Abstract
Background
Toll-like receptor 4 (TLR4), the receptor for endotoxin, mediates hyperinflammatory response and contributes to high mortality during both endotoxin shock and severe sepsis. However, little is known about the role of TLR4 in the pathogenesis of low grade polymicrobial sepsis, which is often associated with immunosuppression.
Methods
Low grade polymicrobial sepsis was generated by cecum ligation and puncture (CLP). Mortality was monitored in wild type (C57BL/10ScSn) and TLR4def (C57BL/10ScCr) mice. Ex vivo heart and individual cardiomyocyte function were assessed in Langendorff (Hugo Sachs Elektronik-Harvard Apparatus, Holliston, MA) and IonOptix systems (IonOptix, Milton, MA), respectively. Serum chemistry was tested for liver and kidney injury. Cytokines were examined using a multiplex immunoassay. Neutrophil migratory and phagocytic functions were assessed using flow cytometry. Reactive oxygen species (ROS) were measured using redox sensitive dichlorodihydrofluorescein dye.
Results
Following CLP, wild type mice developed bacterial peritonitis with mild cardiac dysfunction (n=3 in sham and 8 in CLP) and a mortality of 23% within 14 days (n=22). In comparison, septic TLR4def mice had deleterious cardiac dysfunction (n=6 in sham and 10 in CLP), kidney and liver injury (n=7), and much higher mortality at 81% (n=21). The deleterious effects observed in septic TLR4def mice were associated with increased local and systemic cytokine response, reduced neutrophil migratory and phagocytic function, increased ROS generation in leukocytes and impaired bacterial clearance.
Conclusions
TLR4 plays an essential role in host defense against low grade polymicrobial sepsis by mediating neutrophil migratory/phagocytic functions, attenuating inflammation, reducing ROS generation and enhanced bacterial clearance.
Introduction
Sepsis has an estimated prevalence of 751,000 cases each year 1. Between 1979 and 2000, there was a steady increase in the incidence of sepsis 2. Even though the total in-hospital mortality rate fell to 17.9 percent during the period from 1995 through 2000, the total number of sepsis-related deaths continued to rise 2. Myocardial depression and associated hemodynamic collapse are among the major causes of death in severe sepsis 3.
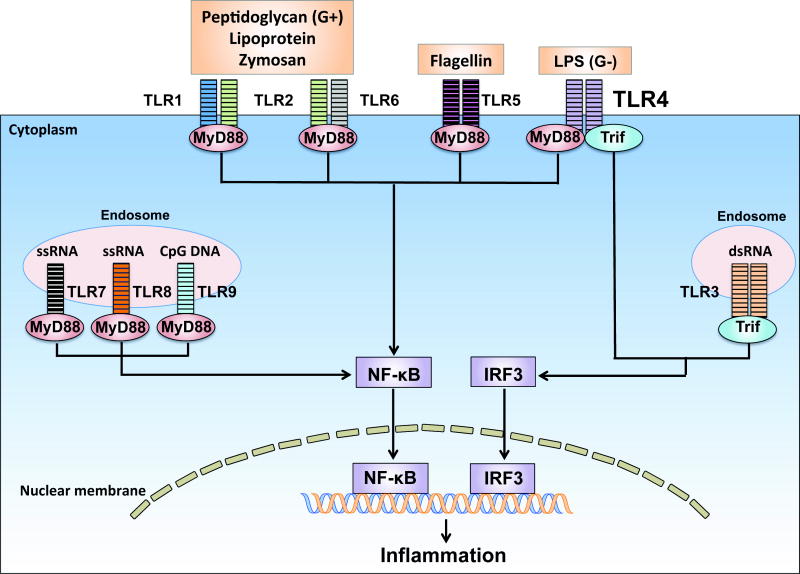
Toll-like receptors (TLRs) are an important member of the innate immunity and represent the first line of host defense against pathogen invasion 4. As illustrated in Fig. 1, various TLRs detect different pathogens through the pathogen-associated molecular patterns recognition. All TLRs with exception of TLR3 signal through MyD88 4. TLR4 also signals via Trif 4. TLRs such as TLR2, TLR3, TLR4, TLR5, TLR7, and TLR9 have been identified in cardiomyocytes 5. Natural deletion of TLR4, a receptor for lipopolysaccharide (endotoxin) 6, protects against lipopolysaccharide-induced cardiac dysfunction 7,8. We have demonstrated that genetic deletion of MyD88 or Trif, two adaptors downstream of TLR4, confers a profound protection with markedly improved cardiac function and survival in an endotoxin shock model 9. These findings establish that TLR4 signaling is responsible for myocardial depression and mortality during endotoxin shock.
Fig. 1. Pathogen sensing by Toll-like receptors.
Toll-like receptors (TLRs) are pattern-recognition receptors. All TLRs are transmembrane proteins. Some TLRs such as TLR1, 2, 4, 5 and 6 are expressed on the cell surface whereas others such as TLR3, 7, 8 and 9 are located almost exclusively in intra-cellular compartments such as endosomes. Different TLRs recognize different microbial components 49. For example, TLR4 senses lipopolysaccharide (LPS), a wall component of Gram-negative (G−) bacteria such as Escherichia coli. TLR2 recognizes lipoprotein, a wall component of Gram-positive (G+) bacteria such as Staphylococcus aureus or Streptococcus pneumoniate. TLR2 heterodimerizes either with TLR1 to recognize triacylated lipopeptide or with TLR6 to recognize diacylated lipopeptides. TLR5 senses bacterial flagellin, a protein component of flagella. TLR3 recognizes viral double-stranded RNA (dsRNA), whereas TLR7 and 8 are the sensors for single stranded RNA (ssRNA). Finally, TLR9 senses bacterial CpG-rich hypomethylated DNA (CpG DNA) motifs. With the exception of TLR3, all TLR members signal through the adaptor myeloid differentiation primary-response gene 88 (MyD88) to recruit the downstream kinases. TLR3 signals through the adaptor TIR-domain-containing adaptor protein inducing interferon-β (IFN-β)-mediated transcription-factor (Trif). TLR4 signals through both MyD88 and Trif dependent pathways. Activation of these signaling pathways ultimately activates the transcription factors such as nuclear factor-κB (NF-κB) and IFN regulatory factor 3 (IRF3), which leads to production of diverse proinflammatory cytokines 50.
The pathogenesis of bacterial sepsis has been described as immunological imbalance characterized by early hyper-inflammatory response featured by pro-inflammatory cytokine storm and late immunosupressive phase characterized by a shift to anti-inflammatory cytokines, T cell anergy, and immune cell death 10. While hyperinflammatory response associated with endotoxin shock or severe sepsis could be lethal, immunosupression is believed to be the predominant cause for morbidity and mortality of many intensive care unit septic patients who have survived the initial hyperinflammatory attack 11. Death in the immunosuppressed septic patients is typically due to failure to control the primary infection and the acquisition of secondary hospital acquired infections 11–13. Therefore, an effective host defense is crucial for the survival of septic patients, particularly for those immunosuppressed patients. In an animal model of low grade polymicrobial sepsis, as defined by relatively low mortality, followed by a second hit of bacterial challenge, Muenzer et al 14 demonstrate that immunosuppresion created by the low grade sepsis model increases susceptibility to the secondary bacterial infection.
While the role of TLR4 signaling in endotoxin shock is well defined, its role in bacterial sepsis is less clear. The reports on the role of TLR4 in severe bacterial sepsis have been somewhat conflicting. Both protective and contributory roles have been proposed for TLR4 in severe lethal bacterial sepsis15,16. Neverthelss, the role of TLR4 signaling in low grade polymicrobial sepsis is unclear. Our overall hypothesis was that intact TLR4 signaling is essential for host immune defense against polymicrobial infection during low grade sepsis. Specifically, we hypothesized that mice lacking TLR4 would have impaired neutrophil function and higher bacterial load as compared with mice possessing TLR4. Consequently, we anticipated that these TLR4-deficient mice would have higher mortality, worsening cardiac function, and deleterious kidney and hepatic injury.
Materials And Methods
Animals
Eight-12 week-old age and gender matched mice were used for the studies. Wild-type (WT) (C57BL/10ScSn) and TLR4def mice (C57BL/10ScCr) were purchased from the Jackson Laboratory (Bar Harbor, ME). C57BL/10ScCr is also referred to as C57BL/10ScNJ (stock no. 003752) with WT IL-12Rβ2 allele. C57BL/10ScCr mice have a deletion of the tlr4 gene, which results in the absence of both TLR4 messenger RNA and protein and thus a defective response to lipopolysaccharide. C57BL/10ScCr mice differs from C3H/HeJ mice with a point tlr4 mutation that causes an amino acid substitution 6. C57BL/10ScSn mice were used as the appropriate WT controls for the TLR4def mice. All mice were housed and maintained in temperature-controlled, air-conditioned facilities with 12 h/12 h light/dark cycles and fed with the same bacteria-free diet (Prolab Isopro RMH 3000, LabDiet, Brentwood, MO). All animal experiments were performed with the approval of the Subcommittee on Research Animal Care of Massachusetts General Hospital (Charlestown, Massachusetts). Simple randomization method was used to assign animals to various experimental conditions. Different groups were processed identically throughout the whole experiment. For example, 1) all mice used were gender and age matched, 2) all mice were the same inbred strains. Except for the sex difference, mice of an inbred strain were genetically alike, and 3) mice were housed on the same shelves in the same rooms before and after surgery.
Mouse model of low grade polymicrobial sepsis
In brief, cecum was ligated 1.0 cm from the tip and punctured through to through with an 18-gauge needle. A small amount of fecal materials was squeezed gently to expel before the ligated cecum was returned to the abdominal cavity. The sham-operated mice underwent laparotomy but without cecum ligation and puncture (CLP). After surgery, prewarmed normal saline (0.05 ml/gram body weight) was administered subcutaneously. The low grade of sepsis in C57BL/10ScSn mice was defined by low mortality rate (− 20%) 17, modest cytokine responses, and mild organ dysfunction. Of note, all surgeries were performed by operators blinded to the strain information.
Endotoxin shock model
Endotoxin shock model was induced as previously described 7,9. Mice were administered with lipopolysaccharide (15 mg/kg body weight) (Escherichia coli 0111:B4, Sigma, St Louis, MO) by intra-peritoneal injection followed by administration of 1 ml of pre-warmed normal saline.
Langendorff system
Left ventricular (LV) function was assessed in a Langendorff perfusion system (Hugo Sachs Elektronik-Harvard Apparatus, Holliston, MA) as described previously 18,19. Briefly, mice were heparinized (1000 IU/kg, subcutaneously) and euthanized. After the heart was excised, the aorta was quickly cannulated and retrograde-perfused at a constant flow rate (3 ml/min) at 37 °C with modified Krebs-Henseleit buffer (NaCl 118.5 mM, NaHCO3 25 mM, D-Glucose 11.2 mM, KCl 4.7 mM, MgSO4 1.2 mM, KH2PO4 1.2 mM, Sodium Pyruvate 2 mM, CaCl2 2 mM). The heart was paced at 7 Hz (420 beats/min). After 20 min of coronary perfusion, LV end systolic pressure, LV end-diastolic pressure, dP/dtmax (maximal first derivative of LV developed pressure) were contiuously recorded for up to 30 min. LVDP (LV developed pressure) was calculated as follows: LVDP = LV end-systolic pressure-LV end-diastolic pressure.
Cardiomyocyte sarcomere shortening and intracellular calcium measurement
Sarcomere shortening and Ca2+ transients were recorded simultaneously on an IonOptix system (IonOptix, Milton, MA) as described previously18,20,21. Adult cardiomyocytes were incubated with membrane permeable fluorescent indicator fura-2 AM (1 μM) (Invitrogen, Carlsbad, CA) and probenecid (0.5 mM), placed in a flow chamber that was perfused with 1.2 mM Ca2+ Tyrode solution (NaCl 137 mM, KCl 5.4 mM, HEPES, 0.5 mM, MgCl2 0.5 mM, Glucose 5.5 mM), and electrically paced at 1, 2, 4, and 6 Hz via platinum wires. The Ca2+ transients and sarcomere shortening were analyzed based on single-cell-averaged tracing. The final values were derived from 14 to 15 individual cells in each group and calculated for statistical analysis. Four mice from each group were used to prepare cardiomyocytes for the functional studies.
Echocardiographic assessment of cardiac function
Transthoracic echocardiographic images were obtained one day before (baseline) and again at 6 hours after lipopolysaccharide administration. In brief, 30 mins prior to echocardiographic measurements, 1 ml of pre-warmed normal saline was injected to each mouse and mouse cages were warmed to 30 °C under light for 15–20 minutes. Mice were lightly anesthetized with ketamine (20 mg/kg). All images were collected using a 13.0-MHz linear probe (Vivid 7, GE Medical System, Milwaukee, WI) as described previously 9. M-mode images were obtained from a parasternal short-axis view at the mid-ventricular level with a clear view of papillary muscle. LV internal diameters at end-diastole and -systole were measured. The fractional shortening was defined as (LV internal diameters at end-diastole − LV internal diameters at end-systole)/(LV internal diameters at end-diastole) × 100%. The values of three consecutive cardiac cycles were averaged. Of note, echocardiographic measurements were performed and analyzed by an operator blinded to strain information.
Flow cytometry analysis of peritoneal neutrophils
Twenty-four hours after surgeries, 5 ml of normal saline was injected into the peritoneal cavity and mixed thoroughly by gentle massage to the abdomen. Three ml of the peritoneal lavage fluid was collected and centrifuged. The supernatants were saved for cytokine measurements and the cell pellets were resuspended and manually counted. A fraction of cells (5 × 105) from the peritoneal lavage was labeled with Gr-1 (anti-Ly-6C/Ly-6G) (BD Biosciences, San Jose, CA) and gated on Gr-1 for neutrophil percentage in the recruited peritoneal cells. Total neutrophil numbers in the peritoneum were calculated based on the total cell numbers and the percentage of Gr-1+ neutrophils.
Phagocytosis assay
Phagocytosis assay was performed as described previously 22,23. Briefly, 4 × 105 cells were incubated with serum-opsonized yellow-green fluorescent polystyrene microspheres (FITC) (FluoSpheres; Invitrogen) at 37 °C for 30 min. Cells were then washed, stained with allophycocyanin-labeled Gr-1 antibody, and analyzed with flow cytometry for phagocytic neutrophils (FITC+/allophycocyanin+), which were expressed as percentage of Gr-1+ neutrophils.
Bacterial counts in the peritoneal exudates and blood
Twenty-four hours after sham or CLP procedures, five ml of sterile normal saline was injected into the peritoneal space and mixed thoroughly by gentle massage to the abdomen. Three ml of the peritoneal lavage was collected. Blood was collected through cardiac puncture and anticoagulated with lithium heparin. Samples were serially diluted, plated on Trypticase™ soy agar with 5% sheep blood (BD Company, Sparks, MD), and incubated at 37°C for 14–16 hours. Colony forming units (CFU) were counted and expressed as log10 of CFU/ml of blood or lavage fluid as we described previously 18,23.
Serum chemistry
Serum blood urea nitrogen (BUN) and creatinine were examed for kidney injury and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) for liver injury. Blood was collected 24 hours after sham or CLP surgery. Serum was prepared and stored at −80 °C until analysis. Serum chemistry was measured by DRI-CHEM 7000 Chemistry Analyzer (HESKA, Des Moines, IA) according to the manufacturer’s instruction.
Multiplex cytokine immunoassays
Twenty-four hours after sham or CLP procedures, blood was collected through cardiac puncture and anticoagulated with lithium heparin. Plasma were harvested after centrifuged at 1000g for 10 minutes at 4°C and stored at −80 °C. Five ml of saline was administered intraperitonealy and peritonal lavage fluid was harvested after gentle abdominal massage. Cell-free supernatant of the peritoneal lavage fluid was collected and stored at −80 °C. Cytokine concentrations were determined using a fluorescent bead-based multiplex immunoassay (Luminex Co., Austin, TX) as previously described 18,23. Briefly, antibody for each cytokine was covalently immobilized to a set of fluorescent microspheres by manufacturer (Millipore, Billerica, MA). After overnight incubation, cytokines bound on the surface of microspheres were detected by a cocktail of biotinylated antibodies. Following binding of streptavidin-phycoerythrin conjugates, the reporter fluorescent signal was measured using a Luminex 200™ reader. Final cytokine concentrations were calculated based on a standard cytokine curve obtained in each experiment.
Detection of intracellular reactive oxygen specice (ROS)
Peritoneal cells were harvested 12 h after sham and CLP procedures and incubated with the redox sensitive dye dichlorodihydrofluorescein diacetate (Molecular Probes, Grand Island, NY), at 37 °C for 30 min. Dichlorodihydrofluorescein diacetate was measured in FITC channel of flow cytometry and quantified by mean fluorescence intensity.
Mortality Study
Following sham or CLP procedures, mice were observed every 24 hours for up to 14 days. Mice administered with saline or lipopolysaccharide were monitored every 6 hours for up to 48 hours. Mouse mortality was monitored by operators blinded to strain information.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA). The distributions of the continuous variables were expressed as the mean ± SE. Isolated heart function assessed by Langendorff and cardiomyocytes function were analyzed by two-way ANOVA. Serum chemistry data, neutrophil migration and ROS production were analyzed by two-way ANOVA analysis of variance with Bonferroni adjusted p-value for post-hoc analysese between groups. Analysis of echocardiographic measurements was performed by two-way ANOVA with repeated measurements with Bonferroni post-hoc test. CFU was applied on the log10 scale and based on Student’s t-test. The survival data were analyzed with a log-rank test. Student’s t test was used for statistical analysis between groups of all other data. Of note, these specific comparisons were made based on a priori hypotheses rather than pure statistic considerations. The null hypothesis was rejected for P < 0.05 with the two-tailed test.
Results
TLR4def mice have increased mortality in low grade polymicrobial sepsis
We subjected WT (C57BL/10ScSn) and TLR4def (C57BL/10ScCr) mice to a CLP model of low grade polymicrobial sepsis. We found that WT mice developed low grade sepsis with an accumulated mortality of 14% on day 2 and 23% on day 14 (Fig. 2A). TLR4def mice had a marked increase in the mortality (48% on day 2, and 81% on day 14) compared with the WT control (P < 0.001). In stark contrast, these TLR4def mice were totally resistant to endotoxin-induced shock (Fig. 2B). After a lethal dose of lipopolysaccharide administration (15 mg/kg), WT mice had 100% mortality within 28 h whereas TLR4def mice were completely protected with no mortality for up to 48 h. These data suggest that TLR4 signaling confers a potent survival benefit in low grade polymicrobial sepsis and absence of TLR4 signaling leads to increased mortality.
Fig. 2. TLR4 deficiency leads to improved survival in endotoxin shock but decreased survival in low grade polymicrobial sepsis.
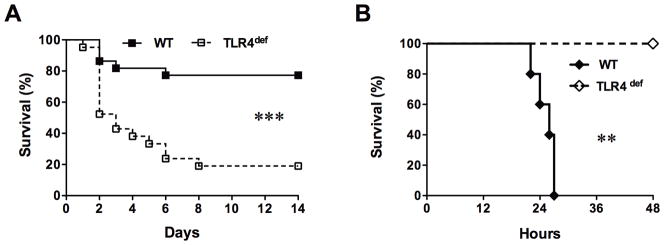
Mice were subjected to CLP or lipopolysaccharide administration and observed for mortality. A. Survival curve in CLP model. n = 22 in WT group, n=21 in TLR4-def group. *** P=0.0001; B. Survival curve in endotoxin shock model. n=5 in each group. ** P=0.003. CLP, cecum ligation and puncture; TLR, Toll-like receptor; WT, wild type. Log-rank test was used to analyze statistic difference in survival between the two groups.
TLR4def mice have worse LV dysfunction and cardiomyocyte functional impairement in low grade polymicrobial sepsis
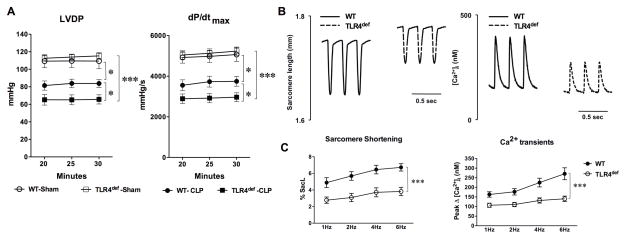
The hearts isolated from both sham and CLP mice were perfused in a Langendorff system. There was no difference in LV contractile function between WT and TLR4def groups after the sham procedure (Fig. 3A). Twenty four hours after CLP, however, there was a reduction in LV contractile function in WT mice as demonstrated by moderate but significant decrease in LVDP (24% reduction, 109±4 vs 83±2 mmHg) and dP/dtmax (26% reduction, 4991±140 vs 3679±101 mmHg/s). Compared to septic WT mice, septic TLR4def mice had significantly worse LV contractile dysfunctions (42% reduction in LVDP and 43% reduction in dP/dtmax at 30 min of perfusion) (Fig. 3A). Consistent with the mortality data above, TLR4def mice were largely protected with near normal LV function in response to endotoxin shock (table in Supplemental Digital Content 1 and Figure in Supplemental Digital Content 1). To further test the impact of TLR4 deficiency on sepsis-induced cardiac dysfunction, we isolated adult cardiomyocytes 24 h after CLP surgery and examined cardiomyocyte function as measured by sarcomere shortening and Ca2+ transients. As shown in Fig. 3B–C, compared with septic WT cardiomyocytes, septic TLR4def cardiomyocytes had attenuated sarcomere shortening (43% reduction, 6.7±0.4 vs 3.8±0.5%) and peak change in [Ca2+]i (48% reduction, 271±31 vs 140±14 nM). These data suggest a critical role of TLR4 in preserving cardiomyocyte function during low grade polymicrobial sepsis.
Fig. 3. TLR4-deficient mice have deleterious LV function compared with WT mice after CLP surgery.
A. LV function measured in a Langendorff perfusion system. Twenty-four hours after CLP or sham surgery, the heart was excised and perfused in a Langendorff system. Left ventricle (LV) function was measured. Each error bar represents the mean ± SE, n=4 in WT-sham group, n=6 in TLR4def-sham group, n=8 in WT-CLP group, n=10 in TLR4def-CLP group. Two-way ANOVA was used for statistical analysis. * P < 0.05. *** P < 0.001. Detailed P values in LVDP assessement: WT-CLP vs. WT-sham, P=0.013; WT-CLP vs. TLR4def-CLP, P=0.034. Detailed P values in dP/dtmax assessement: WT-CLP vs. WT-sham, P=0.012; WT-CLP vs. TLR4def-CLP, P=0.041. LVDP=left ventricle developed pressure; dP/dt max=the maximal rate of left ventricle pressure development; B–C. Sarcomere shortening and Ca2+ transients in isolated adult cardiomyocytes. B, Representative tracing of sarcomere shortening and Ca2+ transients in isolated cardiomyocytes 24 h after CLP surgery. C, Accumulated data of sarcomere shortening and Ca2+ transients. The data in each group were recorded from 14 to 15 single adult cardiomyocytes isolated from four mice. Two-way ANOVA was used for statistical analysis. ***P < 0.0001. WT, wild type; TLR, Toll-like receptor; CLP, cecum ligation and puncture; SacL, Sarcomere length.
TLR4 deficiency deteriorates organ injury during low grade sepsis
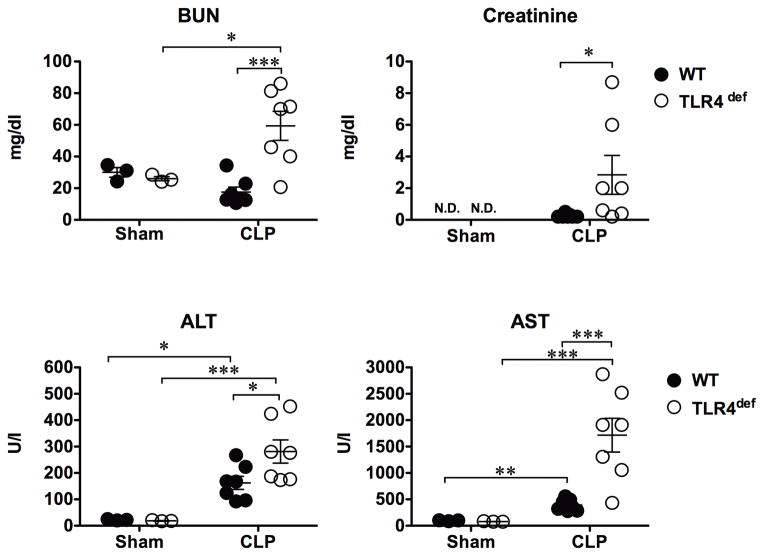
We measured the serum levels of BUN, creatinine, ALT and AST. As shown in Fig. 4, in WT mice, the low grade model of sepsis did not cause any detectable acute kidney injury at 24 h with normal BUN and creatinine levels, but induced liver injury as evidenced by an increase in both ALT and AST (ALT: 22±1.4 vs 162±25, U/I, P=0.007; AST: 96±5.6 vs 392.4±41.4 U/I, P=0.002). In comparison, septic TLR4def mice had significantly worse kidney and liver injury as demonstrated by markedly elevated BUN/creatinine and ALT/AST (BUN:18±3 vs 59±9 mg/dl, P=0.001; creatinine: 0.3±0.04 vs 2.8±1.2 mg/dl, P=0.057; ALT: 162±25 vs 281±44 U/I, P=0.036, AST: 392±41 vs 1715±320 U/I, P=0.015, WT vs TLR4def, respectively).
Fig. 4. TLR4-deficient mice develop worse kidney and liver injury during low grade sepsis.
Mice were subjected to sham or CLP surgery. Serum was collected 24 h after surgery. Each error bar represents the mean ± SE. Two-way ANOVA was used for statistical analysis and Bonferroni post hoc tests was applied for difference between groups. * P<=0.05, ** P<0.01, *** P<0.001, n=3 in sham group, n=7 in CLP group. N.D., not detectable; BUN, blood urea nitrogen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CLP, cecum ligation and puncture; WT, wild type; TLR, Toll-like receptor.
TLR4def mice have higher bacterial loading during low grade polymicrobial sepsis
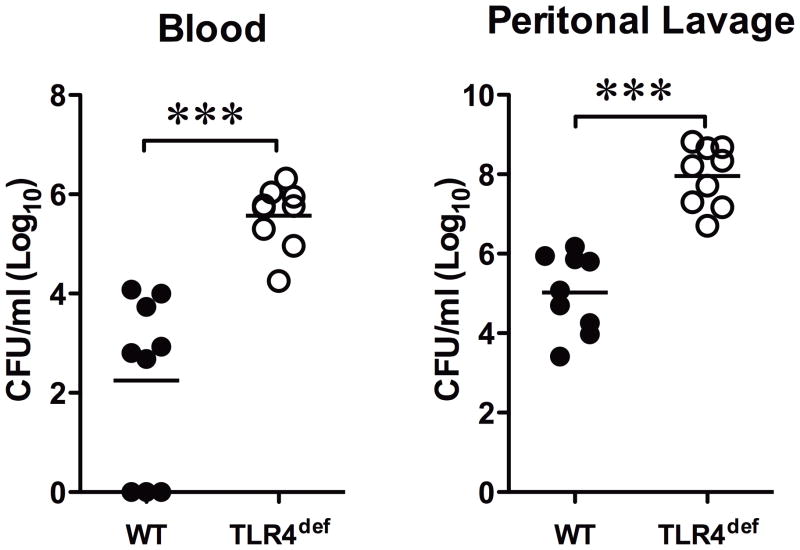
We examined the bacterial loading in the blood and the peritoneal lavage from the mice subjected to CLP surgery. As indicated in Fig. 5, 24 h after CLP, TLR4def mice displayed a significant increase in the bacterial counts both in the blood and the peritoneal fluid (Log10 scale, 5.6±0.2 and 7.9±0.3, respectively,) when compared with WT mice (2.2±0.6 and 5.0±0.3, respectively,).
Fig. 5. TLR4 deficient mice have higher bacterial load in the blood and peritoneal space compared with WT mice following low grade polymicrobial infection.
Both the blood and peritoneal lavage were harvested 24 h after the CLP surgery. After serial dilutions, the samples were incubated on agar plates at 37°C for 14–16 hours. Bacterial colony forming units (CFU) were counted. Each data point represents the CFU from one mouse and was plotted in a Log10 scale. Horizontal bars indicate the mean value of the CFU in each mouse group and t-test was applied for statistical difference analysis. *** P<0.001, Detailed P value: P=0.0001 in both blood and lavage CFU comparison between groups. n = 9. CLP, cecum ligation and puncture; TLR, Toll-like receptor; WT, wild type.
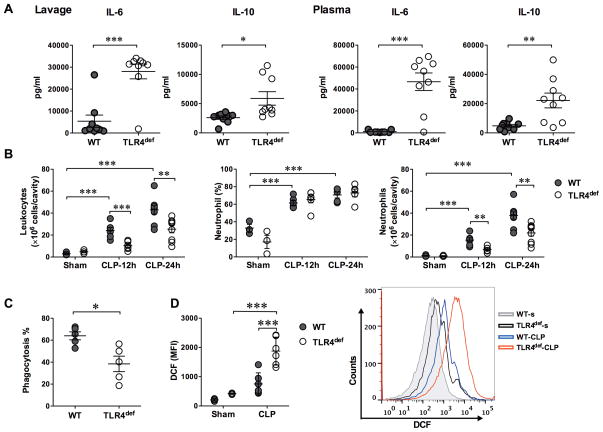
TLR4def mice have increased local and systemic cytokine responses, impaired neutrophil functions, and increased leukocyte ROS generation
Interleukin (IL)-6 is a pro-inflammatory cytokine during sepsis 24,25, whereas IL-10 is anti-inflammatory 25. To determine the impact of TLR4 signaling on cytokine responses during low grade polymicrobial sepsis, we harvested blood and peritoneal lavage 24 h after CLP. Compared with septic WT mice, TLR4def mice exhibited markedly elevated levels of local and systemic IL-6 (28,040 pg/ml in the peritoneal lavage and 46,651 pg/ml in the plasma) and IL-10 (5,893 pg/ml in the lavage and 22,171 pg/ml in the plasma). In contrast, septic WT mice had significantly lower pro-inflammatory cytokines IL-6 (5,394 pg/ml in lavage, P=0.0001 vs. TLR4def group, and 1,192 pg/mL in plasma, P=0.001 vs. TLR4def group) and IL-10 (2,611 pg/ml in lavage, P=0.022 vs. TLR4def group, and 4,826 pg/ml in plasma, P=0.001 vs. TLR4def group)(Fig. 6A). Innate immune cells such as neutrophils play a pivotal role in the host defense as well as the pathogenesis of sepsis 26. To determine the impact of TLR4 signaling on neutrophil migratory function, we next examined neutrophil recruitment into the peritoneal space after CLP and the neutrophil phagocytic function ex vivo to engulf opsonized fluorescent beads. In the sham-operated mice, both WT and TLR4def, there was a small number of leukocytes present in the peritoneal space and less than 33% of these leukocytes were Gr-1+ neutrophils (Fig. 6B). Twelve to 24 h after CLP, there was a marked and time-dependent increase in the number of neturophils recruited into the infectious peritioneal space of WT mice (37.8±3.4 × 106 at 24 h). The percentage of neutrophils was also increased to near 70%. In comparison, there was significantly fewer number of neutrophils in the peritoneal space of the septic TLR4def mice (21.9±2.9 ×106, P=0.003 vs. WT-CLP) at 24 h (Fig. 6B). Moreover, neutrophils isolated from TLR4def mice had significantly lower phagocytic function compared with that of WT mice (64.0±3.6% vs 38.5±7.1%, P=0.012) (Fig. 6C). ROS, especially intracellular ROS, plays an important role in regulating cytokine production 27,28 and is associated with organ injury during sepsis 28,29. ROS generation was assessed using redox sensitive dye dichlorodihydrofluorescein by flow cytometry. As illustrated in Fig. 6D, there was a much higher level of the intracellular ROS production in the peritoneal cells of TLR4def septic mice when compared with that of WT mice (P=0.001). Together, these data suggest that TLR4 plays a vital role in maintaining normal neutrophil migratory and phagocytic function and in controlling ROS generation during low grade polymicrobial sepsis.
Fig. 6. TLR4 deficiency lead to increase inflammation, impaired neutrophil recruitment and phagocytic function, and increased leukocyte ROS production during low grade polymicrobial sepsis.
A. Cytokines in the lavage and plasma of WT and TLR4def mice following CLP. Twenty-four hours after CLP procedure, the peritoneal lavage and plasma were collected from the septic mice. IL-6 and IL-10 were measured using a multiplex fluorescent bead-based immunoassay. t test was applied for difference between groups. n=9 in each group. B. Leukocytes in the peritoneal space following sham or CLP procedures. Total peritoneal cells were manually counted by hemacytometer. Neutrophils were calculated based on the total peritoneal cell numbers multiplied by the percentage of neutrophils as measured by flow cytometry. Two-way ANOVA was used for statistic analysis and Bonferroni adjusted p-value was applied for post-hoc analyses between groups. n=3 in sham groups, n=6 in CLP-12h groups, n= 9 in CLP-24h groups. C. Percentages of phagocytic neutrophils. t-test was applied for difference between the two groups. n=5 in each group. D. ROS production in peritoneal cells. Intracellular ROS of the peritoneal cells was measured by flow cytometry with DCF stain 12 h after surgery. Two-way ANOVA was used for statistic analysis and Bonferroni post hoc tests was applied for difference between groups. n=3 in sham groups, n=6 in CLP groups, * P<0.05, ** P<0.01, *** P<0.001. WT, wild type; TLR, Toll-like receptor; IL, interleukin; DCF, dichlorodihydrofluorescein; MFI, mean fluorescence intensity; CLP, cecum ligation and puncture; WT-s, WT-sham; TLR4def-s, TLR4def-sham; ROS, Reactive oxygen species.
Discussion
In the current study, we demonstrated an important role of TLR4 in the host defense mechanism in a clinically relevant mouse model of sepsis. In a low grade model of polymicrobial sepsis, we found that TLR4 deficiency deteriorated cardiac function and induced hepatic and renal injury with markedly increased mortality. These deleterious effects of TLR4-deficiency were associated with grossly impaired neutrophil migratory and phagocytic functions and attenuated bacterial clearance. These data suggest that in low grade polymicrobial sepsis, TLR4 signaling appears to be critical for maintaining normal host immune defense against bacterial invasion and protecting the vital organs from polymicrobial infection-induced injury.
Cardiac dysfunction represents a clinical feature of sepsis and contributes to its mortality. Several mechanisms have been proposed responsible for myocardial dysfunction during sepsis, including cardiosuppressive cytokines such as IL-6 and tumor necrosis factor α, mitochondrial dysfunction and alterations of myocardial calcium homeostasis30,31. It has been well documented that TLR4, in particular those of bone marrow-derived immune cells, mediates cardiac dysfunction induced by endotoxin7,32–34, a wall-component of gram-negative bacteria. Consistent with these previous findings, we found that systemic TLR4 deficiency in C57BL/10ScCr mice conferred a profound protection against endotoxin-induced cardiac dysfunction and prevented mortality. This is because during endotoxin shock, the key underlying pathology is systemic hyperinflammation featured by cytokine storm (e.g., IL-6 and tumor necrosis factor α), which leads to cardiovascular collapse and death. Lack of TLR4 would effectively block endotoxin-induced cytokine storm and thus confer survival benefit. In contrast, in the mouse model of low grade peritonitis sepsis, which involves multiple live bacterial infection and only modest systemic cytokine response and low mortality, we found that TLR4 deficiency deteriorated cardiac function as demonstrated ex vivo in isolated heart and isolated adult cardiomyocytes following CLP procedure. There are several possible mechanisms that may explain the deleterious impact of TLR4 deficiency on cardiac function in low grade bacterial sepsis observed in the current study. First, TLR4 is critically involved in the effective host immune defense against bacterial invasion. In our study, WT mice exhibit more robust neutrophil migratory and phagocytic functions and markedly reduced bacterial loading compared with mice lacking TLR4. We speculate that as results of uncontrolled bacterial dissemination in the absence of TLR4, animals lacking TLR4 have more systemic cytokine production including IL-6, a major cardiodepressant 31,35. Moreover, higher bacterial load may lead to more ROS production in cardiac tissue and immune cells as demonstrated in the current study, which could induce tissue injury and adversely impact on cardiomyocyte function. Second, the cardiac protective benefit associated with TLR4 signaling might attribue to a local and direct cardiac “preconditioning-like” effect of TLR4 activation during low grade bacterial infection. Several studies have found that very small doses of lipopolysaccharide pretreatment confers a cardioprotective effect against hypoxic injury 36–38 in part through IRAK-1, MyD88 and nitric oxide synthase 2 signaling pathway 20,39. It is worth noting that the amount of lipopolysaccharide used in these models is extremely small (0.1–0.5 mg/kg) while the dose for endotoxin shock model is many times higher (between 10 mg/kg 32,33 to 15 mg/kg 9). Therefore, it seems possible that a small amount of endotoxin released from bacterial peritonitis to the circulatory system in the early stage of the low grade polymicrobial sepsis could activate TLR4 signaling and initiate a “pre-conditioning-like” cardiac protection against subsequent myocardial depression during polymicrobial sepsis. Supporting this notion are studies that demonstrate the pre-conditioning effect of low dose of endotoxin against both subsequent endotoxin challenges 40 and polymicrobial infection 41. Meng, et al. demonstrate that animals pre-treated with small dose of lipopolysaccharide (0.5 mg/kg) become resistent to subsequent lipopolysaccharide-induced cardiac dysfunction 40. Wheeler, et al show that lipopolysaccharide pretreatment also attenuates systemic inflammatory response and improved bacterial clearance and survival in a CLP model of polymicrobial sepsis 41.
Successful bacterial clearance at the infection site and circulatory system is essential for survival in bacterial sepsis. In current study, we demonstrated that compared with WT mice, TLR4-deficient mice had impaired bacterial clearance in both peritoneal space and in the blood following CLP. There are several possible mechanisms that may be responsible for this. First, TLR4 signaling is important for neutrophil migratory function. The ability of neutrophils to migrate to the infection site is critical for a successful host defense. Our in vivo study demonstrates that compared with WT mice, TLR4-def mice have markedly attenuated neutrophil recruitment in the peritoneal space during both early (12 h) and late (24 h) polymicrobial infection. This is in consistent with a previous in vitro study demonstrating that TLR4 signaling promotes neutrophil migration by reducing chemokine receptor (CXCR2 and MIP2 receptors) internalization and desensitization 42. Our own previous study 43 also demonstrates that signaling via MyD88, the downstream adaptor of TLRs including TLR4, is important for maintaining neutrophil chemokine receptor expression and migratory function. Thus, lack of TLR4-MyD88 signaling may impair neutrophil migratory function. In addition, an intact TLR4 signaling has an anti-apoptotic survival effect in neutrophils. Thus, the relative longer survival life span of the peritoneal neutrophils in WT septic mice may contribute to better neutrophil phagocytic function compared with TLR4def mice 44. Second, TLR4 is important for neutrophil phagocytic function. In our study, neutrophils isolated from septic TLR4-def mice exhibited significantly reduced phagocytic capability compared with those from WT septic mice. Consistent with this, Wheeler et al 41 demonstrate that lipopolysaccharide preconditioning via TLR4 enhances macrophage phagocytosis of both gram-negative bacteria (E. coli) and gram-positive bacteria (S. aureus), suggesting that activation of TLR4 signaling augments bacterial clearance through increased phagocytosis.
The current study establishes that TLR4 signaling is essential for overall survival and organ function in low grade polymicrobial sepsis. However, in high grade lethal models of sepsis, TLR4 signaling appears detrimental and absence of TLR4 actually improves survival 15,16 These observations suggest that the role of TLR4 signaling in the pathogenesis of polymicrobial sepsis is complex and may well depend on the severity of sepsis. Similar observations have been reported for the role of MyD88 and type I interferons, two downstream molecules of TLR4 signaling, in polymicrobial sepsis 9,45–47. Collectively, these studies appear to suggest a common theme, i.e., dual roles for TLR4 signaling in the pathogenesis of bacterial sepsis. In lethal and severe sepsis, where cytokine storm dominates the underlying pathology, TLR4 signaling mediates the harmful hyper-inflammatory responses. In this case, absence of TLR4 signaling attenuates cytokine production, improves hemodynamics, reduces organ injury and promotes survival. In low grade or non-lethal sepsis, on the other hand, the main pathology is immunosuppresion and bacterial invasion. TLR4 signaling provides an essential host immune defense mechanism such as neutrophil migration and phagocytosis as demonstrated in the current study. Absence of TLR4 signaling would weaken the host immune defense, compromise bactereial clearence, deleteriorate organ injury and dysfunction, and reduce the survival.
The current and several previous studies have suggested a potential therapeutic value of TLR4 agonist in enhancing host immunity in bacterial sepsis. For example, Wheeler and colleagues 41 have reported that a low dose of lipopolysaccharide pretreatment attenuates inflammatory cytokine level in the plasma and peritoneal fluid in a CLP model of sepsis. In addition, lipopolysaccharide pretreatment improves bacterial clearance, phagocytosis, and survival during polymicrobial sepsis. Moreover, synthetic Toll-like receptor 4 agonists appear to stimulate innate resistance to infectious challenge 48. Administration of the synthetic TLR4 agonists, namely aminoalkyl gluocosaminide phosphates, provides strong protection against subsequent Listeria challenge in WT mice, but not in inactive TLR4 mutant mice 48. These results support TLR4 agonists as potential immunomodulatory agents in the setting of bacterial sepsis.
In summary, the current study demonstrates that natural deletoin of TLR4 in C57BL/10ScCr mice results in attenuated neutrophil function, decreased bacterial clearance, deleterious cardiac function and kidney/liver injury, and markedly increased mortality during low grade polymicrobial sepsis. These data suggest that TLR4 signaling is pivoital in the host defense by maintaining normal neutrophil functions and effective bacterial clearance and thus protecting the vital organs from sepsis-induced organ injury during the low grade polymicrobial sepsis.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health (Bethesda, Maryland) grant R01-GM080906 and R01-GM097259 (to Dr. Chao) and a mentored research award from International Anesthesia Research Society (San Francisco, California) (to Dr. Zou).
The authors thank Kwun Yee Trudy Poon, MS, Department of Anesthesia, Critical Care and Pain Management of Massachusetts General Hospital, Harvard Medical School, Boston, MA, for the advice on statistical analysis.
Footnotes
The authors declare no competing interests.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans: Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–42. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 5.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res. 2006;72:384–93. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 7.Nemoto S, Vallejo JG, Knuefermann P, Misra A, Defreitas G, Carabello BA, Mann DL. Escherichia coli LPS-induced LV dysfunction: Role of toll-like receptor-4 in the adult heart. Am J Physiol Heart Circ Physiol. 2002;282:H2316–23. doi: 10.1152/ajpheart.00763.2001. [DOI] [PubMed] [Google Scholar]
- 8.Thomas JA, Haudek SB, Koroglu T, Tsen MF, Bryant DD, White DJ, Kusewitt DF, Horton JW, Giroir BP. IRAK1 deletion disrupts cardiac Toll/IL-1 signaling and protects against contractile dysfunction. Am J Physiol Heart Circ Physiol. 2003;285:H597–606. doi: 10.1152/ajpheart.0655.2001. [DOI] [PubMed] [Google Scholar]
- 9.Feng Y, Zou L, Zhang M, Li Y, Chen C, Chao W. MyD88 and Trif signaling play distinct roles in cardiac dysfunction and mortality during endotoxin shock and polymicrobial sepsis. Anesthesiology. 2011;115:555–67. doi: 10.1097/ALN.0b013e31822a22f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: Tilting toward immunosuppression. Nat Med. 2009;15:496–7. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–74. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otto GP, Sossdorf M, Claus RA, Rodel J, Menge K, Reinhart K, Bauer M, Riedemann NC. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care. 2011;15:R183. doi: 10.1186/cc10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torgersen C, Moser P, Luckner G, Mayr V, Jochberger S, Hasibeder WR, Dunser MW. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesth Analg. 2009;108:1841–7. doi: 10.1213/ane.0b013e318195e11d. [DOI] [PubMed] [Google Scholar]
- 14.Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, Hotchkiss RS. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 2010;78:1582–92. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alves-Filho JC, de Freitas A, Russo M, Cunha FQ. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit Care Med. 2006;34:461–70. doi: 10.1097/01.ccm.0000198527.71819.e1. [DOI] [PubMed] [Google Scholar]
- 16.Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, Burns K, Riederer BM, Akira S, Calandra T. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A. 2009;106:2348–52. doi: 10.1073/pnas.0808146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–6. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou L, Feng Y, Chen YJ, Si R, Shen S, Zhou Q, Ichinose F, Scherrer-Crosbie M, Chao W. Toll-like receptor 2 plays a critical role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med. 2010;38:1335–42. doi: 10.1097/CCM.0b013e3181d99e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang E, Feng Y, Zhang M, Zou L, Li Y, Buys ES, Huang P, Brouckaert P, Chao W. Toll-like Receptor 4 Signaling Confers Cardiac Protection Against Ischemic Injury via Inducible Nitric Oxide Synthase- and Soluble Guanylate Cyclase-dependent Mechanisms. Anesthesiology. 2011;114:603–13. doi: 10.1097/ALN.0b013e31820a4d5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu X, Zhao H, Graveline AR, Buys ES, Schmidt U, Bloch KD, Rosenzweig A, Chao W. MyD88 and NOS2 are essential for toll-like receptor 4-mediated survival effect in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2006;291:H1900–9. doi: 10.1152/ajpheart.00112.2006. [DOI] [PubMed] [Google Scholar]
- 21.Ichinose F, Buys ES, Neilan TG, Furutani EM, Morgan JG, Jassal DS, Graveline AR, Searles RJ, Lim CC, Kaneki M, Picard MH, Scherrer-Crosbie M, Janssens S, Liao R, Bloch KD. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circ Res. 2007;100:130–9. doi: 10.1161/01.RES.0000253888.09574.7a. [DOI] [PubMed] [Google Scholar]
- 22.Zou L, Feng Y, Zhang M, Li Y, Chao W. Nonhematopoietic toll-like receptor 2 contributes to neutrophil and cardiac function impairment during polymicrobial sepsis. Shock. 2011;36:370–80. doi: 10.1097/SHK.0b013e3182279868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou L, Feng Y, Li Y, Zhang M, Chen C, Cai J, Gong Y, Wang L, Thurman JM, Wu X, Atkinson JP, Chao W. Complement Factor B Is the Downstream Effector of TLRs and Plays an Important Role in a Mouse Model of Severe Sepsis. J Immunol. 2013;191:5625–35. doi: 10.4049/jimmunol.1301903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect Immun. 2006;74:5227–35. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: Setting the stage. Nat Rev Drug Discov. 2005;4:854–65. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 26.Hoesel LM, Neff TA, Neff SB, Younger JG, Olle EW, Gao H, Pianko MJ, Bernacki KD, Sarma JV, Ward PA. Harmful and protective roles of neutrophils in sepsis. Shock. 2005;24:40–7. doi: 10.1097/01.shk.0000170353.80318.d5. [DOI] [PubMed] [Google Scholar]
- 27.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417–20. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crimi E, Sica V, Slutsky AS, Zhang H, Williams-Ignarro S, Ignarro LJ, Napoli C. Role of oxidative stress in experimental sepsis and multisystem organ dysfunction. Free Radic Res. 2006;40:665–72. doi: 10.1080/10715760600669612. [DOI] [PubMed] [Google Scholar]
- 29.Bayir H. Reactive oxygen species. Crit Care Med. 2005;33:S498–501. doi: 10.1097/01.ccm.0000186787.64500.12. [DOI] [PubMed] [Google Scholar]
- 30.Flierl MA, Rittirsch D, Huber-Lang MS, Sarma JV, Ward PA. Molecular events in the cardiomyopathy of sepsis. Mol Med. 2008;14:327–36. doi: 10.2119/2007-00130.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandes CJ, Jr, Akamine N, Knobel E. Myocardial depression in sepsis. Shock. 2008;30 (Suppl 1):14–7. doi: 10.1097/SHK.0b013e3181818617. [DOI] [PubMed] [Google Scholar]
- 32.Fallach R, Shainberg A, Avlas O, Fainblut M, Chepurko Y, Porat E, Hochhauser E. Cardiomyocyte Toll-like receptor 4 is involved in heart dysfunction following septic shock or myocardial ischemia. J Mol Cell Cardiol. 2010;48:1236–44. doi: 10.1016/j.yjmcc.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res. 2004;95:700–7. doi: 10.1161/01.RES.0000144175.70140.8c. [DOI] [PubMed] [Google Scholar]
- 34.Binck BW, Tsen MF, Islas M, White DJ, Schultz RA, Willis MS, Garcia JV, Horton JW, Thomas JA. Bone marrow-derived cells contribute to contractile dysfunction in endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288:H577–83. doi: 10.1152/ajpheart.00745.2004. [DOI] [PubMed] [Google Scholar]
- 35.Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, McCabe C, Welch SB, Whitney A, O’Gara P, Nadel S, Relman DA, Harding SE, Levin M. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363:203–9. doi: 10.1016/S0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 36.Wang E, Feng Y, Zhang M, Zou L, Li Y, Buys ES, Huang P, Brouckaert P, Chao W. Toll-like receptor 4 signaling confers cardiac protection against ischemic injury via inducible nitric oxide synthase- and soluble guanylate cyclase-dependent mechanisms. Anesthesiology. 2011;114:603–13. doi: 10.1097/ALN.0b013e31820a4d5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown JM, Grosso MA, Terada LS, Whitman GJ, Banerjee A, White CW, Harken AH, Repine JE. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci U S A. 1989;86:2516–20. doi: 10.1073/pnas.86.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng X, Ao L, Brown JM, Meldrum DR, Sheridan BC, Cain BS, Banerjee A, Harken AH. LPS induces late cardiac functional protection against ischemia independent of cardiac and circulating TNF-alpha. Am J Physiol. 1997;273:H1894–902. doi: 10.1152/ajpheart.1997.273.4.H1894. [DOI] [PubMed] [Google Scholar]
- 39.Chao W, Shen Y, Zhu X, Zhao H, Novikov M, Schmidt U, Rosenzweig A. Lipopolysaccharide improves cardiomyocyte survival and function after serum deprivation. J Biol Chem. 2005;280:21997–2005. doi: 10.1074/jbc.M413676200. [DOI] [PubMed] [Google Scholar]
- 40.Meng X, Brown JM, Ao L, Rowland RT, Nordeen SK, Banerjee A, Harken AH. Myocardial gene reprogramming associated with a cardiac cross-resistant state induced by LPS preconditioning. Am J Physiol. 1998;275:C475–83. doi: 10.1152/ajpcell.1998.275.2.C475. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler DS, Lahni PM, Denenberg AG, Poynter SE, Wong HR, Cook JA, Zingarelli B. Induction of endotoxin tolerance enhances bacterial clearance and survival in murine polymicrobial sepsis. Shock. 2008;30:267–73. doi: 10.1097/shk.0b013e318162c190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nature medicine. 2003;9:315–21. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 43.Feng Y, Zou L, Si R, Nagasaka Y, Chao W. Bone marrow MyD88 signaling modulates neutrophil function and ischemic myocardial injury. Am J Physiol Cell Physiol. 2010;299:C760–9. doi: 10.1152/ajpcell.00155.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, Dower SK, Whyte MK. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170:5268–75. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 45.Kelly-Scumpia KM, Scumpia PO, Delano MJ, Weinstein JS, Cuenca AG, Wynn JL, Moldawer LL. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J Exp Med. 2010;207:319–26. doi: 10.1084/jem.20091959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peck-Palmer OM, Unsinger J, Chang KC, Davis CG, McDunn JE, Hotchkiss RS. Deletion of MyD88 markedly attenuates sepsis-induced T and B lymphocyte apoptosis but worsens survival. J Leukoc Biol. 2008;83:1009–18. doi: 10.1189/jlb.0807528. [DOI] [PubMed] [Google Scholar]
- 47.Weighardt H, Kaiser-Moore S, Schlautkotter S, Rossmann-Bloeck T, Schleicher U, Bogdan C, Holzmann B. Type I IFN modulates host defense and late hyperinflammation in septic peritonitis. J Immunol. 2006;177:5623–30. doi: 10.4049/jimmunol.177.8.5623. [DOI] [PubMed] [Google Scholar]
- 48.Cluff CW, Baldridge JR, Stover AG, Evans JT, Johnson DA, Lacy MJ, Clawson VG, Yorgensen VM, Johnson CL, Livesay MT, Hershberg RM, Persing DH. Synthetic toll-like receptor 4 agonists stimulate innate resistance to infectious challenge. Infec Immun. 2005;73:3044–52. doi: 10.1128/IAI.73.5.3044-3052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13:453–60. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 50.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.