Abstract
The protease-activated receptors (PARs) are G protein-coupled receptors (GPCRs) that are uniquely activated by proteolysis. PARs mediate hemostasis, thrombosis, inflammation, embryonic development and progression of certain malignant cancers. The family of PARs include four members: PAR1, PAR2, PAR3 and PAR4. PARs harbor a cryptic ligand sequence within the N-terminus that is exposed following proteolytic cleavage. The newly formed PAR N-terminus functions as a tethered ligand that binds intramolecularly to the receptor to trigger transmembrane signaling. This unique mechanism of activation would indicate that regardless of the activating protease, cleavage of PARs would unmask a tethered ligand sequence that would induce a similar active receptor conformation and signaling response. However, this is not the case. Recent studies demonstrate that PARs can be differentially activated by synthetic peptide agonists, proteases or through dimerization, that ultimately result in distinct cellular responses. In some cases, allosteric modulation of PARs involves compartmentalization in caveolae, plasma membrane microdomains enriched in cholesterol. Here, we discuss the mechanisms that lead to allosteric modulation of PAR signaling.
Keywords: Thrombin, GPCR, G protein, caveolae, palmitoylation
INTRODUCTION
The seven transmembrane superfamily of G protein-coupled receptors (GPCRs) are the most abundant group of signaling receptors in the mammalian genome and one of the most important targets for drug development. GPCRs are dynamic molecules assuming multiple conformational states, many of which are "active" as defined by their ability to modulate cellular activities [1]. The ability of GPCRs to exist as multiple distinct conformations is related to their capacity to be allosterically modulated. Several factors influence allosteric modulation of GPCRs including, but not limited to the binding of ligands to allosteric sites on the receptor, interaction with other transmembrane proteins, the plasma membrane microenvironment, receptor dimerization, and the interaction of proteins with the intracytosolic surfaces of the receptor. The capacity of different ligands or factors to stabilize unique conformations of the same receptor, which result in distinct signaling responses is termed “biased” agonism [2].
Protease-activated receptors (PARs) constitute a unique family of GPCRs that possess their own cryptic ligand sequence, which is revealed upon proteolytic cleavage. The family of PARs is comprised of four members including: PAR1, PAR2, PAR3 and PAR4. PARs are activated predominantly by serine proteases, which cleave the extracellular N-terminus, exposing a new N-terminus that acts as a tethered ligand by binding intramolecularly to the receptor to trigger transmembrane signaling. The mechanism of PAR1 activation by the coagulant protease thrombin has been most extensively studied [3, 4]. Thrombin binds to the PAR1 N-terminal LDPR41-S42FLLRN sequence and cleaves the R41-S42 peptide bond exposing a new N-terminal ligand sequence. A second interaction occurs between thrombin’s anion-binding exosite and an N-terminal acidic region distal to the cleavage site termed the “hirudin”-like domain, based on sequence homology to the leech anticoagulant peptide hirudin [5], which significantly enhances thrombin's affinity for the receptor. Synthetic peptide agonists similar in sequence to the newly exposed N-terminus can activate PAR1 independent of thrombin and proteolytic cleavage, albeit differently than that observed for thrombin in some cases as discussed below. Amongst PARs, only PAR1 and PAR3 contain the hirudin-like domain. PAR4 is also cleaved by thrombin, but it lacks the hirudin-like domain and is a low affinity receptor for thrombin. PAR2 is the only PAR not activated by thrombin.
In addition to thrombin, numerous other proteases have been shown to cleave and activate PAR1 including factor Xa, plasmin, kallikriens, activated protein C (APC) and the matrix metalloprotease-1 (MMP1). PAR4 and to a lesser extent PAR3 can be activated by many but not all of the same proteases. PAR2 is activated by factors VIIa and Xa and by a distinct group of serine proteases including trypsin, tryptase and the membrane-anchored matriptase. Clearly, a diverse group of proteases can activate PARs raising the possibility that different proteases may stabilize distinct active receptor conformations to elicit unique cellular responses in various cell types. Despite a vast literature on PARs, the function of allosteric modulation by different proteases, synthetic ligands or factors remains relatively unexplored. Here, we discuss allosteric modulation of PARs by peptide agonists, proteases, dimerization and by their localization to distinct plasma membrane microdomains.
PARs COUPLE TO MULTIPLE DISTINCT G PROTEIN SUBTYPES
The heterotrimeric G proteins are comprised of α- and βγ- subunits, which are each encoded by distinct genes and are grouped into four different families based on sequence homology and include the Gq/11, Gs, Gi/o and G12/13 subtypes. Once activated, GPCRs act as guanine nucleotide exchange factors and promote GTP exchange for GDP on the α-subunit, which causes dissociation from the βγ subunit and is the rate limiting step for activation of subsequent signaling responses. Similar to other GPCRs, PAR1 is promiscuous and couples to multiple G protein subtypes including Gi/o, Gq/11 and G12/13 to promotes various signaling responses that differ in distinct cell types. This phenomenon has been observed in cells expressing endogenous PAR1 and in cellular systems ectopically expressing PAR1. Previous studies indicated that activation of PAR1 generally inhibits cAMP accumulation and activates Rac1 through Gi, stimulates phospholipase C-mediated phosphoinositide hydrolysis and calcium mobilization via Gq [6, 7], and activates RhoA through G12/13 [8, 9]. Human PAR3 also appears to couple to Gq, whereas activated PAR4 can elicit signaling responses through Gq, Gi, and G12/13 depending on the cell type. Activated PAR2 also differentially couples to distinct G protein subtypes including Gq, Gi, and G12/13 that is cell type-dependent. PAR2 further promotes sustained signaling through stable interactions with β-arrestins, independent of heterotrimeric G proteins. β-arrestins function as scaffolds that elicit extracellular signal-regulated protein kinase-1, 2 (ERK1, 2) activation from intracellular compartments [10, 11]. The mechanisms that specify activated PAR coupling to distinct G protein subtypes remain poorly understood.
Verrall et al. previously demonstrated that the PAR1 second intracellular loop specifies coupling to Gq signaling when examined by ectopic expression of chimeric receptors in COS7 cells [12]. The PAR1 second intracellular loop harbors a highly conserved glutamic acid (D), aspartic acid (R) and phenylalanine (F), termed DRF or DRY motif that is present in most GPCRs and is critical for receptor interaction with G proteins. Mutation of DR to RD virtually ablated activated PAR1 coupling to G protein signaling when exogenously expressed in Xenopus oocytes [13]. More recent studies have examined the capacity of activated PAR1 to couple to various G protein subtypes in living cells using bioluminescence resonance energy transfer (BRET). BRET entails expressing a PAR1 fused to the energy acceptor yellow fluorescent protein (YFP) at the carboxyl tail and the G protein with the energy donor Renilla Luciferase (Rluc) engineered within a helical domain that does not appear to perturb G protein function. If the Rluc- and YFP-fused proteins are in close enough proximity (~10 nm), then energy transfer from Rluc to YFP occurs and causes the latter to emit fluorescensce [14, 15]. Using BRET analysis, Ayoub et al. showed that unactivated PAR1 basally associates with Gαi in COS7 cells suggesting that it is preassembled or localized together with Gαi in a plasma membrane microdomain and is poised to signal following activation [16]. After incubation with thrombin or peptide agonists, activated PAR1 resulted in rapid stimulation of Gαi activation with a t½ value of 5 sec, as determined by an increased in BRET that was abolished by pertussis toxin or PAR1 antagonists. The agonist-induced change in BRET between PAR1-YFP and Gαi-Rluc likely involves movement within the preassembled complex that results in more efficient energy transfer. Moreover, activated PAR1 coupling to Gαi signaling exhibited desensitization with an attenuation of BRET that is correlated with the recruitment of β-arrestin-1. In contrast to Gαi, Ayoub et al. also showed that activated PAR1 slowly recruits Gα12 to a population of receptors not preassembled with Gα12 or Gαi [15]. The PAR1 and Gα12 complex forms after several minutes of activation and is maintained for almost one hour, but whether the complex remains active is not known. At face value, these findings suggest the existence of two distinct PAR1 populations, one that is preassembled with Gαi and a second that slowly recruits Gα12. The molecular basis for differences in G protein subtype activation by PAR1 is not known and could contribute to biased agonism.
DIFFERENTIAL ACTIVATION OF PARS BY SYNTHETIC PEPTIDE AGONISTS
Previous studies indicated that activation of PARs with synthetic peptide agonists fails to recapitulate the full repertoire of responses when compared with activation by native proteases, suggesting that PARs are capable of being differentially activated. This has been most studied for PAR1 and PAR2 in various cellular contexts including human endothelial cells. The activation of PAR1 with synthetic peptide agonists SFLLRN or TFLLRNPNDK promotes preferential coupling to Gq signaling. In contrast, thrombin activation of PAR1 results in coupling to G12/13 signaling when examined in human dermal microvascular endothelial cells [17]. The differential signaling responses observed with thrombin and peptide agonist were revealed at low agonist concentrations and were generally masked at high agonist concentrations. In human brain microvascular endothelial cells, thrombin activation of PAR1 induced rapid and transient calcium responses and triggered endothelial barrier permeability, whereas agonist peptide caused sustained calcium responses with minimal effects on endothelial barrier permeability [18]. In addition, mutations within the PAR1 extracellular loops (ECLs) impaired peptide agonist activation of the receptor but not thrombin [19], suggesting that the activation mechanisms are not necessarily the same. Similar to PAR1, activation of PAR2 is thought to occur through cleavage and tethered ligand interactions with the surface of the ECL2 domain [20]. Activation of PAR2 ECL2 mutants with peptide agonists was also impaired compared to receptor activation with trypsin, a natural activating protease [21]. Additional studies comparing the activation of PAR2 tethered ligand mutants with trypsin and soluble peptide mimetics harboring similar mutations revealed different modes of activation by tethered versus soluble agonist peptides [22]. Moreover, PAR2 tethered ligands and soluble peptide agonist variants elicited distinct cellular responses. These findings suggest that tethered versus soluble agonist peptides differ in their capacity to stabilize distinct active receptor conformations and promote biased signaling.
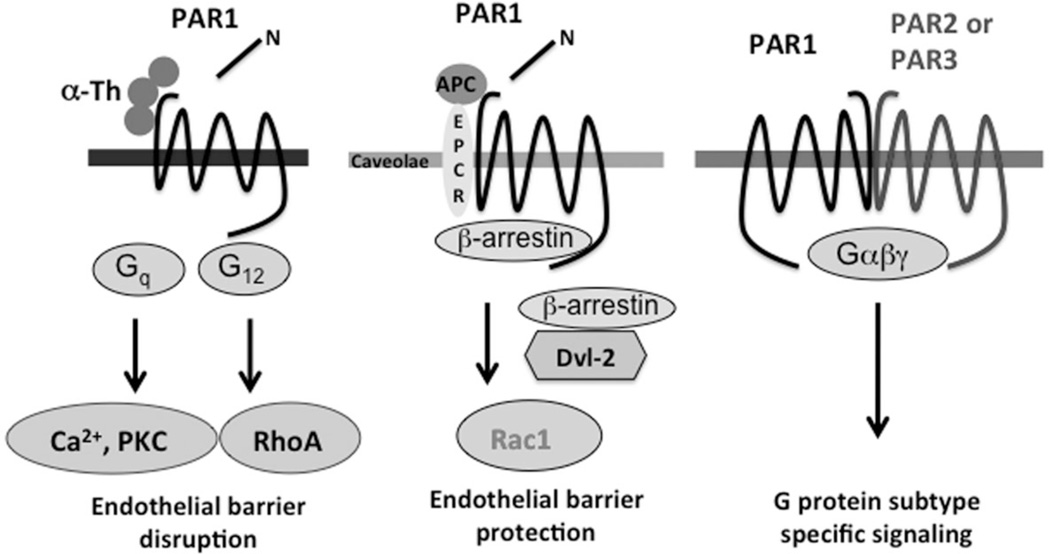
PAR3 and PAR4 also exhibit differences in their activation mechanisms compared to other PARs. The initial characterization of human PAR3 indicated that it was equally responsive to thrombin comparable to PAR1 when expressed ectopically in Xenopus oocytes [23]. However, in this system synthetic peptide agonists failed to activate PAR3. Thus, the proteolytic cleavage of PAR3 appears to be crucial for proper signaling when exogenously expressed. In human smooth muscle cells, both thrombin and the PAR3-activating peptide TFRGAP induced calcium mobilization, a response desensitized by prior thrombin exposure indicating that PAR3 is a functional thrombin receptor in human smooth muscle cells [24]. Thrombin signaling in human lung epithelial cells is also mediated by endogenous PAR3, since these cells lack PAR1 and PAR4 expression [25]. Human PAR3 was shown to heterodimerize with PAR1 in human pulmonary artery endothelial cells and dimerization allosterically modulates PAR1 signaling towards preferential coupling to G13 activation (Figure 1) [26]. PAR1 also appears to dimerize with PAR2. Thrombin cleavage of the PAR1 N-terminus unmasks a tethered ligand domain that binds to and activates PAR2 in trans to promote distinct signaling responses [27, 28]. Transactivation of PAR2 by PAR1 has been implicated in endothelial barrier protection during sepsis [28], and switches the signaling responses of thrombin from G12/13-mediated barrier disruptive to Gi-induced barrier protective. Thus, dimerization of PARs modulates G protein coupling specificity and signaling responses, and ultimately promotes biased agonism.
Figure 1. Allosteric modulation of PAR1 signaling.
In endothelial cells activation of PAR1 with thrombin promotes coupling to G12 and RhoA activation the promotes endothelial barrier disruption. In contrast, activation of PAR1 with activated protein C (APC) leads to Rac1 activation presumably through Gi coupling and is critical for maintaining endothelial barrier integrity. The biased agonism promoted by APC activation of PAR1 requires compartmentalization in caveolae - lipid rafts enriched in cholesterol, sphingolipids and caveolin-1. Recent work suggests that PARs interact with each other and result in distinct signaling responses compared to activation of monomeric PARs.
The signaling capacity of PAR3 is species dependent. In murine platelets, activated PAR3 fails to signal and instead functions as a cofactor for the cleavage and activation of PAR4 by thrombin [29]. As mentioned above, PAR4 lacks the hirudin-like sequence and is a low affinity receptor for thrombin [30]. PAR4 is less sensitive to synthetic peptide agonists compared to other PARs. The synthetic peptide agonist that mimics the PAR4 native tethered ligand sequence GYPGQV lacks potency and requires high agonist concentrations to drive receptor activation. However, an agonist peptide variant AYPGKF is ten times more potent at activating PAR4 than the natural peptide agonist and is comparable to thrombin at triggering cellular responses in some cell types [31]. Moreover, activation of PAR4 by thrombin or peptide agonists exhibits a slower onset, but sustained signaling response compared to PAR1, which displays rapid and transient coupling to G protein signaling [32]. PAR4 also differs from PAR1 in G protein coupling specificity. In human platelets, activated PAR4 couples predominantly to Gq and G12/13 but not Gi/o [33], whereas in human endothelial cells PAR4 preferentially couples to Gi/o and not Gq signaling [34]. The differential activation of PAR3 and PAR4 by tethered versus peptide agonist and the subsequent activation of diverse signaling responses suggest the existence of multiple distinct active receptor conformations.
DIFFERENTIAL ACTIVATION OF PARS BY PROTEASES
The proteolytic activation of PARs reveals a cryptic tethered ligand sequence that binds intramolecularly to the receptor to trigger transmembrane signaling and thus, it was expected that cleavage of PARs with different proteases would activate similar signaling cascades and exhibit linear efficacy. The phenomenon of linear efficacy assumes that the capacity of an agonist to activate a signaling response is linearly related to subsequent receptor behaviors like phosphorylation, desensitization and internalization [35]. However, this is not the case for the activation of PAR1 with different proteases. The best example of differential activation of PARs by proteases occurs with thrombin versus activated protein C (APC), which results in distinct regulation of endothelial barrier permeability (Figure 1) [36]. Thrombin activation of PAR1 promotes coupling to Gq and G12/13 in endothelial cells and causes disassembly of adherens junctions and reorganization of the actin cytoskeleton via RhoA signaling and results in rapid and transient disruption of the endothelial barrier [37]. Conversely, APC cleaves and activates PAR1 in endothelial cells to stabilize the endothelial barrier. Moreover, prolonged incubation with APC protects against thrombin-induced endothelial barrier disruption [38, 39]. APC requires PAR1 for its cytoprotective functions since loss of PAR1 signaling by anti-PAR1 blocking antibodies or siRNA-mediated knockdown of PAR1 expression abolished the ability of APC to protect against thrombin-induced endothelial barrier permeability [38, 40]. APC-activated PAR1 is also linked to endothelial barrier protection in vivo [41]. In contrast to thrombin-activated PAR1, endothelial cells stimulated with APC do not exhibit changes in RhoA signaling but rather APC-activated PAR1 appears to couple preferentially to Rac1 activation [39, 40]. The endothelial barrier is maintained by basal Rac1 activity through modulation of the actin cytoskeleton and enhanced junctional linkages, whereas increased Rac1 activity is thought to counteract RhoA signaling and is important for restoring endothelial barrier [37]. The capacity of APC to enhance endothelial barrier integrity is suggested to involve transactivation of the Gi coupled sphingosine-1-phosphate (S1P) receptor [38, 39]. The S1P receptor activates Rac1 and is a key regulator of basal endothelial barrier integrity examined in cultured cells in vitro and in vivo, where plasma S1P levels are high [42]. The relative contribution of APC-activated PAR1 toward direct activation of Rac1 versus S1P receptor transactivation in APC cytoprotective signaling in endothelial cells has not been thoroughly examined.
As discussed above, activated PAR1 couples to multiple G protein subtypes including Gi, Gq, G12/13 even in the same cell. While the mechanisms that specify PAR1 coupling to distinct heterotrimeric G protein subtypes are not known, differential coupling to specific G protein subtypes may impart protease-selective or biased PAR1 signaling. Although thrombin and APC cleave PAR1 at the same LDPR41-S42FLLRN site, thrombin cleaves PAR1 with considerably greater efficiency compared to APC [43], but differences in rates of PAR1 cleavage by thrombin versus APC are not responsible for the distinct cellular responses elicited by the proteases [40, 44]. In contrast to thrombin, APC-activated PAR1 fails to stimulate Gq- or G12/13-dependent signaling responses. Rather, APC-activated PAR1 requires Gi to maintain endothelial barrier integrity based on studies using pertussis toxin which ADP-ribosylates the Gα subunits of Gi/o proteins, rendering them inactive [45]. However, this study did not discriminate between the effects of pertussis toxin on Gi/o inactivation directly on APC-mediated intracellular signaling versus S1P receptor activation, which couples to Gi to maintain basal endothelial barrier integrity. Thus, whether APC-activated PAR1 couples directly to Gi to promote Rac1 activation is not known. In addition, the role of other mediators in APC-induced biased PAR1 signaling has not been investigated.
The compartmentalization of PAR1 in caveolae is important for biased signaling induced by APC. Caveolae are lipid rafts enriched in cholesterol and sphingolipids that form 60–80 nm invaginations that require the structural protein caveolin-1 for formation and facilitate receptor-effector coupling [46]. Studies using sucrose gradient fractionation indicate that PAR1 together with the APC co-factor endothelial protein C receptor (EPCR) are present in caveolin-1 enriched fractions [47, 48]. The use of cholesterol depleting agents or siRNA-mediated caveolin-1 knockdown further suggested that APC cytoprotective signaling requires compartmentalization in caveolae [40, 48]. Interestingly, after APC treatment the majority of PAR1 remains intact on the cell surface as determined by its susceptibility to thrombin cleavage [40]. These findings suggest that APC only cleaves and activates a small population of cell surface PAR1. Furthermore, APC-activated PAR1 remains on the cell surface and fails to internalize in contrast to thrombin activated PAR1 [40, 49]. These studies suggest that in endothelial cells APC targets a distinct subpopulation of PAR1 residing in caveolae that undergo differential activation and signal termination processes compared to thrombin-activated PAR1. A more recent study indicates that another coagulant protease factor VIIa binds to EPCR to facilitate PAR1 activation, cytoprotective Rac1 signaling and endothelial barrier protection [50]. Activation of PAR2 is also differentially regulated based on its localization to caveolae. Tissue-factor-VIIa mediated activation of PAR2 requires intact caveolae, whereas activation of PAR2 with synthetic peptide agonists can occur in the absence of caveolae as demonstrated by cholesterol depletion [51]. Whether PAR2 compartmentalization is important for eliciting distinct signaling responses was not determined. Thus, the localization of PARs in plasma membrane microdomains restricts activation to a subset of signaling effectors important for biased agonism.
The matrix metalloprotease-1 (MMP1), also known as interstitial collagenase, can cleave and activate PAR1 at distinct cleavage sites in various cell types resulting in differential activation. In invasive breast carcinoma, MMP1 was shown to cleave PAR1 at the canonical N-terminal LDPR41-S42FLLRN site generating a tethered ligand that induced a modest elevation of intracellular calcium compared to thrombin, to stimulate breast cancer cell motility [52]. Thus, MMP1 activity generated from stromal fibroblasts appears to induce cancer cell migration through the activation of PAR1 via a proteolytic cleavage mechanism similar to that reported for thrombin [3]. Interestingly, however, substrate specificity analysis of MMP1 cleavage of the PAR1 exodomain in vitro using the amino terminus of PAR1, spanning residues A26 through L103 and exogenous MMP1 indicated that the SFL44-L45RN peptide bond is the preferred site of cleavage [53]. More recently, Trivedi et al. showed that collagen induced MMP1 activation of PAR1 on the surface of platelets and promoted preferentially coupling to RhoA activation [54]. Remarkably, MMP1 appears to cleave the PAR1 N-terminal exodomain at LD39-P40RSFLLRN site, which is two residues proximal to the canonical thrombin cleavage site. These findings suggest that endogenous proteases can differentially activate PAR1 signaling by generating different tethered ligands that presumably stabilize distinct active PAR1 conformations.
MOLECULAR BASIS OF PAR BIASED AGONISM
The observation that APC associates with its co-factor EPCR in caveolae and activates PAR1 to facilitate cytoprotective signaling indicates that receptor compartmentalization is one mechanism that contributes to differential signaling [40, 48]. However, the mechanisms responsible for targeting PAR1 to caveolae and facilitating biased signaling are largely unknown. It is possible that modification of PAR1 by palmitoylation is important for targeting the receptor to caveolae. Palmitoylation is a post-translational modification in which palmitate, a 16-carbon fatty acid, is added to a cysteine residue via a thioester linkage. This modification is a dynamic process whereby the palmitoyl group is added enzymatically through palmitoyl acyl transferases (PATs) and removed by palmitoyl-protein thioesterases (PPTs). The first PATs were identified in yeast and found to have a conserved aspartate-histidine-histidine-cysteine (DHHC) motif [55]. Recently, twenty-three putative PAT DHHC proteins were identified in the human genome and appear to localize to distinct subcellular compartments [55].
A number of GPCRs have been reported to undergo palmitoylation on conserved cysteine residues within the carboxyl terminal tail. The modification of GPCRs by palmitoylation appears to have multiple functions in receptor signaling and trafficking [56]. Palmitoylation of some GPCRs is important for proper maturation and trafficking of the receptors within the biosynthetic pathway. In addition, GPCR palmitoylation can influence the efficiency and specificity of receptor coupling to G proteins. Palmitoylation also regulates GPCR localization to lipid rafts and endocytic trafficking. Palmitoylation of the D1 receptor specifies internalization through caveolae rather than clathrin-coated pits [57], whereas palmitoylation of the 5-hydroxytryptamine-1A receptor is important for retention in lipid rafts [58] and is crucial for coupling to Gi signaling [59]. The localization of the µ-opiate receptor (MOR) to lipid rafts requires stable interaction with Gi and regulates agonist selective signaling [60], but itself does not appear to be palmitoylated, indicating that palmitoylation is not absolutely required for lipid raft localization of some GPCRs. The activation of MOR with etorphine results in β-arrestin recruitment, translocation out of lipid rafts and dissociation from Gi, whereas morphine induces MOR coupling to Gi with minimal effects on β-arrestin recruitment, phosphorylation or internalization. Modification of the endothelin-1 receptor with palmitoylation is important for Gq signaling but not for stimulation of Gs activation of adenylyl cyclase [61], suggesting that receptor palmitoylation affects G protein coupling specificity.
The palmitoylation of GPCRs within the carboxyl tail was predicted to result in the creation of a fourth intracellular loop. Indeed, the X-ray crystal structure for rhodopsin confirmed the presence of a fourth intracellular loop that exists as an α-helix and is often referred to as the 8th helix [62]. Moreover, palmitoylation of rhodopsin is also important for activation of the G protein transducin [63]. No studies to date have shown that PARs are posttranslationally modified by palmitoylation to our knowledge. However, sequence alignments of PAR carboxyl tails indicate the presence of two highly conserved juxtamembrane cysteine residues in PAR1 and a single cysteine residue in PAR2 [64]. There are no cysteine residues present in human PAR3 or PAR4 carboxyl terminal tails making it unlikely that these receptors are modified by palmitoylation. A structural model of PAR1 based on the X-ray structure of rhodopsin indicates that palmitoylation of cysteine (C) residues C387 and C388 would likely create an 8th helix that appears to be important for receptor coupling to Gq signaling [65]. Mutagenesis of the PAR1 C387 and C388 residues to serine (S) failed to affect the maximal signaling response induced by thrombin or SFLLRN as assessed by monitoring Gq-mediated phosphoinositide hydrolysis, but caused a shift in the concentration effect curves. Interestingly, the change in EC50 response was considerably greater with agonist peptide compared to thrombin, suggesting differences in intra- versus inter-molecular activation mechanisms. Whether such changes in PAR1 are actually due to palmitoylation was not determined in this study and is further complicated by the finding that serine can be used as an alternative palmitoylation site as shown previously for the human transferrin receptor [66]. Thus, it remains to be determined whether PARs are modified by palmitoylation and whether altering the status of palmitoylation has any consequence on G protein coupling specificity.
CONCLUSIONS
The findings that small molecules can function as allosteric modulators of GPCRs by binding to distinct sites on the receptor to modulate some, but not all signaling responses provides an important opportunity for new PAR drug development [35]. PARs couple to diverse signaling pathways and this raises the possibility that certain drugs could be developed to selectively block some but not all signaling pathways. The development of such allosteric modulators for PARs would have critical functions in modulating thrombosis, hemostasis, inflammation and progression of certain malignant cancers without global disruption of PAR function.
Two allosteric modulators of PAR1 have been described in the literature that appear to specifically regulate cell proliferation, fibrosis and thrombosis through inhibition of the Gq signaling pathway. A previous study showed that the small molecule benzimidazole derivate Q94 selectively blocks thrombin-activated PAR1 coupling to Gq signaling important for mouse lung fibroblast proliferation and fibroblast-to-myofibroblast differentiation [67, 68]. In recent work, Dowal et al. identified an inhibitory molecule termed J5F that appears to require the putative PAR1 8th helix to inhibit Gq signaling stimulated by SFLLRN in platelets [69]. Remarkably, however, the same compound J5F failed to affect SFLLRN-mediated signaling to G12 when examined in MDCK epithelial cells. J5F was also shown to inhibit mouse PAR4 signaling based on the observed decrease in arteriolar thrombus formation using a laser induction model. Mouse PAR4 possesses a carboxyl tail cysteine residue that is not conserved in human PAR4, suggesting that palmitoylation and formation of the 8th helix may be important for J5F effects in vivo. Clearly, more work is needed to delineate the mechanisms by which activated PARs couple to distinct signaling pathways and the contribution of posttranslational modifications to this process. This new information will facilitate the development of novel therapeutics that can be used to selectively modulate PAR specific signaling responses important for various diseases.
ACKNOWLEDGEMENTS
This work is also supported by the National Institutes of Health Grants HL073328 and GM090689 and a UC Tobacco-Related Disease Research Exploratory Award to J. Trejo I. Canto is supported by a National Institutes of Health T32 Pharmacological Sciences Training Grant from NIGMS and a Cornelius Hopper Diversity Award Supplement. U.J.K. Soh is supported by a UC Tobacco-Related Disease Research Postdoctoral Fellowship Award.
ABBREVIATIONS
- ADP
adenosine diphosphate
- APC
activated protein C
- BRET
bioluminescence resonance energy transfer
- cAMP
cyclic adenosine monophosphate
- DHHC
aspartate-histidine-histidine-cysteine
- EC50
half-maximal effective concentration
- ECL
extracellular loop
- EPCR
endothelial protein C receptor
- ERK1,2
extracellular signal-regulated protein kinase-1,2
- GPCR
G protein-coupled receptor
- MDCK
Madin-Darby canine kidney
- MMP-1
matrix metalloprotease-1
- MOR
µ-opiate receptor
- PAR
protease-activated receptor
- PATs
palmitoyl acyl transferases
- PPTs
palmitoyl-protein thioesterases
- Rluc
Renilla luciferase
- S1P
sphingosine-1-phosphate
- t1/2
half-life
- YFP
yellow fluorescent protein
REFERENCES
- 1.Kenakin T. Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther. 2011;336(2):296–302. doi: 10.1124/jpet.110.173948. [DOI] [PubMed] [Google Scholar]
- 2.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional Selectivity and Classical Concepts of Quantitative Pharmacology. J. Pharmacol. Exp. Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 3.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 4.Vu TK, Wheaton VI, Hung DT, Coughlin SR. Domains specifying thrombin-receptor interaction. Nature. 1991;353:674–677. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- 5.Rydel TJ, Ravichandran KG, Tulinsky A, Bode W, Huber R, Roitsch C, Fenton Jd. The structure of a complex of recombinant hirudin and human alpha-thrombin. Science. 1990;249(4966):277–280. doi: 10.1126/science.2374926. [DOI] [PubMed] [Google Scholar]
- 6.Baffy G, Yang L, Raj S, Manning DR, Williamson JR. G protein coupling to the thrombin receptor in Chinese hamster lung fibroblasts. J. Biol. Chem. 1994;269:8483–8487. [PubMed] [Google Scholar]
- 7.Offermanns S, Toombs CF, Hu YH, Simon MI. Defective platelet activation in G alpha(q)-deficient mice. Nature. 1997;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 8.Offermanns S, Laugwitz K-L, Spicher K, Schultz G. G proteins of the G12 family are activated via thromboxane A2 and thrombin receptors in human platelets. Proc. Natl. Acad. Sci. USA. 1994;91:504–508. doi: 10.1073/pnas.91.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez I, Mak EC, Ding J, Hamm HE, Lomasney JW. A novel bifunctional phospholipase C that is regulated by G alpha 12 and stimulates the ras/mitogen-activated protein kinase pathway. J. Biol. Chem. 2001;276:2758–2765. doi: 10.1074/jbc.M008119200. [DOI] [PubMed] [Google Scholar]
- 10.DeFea KA, Zalevski J, Thoma MS, Dery O, Mullins RD, Bunnett NW. β-Arrestin-dependent Endocytosis of Proteinase-activated Receptor-2 Is Required for Intracellular Targeting of Activated ERK1/2. J. Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stalheim L, Ding Y, Gullapalli A, Paing MM, Wolfe BL, Morris DR, Trejo J. Multiple independent functions of arrestins in regulation of protease-activated receptor-2 signaling and trafficking. Mol. Pharm. 2005;67:1–10. doi: 10.1124/mol.104.006072. [DOI] [PubMed] [Google Scholar]
- 12.Verrall S, Ishii M, Chen M, Wang L, Tram T, Coughlin SR. The thrombin receptor second cytoplasmic loop confers coupling to Gq-like G proteins in chimeric receptors. Additional evidence for a common transmembrane signaling and G protein coupling mechanism in G protein-coupled receptors. J. Biol. Chem. 1997;272(11):6898–6902. doi: 10.1074/jbc.272.11.6898. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Ishii M, Wang L, Ishii K, Coughlin SR. Thrombin receptor activation: Confirmation of the intramolecular tethered liganding hypothesis and discovery of an alternative intermolecular liganding mode. J. Biol. Chem. 1994;269:16041–16045. [PubMed] [Google Scholar]
- 14.Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M. Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET) Proc Natl Acad Sci U S A. 2000;97(7):3684–3689. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayoub MA, Trinquet E, Pfleger KD, Pin JP. Differential association modes of the thrombin receptor PAR1 with Galphai1, Galpha12, and beta-arrestin 1. FASEB J. 2010;24(9):3522–3535. doi: 10.1096/fj.10-154997. [DOI] [PubMed] [Google Scholar]
- 16.Ayoub MA, Maurel D, Binet V, Fink M, Prezeau L, Ansanay H, Pin JP. Real-time analysis of agonist-induced activation of protease-activated receptor-1/Galphai protein complex measured by bioluminescence resonance energy transfer in living cells. Mol Pharm. 2007;71:704–712. doi: 10.1124/mol.106.030304. [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin JN, Shen L, Holinstat M, Brooks JD, DiBenedetto E, Hamm HE. Functional Selectivity of G Protein Signaling by Agonist Peptides and Thrombin for the Protease-activated Receptor-1. J. Biol. Chem. 2005;280:25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- 18.Kim YV, Di Cello F, Hillaire CS, Kim KS. Differential Ca2+ signaling by thrombin and protease-activated receptor-1-activating peptide in human brain microvascular endothelial cells. Am. J. Physiol. Cell Physiol. 2004;286(1):C31–C42. doi: 10.1152/ajpcell.00157.2003. [DOI] [PubMed] [Google Scholar]
- 19.Blackhart BD, Ruslim-Litrus L, Lu CC, Alves VL, Teng W, Scarborough RM, Reynolds EE, Oksenberg D. Extracellular mutations of protease-activated receptor-1 result in differential activation by thrombin and thrombin receptor agonist peptide. Mol Pharm. 2000;58:1178–1187. doi: 10.1124/mol.58.6.1178. [DOI] [PubMed] [Google Scholar]
- 20.Lerner DJ, Chen M, Tram T, Coughlin SR. Agonist recognition by proteinase-activated receptor 2 and thrombin receptor. Importance of extracellular loop interactions for receptor function. J. Biol. Chem. 1996;271(24):13943–13947. [PubMed] [Google Scholar]
- 21.Al-Ani B, Wijesuriya SJ, Hollenberg MD. Proteinase-activated receptor 2: differential activation of the receptor by tethered ligand and soluble peptide analogs. J. Pharmacol. Exp. Ther. 2002;302(3):1046–1054. doi: 10.1124/jpet.302.3.1046. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran R, Mihara K, Mathur M, Rochdi MD, Bouvier M, Defea K, Hollenberg MD. Agonist-biased signaling via proteinase activated receptor-2: differential activation of calcium and mitogen-activated protein kinase pathways. Mol. Pharmacol. 2009;76(4):791–801. doi: 10.1124/mol.109.055509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishihara H, Connolly AJ, Zeng D, Kahn ML, Zheng YW, Timmons C, Tram T, Coughlin SR. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 24.Bretschneider E, Spanbroek R, Lotzer K, Habenicht AJ, Schror K. Evidence for functionally active protease-activated receptor-3 (PAR-3) in human vascular smooth muscle cells. Thromb. Haemost. 2003;90(4):704–709. doi: 10.1160/TH03-04-0203. [DOI] [PubMed] [Google Scholar]
- 25.Seminario-Vidal L, Kreda S, Jones L, O'Neal W, Trejo J, Boucher RC, Lazarowski ER. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of Rho- and Ca2+-dependent signaling pathways. J. Biol. Chem. 2009;284:20638–20648. doi: 10.1074/jbc.M109.004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin JN, Patterson MM, Malik AB. Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proc Natl Acad Sci U S A. 2007;104:5662–5667. doi: 10.1073/pnas.0700763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien PJ, Prevost N, Molino M, Hollinger MK, Woolkalis MJ, Woulfe DS, Brass LF. Thrombin Responses in Human Endothelial Cells. Contributions from receptors other than PAR1 include transactivation of PAR2 by thrombin-cleaved PAR1. J. Biol. Chem. 2000;275:13502–13509. doi: 10.1074/jbc.275.18.13502. [DOI] [PubMed] [Google Scholar]
- 28.Kaneider NC, Leger AJ, Agarwal A, Nguyen N, Perides G, Derian C, Covic L, Kuliopulos A. "Role reversal" for the receptor PAR1 in sepsis-induced vascular damage. Nat. Immunol. 2007;12:1303–1312. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakanishi-Matsui M, Zheng Y-W, Weiss EJ, Sulciner D, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- 30.Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV, Jr, Tam C, Coughlin SR. A dual thrombin receptor system for platelet activation. Nature. 1998;394(6694):690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- 31.Faruqi TR, Weiss EJ, Shapiro MJ, Huang W, Coughlin SR. Structure-function analysis of protease-activated receptor-4 tethered ligand peptides. Determinants of specificity and utility in assays of receptor function. J. Biol. Chem. 2000;275:19728–19734. doi: 10.1074/jbc.M909960199. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro MJ, Weiss EJ, Faruqi TR, Coughlin SR. Protease-activated receptors 1 and 4 are shut off with distinct kinetics after activation by thrombin. J. Biol. Chem. 2000;275:25216–25221. doi: 10.1074/jbc.M004589200. [DOI] [PubMed] [Google Scholar]
- 33.Voss B, McLaughlin JN, Holinstat M, Zent R, Hamm HE. PAR1, but not PAR4, activates human platelets through a Gi/o/phosphoinositide-3 kinase signaling axis. Mol. Pharmacol. 2007;71(5):1399–1406. doi: 10.1124/mol.106.033365. [DOI] [PubMed] [Google Scholar]
- 34.Hirano K, Nomoto N, Hirano M, Momota F, Hanada A, Kanaide H. Distinct Ca2+ requirement for NO production between proteinase-activated receptor 1 and 4 (PAR1 and PAR4) in vascular endothelial cells. J. Pharmacol. Exp. Ther. 2007;322(2):668–677. doi: 10.1124/jpet.107.121038. [DOI] [PubMed] [Google Scholar]
- 35.Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat. Rev. Drug Discov. 2005;4(11):919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- 36.Russo A, Soh UJ, Trejo J. Proteases display biased agonism at protease-activated receptors: location matters! Mol. Interv. 2009;9(2):87–96. doi: 10.1124/mi.9.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci. STKE. 2007;(412):re8. doi: 10.1126/stke.4122007re8. [DOI] [PubMed] [Google Scholar]
- 38.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine-1-phosphate receptor-1 crossactivation. Blood. 2005;105(8):3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 39.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JGN. Activated Protein C Mediates Novel Lung Endothelial Barrier Enhancement. J. Biol. Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 40.Russo A, Soh JK, Paing MM, Arora P, Trejo J. Caveolae are required for protease-selective signaling by protease-activated receptor-1. Proc. Natl. Acad. Sci. U S A. 2009;106:6393–6397. doi: 10.1073/pnas.0810687106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niessen F, Furlan-Freguia C, Fernandez JA, Mosnier LO, Castellino FJ, Weiler H, Rosen H, Griffin JH, Ruf W. Endogenous EPCR/aPC-PAR1 signaling prevents inflammation-induced vascular leakage and lethality. Blood. 2009;113(12):2859–2866. doi: 10.1182/blood-2008-12-192385. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Invest. 2009;119(7):1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ludeman MJ, Kataoka H, Srinivasas Y, Esmon NL, Esmon CT, Coughlin SR. PAR1 Cleavage and Signaling in Response to Activated Protein C and Thrombin. J. Biol. Chem. 2005;280:13122–13128. doi: 10.1074/jbc.M410381200. [DOI] [PubMed] [Google Scholar]
- 44.Riewald M, Ruf W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J. Biol. Chem. 2005;280(20):19808–19814. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- 45.Bae J-S, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor-1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parton RG, Richards AA. Lipid Rafts and Caveolae as Portals for Endocytosis: New Insights and Common Mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 47.Bae JS, Yang L, Rezaie AR. Lipid raft localization regulates the cleavage specificity of protease activated receptor-1 in endothelial cells. J. Thomb. Haemost. 2008;6:954–961. doi: 10.1111/j.1538-7836.2008.02924.x. [DOI] [PubMed] [Google Scholar]
- 48.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc. Natl. Acad. Sci. U S A. 2007;104(8):2867–2872. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuepbach RA, Feistritzer C, Brass LF, Riewald M. Activated protein C-cleaved protease activated receptor-1 is retained on the endothelial cell surface even in the presence of thrombin. Blood. 2008;111(5):2667–2673. doi: 10.1182/blood-2007-09-113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sen P, Gopalakrishnan R, Kothari H, Keshava S, Clark CA, Esmon CT, Pendurthi UR, Rao LV. Factor VIIa bound to endothelial cell protein C receptor activates protease activated receptor-1 and mediates cell signaling and barrier protection. Blood. 2011;117(11):3199–3208. doi: 10.1182/blood-2010-09-310706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Awasthi V, Mandal SK, Papanna V, Rao LVM, Pendurthi UR. Modulation of Tissue Factor-Factor VIIa Signaling by Lipid Rafts and Caveolae. Arterioscler. Thromb. Vasc. Biol. 2008;27:1447–1455. doi: 10.1161/ATVBAHA.107.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 Is a Matrix Metalloprotease-1 Receptor that Promotes Invasion and Tumorigenesis of Breast Cancer Cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 53.Nesi A, Fragai M. Substrate Specificities of Matrix Metalloproteinase 1 in PAR-1 Exodomain Proteolysis. Chembiochem. 2007;12:1367–1369. doi: 10.1002/cbic.200700055. [DOI] [PubMed] [Google Scholar]
- 54.Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O'Callaghan K, Covic L, Kuliopulos A. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137(2):332–343. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell. Biol. 2007;8:74–94. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 56.Chini B, Parenti M. G-protein-coupled receptors, cholesterol and palmitoylation: facts about fats. J. Mol. Endocrinol. 2009;42(5):371–379. doi: 10.1677/JME-08-0114. [DOI] [PubMed] [Google Scholar]
- 57.Kong MM, Verma V, O'Dowd BF, George SR. The role of palmitoylation in directing dopamine D1 receptor internalization through selective endocytic routes. Biochem. Biophys. Res. Commun. 2011;405(3):445–449. doi: 10.1016/j.bbrc.2011.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Renner U, Glebov K, Lang T, Papusheva E, Balakrishnan S, Keller B, Richter DW, Jahn R, Ponimaskin E. Localization of the mouse 5-hydroxytryptamine(1A) receptor in lipid microdomains depends on its palmitoylation and is involved in receptor-mediated signaling. Mol. Pharmacol. 2007;72(3):502–513. doi: 10.1124/mol.107.037085. [DOI] [PubMed] [Google Scholar]
- 59.Papoucheva E, Dumuis A, Sebben M, Richter DW, Ponimaskin EG. The 5-hydroxytryptamine(1A) receptor is stably palmitoylated, and acylation is critical for communication of receptor with Gi protein. J. Biol. Chem. 2004;279(5):3280–3291. doi: 10.1074/jbc.M308177200. [DOI] [PubMed] [Google Scholar]
- 60.Zheng H, Chu J, Qiu Y, Loh HH, Law P-Y. Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc. Natl. Acad. Sci. U S A. 2008;105:9421–9426. doi: 10.1073/pnas.0802253105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horstmeyer A, Cramer H, Sauer T, Muller-Esterl W, Schroeder C. Palmitoylation of endothelin receptor A. Differential modulation of signal transduction activity by post-translational modification. J. Biol. Chem. 1996;271(34):20811–20819. doi: 10.1074/jbc.271.34.20811. [DOI] [PubMed] [Google Scholar]
- 62.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289(5480):739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 63.Natochin M, Gasimov KG, Moussaif M, Artemyev NO. Rhodopsin determinants for transducin activation: a gain-of-function approach. J. Biol. Chem. 2003;278(39):37574–37581. doi: 10.1074/jbc.M305136200. [DOI] [PubMed] [Google Scholar]
- 64.Arora P, Ricks TK, Trejo J. Protease-activated receptor signalling, endocytic sorting and dysregulation in cancer. J. Cell Sci. 2007;120:921–928. doi: 10.1242/jcs.03409. [DOI] [PubMed] [Google Scholar]
- 65.Swift S, Leger AJ, Talavera J, Zhang L, Bohm A, Kuliopulos A. Role of the PAR1 receptor 8th helix in signaling: the 7-8-1 receptor activation mechanism. J. Biol. Chem. 2006;281(7):4109–4116. doi: 10.1074/jbc.M509525200. [DOI] [PubMed] [Google Scholar]
- 66.Jing SQ, Trowbridge IS. Nonacylated human transferrin receptors are rapidly internalized and mediate iron uptake. J. Biol. Chem. 1990;265(20):11555–11559. [PubMed] [Google Scholar]
- 67.Deng X, Mercer PF, Scotton CJ, Gilchrist A, Chambers RC. Thrombin induces fibroblast CCL2/JE production and release via coupling of PAR1 to Galphaq and cooperation between ERK1/2 and Rho kinase signaling pathways. Mol. Biol. Cell. 2008;19(6):2520–2533. doi: 10.1091/mbc.E07-07-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scotton CJ, Krupiczojc MA, Konigshoff M, Mercer PF, Lee YC, Kaminski N, Morser J, Post JM, Maher TM, Nicholson AG, Moffatt JD, Laurent GJ, Derian CK, Eickelberg O, Chambers RC. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J. Clin. Invest. 2009;119(9):2550–2563. doi: 10.1172/JCI33288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dowal L, Sim DS, Dilks JR, Blair P, Beaudry S, Denker BM, Koukos G, Kuliopulos A, Flaumenhaft R. Identification of an antithrombotic allosteric modulator that acts through helix 8 of PAR1. Proc. Natl. Acad. Sci. U S A. 2011;108(7):2951–2956. doi: 10.1073/pnas.1014863108. [DOI] [PMC free article] [PubMed] [Google Scholar]