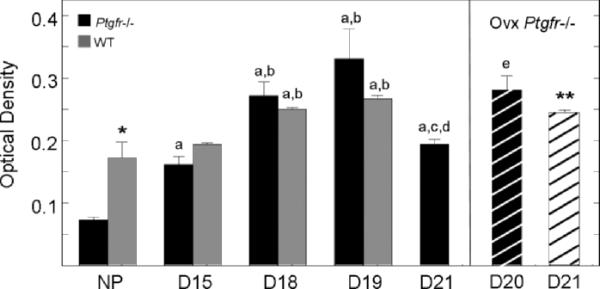
In addition to morphology, OD measures indicated that collagen structure of the cervix varied with respect to pregnancy. In sections from the cervices of nonpregnant and pregnant Day 15 mice, optical density was low in both wild-type controls and Ptgfr−/− strains, that is, birefringence of polarized light indicated dense collagen content and complex structure (Fig. 4). By Day18 of pregnancy, mean OD of stain in the cervix increased (P < 0.05, Kruskal-Wallis). In both strains, OD remained high on Day 19 after breeding, an indication of a reduced collagen matrix in the cervix even though wild-type controls had given birth. With continued pregnancy in Ptgfr−/− mice, OD decreased by Day 21 after breeding. This decrease was blocked by ovariectomy. Optical density remained high in ovariectomized Ptgfr−/− mice on Day 20 of pregnancy, and the next day, Day 21 (after birth), equivalent to levels in postpartum wild-type controls. Thus, removal of the ovary from postterm Ptgfr−/− mice blocked restructuring of the collagen matrix, ripened the cervix, and induced birth.
FIG. 4.
Mean OD ± SEM normalized to cell density of picrosirius red-stained sections of cervix from nonpregnant or pregnant Ptgfr−/− and wild-type mice (n = 3–5 per group; three sections were analyzed per mouse). Analysis of a reversed grayscale image by National Institutes of Health ImageJ software indicated higher mean OD on D18 and PP compared with NP and D15 groups. Higher OD values denote regions of reduced birefringence, indicative of reduced collagen and diffuse structure. Symbols denote P < 0.05 when a specific group in the same strain was compared to NP mice (a), or groups D15 (b), D18 (c), D19 (d), or D21 (e) of pregnancy (for Ptgfr−/− mice, ANOVA F = 21.59, df = 4; for wild-type mice, chi-square=13.15, df = 3). *P < 0.05 to NP Ptgfr−/− when compared controls; **P < 0.05 when compared to D19 WT controls.
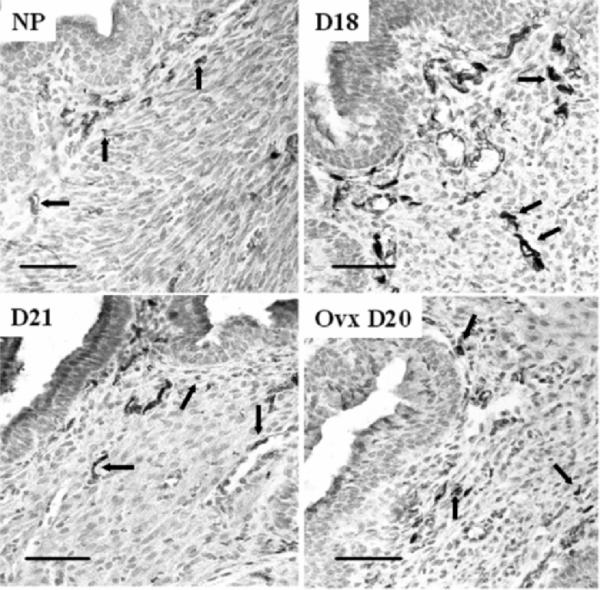
As for nerve fibers in the cervix, peripherin immunoreactivity was evident as punctuate dark (brown in tissue sections) stain that resembled fibers in cross-section, or as thin lines in subepithelial stroma, or around blood vessels (Fig. 5). By Day 18 of pregnancy, many more fibers were apparent in both Ptgfr−/− mice, as well as in wild-type controls, compared with those in cervices from nonpregnant or pregnant Day 15 groups. In all peripartum mice, stained fibers were thicker, longer, and commonly in bundles. By Day 21 after breeding, prolonged pregnancy in Ptgfr−/− mice was associated with diminished innervation, resembling that in nonpregnant mice. Ovariectomy did not appear to influence the morphology or distribution of nerve fibers in Ptgfr−/− mice either before birth or postpartum.
FIG. 5.
Photomicrograph of peripherin-stained nerve fibers in the cervix of nonpregnant and pregnant Ptgfr−/− mice. Groups are the same as those described in Figure 3. Luminal epithelium is in the upper left portion of each panel. Stained fibers are dark in between light gray hematoxylincounterstained cells (i.e., dark brown cells among violet-colored nuclei before conversion to grayscale). Arrows denote examples of fibers that are sparse with varicosities or cut in the plane of section in nonpregnant (NP) and pregnant D21 mice compared with the prevalent thick or bundled fibers on Day 18 of pregnancy. Morphology and density of peripherin fibers were similar in cervices from wild-type controls (data not shown). Bars = 50 μm.
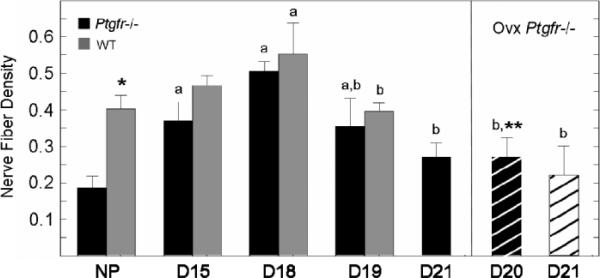
The density of nerve fibers in the cervix of Ptgfr−/− mice directly corresponded to that in wild-type controls. In both strains, the nerve fiber density increased by Day 15 of pregnancy to peak by Day 18 compared with that in nonpregnant mice (Fig. 6). Thereafter, nerve fiber density declined to levels found in nonpregnant mice, whether postpartum in wild-type controls or with continued pregnancy in Ptgfr−/− mice. Ovariectomy of pregnant Ptgfr−/− mice on Day 19 did not prevent the decline in density of nerve fibers in the cervix either on Day 20 of pregnancy, before birth, or on Day 21, 1 day postpartum.
FIG. 6.
Mean density of peripherin-stained nerve fibers in cervix of Ptgfr−/− and wild-type mice (±SEM, n = 3–5 per group) adjusted for hypertrophy of pregnancy (cell nuclei number/μm3 ± 103). Under bright-field microscopy, boxes in an eyepiece reticle grid (10 ± 10 boxes) that contained immunoreactive nerve fibers were counted, and area of innervated tissue was calculated as described previously in [41] and in Materials and Methods. Group designations are the same as in previous figures. P < 0.05 with respect to group NP (a) or D18 (b) of pregnancy same strain (ANOVA F > 5.2, df = 4), or asterisk (*) compared with wild-type mice same group (t = 4.23), or double asterisk the (**) vs. wild-type mice from D18 group (F = 5.2, df = 3).
DISCUSSION
The present findings confirm that PGF2alpha receptors are crucial for parturition. However, evidence did not support the hypothesis that delayed parturition in pregnant Ptgfr−/− mice results from a defect in specific aspects of cervical ripening. Rather, macrophages are recruited into the cervix in pregnant Ptgfr−/− mice with the same time course as in wild-type controls and parallel previous findings in pregnant C3H/HeN mice [35]. Immigration of macrophages into the cervix peaked by Day 18 of pregnancy in Ptgfr−/− mice relative to that earlier in pregnancy and in nonpregnant controls. Even though parturition did not occur, the population of macrophages declined to the baseline seen in nonpregnant mice. This pattern of macrophage traffic in the cervix occurred, as previously reported, with sustained production of progesterone by the corpus luteum [38]. Thus, the actions of PGF2alpha cannot account for trafficking of macrophages into or out of the cervix of pregnant Ptgfr−/− mice. Whether prostaglandins are involved in the final stage of cervical dilation before birth [14] remains to be determined.
In the absence of PGF2alpha receptor-mediated actions, restructuring of the cervix appeared to coincide with immigration of macrophages into the cervical stroma. The stimulus for recruitment of macrophages may be amplified because the peak in resident macrophages in cervices of Day 18 pregnant Ptgfr−/− mice was several times higher than that in current or previous controls on the same day after breeding. These increases in macrophage numbers correlated with increased OD, an indication of decreased collagen structure (i.e., reduced birefringence reflecting collagen fibers that are fragmented, loosely packed, and dilated). The time course of cervical restructuring as pregnancy neared normal term in wild-type controls was directly comparable to that in Ptgfr−/− mice that did not progress to birth, as well as that previously reported for remodeling of the prepartum cervix in C3H/HeN mice [35, 41] and in the pregnant rat [27, 44]. With evidence that birefringence was reduced due to collagen disarray and decreased content in cervices from both controls that gave birth and pregnant Ptgfr−/− mice that did not deliver, the findings suggest that this phase of cervical remodeling may be independent of actions mediated by PGF2alpha receptors. To complete the ripening process, activities mediated by PGF2alpha are necessary for dilation and effacement, as well as to discontinue quiescence of the uterine myometrium [15].
An accurate estimate of resident immune cells in the cervix depends upon the consideration that there is a significant hypertrophy of reproductive tissues with pregnancy. In a variety of viviparous species, the volume of the cervix increases with the progression of pregnancy [45–47]. The present investigation of Ptgfr−/− mice and wild-type controls replicates the finding that the cervix from nonpregnant mice contains more than twice the number of cell nuclei per area compared with that seen in pregnant mice near term [35, 41]. Taking cell density in the cervix into consideration, variability in the density of macrophages per volume of tissue among individuals and within groups was reduced. Of particular significance is that enhanced numbers of resident macrophages in the cervix on Day 18 versus Day 19 of pregnancy in Ptgfr−/− mice were independent of a change in cell nuclei density. Rather, traffic of immune cells into the cervix was likely to have increased. The present findings thus are consistent with reports that immune cells, and macrophages in particular, have an enhanced presence in the cervix during the peripartum period [19]. Although one report has indicated no change in neutrophil or eosinophil numbers in the uterine cervix from pregnant mice before birth [48], morphologically identified cells were counted in a suspension of tissue with a hemacytometer. Compared to the stereological analysis of resident immune cells in cervix sections in the present study in mice or by others in humans [17, 36], it is not known whether this approach accurately reflects the census of immune cells in the cervix given the dramatic hypertrophy of this tissue with pregnancy.
Certainly, withdrawal of progesterone is critical for ripening of the cervix and parturition [9, 15]. As term approaches in mice, progesterone in circulation declines from the peak on Day 16 of pregnancy to a baseline by the day of birth [6]. The withdrawal from high systemic concentrations of progesterone coincides with restructuring of collagen and immigration of macrophages in the cervix of wild-type controls by Day 18 of pregnancy in the present study. However, this same pattern of remodeling and immigration occurred in Ptgfr−/− mice, even though progesterone in circulation remained elevated [4, 38]. These findings raise the possibility that recruitment of macrophages during this phase of cervical restructuring may be independent of a change in progesterone in systemic circulation.
Progesterone is crucial for cervical ripening. Ovariectomy of pregnant Ptgfr−/− mice removed the major source for progesterone production and prevented restructuring of collagen fibers as in ovary-intact Ptgfr−/− mice with delayed birth on Day 21 of pregnancy. Rather, the collagen matrix remained remodeled while progesterone declined. Only after birth was an increase in macrophages found in the cervices of ovariectomized Ptgfr−/− mice. Conceivably, this progesterone withdrawal may promote final ripening of the cervix by activating, but not necessarily recruiting, macrophages. In guinea pigs, progesterone withdrawal induces parturition in association with collagenolysis, decreased collagen content, and infiltration of leukocytes in the cervix [49]. By contrast, treatment with progesterone delays parturition in ovariectomized pregnant Ptgfr−/− mice [40] and inhibits remodeling of the cervix in ovariectomized pregnant rats [50]. Moreover, recent findings in mice indicate that inflammation-induced preterm ripening of the cervix and immigration of macrophages can be prevented by treatment with medroxyprogesterone acetate [51]. It remains to be determined whether progesterone treatments affect macrophage traffic in the cervix or can forestall premature ripening of the cervix in patients at risk for preterm delivery [52–54].
In addition to systemic withdrawal of progesterone, findings by Condon et al. raise the possibility that a local progesterone withdrawal may be a common unifying mechanism to promote uterine contractility and the onset of labor in mice [7], as has been suggested to occur in the uterus of parturient women [9]. Whether a shift to a predominantly inhibitory progesterone receptor isoform is part of the mechanism that regulates ripening of the cervix at term remains to be explored. However, support for this contention is suggested by evidence that PGF2alpha promotes the expression of inhibitory progesterone receptor isoforms in myometrium from women with postpartum or chorioamnionitis [7, 11, 12]. Collectively, these findings raise the possibility that prostaglandin actions on the late-term pregnant cervix may be upstream from local progesterone withdrawal, which then promotes remodeling of the cervix in preparation for birth.
With actions by prostaglandins and withdrawal of progesterone as critical components of a final common mechanism for parturition, innervation has been proposed to influence processes that remodel the cervix. In the present study, the density of nerve fibers in the cervix of pregnant Ptgfr−/− as well as wild-type mice increased by Day 18 after breeding compared with nonpregnant controls. Increased innervation coincided with an expanded presence of macrophages. These findings parallel previous results in peripartum C3H/HeN mice [41]. The hypertrophy of innervation in the cervix near term may, acting through neuromediators or neuropeptides, promote sensory and effector functions that are proinflammatory (i.e., serve as chemoattractants for immune cells or to increase vascular permeability) [41, 55]. However, hypertrophy of nerve fibers alone was not sufficient to activate the cascade of processes in pregnant Ptgfr−/− mice with delayed parturition. The relationship of increased nerve fiber density, neural activities, and immune cell recruitment with the final common mechanism that ripens the cervix has yet to be defined.
In summary, both wild-type controls and mice lacking the prostaglandin F2alpha receptor demonstrate an increased presence of macrophages and nerve fibers, as well as reduced collagen structure in the cervix late in pregnancy. These findings are consistent with the hypothesis that recruitment of macrophages and hypertrophy of innervation are important for cervical remodeling before term. Neither the lack of PGF2alpha receptor-mediated effects nor absence of progesterone withdrawal prevented processes that promote restructuring of the cervix. The next phase of the final common pathway that initiates parturition appears to require prostaglandins and progesterone withdrawal to activate inflammatory processes to complete cervical ripening for birth. Whether effects of prostaglandins upon proinflammatory signals that initiate parturition in mice are solely mediated by a systemic reduction in progesterone following luteolysis, or reflect a local progesterone withdrawal in the cervix, remain to be determined.
ACKNOWLEDGMENTS
We thank Long Tran and Tom Lechuga for their technical expertise and Drs. Grenith Zimmerman and Floyd Petersen for their consultations for statistical analyses. We also appreciate the encouragement and support of Gerald and Susan Ebner, as well as Dennis and Franziska Shepard.
Footnotes
Supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan to Y.S., HD054931 to S.Y., and the Dean of Loma Linda University School of Medicine.
REFERENCES
- 1.Peri KG, Quiniou C, Hou X, Abran D, Varma DR, Lubell WD, Chemtob S. THG113: a novel selective FP antagonist that delays preterm labor. Semin Perinatol. 2002;26:389–397. doi: 10.1053/sper.2002.37307. [DOI] [PubMed] [Google Scholar]
- 2.Hirst JJ, Parkington HC, Young IR, Palliser HK, Peri KG, Olson DM. Delay of preterm birth in sheep by THG113.31, a prostaglandin F2alpha receptor antagonist. Am J Obstet Gynecol. 2005;193:256–266. doi: 10.1016/j.ajog.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Gross GA, Imamura T, Luedke C, Vogt SK, Olson LM, Nelson DM, Sadovsky Y, Muglia LJ. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci U S A. 1998;95:11875–11879. doi: 10.1073/pnas.95.20.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuboi K, Sugimoto Y, Iwane A, Yamamoto K, Yamamoto S, Ichikawa A. Uterine expression of prostaglandin H2 synthase in late pregnancy and during parturition in prostaglandin F receptor-deficient mice. Endocrinology. 2000;141:315–324. doi: 10.1210/endo.141.1.7236. [DOI] [PubMed] [Google Scholar]
- 5.Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- 6.Virgo BB, Bellward GD. Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology. 1974;95:1486–1490. doi: 10.1210/endo-95-5-1486. [DOI] [PubMed] [Google Scholar]
- 7.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Upregulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of NF-{kappag}B may contribute to the onset labor through inhibition of PR f of function. Mol Endocrinol. 2006;20:764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 8.Haluska GJ, Wells TR, Hirst JJ, Brenner RM, Sadowsky DW, Novy MJ. Progesterone receptor localization and isoforms in myometrium, decidua, and fetal membranes from rhesus macaques: evidence for functional progesterone withdrawal at parturition. J Soc Gynecol Investig. 2002;9:125–136. [PubMed] [Google Scholar]
- 9.Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87:2924–2930. doi: 10.1210/jcem.87.6.8609. [DOI] [PubMed] [Google Scholar]
- 10.Ekman-Ordeberg G, Stjernholm Y, Wang H, Stygar D, Sahlin L. Endocrine regulation of cervical ripening in humans—potential roles for gonadal steroids and insulin-like growth factor-I. Steroids. 2003;68:837–847. doi: 10.1016/j.steroids.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Madsen G, Zakar T, Ku CY, Sanborn BM, Smith R, Mesiano S. Prostaglandins differentially modulate progesterone receptor-A and -B expression in human myometrial cells: evidence for prostaglandin-induced functional progesterone withdrawal. J Clin Endocrinol Metab. 2004;89:1010–1013. doi: 10.1210/jc.2003-031037. [DOI] [PubMed] [Google Scholar]
- 12.Goldman S, Weiss A, Almalah I, Shalev E. Progesterone receptor expression in human decidua and fetal membranes before and after contractions: possible mechanism for functional progesterone withdrawal. Mol Hum Reprod. 2005;11:269–277. doi: 10.1093/molehr/gah161. [DOI] [PubMed] [Google Scholar]
- 13.Danforth DN, Veis A, Breen M, Weinstein HG, Buckingham JC, Manalo P. The effect of pregnancy and labor on the human cervix: changes in collagen, glycoproteins, and glycosaminoglycans. Am J Obstet Gynecol. 1974;120:641–651. doi: 10.1016/0002-9378(74)90608-5. [DOI] [PubMed] [Google Scholar]
- 14.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–80. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 15.Hertelendy F, Zakar T. Prostaglandins and the myometrium and cervix. Prostaglandins Leukot Essent Fatty Acids. 2004;70:207–222. doi: 10.1016/j.plefa.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Novy MJ, Liggins GC. Role of prostaglandins, prostacyclin, and thromboxanes in the physiologic control of the uterus and in parturition. Semin Perinatol. 1980;4:45–66. [PubMed] [Google Scholar]
- 17.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 18.Sennstrom MB, Ekman G, Westergren-Thorsson G, Malmstrom A, Bystrom B, Endresen U, Mlambo N, Norman M, Stabi B, Brauner A. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod. 2000;6:375–381. doi: 10.1093/molehr/6.4.375. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol. 2005;66:161–173. doi: 10.1016/j.jri.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Ledingham MA, Thomson AJ, Jordan F, Young A, Crawford M, Norman JE. Cell adhesion molecule expression in the cervix and myometrium during pregnancy and parturition. Obstet Gynecol. 2001;97:235–242. doi: 10.1016/s0029-7844(00)01126-1. [DOI] [PubMed] [Google Scholar]
- 21.Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorpho-nuclear leukocytes during cervical dilation. Am J Obstet Gynecol. 1980;138:273–281. doi: 10.1016/0002-9378(80)90248-3. [DOI] [PubMed] [Google Scholar]
- 22.Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66:445–449. doi: 10.1095/biolreprod66.2.445. [DOI] [PubMed] [Google Scholar]
- 23.Goshowaki H, Ito A, Mori Y. Effects of prostaglandins on the production of collagenase by rabbit uterine cervical fibroblasts. Prostaglandins. 1988;36:107–114. doi: 10.1016/0090-6980(88)90106-2. [DOI] [PubMed] [Google Scholar]
- 24.Norwitz ER, Starkey PM, Lopez Bernal A, Turnbull AC. Identification by flow cytometry of the prostaglandin-producing cell populations of term human decidua. J Endocrinol. 1991;131:327–334. doi: 10.1677/joe.0.1310327. [DOI] [PubMed] [Google Scholar]
- 25.Norwitz ER, Lopez BA, Starkey PM. Tumor necrosis factor-alpha selectively stimulates prostaglandin F2 alpha production by macrophages in human term decidua. Am J Obstet Gynecol. 1992;167:815–820. doi: 10.1016/s0002-9378(11)91595-6. [DOI] [PubMed] [Google Scholar]
- 26.Rath W, Osmers R, Adelmann-Grill BC, Stuhlsatz HW, Szevereny M, Kuhn W. Biochemical changes in human cervical connective tissue after intracervical application of prostaglandin E2. Prostaglandins. 1993;45:375–384. doi: 10.1016/0090-6980(93)90114-m. [DOI] [PubMed] [Google Scholar]
- 27.Yu SY, Tozzi CA, Babiarz J, Leppert PC. Collagen changes in rat cervix in pregnancy—polarized light microscopic and electron microscopic studies. Proc Soc Exp Biol Med. 1995;209:360–368. doi: 10.3181/00379727-209-43908. [DOI] [PubMed] [Google Scholar]
- 28.Calder AA. Prostaglandins and biological control of cervical function. Aust N Z J Obstet Gynaecol. 1994;34:347–351. doi: 10.1111/j.1479-828x.1994.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 29.Kelly RW. Inflammatory mediators and cervical ripening. J Reprod Immunol. 2002;57:217. doi: 10.1016/s0165-0378(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 30.Hollingsworth M, Isherwood CN. Changes in the extensibility of the cervix of the rat in late pregnancy produced by prostaglandin F2alpha, ovariectomy and steroid replacement [proceedings]. Br J Pharmacol. 1977;61:501P–502P. [PMC free article] [PubMed] [Google Scholar]
- 31.Liggins GC. Cervical ripening as an inflammatory reaction. In: Ellwood DA, Anderson ABM, editors. The Cervix in Pregnancy and Labour. Clinical and Biochemical Investigations. Churchill Livingstone; London: 1981. pp. 1–12. [Google Scholar]
- 32.Busiek DF, Baragi V, Nehring LC, Parks WC, Welgus HG. Matrilysin expression by human mononuclear phagocytes and its regulation by cytokines and hormones. J Immunol. 1995;154:6484–6491. [PubMed] [Google Scholar]
- 33.Osmers R, Rath W, Adelmann-Grill BC, Fittkow C, Kuloczik M, Szeverenyi M, Tschesche H, Kuhn W. Origin of cervical collagenase during parturition. Am J Obstet Gynecol. 1992;166:1455–1460. doi: 10.1016/0002-9378(92)91619-l. [DOI] [PubMed] [Google Scholar]
- 34.Hunt JS, Manning LS, Mitchell D, Selanders JR, Wood GW. Localization and characterization of macrophages in murine uterus. J Leukoc Biol. 1985;38:255–265. doi: 10.1002/jlb.38.2.255. [DOI] [PubMed] [Google Scholar]
- 35.Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod. 1999;61:879–883. doi: 10.1095/biolreprod61.4.879. [DOI] [PubMed] [Google Scholar]
- 36.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- 37.Hunt JS, Petroff MG, Burnett TG. Uterine leukocytes: key players in pregnancy. Semin Cell Dev Biol. 2000;11:127–137. doi: 10.1006/scdb.2000.0158. [DOI] [PubMed] [Google Scholar]
- 38.Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- 39.Kabashima K, Saji T, Murata T, Nagamachi M, Matsuoka T, Segi E, Tsuboi K, Sugimoto Y, Kobayashi T, Miyachi Y, Ichikawa A, Narumiya S. The prostaglandin E receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883–893. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuboi K, Iwane A, Nakazawa S, Sugimoto Y, Ichikawa A. Role of prostaglandin H2 synthase 2 in murine parturition: study on ovariectomy-induced parturition in prostaglandin F receptor-deficient mice. Biol Reprod. 2003;69:195–201. doi: 10.1095/biolreprod.102.013870. [DOI] [PubMed] [Google Scholar]
- 41.Kirby LS, Kirby MA, Warren JW, Tran LT, Yellon SM. Increased innervation and ripening of the prepartum murine cervix. J Soc Gynecol Investig. 2005;12:578–585. doi: 10.1016/j.jsgi.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Leppert PC, Kokenyesi R, Klemenich CA, Fisher J. Further evidence of a decorin-collagen interaction in the disruption of cervical collagen fibers during rat gestation. Am J Obstet Gynecol. 2000;182:805–811. doi: 10.1016/s0002-9378(00)70329-2. [DOI] [PubMed] [Google Scholar]
- 43.Rasband WS. ImageJ. U.S. National Institutes of Health; Bethesda, Maryland: Jun 30, 2004. pp. 1997–2007. World Wide Web (URL: http://rsb.info.nih.gov/ij/) [Google Scholar]
- 44.Luque EH, Munoz de Toro MM, Ramos JG, Rodriguez HA, Sherwood OD. Role of relaxin and estrogen in the control of eosinophilic invasion and collagen remodeling in rat cervical tissue at term. Biol Reprod. 1998;59:795–800. doi: 10.1095/biolreprod59.4.795. [DOI] [PubMed] [Google Scholar]
- 45.Fosang AJ, Handley CJ, Santer V, Lowther DA, Thorburn GD. Pregnancy-related changes in the connective tissue of the ovine cervix. Biol Reprod. 1984;30:1223–1235. doi: 10.1095/biolreprod30.5.1223. [DOI] [PubMed] [Google Scholar]
- 46.Kavanagh J, Kelly AJ, Thomas J. Hyaluronidase for cervical priming and induction of labour. Cochrane Database Syst Rev. 2001:CD003097. doi: 10.1002/14651858.CD003097. [DOI] [PubMed] [Google Scholar]
- 47.Varayoud J, Ramos JG, Joazeiro PP, Montes GS, Munoz de Toro MM, Luque EH. Characterization of fibroblastic cell plasticity in the lamina propria of the rat uterine cervix at term. Biol Reprod. 2001;65:375–383. doi: 10.1095/biolreprod65.2.375. [DOI] [PubMed] [Google Scholar]
- 48.Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod. 2006;74:236–245. doi: 10.1095/biolreprod.105.044891. [DOI] [PubMed] [Google Scholar]
- 49.Chwalisz K, Hegele-Hartung C, Schulz R, Qing SS, Louton PT, Elger W. Progesterone control of cervical ripening—Experimental studies with the progesterone antagonists onapristone, lilopristone and mifepristone. In: Leppert PC, Woessner JF Jr., editors. The Extracellular Matrix of the Uterus, Cervix and Fetal Membranes: Synthesis, Degradation and Hormonal Regulation. Perinatology Press; Ithaca: 1991. pp. 119–131. [Google Scholar]
- 50.Luque EH, Ramos JG, Rodriguez HA, Munoz de Toro MM. Dissociation in the control of cervical eosinophilic infiltration and collagenolysis at the end of pregnancy or after pseudopregnancy in ovariectomized steroid-treated rats. Biol Reprod. 1996;55:1206–1212. doi: 10.1095/biolreprod55.6.1206. [DOI] [PubMed] [Google Scholar]
- 51.Yellon SM, Ebner CA, Elovitz MA. MPA blocks effects of LPS-induced preterm birth on ripening, immigrations of immune cells, and density of nerve fibers in the cervix. Reprod Sci. 2007;14(suppl 1):143A. doi: 10.1177/1933719108325757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Renzo GC, Mattei A, Gojnic M, Gerli S. Progesterone and pregnancy. Curr Opin Obstet Gynecol. 2005;17:598–600. doi: 10.1097/01.gco.0000191899.84567.4d. [DOI] [PubMed] [Google Scholar]
- 53.Spong CY, Meis PJ, Thom EA, Sibai B, Dombrowski MP, Moawad AH, Hauth JC, Iams JD, Varner MW, Caritis SN, O'Sullivan MJ, Miodovnik M, et al. Progesterone for prevention of recurrent preterm birth: impact of gestational age at previous delivery. Am J Obstet Gynecol. 2005;193:1127–1131. doi: 10.1016/j.ajog.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 54.Sanchez-Ramos L, Kaunitz AM, Delke I. Progestational agents to prevent preterm birth: a meta-analysis of randomized controlled trials. Obstet Gynecol. 2005;105:273–279. doi: 10.1097/01.AOG.0000150559.59531.b2. [DOI] [PubMed] [Google Scholar]
- 55.Hertelendy F, Zakar T. Prostaglandins and the myometrium and cervix. Prostaglandins Leukot Essent Fatty Acids. 2004;70:207–222. doi: 10.1016/j.plefa.2003.04.009. [DOI] [PubMed] [Google Scholar]