Abstract
A 21 year old male student presented in 1980 as an Olympic athlete with a 12 year history of jaundice, pallor, and darkened urine induced by the atraumatic exercise of swimming (1). Physical examination at that time was remarkable only for moderate scleral icterus without hepatosplenomegaly. Hematological examination revealed moderate macrocytosis (MCV 102 fL) without anemia (Hct 50%, Hb 17 g/dL, 9% reticulocytes). The peripheral blood smear showed occasional target cells. Red cell osmotic fragility was decreased. Red cell Na content was increased and K content was decreased, with reduced total monovalent ion content. Passive red cell permeability of both Na and K were increased. A supervised 2.5 hr swimming workout increased free plasma Hb from <5 to 45 mg/dL and decreased serum haptoglobin from 25 to 6 mg/dL. The post-exercise urine sediment was remarkable for hemosiderin-laden tubular epithelial cells, without frank hemoglobinuria. The circulating 15 day erythrocyte half-life measured after 6 days without exercise was further shortened to 12 days after resumption of twice-per-day swimming workouts for 1 week. The patient’s red cells were hypersensitive to in vitro shear stress applied by cone-plate viscometer.
Keywords: hereditary xerocytosis, hemolytic anemia, iron overload, dehydrated stomatocytosis, PIEZO1, cation channel
The diagnosis of xerocytosis was made (1). Hereditary xerocytosis (HX) is a rare, autosomal dominant congenital hemolytic anemia characterized by macrocytic stomatocytosis, and decreased red cell osmotic fragility due to a defect in cation permeability. This defect results in dehydrated erythrocytes with a deficit in intracellular potassium incompletely compensated by intracellular sodium (2, 3).
Recent studies have helped elucidate the molecular basis of HX and other primary disorders of erythrocyte hydration. HX is one of two types of autosomal dominant stomatocytosis. The other is overhydrated stomatocytosis (OHSt), also known as erythrocyte hydrocytosis. OHSt is associated with high sodium content, decreased MCH, increased osmotic fragility, and missense mutations in the red cell membrane gene products SLC4A1/AE1, SLC2A1/GLUT1, and RHAG. HX, sometimes referred to as dehydrated stomatocytosis (DHSt), is caused by missense mutations in the ATP binding cassette transporter ABCB6 (4) and in the red cell membrane mechanosensitive cation channel, PIEZO1 (5–7). Familial pseudohyperkalemia, a related benign condition, is characterized by temperature-dependent loss of erythrocyte K, and is an incompletely penetrant manifestation of mutations in either HX gene (4).
The clinical presentation of hereditary xerocytosis (HX) exhibits marked heterogeneity, ranging from supernormal hemoglobin values to severe anemia. While many patients are asymptomatic, cholelithiasis and, less commonly, perinatal edema and pulmonary arteriolar thromboses, can occur in some patients. Most patients with HX have evidence of iron overload of variable severity. Splenectomy can be beneficial in hereditary spherocytosis, but is contraindicated in HX due to elevated risk of venous thrombosis and thromboembolism.
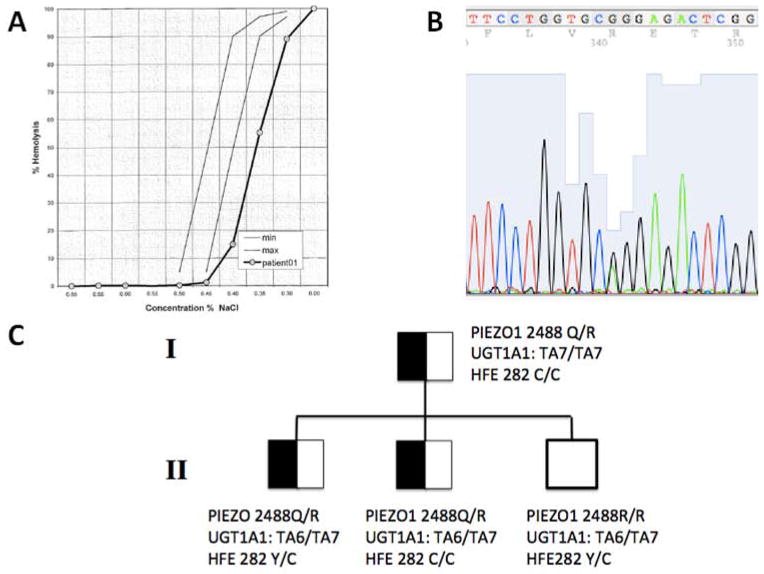
In March 2013, the now 54-year-old patient returned to our clinic for follow-up. He had been well except for a history of episodic jaundice and darkening of the urine in the setting of upper respiratory infections, consistent with Gilbert Syndrome. Incidental findings were splenomegaly of 14 cm on MRI and mild thrombocytopenia (152,000 cells/μL). Physical exam revealed a palpable spleen tip without jaundice. Hematological indices (Table 1) showed normal Hb, elevated reticulocytes, increased MCV, MCH, and MCHC, with borderline reduction in platelet count (not shown) and reduced osmotic fragility (Fig. 1A) that was preserved after overnight storage at 4°C. Hemoglobin electrophoresis was normal. Serum lactate dehydrogenase (LDH) activity was entirely normal. Total bilirubin was 4.9 mg/dL (direct bilirubin 0.2 mg/dL). Plasma iron, serum ferritin, and total iron binding capacity were elevated at 178 mcg/dL, 606 ng/mL, and 218 mcg/dL, respectively with a calculated transferrin saturation of 82 % (Table 1).
Table 1.
Genetic, hematologic, and iron parameters in proband and sons.
| Reference values | I.1 (proband) | II. 1 (affected son 1) | II.2 (affected son 2) | II.3 (unaffected son 3) | |
|---|---|---|---|---|---|
| Age (yr) | 54 | 27 | 24 | 17 | |
| Genetics | |||||
| PIEZO1 | R2488Q/WT | R2488Q/WT | R2488Q/WT | WT/WT | |
| UGT1A1 | TA7/TA7 | TA6/TA7 | TA6/TA7 | TA6/TA7 | |
| HFE | WT/WT | C282Y/WT | WT/WT | C282Y/WT | |
| Hematology | |||||
| Hemoglobin (g/dL) | 11.4 – 15.1 | 15.1 | 16.2 | 15.7 | 15.5 |
| MCV (fL) | 81 – 95.5 | 103 | 97.3 | 94.8 | 93.3 |
| MCH (pg) | 27 – 32.8 | 36.8 | 33 | 32.7 | 31.5 |
| MCHC (g/dL) | 32.5 – 35.2 | 35.6 | 33.9 | 34.5 | 33.8 |
| Reticulocyte (%) | 0.8 – 2.2 | 5.6 | 4.3 | 5.0 | 1.7 |
| Absolute Reticulocyte (M cells/μL) | 0.043 – 0.085 | 0.185 | 0.099 | 0.101 | 0.063 |
| Chemistry | |||||
| LDH (unit/L) | 100 – 210 | 137 | 140 | 199 | 220 |
| Total Bilirubin (mg/dL) | 0.3 – 1.2 | 4.94 | 1.65 | 2.1 | 0.32 |
| Direct Bilirubin (mg/dL) | 0.1 – 0.4 | 0.2 | 0.24 | 0.26 | 0.09 |
| Indirect Bilirubin (mg/dL) | 0.2 – 0.8 | 4.74 | 1.41 | 1.84 | 0.23 |
| Haptoglobin (mg/dL) | 33 – 183 | 10 | 6 | 35 | 31 |
| Iron (mcg/dL) | 65 – 175 | 178 | 151 | 177 | 85 |
| Ferritin (ng/dL) | 10 – 320 | 606 | 363 | 275 | 44 |
| Transferrin (mg/dL) | 200 – 400 | 179 | 221 | 244 | 259 |
| TIBC (mcg/dL) | 250 – 420 | 218 | 266 | 309 | 309 |
| Transferrin saturation (%) | 82 | 57 | 57 | 28 | |
| Hepcidin (ng/mg creatinine) | 71 – 1762 | 47 | 24 | 38 | 17 |
| Hepcidin/ferritin ratio | N/A | 0.078 | 0.066 | 0.14 | 0.39 |
| sTfR (μg/mL) | 2.2 – 5.0 | 8.6 | 8.1 | 10.2 | 4.0 |
| GDF 15 (pg/ml) | 200 – 500 | 677 | 304 | 269 | 302 |
Figure 1. HX osmotic Fragility and mutation detection.
Osmotic fragility curve of proband’s red blood cells. Gray lines represent normal limits. B. Sanger DNA sequence of the proband’s PIEZO1 cDNA (sense strand) showing the heterozygous mutation (asterisk) encoding missense substitution R2488Q. C. Partial pedigree of the family, with proband (I.1) and his three sons (II.1, II.2, and II.3). Genotypes are shown for PIEZO1 aa2488, UGT1A1 TA6/7 polymorphism, and HFE aa282.
The clinical picture again strongly suggested HX. In view of interim advances in the understanding of RBC cation leak disorders, the clinical diagnosis would be strengthened by ion transport studies demonstrating a potassium leak. Molecular diagnosis requires candidate gene testing of the patient and closely related family members for missense mutations in the disease genes PIEZO1 and/or ABCB6. The patient’s iron overload might be due to HX itself, but the apparent absence of ineffective erythropoiesis encourages screening for hemochromatosis-causing gene variants and for Gilbert Syndrome to account for the hyberbilirubinemia.
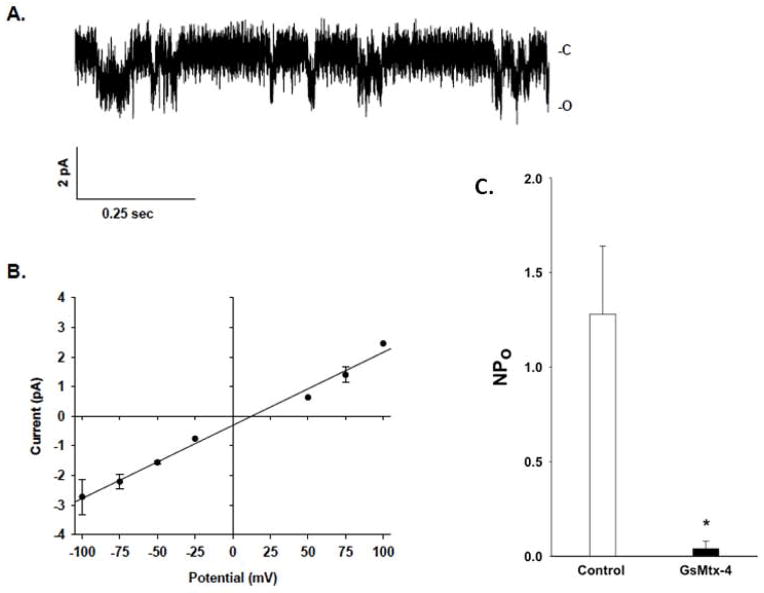
Further investigation showed that hyperdense, dehydrated patient red cells (>41 g Hb/dL) increased from the already elevated level of 3.0% to 7.4% after overnight cold storage. This was equally accompanied by an increase in red cell Na content from the baseline elevated value of 39.2 to 55.5 mmol/kg Hb (control 31.5), and by K content reduction from the already low value of 176 mmol/kg Hb to 166 (control 238). These findings were further corroborated by a reduced osmotic fragility (Fig. 1A). The ouabain-sensitive Na+ pump activity of the proband’s red cells was increased, as was hypotonic swelling-activated K-Cl cotransport activity and Na/H exchange activity (not shown). On-cell patch recordings revealed substantially increased cationpermeable ion channel activity (Fig. 2A), with a single channel conductance of 25.6 pS (Fig. 2B). NPo of 1.28±0.36 was reduced to 0.04±0.04 in the presence of the mechanosensitive cation channel inhibitor, GsMTx4 (1 μM, Fig. 2C, n=4), a value similar to that recorded in control erythrocytes (not shown) (4, 7, 8).
Figure 2. HX red cell channel activity.
A. Representative trace from cell-attached patch on the proband’s erythrocyte. The negative of the command potential applied to the pipette (−Vp) = −100mV. “C” indicates closed level. “O” indicates open channel. B. Composite single channel current voltage relationship from 4 patches. Mean slope conductance was 25.6 pS (r2 = 0.99). C. NPo values from the proband’s erythrocyte cell-attached patches in the absence (left open bar) and presence (right filled bar) of 1 μM GsMTx-4 (*, p<0.05).
The elevated red cell Na content and reduced red cell K content are consistent with a cation leak stomatocytosis. The lack of effect of cold storage on intracellular cation content is more suggestive of HX than OHSt. The reduced osmotic fragility, together with the elevated hemoglobin indices, strongly suggest HX. Furthermore the elevated cation channel activity and sensitivity to GsMTx4 are consistent with a gain-of-function mutation in PIEZO1 (9) similar to those previously reported (4, 5, 10, 11). The increased sodium pump activity likely represents a compensatory response to elevated intracellular Na content resulting from increased cation channel activity. The elevated K-Cl cotransport activity in response to hypotonic swelling might equally represent a compensatory response to the moderately elevated MCV. Nevertheless, the elevated Na/H exchange activity observed in this and other cases remains unexplained.
The clinical phenotype as well as GsMTx4-sensitive cation channel activity prompted examination of PIEZO1 as the most likely candidate gene (6). Indeed, the patient’s PIEZO1 cDNA revealed the heterozygous variant, c.G7463A, encoding the missense substitution p.R2488Q (Fig. 1B), and the mutation was confirmed present in patient genomic DNA. Two of the patient’s three sons shared this heterozygous variant (Fig. 1C).
We previously described this same heterozygous PIEZO1 variant in cis with a second, heterozygous missense variant c.2152G>Z; p.G718S (6). The absence of this second variant in the present family conclusively demonstrates that the R2488Q variant is the HX disease-associated allele. In contrast to the present case, the family cosegregating the G718S variant also exhibited familial pseudohyperkalemia, suggesting that this second missense variant could further exacerbate the potassium leak phenotype.
Several HX-associated PIEZO1 mutations (p.R2456H, p.M2255R, p.A2020T, p.R1358P, p.T2127M, and p.E2496ELE) have been studied functionally in transiently transfected HEK-293 cells (7, 10). Figure 3 shows the properties of our patient’s mutation p.R2488Q. HEK-293 cells overexpressing the mutant PIEZO1 polypeptide revealed a prolonged inactivation time constant of mechanically stimulated whole cell currents. The mean current trace from cells expressing mutant PIEZO1 R2488Q exhibited an inactivation time constant of 33 ms (n=10), compared to the wildtype value of 22 ms (n=15). Mean values of the inactivation time constants measured from current traces of the same individual cells also revealed prolongation in cells expressing the mutant (53±11.6 ms vs. the control wildtype value of 29±4.8 ms (mean±s.e.m.; p<0.05). These biophysical properties of the mutant R4288Q polypeptide resemble those reported for multiple heterozygous PIEZO1 mutations found in other HX families (7, 10).
Figure 3. The effect of PIEZO1 mutation R2488Q on mechanically activated whole-cell currents.
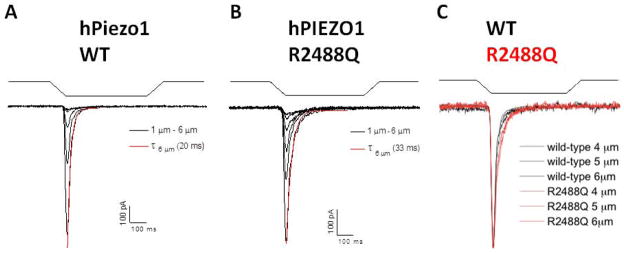
A. Whole-cell currents were elicited by applying indenting stimuli to the surface of HEK-293 cells transiently transfected with cDNA encoding wild-type hPiezo1. The stimuli (waveforms above current traces) consisted of indentation steps of 500 ms duration, applied in successive, 1 μm depth increments from 1–6 μm. Shown in black are mean whole-cell current traces elicited from cells transfected with wild-type PIEZO1 (n=15). The inactivation phase of the mean trace at 6 μm indentation depth was curve-fitted (red) using a mono-exponential equation ( ), yielding the time constant of inactivation (τ6 μm). B. Mean whole cell current traces elicited from cells transfected with PIEZO1 mutant R2488Q (n=10), and the curve-fit of the mean data at 6 μm indentation (red). C. Superposed, normalized mean current traces elicited from indentations of 4, 5, and 6 μm (taken from panels A and B) with R2488Q currents shown in red. The inactivation half-times of whole-cell currents in cells expressing the PIEZO1 R2488Q mutant were longer than in cells expressing wild-type PIEZO1 protein.
The prolonged inactivation time that characterizes the mutant PIEZO1 polypeptides found in HX could account in part for the increased Na content and decreased K content of HX red cells. It is also possible that increased PIEZO1 activity in red cells regulates other erythroid cation channels to eventuate in the observed hematological indices.
Maturation and senescence of circulating RBCs were evaluated by single-RBC volume and hemoglobin measurements and a mathematical model of erythrocyte population dynamics to estimate in vivo rates of change in RBC volume and hemoglobin (12). The proband’s circulating RBC population was abnormally homogeneous: coefficients of variation for RBC volume (RDW), hemoglobin mass (HDW), and concentration (CHDW) were low-normal or below the normal range (data not shown). The proband’s reticulocytes were similarly homogeneous (rRDW, rHDW, rCHDW).
Normal RBCs undergo rapid volume and hemoglobin concentration reduction during their first several days of circulation, followed by a slow phase of continued reduction in both quantities until clearance. Modeling predicted nearly two-fold acceleration of both phases of proband RBC maturation, consistent with the previously measured short RBC lifespan.
Additional genetic tests for iron overload (HFE mutations C282Y and H63D) and for Gilbert’s hyperbilirubinemia were performed (Table 1). The proband and one of his HX-affected sons were wildtype for the disease associated C282Y allele in the hemochromatosis protein HFE, whereas the other HX-affected son was heterozygous for the HFE C282Y mutation (Fig. 1C). A second major HFE disease allele, H63D, was wildtype in all four family members. The proband was also homozygous for the UGT1A1 variant, consistent with Gilbert Syndrome, while his two PIEZO1 mutant sons were, as expected, heterozygous.
Further hematological evaluation of the proband’s three sons revealed absence of anemia, with hemoglobin values ranging from 15.1–16.2 g/dL. The proband’s HX-affected sons had elevated MCH (33 and 32.7 pg) and reticulocytes (4.3% (0.099×106 cells/uL) and 5% (0.101 × 106 cells/uL), whereas their unaffected brother had normal values for both. Indirect bilirubinemia of 1.41 and 1.84 mg/dL in the two HX-affected sons was 6 times higher than that of their unaffected brother. Transferrin saturation was elevated at 57% in both HX-affected sons. (Please see supplementary information for complete methods.)
The persistence of a chronic hemolytic process reducing red cell life span, in the setting of elevated values of transferrin saturation prompted recommendation of monthly therapeutic phlebotomies for the proband and his 27 year old HX-affected son, with a target ferritin level of < 50 ng/dL. Volume indices have also been proposed as clinical target values in hemochromatosis (13).
DISCUSSION
The molecular diagnosis presented here confirms a previously proposed case of HX and highlights the clinical variation among and within HX families. Our patient and two of his three sons carry a heterozygous R2488Q missense mutation in PIEZO1; all are healthy and none is anemic. They do, however, share features commonly found in patients with HX, an elevated MCH and excess iron load. PIEZO1 knockout reduces erythrocyte volume in a zebrafish model (14). Its activation might, perhaps, exert independent trophic effects on hemoglobin synthesis and erythroid cell volume reflected in elevation of MCH and MCV. Furthermore, reticulocytosis and a young mean erythrocyte age independently increase MCH and MCV as do high iron stores. As iron absorption increases, transferrin saturation rises and provides more iron for hemoglobin synthesis and comcomitant erythrocyte volume (15). Other unknown modifiers may also influence the macrocytic phenotype in this curious disorder (15–17), possibly including other polymorphic variations in PIEZO1.
HX itself confers chronic iron overload (18). In other types of hemolytic anemia such as thalassemia intermedia, ineffective erythropoiesis can cause severe hepcidin deficiency and concomitant hemochromatosis (19). However, our patient and his affected sons exhibit no evidence of ineffective erythropoiesis. The elevated levels of soluble transferrin receptor (STfR; 8.1 – 10.2mg/dL; normal 2.2 – 5.0mg/dL) in all three affected family members reflects increased marrow erythroblast input from progenitors and excess production of immature reticulocytes into the peripheral blood. This is itself not indicative of increased ineffective erythropoiesis (defined as excessive death of marrow erythroblasts). Elevated serum LDH typically accompanies such increased death of immature erythroid precursors. The low or normal LDH values seen in this family indicate that while total erythropoiesis is surely elevated in affected family members with mutant PIEZO1, ineffective erythropoiesis, as ordinarily defined, is not present.
Hereditary spherocytosis (HS) is another type of hemolytic anemia that does not demonstrate excessive ineffective erythropoiesis. HS has been (infrequently) associated with iron overload, but only very rarely in the absence of a concomitant HFE mutation (20–23). Our patient and one HX-affected son were wildtype for the hemochromatosis protein HFE, whereas the other HX-affected son was heterozygous for HFE variant C282Y (Fig. 1C). Therefore iron loading in these cases is not caused by HFE mutation.
Despite the absence of ineffective erythropoiesis and a common HFE mutation, hepcidin levels and hepcidin/ferritin ratios are low in our patient and his two affected sons (Table 1). The hepcidin regulatory signal accompanying accelerated erythropoiesis remains undefined. GDF15 is unlikely to correlate with hepcidin levels (24). Venisection or erythropoietin administration to mice are followed within hours by release of erythroferrone, a putative hepcidin regulator derived from marrow erythroblasts in vitro (25). Activated PIEZO1 might induce marrow erythroblasts to release such a circulating product that would in turn down-regulate hepcidin synthesis in hepatocytes. Alternatively, activated PIEZO1 on hepatocytes might directly down-regulate hepcidin synthesis in the liver (26). Further studies in animal models are needed to test both hypotheses.
Supplementary Material
Acknowledgments
Support was from NIH grants T32HL007574 (NA), R01HL054887 (FS), R34HL108757 (DN), MIUR and Telethon (Italy) GGP09044 (AI), and the Doris Duke Charitable Foundation 2011094 (SA) and 2013010 (NA). The authors would like to thank Samuel Lux for his helpful comments.
Footnotes
The remaining authors declare no conflicts of interest.
References
- 1.Platt OS, Lux SE, Nathan DG. Exercise-induced hemolysis in xerocytosis. Erythrocyte dehydration and shear sensitivity. J Clin Invest. 1981;68:631–638. doi: 10.1172/JCI110297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller DR, Rickles FR, Lichtman MA, La Celle PL, Bates J, Weed RI. A new variant of hereditary hemolytic anemia with stomatocytosis and erythrocyte cation abnormality. Blood. 1971;38:184–204. [PubMed] [Google Scholar]
- 3.Glader BE, Fortier N, Albala MM, Nathan DG. Congenital hemolytic anemia associated with dehydrated erythrocytes and increased potassium loss. N Engl J Med. 1974;291:491–496. doi: 10.1056/NEJM197409052911003. [DOI] [PubMed] [Google Scholar]
- 4.Andolfo I, Alper SL, Delaunay J, Auriemma C, Russo R, Asci R, Esposito MR, Sharma AK, Shmukler BE, Brugnara C, et al. Missense mutations in the ABCB6 transporter cause dominant familial pseudohyperkalemia. Am J Hematol. 2013;88:66–72. doi: 10.1002/ajh.23357. [DOI] [PubMed] [Google Scholar]
- 5.Zarychanski R, Schulz VP, Houston BL, Maksimova Y, Houston DS, Smith B, Rinehart J, Gallagher PG. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120:1908–1915. doi: 10.1182/blood-2012-04-422253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andolfo I, Alper SL, De Franceschi L, Auriemma C, Russo R, De Falco L, Vallefuoco F, Esposito MR, Vandorpe DH, Shmukler BE, et al. Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood. 2013;121:3925–3935. S3921–3912. doi: 10.1182/blood-2013-02-482489. [DOI] [PubMed] [Google Scholar]
- 7.Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Feneant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1–8. doi: 10.1038/ncomms2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandorpe DH, Xu C, Shmukler BE, Otterbein LE, Trudel M, Sachs F, Gottlieb PA, Brugnara C, Alper SL. Hypoxia activates a Ca2+-permeable cation conductance sensitive to carbon monoxide and to GsMTx-4 in human and mouse sickle erythrocytes. PLoS One. 2010;5:e8732. doi: 10.1371/journal.pone.0008732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50:6295–6300. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci U S A. 2013;110:E1162–1168. doi: 10.1073/pnas.1219777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shmukler BE, Vandorpe DH, Rivera A, Auerbach M, Brugnara C, Alper SL. Dehydrated stomatocytic anemia due to the heterozygous mutation R2456H in the mechanosensitive cation channel PIEZO1: a case report. Blood Cells Mol Dis. 2014;52:53–54. doi: 10.1016/j.bcmd.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Golub MS, Hogrefe CE, Malka R, Higgins JM. Developmental plasticity of red blood cell homeostasis. Am J Hematol. 2014;89:459–466. doi: 10.1002/ajh.23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolan CD, Conry-Cantilena C, Mason G, Rouault TA, Leitman SF. MCV as a guide to phlebotomy therapy for hemochromatosis. Transfusion. 2001;41:819–827. doi: 10.1046/j.1537-2995.2001.41060819.x. [DOI] [PubMed] [Google Scholar]
- 14.Faucherre A, Kissa K, Nargeot J, Mangoni ME, Jopling C. Piezo1 plays a role in erythrocyte volume homeostasis. Haematologica. 2014;99:70–75. doi: 10.3324/haematol.2013.086090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews NC. Genes determining blood cell traits. Nat Genet. 2009;41:1161–1162. doi: 10.1038/ng1109-1161. [DOI] [PubMed] [Google Scholar]
- 16.Chami N, Lettre G. Lessons and Implications from Genome-Wide Association Studies (GWAS) Findings of Blood Cell Phenotypes. Genes (Basel) 2014;5:51–64. doi: 10.3390/genes5010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Harst P, Zhang W, Mateo Leach I, Rendon A, Verweij N, Sehmi J, Paul DS, Elling U, Allayee H, Li X, et al. Seventy-five genetic loci influencing the human red blood cell. Nature. 2012;492:369–375. doi: 10.1038/nature11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assis RA, Kassab C, Seguro FS, Costa FF, Silveira PA, Wood J, Hamerschlak N. Iron overload in a teenager with xerocytosis: the importance of nuclear magnetic resonance imaging. Einstein (Sao Paulo) 2013;11:528–532. doi: 10.1590/S1679-45082013000400022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasricha SR, Frazer DM, Bowden DK, Anderson GJ. Transfusion suppresses erythropoiesis and increases hepcidin in adult patients with β-thalassemia major: a longitudinal study. Blood. 2013;122:124–133. doi: 10.1182/blood-2012-12-471441. [DOI] [PubMed] [Google Scholar]
- 20.Edwards CQ, Skolnick MH, Dadone MM, Kushner JP. Iron overload in hereditary spherocytosis: association with HLA-linked hemochromatosis. Am J Hematol. 1982;13:101–109. doi: 10.1002/ajh.2830130202. [DOI] [PubMed] [Google Scholar]
- 21.Mohler D, Wheby M. Patients with hereditary spherocytosis may have clinically significant iron overload when they are also heterozygous for hemochromatosis. Trans Am Clin Climatol Assoc. 1985;96:34–40. [PMC free article] [PubMed] [Google Scholar]
- 22.Fargion S, Cappellini MD, Piperno A, Panajotopoulos N, Ronchi G, Fiorelli G. Association of hereditary spherocytosis and idiopathic hemochromatosis. A synergistic effect in determining iron overload. Am J Clin Pathol. 1986;86:645–649. doi: 10.1093/ajcp/86.5.645. [DOI] [PubMed] [Google Scholar]
- 23.Ichiche M, Lacor P, Hoorens A, Vanden Brande J, Brussaard H, Vanstraelen D. Congenital spherocytosis with hereditary hemochromatosis without pathogenic mutations in the HFE gene. Eur J Intern Med. 2004;15:460–462. doi: 10.1016/j.ejim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Fertrin K, Lanaro C, Franco-Penteado C, de Albuquerque D, Bandeira de Mello M, Pallis F, Bezerra M, Hatzlhofer B, Olbina G, Saad T, et al. Erythropoiesis-driven regulation of hepcidin in human red cell disorders is better reflected through concentrations of soluble transferrin receptor rather than growth differentiation factor 15. Am J Hematol. 2014;89:385–390. doi: 10.1002/ajh.23649. [DOI] [PubMed] [Google Scholar]
- 25.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Scripps Research Institute. BioGPS. La Jolla, CA: Amazon’s Elastic Compute Cloud (EC2); 2014. (URL: http://biogps.org/#goto=genereport&id=9780) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.