Abstract
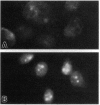
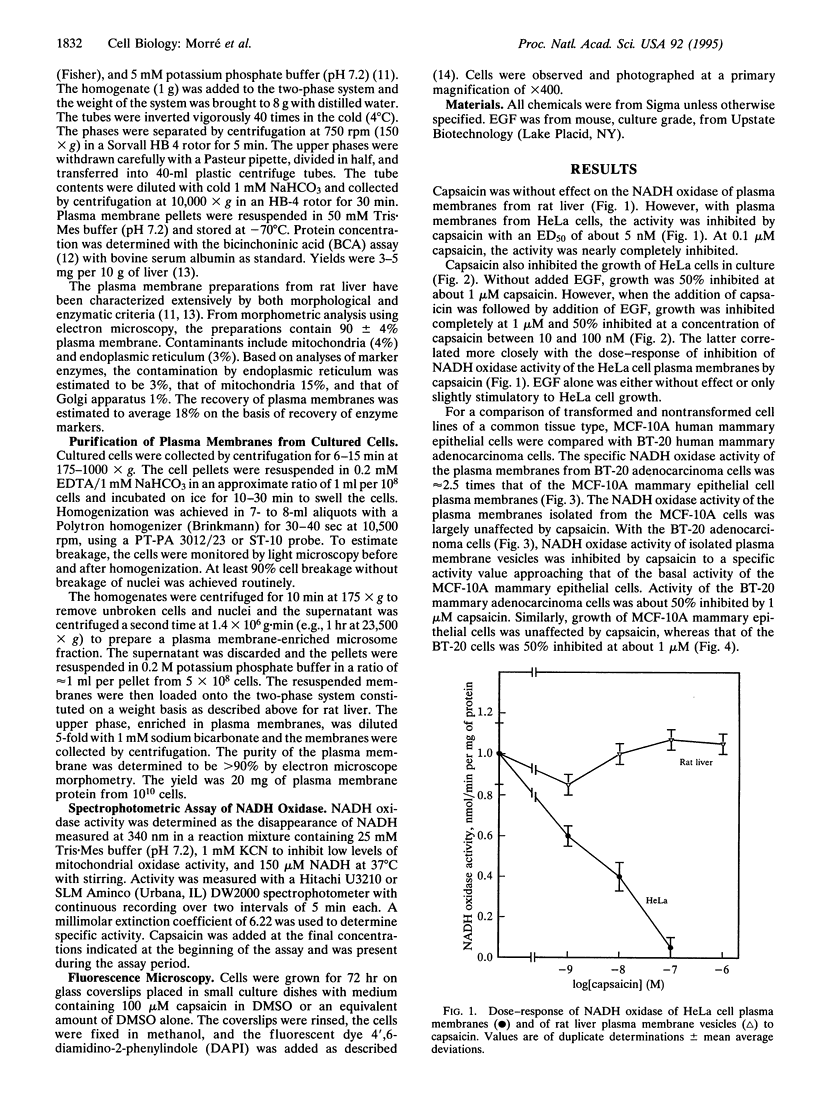
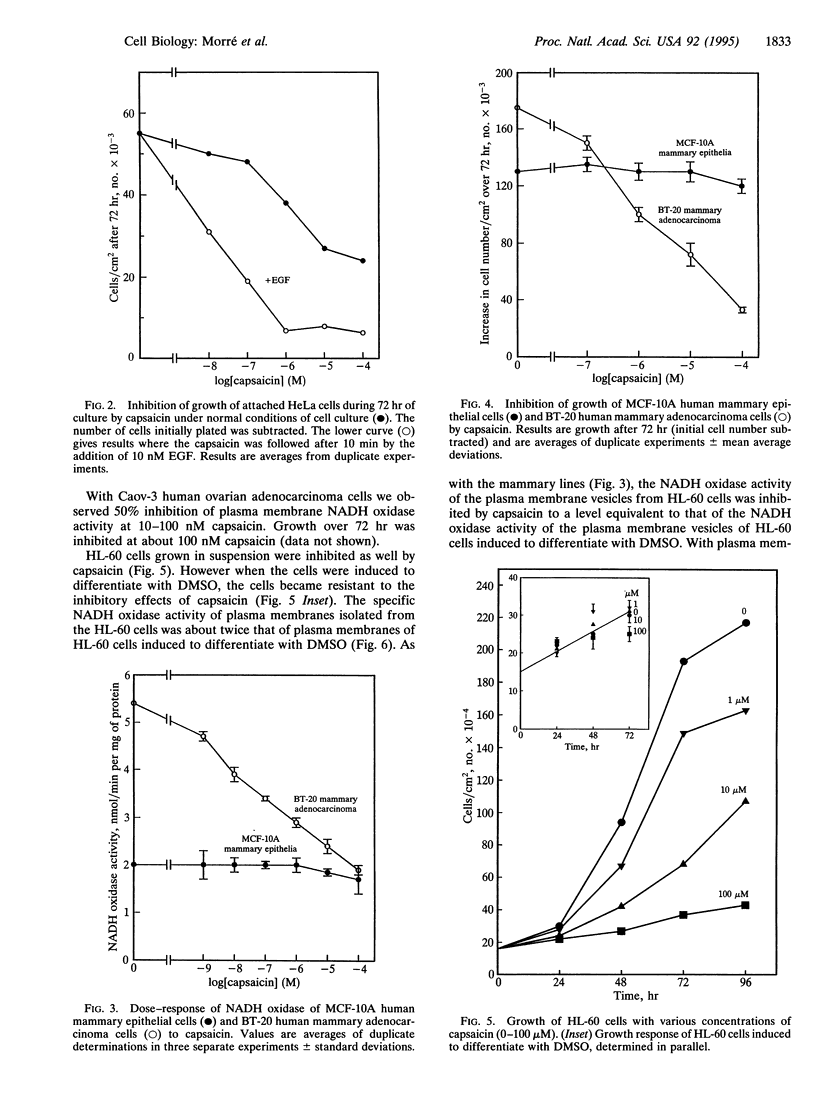
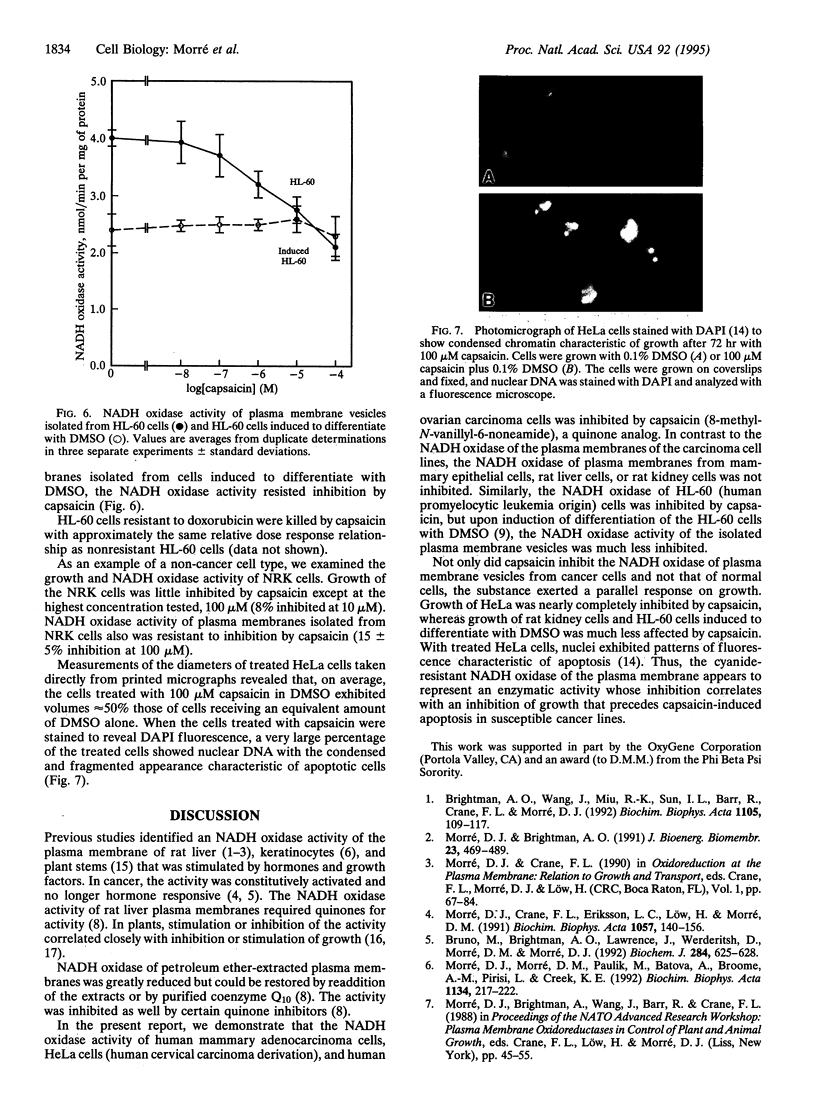
A hormone- and growth factor-stimulated NADH oxidase of the mammalian plasma membrane, constitutively activated in transformed cells, was inhibited preferentially in HeLa, ovarian carcinoma, mammary adenocarcinoma, and HL-60 cells, all of human origin, by the naturally occurring quinone analog capsaicin (8-methyl-N-vanillyl-6-noneamide), compared with plasma membranes from human mammary epithelial, rat liver, normal rat kidney cells, or HL-60 cells induced to differentiate with dimethyl sulfoxide. With cells in culture, capsaicin preferentially inhibited growth of HeLa, ovarian carcinoma, mammary adenocarcinoma, and HL-60 cells but was largely without effect on the mammary epithelial cells, rat kidney cells, or HL-60 cells induced to differentiate with dimethyl sulfoxide. Inhibited cells became smaller and cell death was accompanied by a condensed and fragmented appearance of the nuclear DNA, as revealed by fluorescence microscopy with 4',6-diamidino-2-phenylindole, suggestive of apoptosis. The findings correlate capsaicin inhibition of cell surface NADH oxidase activity and inhibition of growth that correlate with capsaicin-induced apoptosis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brightman A. O., Barr R., Crane F. L., Morré D. J. Auxin-Stimulated NADH Oxidase Purified from Plasma Membrane of Soybean. Plant Physiol. 1988 Apr;86(4):1264–1269. doi: 10.1104/pp.86.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman A. O., Wang J., Miu R. K., Sun I. L., Barr R., Crane F. L., Morré D. J. A growth factor- and hormone-stimulated NADH oxidase from rat liver plasma membrane. Biochim Biophys Acta. 1992 Mar 23;1105(1):109–117. doi: 10.1016/0005-2736(92)90168-l. [DOI] [PubMed] [Google Scholar]
- Bruno M., Brightman A. O., Lawrence J., Werderitsh D., Morré D. M., Morre D. J. Stimulation of NADH oxidase activity from rat liver plasma membranes by growth factors and hormones is decreased or absent with hepatoma plasma membranes. Biochem J. 1992 Jun 15;284(Pt 3):625–628. doi: 10.1042/bj2840625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979 Apr 1;149(4):969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenders J. W., Morre H. L., Smits P., Thien T. The effects of caffeine on the postprandial fall of blood pressure in the elderly. Age Ageing. 1988 Jul;17(4):236–240. doi: 10.1093/ageing/17.4.236. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Brightman A. O. NADH oxidase of plasma membranes. J Bioenerg Biomembr. 1991 Jun;23(3):469–489. doi: 10.1007/BF00771015. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Crane F. L., Eriksson L. C., Löw H., Morré D. M. NADH oxidase of liver plasma membrane stimulated by diferric transferrin and neoplastic transformation induced by the carcinogen 2-acetylaminofluorene. Biochim Biophys Acta. 1991 Mar 1;1057(1):140–146. doi: 10.1016/s0005-2728(05)80094-5. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Morré D. M., Paulik M., Batova A., Broome A. M., Pirisi L., Creek K. E. Retinoic acid and calcitriol inhibition of growth and NADH oxidase of normal and immortalized human keratinocytes. Biochim Biophys Acta. 1992 Apr 7;1134(3):217–222. doi: 10.1016/0167-4889(92)90179-f. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Morré D. M. Preparation of mammalian plasma membranes by aqueous two-phase partition. Biotechniques. 1989 Oct;7(9):946-8, 950-4, 956-8. [PubMed] [Google Scholar]
- Navas P., Nowack D. D., Morré D. J. Isolation of purified plasma membranes from cultured cells and hepatomas by two-phase partition and preparative free-flow electrophoresis. Cancer Res. 1989 Apr 15;49(8):2147–2156. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sun I. L., Sun E. E., Crane F. L., Morré D. J., Lindgren A., Löw H. Requirement for coenzyme Q in plasma membrane electron transport. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11126–11130. doi: 10.1073/pnas.89.23.11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolvetang E. J., Johnson K. L., Krauer K., Ralph S. J., Linnane A. W. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994 Feb 14;339(1-2):40–44. doi: 10.1016/0014-5793(94)80380-3. [DOI] [PubMed] [Google Scholar]