Abstract
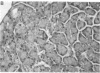
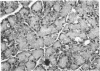
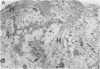
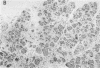
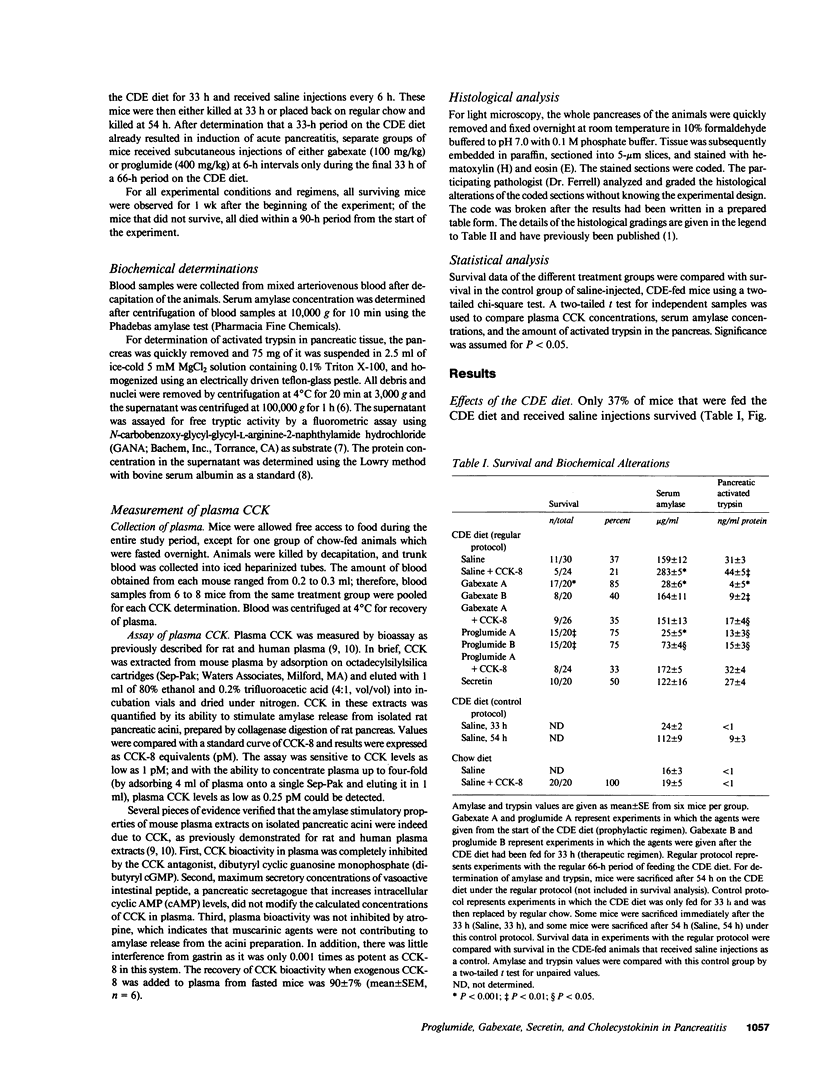
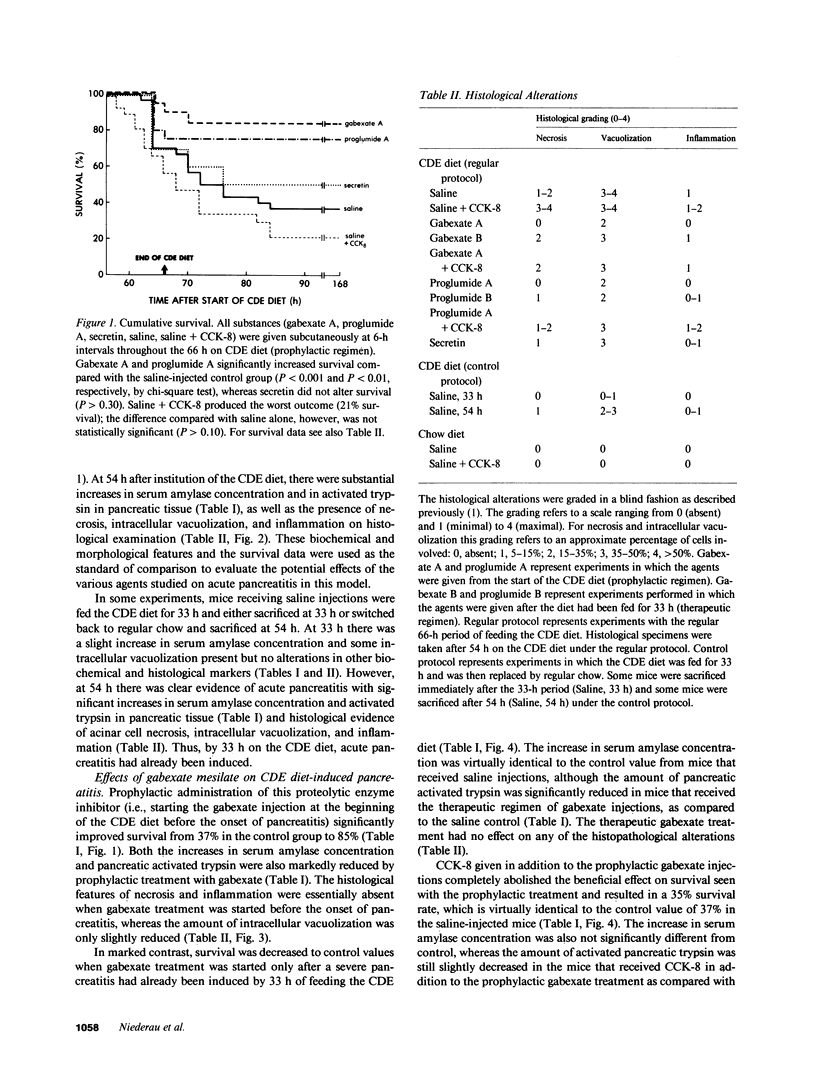
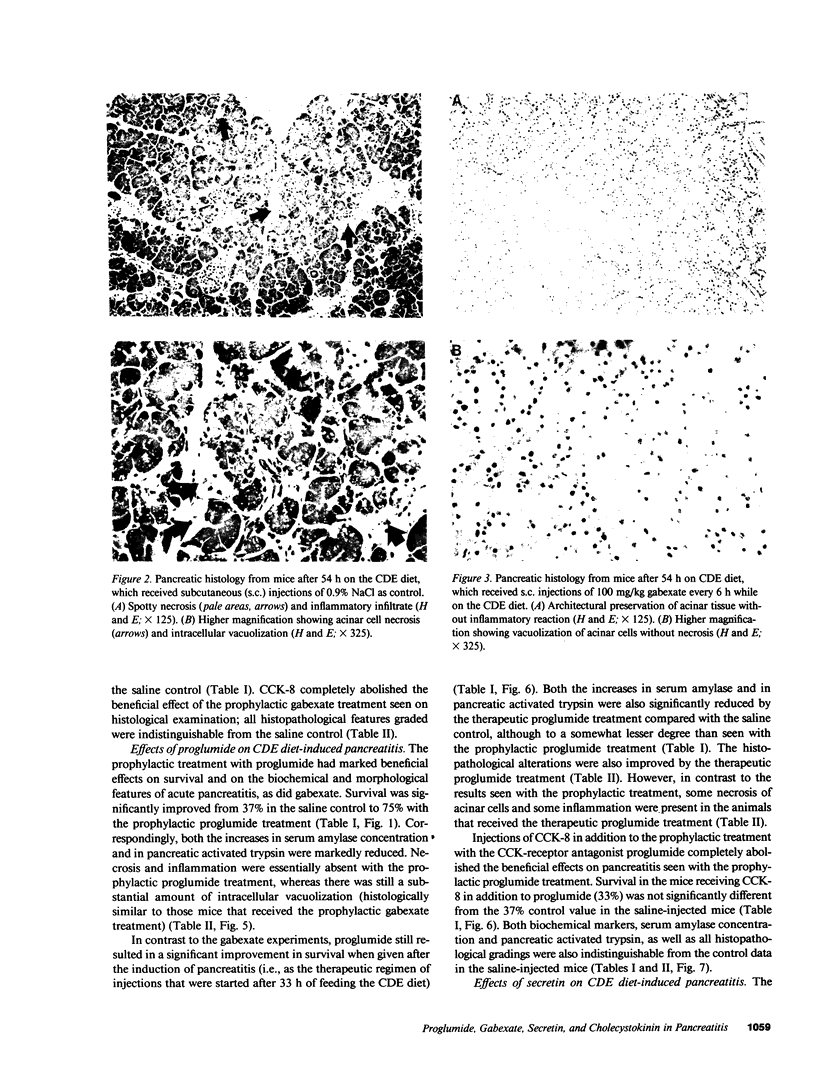
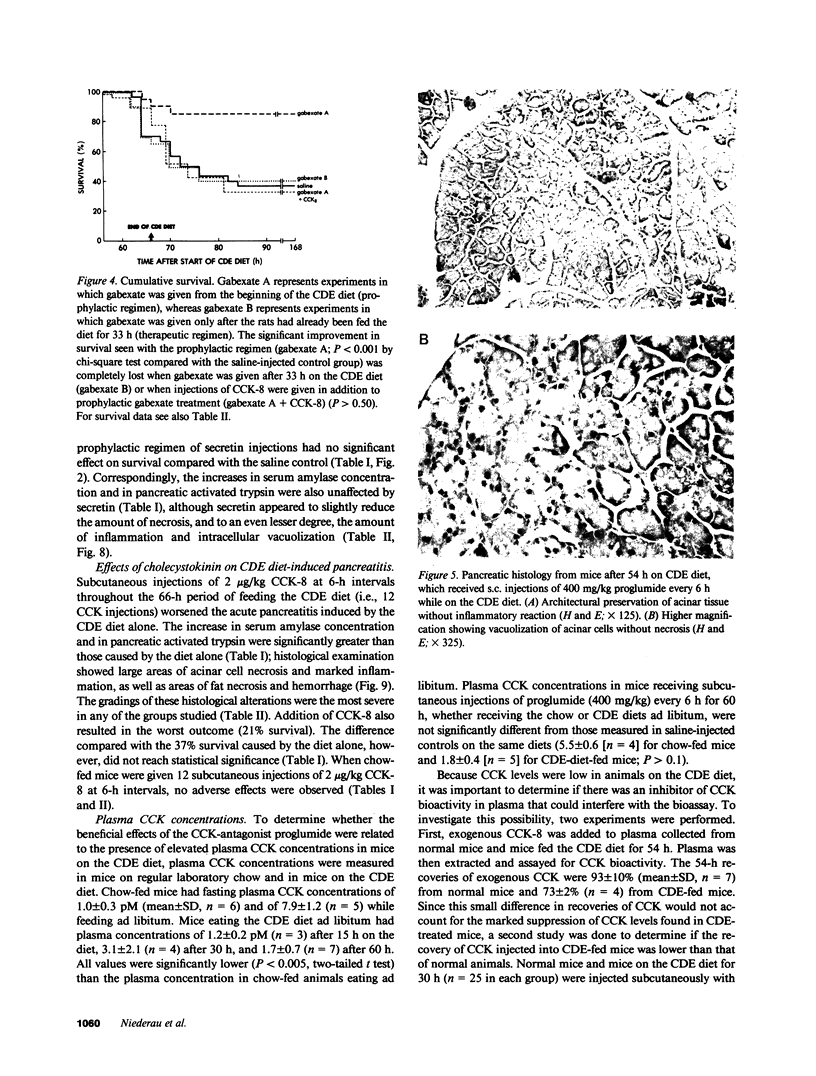
The effects of the cholecystokinin (CCK)-receptor antagonist proglumide, the protease inhibitor gabexate, and the hormones secretin and cholecystokinin-octapeptide (CCK-8) were studied in a model of acute hemorrhagic pancreatitis induced by feeding mice a choline-deficient, ethionine-supplemented (CDE) diet. Injections of gabexate and proglumide from initiation of CDE diet (before induction of pancreatitis) increased survival from 37% (diet alone) to 85 and 75%, respectively, and also ameliorated histological alterations and increases in serum amylase concentration and pancreatic activated trypsin. Secretin had no major beneficial effect. When proglumide or gabexate were given after induction of pancreatitis, proglumide still increased survival to 75%, whereas gabexate no longer did. Injection of nontoxic doses of CCK-8 before proglumide or gabexate injections completely abolished all beneficial effects and also increased the severity of pancreatitis due to CDE diet alone. Blockade of CCK receptors and early inhibition of protease activity may be beneficial in severe acute pancreatitis. Cholecystokinin appears to play a contributory role in the development of pancreatitis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
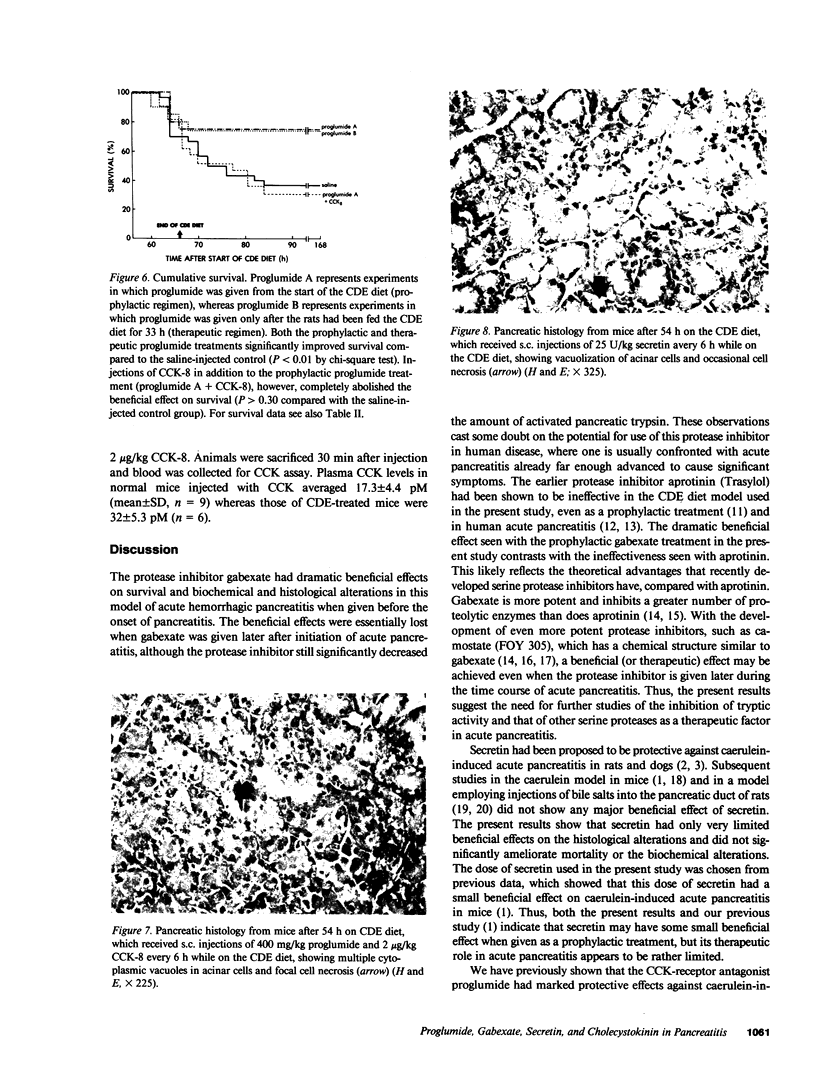
- Cameron J. L., Mehigan D., Zuidema G. D. Evaluation of atropine in acute pancreatitis. Surg Gynecol Obstet. 1979 Feb;148(2):206–208. [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Monaghan R. L., Birnbaum J., Stapley E. O., Goetz M. A., Albers-Schönberg G., Patchett A. A., Liesch J. M., Hensens O. D. A potent nonpeptide cholecystokinin antagonist selective for peripheral tissues isolated from Aspergillus alliaceus. Science. 1985 Oct 11;230(4722):177–179. doi: 10.1126/science.2994227. [DOI] [PubMed] [Google Scholar]
- Coelle E. F., Taylor I. L., Lewin K., Adham N. Beneficial effect of pancreatic polypeptide in experimental pancreatitis. Dig Dis Sci. 1983 Dec;28(12):1083–1088. doi: 10.1007/BF01295806. [DOI] [PubMed] [Google Scholar]
- Dürr H. K., Maroske D., Zelder O., Bode J. C. Glucagon therapy in acute pancreatitis. Report of a double-blind trial. Gut. 1978 Mar;19(3):175–179. doi: 10.1136/gut.19.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evander A., Lundquist I., Ihse I. Influence of gastrointestinal hormones on the course of acute experimental pancreatitis. Hepatogastroenterology. 1982 Aug;29(4):161–166. [PubMed] [Google Scholar]
- Gilliland L., Steer M. L. Effects of ethionine on digestive enzyme synthesis and discharge by mouse pancreas. Am J Physiol. 1980 Nov;239(5):G418–G426. doi: 10.1152/ajpgi.1980.239.5.G418. [DOI] [PubMed] [Google Scholar]
- Göke B., Stöckmann F., Müller R., Lankisch P. G., Creutzfeldt W. Effect of a specific serine protease inhibitor on the rat pancreas: systemic administration of camostate and exocrine pancreatic secretion. Digestion. 1984;30(3):171–178. doi: 10.1159/000199102. [DOI] [PubMed] [Google Scholar]
- Imrie C. W., Benjamin I. S., Ferguson J. C., McKay A. J., Mackenzie I., O'Neill J., Blumgart L. H. A single-centre double-blind trial of Trasylol therapy in primary acute pancreatitis. Br J Surg. 1978 May;65(5):337–341. doi: 10.1002/bjs.1800650514. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Murphy R. B., Trampota M., Schneider L. H., Jones S. W., Howard J. M., Gardner J. D. Proglumide analogues: potent cholecystokinin receptor antagonists. Am J Physiol. 1985 Aug;249(2 Pt 1):G214–G220. doi: 10.1152/ajpgi.1985.249.2.G214. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lankisch P. G., Göke B., Fölsch U. R., Winckler K., Otto J., Creutzfeldt W. Influence of secretin on the course of acute experimental pancreatitis in rats. Digestion. 1983;26(4):187–191. doi: 10.1159/000198888. [DOI] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Rosen M. S., Taplitz R. A., Williams J. A. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985 Apr;75(4):1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Williams J. A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984 Sep;87(3):542–549. [PubMed] [Google Scholar]
- Lombardi B., Estes L. W., Longnecker D. S. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline-deficient diet. Am J Pathol. 1975 Jun;79(3):465–480. [PMC free article] [PubMed] [Google Scholar]
- Manabe T., Steer M. L. Protease inhibitors and experimental acute hemorrhagic pancreatitis. Ann Surg. 1979 Jul;190(1):13–17. doi: 10.1097/00000658-197907000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka K., Green G. M. Effect of partial exclusion of pancreatic juice on rat basal pancreatic secretion. Gastroenterology. 1984 Jan;86(1):114–119. [PubMed] [Google Scholar]
- Niederau C., Ferrell L. D., Grendell J. H. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology. 1985 May;88(5 Pt 1):1192–1204. doi: 10.1016/s0016-5085(85)80079-2. [DOI] [PubMed] [Google Scholar]
- Niederau C., Niederau M., Williams J. A., Grendell J. H. New proglumide-analogue CCK receptor antagonists: very potent and selective for peripheral tissues. Am J Physiol. 1986 Jun;250(6 Pt 1):G856–G860. doi: 10.1152/ajpgi.1986.250.6.G856. [DOI] [PubMed] [Google Scholar]
- Renner I. G., Wisner J. R., Jr Ceruletide-induced acute pancreatitis in the dog and its amelioration by exogenous secretin. Int J Pancreatol. 1986 May;1(1):39–49. doi: 10.1007/BF02795238. [DOI] [PubMed] [Google Scholar]
- Renner I. G., Wisner J. R., Jr, Rinderknecht H. Protective effects of exogenous secretin on ceruletide-induced acute pancreatitis in the rat. J Clin Invest. 1983 Sep;72(3):1081–1092. doi: 10.1172/JCI111033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H., Renner I. G., Carmack C. Activation of human pancreatic juice. Clin Chim Acta. 1976 Dec 1;73(2):369–372. doi: 10.1016/0009-8981(76)90185-6. [DOI] [PubMed] [Google Scholar]
- Takasugi S., Toki N. Inhibitory effects of native and synthetic protease inhibitors on plasma proteases in acute pancreatitis. Hiroshima J Med Sci. 1980 Dec;29(4):189–194. [PubMed] [Google Scholar]
- Takasugi S., Yonezawa H., Ikei N., Kanno T. Prevention of acute experimental pancreatitis in rats and dogs by intraduodenal infusion of a synthetic trypsin inhibitor. Digestion. 1982;24(1):36–41. doi: 10.1159/000198772. [DOI] [PubMed] [Google Scholar]
- Tamura Y., Hirado M., Okamura K., Minato Y., Fujii S. Synthetic inhibitors of trypsin, plasmin, kallikrein, thrombin, C1r-, and C1 esterase. Biochim Biophys Acta. 1977 Oct 13;484(2):417–422. doi: 10.1016/0005-2744(77)90097-3. [DOI] [PubMed] [Google Scholar]