Abstract
Purpose
Pre-clinical studies suggest that anti-angiogenic agents may be toxic to the developing growth plate. The purpose of this study was to evaluate the incidence of growth plate abnormalities in children with refractory cancer undergoing anti-angiogenic therapy.
Materials and methods
Targeted radiographic studies from 53 subjects enrolled on six separate Children’s Oncology Group Phase 1 and Pilot Consortium clinical trials evaluating new anti-cancer agents agents interfering with angiogenesis were reviewed. Subjects received tyrosine kinase inhibitors with anti-angiogenic effects (n=35), monoclonal antibodies targeting VEGF (n=13), or angiopoietin (n=5). Radiographs of their distal femur/proximal tibia were obtained at baseline. Follow-up radiographs were obtained after odd-numbered treatment cycles in patients with open growth plates who did not experience disease progression prior to cycle 3.
Results
Baseline and follow-up growth plate radiographs were acquired in 48/53 (90%) of patients. Five patients (9.4%), all of whom received a specific VEGF/VEGFR blocking agent [sunitinib (n=1) or pazopanib (n=4)], had growth plate abnormalities. Four patients had growth plate widening that was apparent on at least two successive radiographs, but was not confirmed by MRI. The fifth patient had progressive growth plate widening and evidence of physeal cartilage hypertrophy on MRI. Subsequent off treatment radiographs showed that the growth plate changes were reversible.
Conclusion
Growth plate abnormalities occur in a small, but relevant number of patients undergoing anti-angiogenic therapy. These results support the need for growth plate monitoring in children with open growth plates who are receiving anti-angiogenic therapy, and for improved methods to assess toxicity of anti-angiogenic agents to the developing skeleton.
Keywords: Developing Growth Plate, Physeal Toxicity, Anti-angiogenic Therapy
Introduction
Angiogenesis, one of the hallmarks of cancer, plays a critical role in tumorigenesis as well as tumor growth and metastasis [1, 2]. Vascular endothelial growth factor (VEGF) is one of the best-characterized pro-angiogenic factors and its expression correlates with both advanced disease stage and poor prognosis in many malignancies [3–5]. VEGF is a cytokine that acts as a powerful mitogen on endothelial cells, promoting the formation of new vessels required for normal and neoplastic tissue growth. VEGF receptors are expressed on the vascular endothelium of most tumor types; as such interference with VEGF-mediated angiogenesis may be beneficial in controlling the growth and metastatic spread of many types of tumor [6]. Indeed, studies in both animal models and in early human clinical trials have demonstrated the potential efficacy of anti-VEGF approaches to cancer treatment [7–9].
Neovascularization also plays an important role in normal physiologic growth and development. In particular, in the developing skeleton, the physeal growth plates are active sites of new blood vessel growth and proliferation as cartilage and new bone are deposited along the zone of provisional calcification in the growing bone [10, 11]. Blood vessels arising in the juxtaphyseal metaphysis deliver essential growth factors, vitamins and minerals such as Ca+2 and PO4−3 and are crucial to the coordinated program of chondrocyte proliferation, maturation, apoptosis and matrix mineralization central to the process of endochondral ossification [12]. Disruption of blood flow to the developing physis, for example following injury or stress [13], impairs endochondral ossification resulting in chondrocyte hypertrophy and growth plate widening. In preclinical studies evaluating toxicity of anti-angiogenic agents targeted to VEGF, inhibition of new blood vessel formation was shown to suppress trabecular bone formation along the zone of provisional calcification in the epiphyseal growth plate [11]. Growth plate abnormalities were observed in animals being treated with the anti-VEGF compounds bevacizumab [14] and aflibercept [15], and the receptor tyrosine kinase inhibitors (TKI) sorafenib [16], sunitinib [17], and papazonib [18]. The angiopoietin neutralizing peptide-antibody Fc fusion protein AMG 386 [19], has also been shown in animal models to produce growth plate changes at high doses, secondary to its effects on blood vessel growth during development. To date, there are limited data in the clinical literature supporting these preclinical results, including studies of the VEGF-targeted agents bevacizumab [20] and vandetanib [21], and the receptor TKI pazopanib [22].
The purpose of this study was to evaluate the incidence of growth plate abnormalities in children who were receiving anti-angiogenic therapy in the phase 1 setting for the treatment of refractory or recurrent cancer.
Materials and Methods
Patient Enrollment
Clinical trials were conducted through the Children’s Oncology Group Phase 1 and Pilot Consortium. Patient enrollment and eligibility criteria were according to the specific therapeutic trial and have been reported separately [22–28]. Each trial was approved by the respective Institutional Review Boards of the participating sites. Written informed consent was obtained from all patients, parents or guardians, including assent from minor subjects according to institutional guidelines.
The study cohort reported on here includes 53 patients enrolled in six different phase 1 trials of anti-angiogenic agents. The clinical protocols included studies of VEGF-targeted therapies including bevacizumab (ADVL0314, [23]), sorafenib (ADVL0413, [24]), sunitinib (ADVL0612, [25, 26]), pazopanib (ADVL0815) [22], aflibercept (ADVL0714, [27]), and the angiopoietin-targeted peptibody AMG 389 [trebananib] (ADVL1115) [28]. Additional criteria for inclusion in this analytic cohort were the presence of an open growth plate at time of enrollment and receipt of at least one follow-up growth plate radiograph after initiation of treatment with study therapy. The summary results of growth plate toxicity monitoring have been incorporated into primary clinical manuscripts from two of the phase 1 studies included here (ADVL0314 [23] and ADVL0815 [22]), however previously reported data have not been directly reproduced.
Imaging studies
Radiographs of the distal femur and proximal tibia were obtained at baseline in all patients. In patients with open physes who did not experience disease progression, follow-up tibial radiographs were obtained prior to cycle 3, cycle 5, and every 6 months thereafter.
In normal children the physis appears radiographically as a thin, undulating disk that remains relatively uniform in thickness until it eventually closes with skeletal maturity. Focal thickening or irregularity of the physis indicates disruption of the normal process of endochondral ossification [29]. For patients with evidence of growth plate thickening or other suspected physeal changes a follow-up knee MRI was recommended, but not required, to further assess the degree of physeal pathology.
All of the studies were centrally reviewed by one of two board certified pediatric radiologists (SDV, MDN). Radiographs were coded as adequate/inadequate for growth plate assessment, growth plates open without abnormality, growth plates open with abnormality, or growth plates closed. It is important to note that normal growth plates progressively narrow, and ultimately close, during skeletal maturation. For this study, only growth plates that showed unequivocal widening while on therapy were coded as abnormal.
Results
Fifty-three patients were treated on six different phase 1 clinical trials of agents with the potential to adversely affect bone growth along the developing physis. The cohort included 29 boys and 24 girls, with a median age of 10 (range 3–17). Fifteen patients had primary CNS tumors and the remaining 38 patients had either a non-CNS solid tumor (n=36) or a hematologic malignancy(n=2).
Tables I and II summarize the results of the growth plate evaluations. Five of the 53 patients who received anti-cancer agents impacting angiogenesis had a growth plate abnormality for an overall incidence of 9.4%. The five patients with growth plate abnormalities all received anti-VEGFR2 tyrosine kinase inhibitors [sunitinib (n=1) or pazopanib (n=4)) for an incidence of growth plate abnormalities in this subgroup of 35 patients equating to an incidence of 14.3%.
Table I.
Distribution of Growth Plate Abnormalities During Anti-Angiogenic Therapy
| Protocol | Drug | # Pts |
Median Age at Baseline (years) |
Sex (M/F) |
Tumor Type CNS/non- CNS |
Action | Patients With Physeal Abnormality |
Courses of Therapy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–4 | 5–8 | 9–12 | >12 | ||||||||
| ADVL0413 | Sorafenib | 7 | 11.7 | 6/1 | 1/6 | Raf kinase and multi-targeted TK* inhibitor |
0 | 5 | 1 | 0 | 1 |
| ADVL0612 | Sunitinib | 3 | 12 | 2/1 | 2/1 | Multi-targeted TK inhibitor | 1 | 1 | 0 | 1 | 1 |
| ADVL0714 | Aflibercept | 2 | 10 | 1/1 | 0/2 | VEGF inhibitor | 0 | 1 | 0 | 0 | 1 |
| ADVL0815 | Pazopanib | 25 | 11 | 12/13 | 7/18 | Multi-targeted VEGF inhibitor and PDGF-R inhibitor |
4 | 21 | 1 | 2 | 1 |
| ADVL0314 | Bevacizumab | 3 | 5.8 | 0/3 | 0/3 | Anti-VEGF mAb* | 0 | 3 | 0 | 0 | 0 |
| ADVL1115 | Trebananib | 13 | 8 | 8/5 | 5/8 | Angiopoietin-neutralizing peptibody |
0 | 13 | 0 | 0 | 0 |
| Total | 53 | 10 | 29/24 | 15/38 | 5 | 44 | 2 | 3 | 4 | ||
TK = tyrosine kinase; mAb = monoclonal antibody
Table II.
Characteristics of Patients with Growth Plate Widening
| Subject | Agent Received |
Sex | Age (yrs*) |
Diagnosis | Total No. Cycles |
Finding on Knee Radiograph |
MRI Findings |
|---|---|---|---|---|---|---|---|
| 1 | Sunitinib | M | 7 | Ependymoma | 18 | Growth plate widening | Not done |
| 2 | Pazopanib | M | 4 | Malignant Glioma | 4 | Growth plate widening | Not done |
| 3 | Pazopanib | F | 8 | Embryonal Rhabdomyosarcoma |
4 | Growth plate widening | Not done |
| 4 | Pazopanib | F | 11 | Malignant Melanoma | 4 | Growth plate widening | Not done |
| 5 | Pazopanib | F | 11 | Alveolar Rhabdomyosarcoma |
10 | Growth plate widening | Physeal cartilage expansion and growth plate widening |
The reported age is from the time of study enrollment
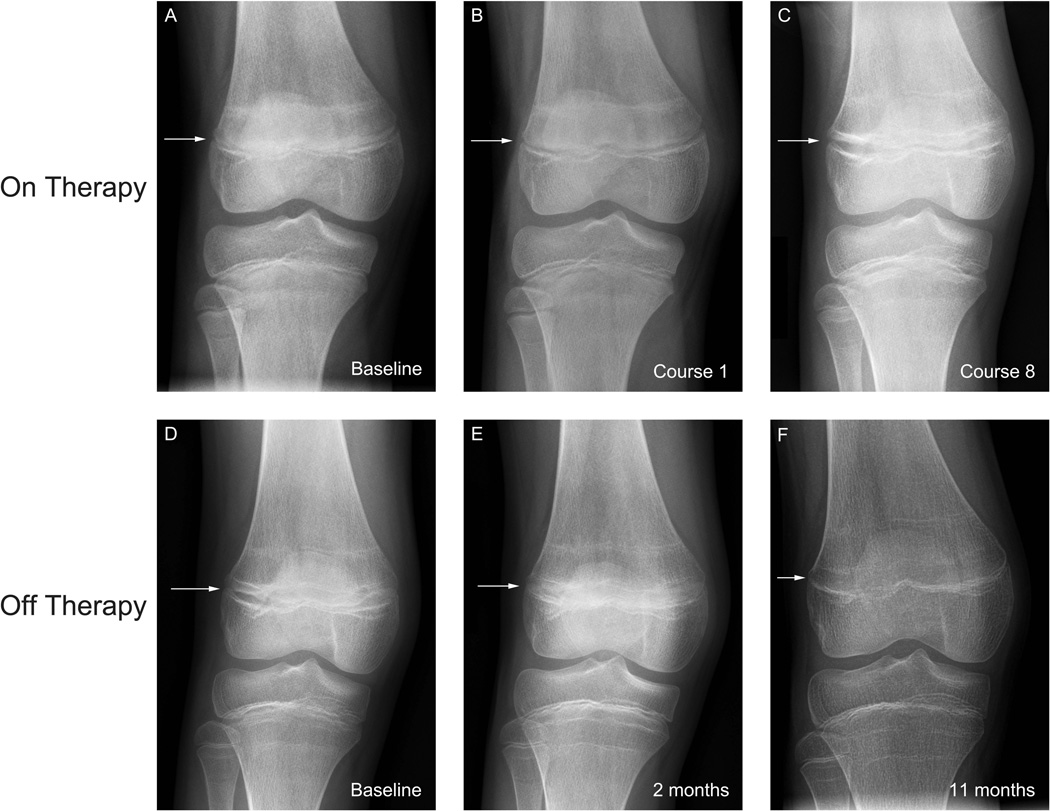
All five patients had growth plate widening that was apparent on plain radiographs of the knee, one of whom had follow-up imaging studies including an MRI. As previously briefly described, this 11 year old female who received 10 cycles of pazopanib therapy was noted to have radiographic evidence of physeal widening that progressed while on therapy [22]. In this report, greater detail (Figures 2–4) of the imaging findings are provided. Sequential imaging of the hands and wrist (Figure 2);the distal femoral and proximal tibial growth plates (Figure 3); as well as an MRI with cartilage sensitive MRI sequences (Figure 4 a, b) are shown in the respective figures. The MRI showed expansion of a high-signal intensity zone within the growth plate, related to expansion of the hypertrophic chrondrocyte layer adjacent to the low-signal intensity zone of provisional calcification. Discrete horizontal bands of hypointensity correspond to the sclerotic transverse metaphyseal bands evident radiographically (Figure 3). When pazopanib therapy was discontinued the growth plate changes rapidly reversed (Fig. 3 d–f), with progressive maturation and near complete fusion of the observed growth plate within the first year later following cessation of anti-angiogenic therapy. Despite the physeal changes, no disruption in longitudinal growth was observed, and this patient continued to meet height milestones, gaining approximately 6 cm while on study (data not shown).
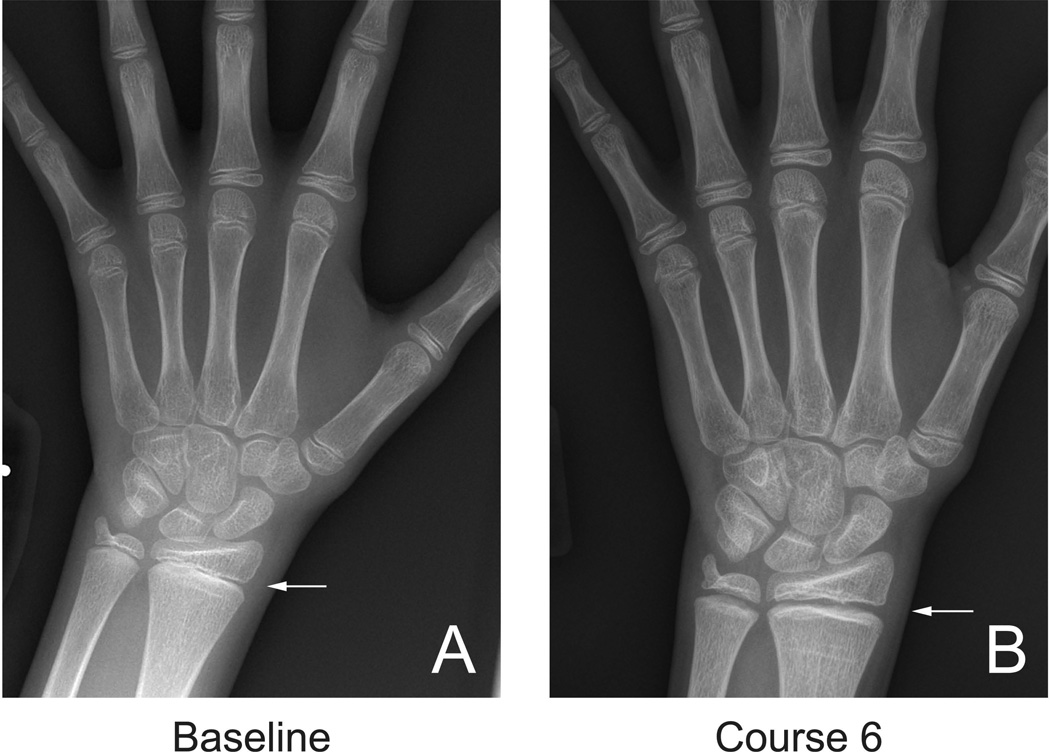
Figure 2.
An 11 yo patient with radiographic changes in the hands and wrist during treatment with pazopanib. Hand/wrist radiographs are not routinely used to monitor growth plate toxicity, however these images obtained at baseline (A) and after 6 cycles of therapy with pazopanib (B) clearly demonstrate that widening of the distal radial physis (arrows) may also occur in response to anti-angiogenic therapy.
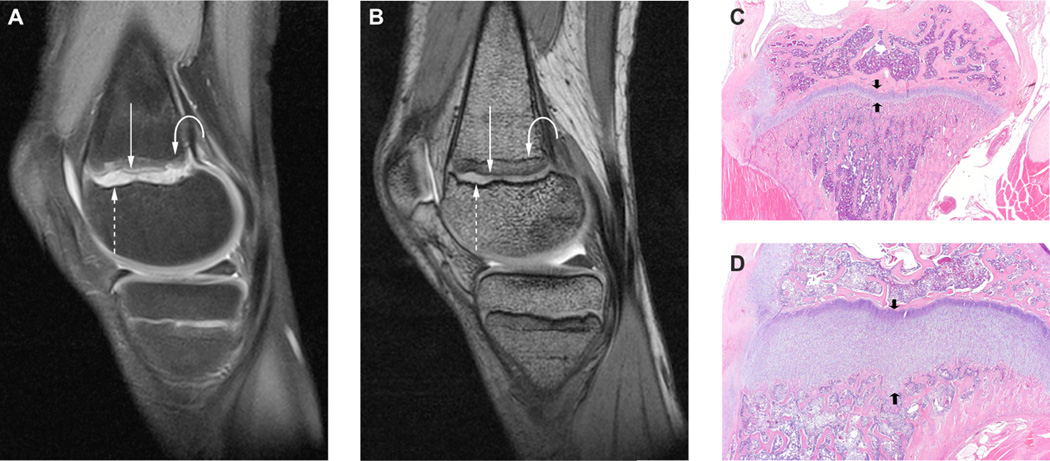
Figure 4.
MRI of 11 yo patient, after receiving 10 courses of pazopanib. (A) Fat-suppressed proton-density (TR 4000; TE 8.0, 2NEX) and (B) 3-D T2/DESS (dual echo steady state, TR 14.3; TE 5.0, NEX 1) MR imaging at 3T demonstrates physeal widening and cartilage hypertrophy corresponding to the radiographic findings (Fig. 3). The high signal intensity zone (dashed arrow) corresponds to the hypertrophic chondrocyte layer adjacent to a low signal rim of epiphyseal cortical bone on the epiphyseal side of the growth plate and the thin hypointense zone of provisional calcification on the metaphyseal side of the physis (solid arrow). Growth recovery lines, reflecting areas of interrupted cartilage-to-bone conversion are seen as discrete horizontal bands of hypointensity (curved arrow) in the juxtaphyseal metaphysis and correspond to the transverse sclerotic lines seen radiographically. (C,D) Preclinical results of H&E stained histologic sections from appendicular bone of control (C) or sunitinib-treated (D) rats (reproduced from Ref. [17], with permission) show hypertrophic chondrocytes in the sunitinib-treated animals with a similar appearance to that shown by the cartilage-sensitive MR imaging (A, B). * Note: Images from this patient were also shown in the primary publication summarizing clinical results of the Phase 1 pazopanib study (ADVL0815) [22]; those images were from either a different study timepoint or MR series. No images have been directly reproduced here.
Figure 3.
An 11 yo patient (also shown in Figs 24; see note * below) with progressive physeal widening during pazopanib therapy (A–C), and reversibility of these changes once therapy was stopped (D–F). A–C: While on therapy the patient received 10 courses of pazopanib, after which therapy was stopped. Note the progressive widening of the distal femoral growth plates (arrows) as therapy continued. D–F: Once therapy stopped, growth plate maturation resumed, the distal femoral physis gradually closed, and had almost completely fused (F) less than one year after stopping anti-angiogenic therapy. Transverse metaphyseal bands of sclerosis reflect growth recovery lines. * Note: Images from this patient were also shown in the primary publication summarizing clinical results of the Phase 1 pazopanib study (ADVL0815) [22]; those images were from either a different study timepoint or MR series. No images have been directly reproduced here.
The majority of the children treated on the phase 1 protocols described in this report developed progessive disease after the first or second cycle of therapy and thus did not have followup growth plate imaging. Because of the relatively small number of patients evaluated, we did not find any correlation between growth plate toxicity and either treatment dose, age, gender, or tumor type.
Discussion
Angiogenesis in general and VEGF in particular are critical for normal embryogenesis and growth. In the developing skeleton, longitudinal bone growth results from the process of endochondral ossification. As the relatively avascular chondrocyte layer along the zone of provisional calcification hypertrophies, osteogenesis begins to occur, coupled with apoptosis of the cartilage layer. This process of bone growth and maturation depends on recruitment of new blood vessels and a variety of angiogenic growth factors and growth factor receptors are expressed along the epiphyseal growth plate [11, 30]. Because of the essential role of angiogenesis in bone growth and preclinical findings in animal models using agents which specifically block VEGF or other associated angiogenic pathways [17, 30], there have been ongoing concerns that targeted anti-angiogenic therapies used to treat pediatric cancer patients would result in toxicity to the developing skeleton. There is only limited data in the clinical literature confirming the preclinical results, with observations of reversible changes to the distal radial growth plate in one patient treated with bevacizumab [20] and variable increases in growth plate volume in 3/13 patients treated with vandetanib [21]. A separate study of neuroblastoma patients undergoing phase 1 therapy with bevacizumab and radiolabeled anti-GD2 antibody found growth plate toxicity [31]. The study presented here extends the current clinical experience using anti-angiogenic agents in skeletally immature patients and demonstrates,growth plate abnormalites in a limited number of patients being treated with a variety of receptor TKI and VEGF-targeted agents.
In our study, 53 patients at potential risk for growth plate toxcity were treated on six different phase 1 clincial trials were evaluated for growth plate disturbance with radiographs of the distal femoral/proximal tibial growth plates. While the majority of the patients had no evidence of growth plate toxicity, five patients had evident physeal abnormalities. One of these five patients also had evident of progressive physeal widening. However, since this patient met height expectations following cessation of the anti-angiogenic therapy and the cartilage MRI sequences resolved, it suggests that physeal changes may be reversible with cessation of therapy. Although it is attractive to speculate that the prolonged course of treatment received by this patient led to the observed growth plate toxicity, the number of patients studied is too small for this observation to be statistically analyzed. Likewise, it is impossible to ascertain whether growth plate changes associated with anti-angiongenic will be fully reversible in all children following cessation of therapy.
Our findings support the earlier preclinical and clinical data suggesting anti-angiogenic agents and therapies targeted to growth factors involved in osteogenesis can be potentially toxic to developing growth plates, and that these changes can be reversible. However, not all patients with open physes who are being treated with anti-angiogenic agents show evidence of growth plate toxicity. Fox et al. did not observe any growth plate changes in a phase 1 study of the VEGF-R inhibitor cediranib [32]. Likewise, in a non-COG phase 1 study of the TKI sorafenib no increases in growth plate volume were observed; although one patient had delayed longitudinal growth, possibly related to concurrent anorexia and 9% weight loss, rather than physeal toxicity per se [33]. Smith et al. have suggested that the patient’s age at the time of drug administration may dictate the degree of skeletal toxicity more than simply the presence of open physes [34]. Despite the small number of patients in whom physeal abnormalities were detected in the six studies described herein, our results do not indicate an association between young age and the effect of anti-angiogenic agents on the developing skeleton.
It is possible that relying on extremity radiographs to monitor patients may have underestimated the extent of growth plate disturbance. However, no agreed upon or validated standard exists for measuring the developing growth plate. Recent experimental work has shown discordance between radiologic and histologic dimensions of the zone of provisional calcification along the developing physis [35]. Others have suggested that MRI may provide a more sensitive and quantitative means of detecting skeletal toxicity in patients undergoing anti-angiogenic therapy, including growth plate abnormalities and osteonecrosis, [36, 37]. The MRI findings presented here showed physeal cartilage expansion and growth plate widening and were comparable to the histologic abnormalities that have been described in preclinical models of antiangiogenic agent toxicity following exposure of animals to VEGF-targeted anti-angiogenics (Figure 4c). These include cartilage hypertrophy, growth plate thickening, and accumulation of hypertrophic chondrocytes adjacent to the zone of provisional calcification [11, 17]. Determining the relative sensitivity and specificity of radiographs versus MRI for assessing physeal abnormalities, as well as the most cost effective strategy, will require future investigation.
Growth plate disturbances similar to those shown here have been described in pediatric athletes sustaining repetitive trauma [13, 29], such as gymnasts (wrist) and little league pitchers (shoulder). The post-traumatic findings of irregular physeal widening and cartilage hypertrophy appear very similar to the physeal abnormalities shown here and are also reversible. Other examples of growth plate toxicity and physeal widening have been reported with infection and following radiation therapy, presumably due to effects on the metaphyseal blood supply. Metabolic disturbances are also well-described and include rickets and hypophosphatasia [29]. The progressive widening of the epiphyseal growth plate that was observed both radiographically and by MRI is consistent with the cumulative effects of anti-angiogenic VEGF-targeted therapy on the peri-physeal neovasculature and developing growth plate. The extent to which disruption of the periphyseal blood supply by anti-angiogenic agents affects the developing physis directly, by impairing blood flow to the fragile new bone forming along the growth plate, or indirectly by affecting the supply of vitamins and minerals and other essential factors necessary for ossification to occur is uncertain.
Anti-angiogenic agents are reasonably well tolerated and have been shown to result in objective and sustained tumor responses in a variety of pediatric cancers [38, 39]. As a result it is likely that maintenance treatment regimens will increasingly include prolonged therapy with anti-angiogenic agents. While our study does not address the potential long-term effects of anti-angiogenic therapy on continued bone growth, skeletal maturation, adult height, and overall bone health, these important considerations should be the focus of future studies as this broad class of anti-tumor agents becomes more common in the routine care of children with cancer.
In summary, this report presents our experience with a large series of pediatric patients receiving anti-angiogenic therapy on a variety of phase I therapeutic trials who were assessed for growth plate abnormalities. Our findings support earlier findings suggesting that a small, but relevant number of children receiving anti-angiogenic therapy may experience growth plate toxicity. Future studies are needed to determine whether there are any factors that may predict which children will develop growth plate toxicity as well as to evaluate the long term impact of growth plate toxicity on overall patient growth and development. The use of MRI to improve both the sensitivity and specificity with which physeal abnormalities are detected should be the focus of future investigation, and could include development of rapid low-cost MRI sequences specific to this purpose.
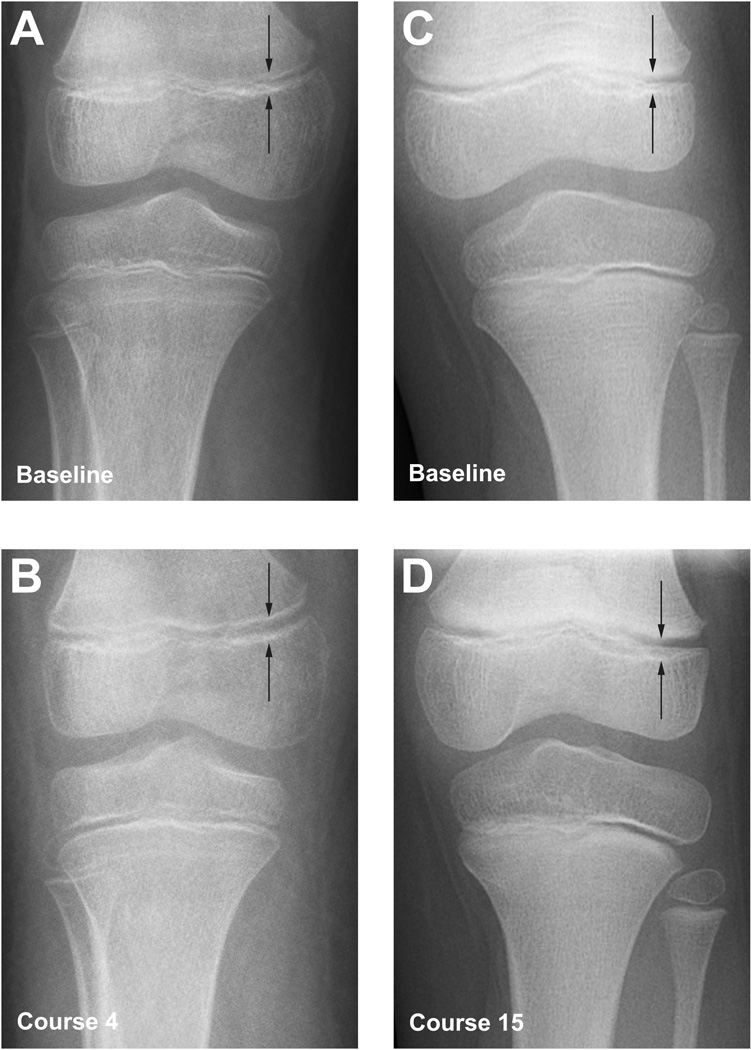
Figure 1.
Radiographic changes to the distal femoral growth plate during anti-angiogenic therapy using multi-targeted tyrosine kinase inhibitors sunitinib and pazopanib directed against VEGF signaling pathways. (A, B) 8 yo patient being treated with pazopanib‥ (C,D) 7 yo patient being treated with sunitinib. Comparing baseline (A,C) to post-treatment course 4 (B) and course 15 (D) radiographs, respectively, both patients show unequivocal growth plate widening (arrows) while on treatment with VEGF-targeted anti-angiogenic agents. Similar findings were seen in three additional patients treated with pazopanib (Figs. 2–4, and data not shown).
Acknowledgments
This was supported by the National Cancer Institute (NCI) grant 5UM1 CA097452-12 (2002-2017) to the COG Phase 1/Pilot Consortium and Cookies for Kids’ Cancer. All authors have contributed to the manuscript in significant ways, have reviewed and agreed upon the manuscript content. The authors wish to thank the following personnel for their contribution: Syed Aamer, Phase 1 Consortium Imaging Center, Children’s Hospital of Los Angeles.
Footnotes
Conflict of Interest Statement Disclosure: “There are no conflicts of interest to declare”.
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 3.Fakhari M, Pullirsch D, Paya K, Abraham D, Hofbauer R, Aharinejad S. Upregulation of vascular endothelial growth factor receptors is associated with advanced neuroblastoma. J Pediatr Surg. 2002;37(4):582–587. doi: 10.1053/jpsu.2002.31614. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs B, Inwards CY, Janknecht R. Vascular endothelial growth factor expression is up-regulated by EWS-ETS oncoproteins and Sp1 and may represent an independent predictor of survival in Ewing's sarcoma. Clinical cancer research. 2004;10(4):1344–1353. doi: 10.1158/1078-0432.ccr-03-0038. [DOI] [PubMed] [Google Scholar]
- 6.Rapisarda A, Melillo G. Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res. 2012;114:237–267. doi: 10.1016/B978-0-12-386503-8.00006-5. [DOI] [PubMed] [Google Scholar]
- 7.Tie J, Desai J. Antiangiogenic therapies targeting the vascular endothelia growth factor signaling system. Crit Rev Oncog. 2012;17(1):51–67. doi: 10.1615/critrevoncog.v17.i1.50. [DOI] [PubMed] [Google Scholar]
- 8.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349(5):427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldner MJ, Neurath MF. Targeting the VEGF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):5–13. doi: 10.1517/14728222.2011.641951. [DOI] [PubMed] [Google Scholar]
- 10.Gerber HP, Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med. 2000;10(5):223–228. doi: 10.1016/s1050-1738(00)00074-8. [DOI] [PubMed] [Google Scholar]
- 11.Hall AP, Westwood FR, Wadsworth PF. Review of the effects of anti-angiogenic compounds on the epiphyseal growth plate. Toxicol Pathol. 2006;34(2):131–147. doi: 10.1080/01926230600611836. [DOI] [PubMed] [Google Scholar]
- 12.Trueta J, Amato VP. The vascular contribution to osteogenesis. III. Changes in the growth cartilage caused by experimentally induced ischaemia. J Bone Joint Surg Br. 1960;42-B:571–587. doi: 10.1302/0301-620X.42B3.571. [DOI] [PubMed] [Google Scholar]
- 13.Laor T, Wall EJ, Vu LP. Physeal widening in the knee due to stress injury in child athletes. AJR Am J Roentgenol. 2006;186(5):1260–1264. doi: 10.2214/AJR.04.1606. [DOI] [PubMed] [Google Scholar]
- 14.Ryan AM, Eppler DB, Hagler KE, Bruner RH, Thomford PJ, Hall RL, Shopp GM, O'Neill CA. Preclinical safety evaluation of rhuMAbVEGF, an antiangiogenic humanized monoclonal antibody. Toxicol Pathol. 1999;27(1):78–86. doi: 10.1177/019262339902700115. [DOI] [PubMed] [Google Scholar]
- 15.Sanofi-Aventis. Investigator's Brochure AVE0005. Tarrytown, NY: 2006. [Google Scholar]
- 16.Bayer. Investigator's Brochure Sorafenib (BAY 43-9006) [Google Scholar]
- 17.Patyna S, Arrigoni C, Terron A, Kim TW, Heward JK, Vonderfecht SL, Denlinger R, Turnquist SE, Evering W. Nonclinical safety evaluation of sunitinib: a potent inhibitor of VEGF, PDGF, KIT, FLT3, and RET receptors. Toxicol Pathol. 2008;36(7):905–916. doi: 10.1177/0192623308326151. [DOI] [PubMed] [Google Scholar]
- 18.GlaxoSmithKline. Document # RR2002/00017/06. Investigator's Brochure: Pazopanib GW786034. [Google Scholar]
- 19.Amgen. Investigator's Brochure AMG 386 (Trebananib) 2011. Section 5.3. [Google Scholar]
- 20.Smith AR, Hennessy JM, Kurth MA, Nelson SC. Reversible skeletal changes after treatment with bevacizumab in a child with cutaneovisceral angiomatosis with thrombocytopenia syndrome. Pediatr Blood Cancer. 2008;51(3):418–420. doi: 10.1002/pbc.21597. [DOI] [PubMed] [Google Scholar]
- 21.Fox E, Widemann BC, Chuk MK, Marcus L, Aikin A, Whitcomb PO, Merino MJ, Lodish M, Dombi E, Steinberg SM, et al. Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res. 2013;19(15):4239–4248. doi: 10.1158/1078-0432.CCR-13-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glade Bender J, Lee A, Reid JM, Baruchel S, Roberts T, Voss SD, Wu B, Ahern C, Ingle AM, Harris P, et al. A Phase I Pharmacokinetic and Pharmacodynamic Study of Pazopanib in Children with Soft Tissue Sarcoma and Other Refractory Solid Tumors: A Children’s Oncology Group Phase I Consortium Report. Journal of clinical oncology. 2013;31(24):3034–3043. doi: 10.1200/JCO.2012.47.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, Kerbel RS, Cooney-Qualter EM, Stempak D, Chen HX, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children's Oncology Group Study. Journal of clinical oncology. 2008;26(3):399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 24.Widemann BC, Kim A, Fox E, Baruchel S, Adamson PC, Ingle AM, Glade Bender J, Burke M, Weigel BJ, Stempak D, et al. A Phase I Trial and Pharmacokinetic Study of the Raf Kinase and Receptor Tyrosine Kinase Inhibitor Sorafenib in Children with Refractory Solid Tumors or Refractory Leukemias. Clinical cancer research. 2012 doi: 10.1158/1078-0432.CCR-11-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DuBois SG, Shusterman S, Reid JM, Ingle AM, Ahern CH, Baruchel S, Glade-Bender J, Ivy P, Adamson PC, Blaney SM. Tolerability and pharmacokinetic profile of a sunitinib powder formulation in pediatric patients with refractory solid tumors: a Children's Oncology Group study. Cancer Chemother Pharmacol. 2012;69(4):1021–1027. doi: 10.1007/s00280-011-1798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois SG, Shusterman S, Ingle AM, Ahern CH, Reid JM, Wu B, Baruchel S, Glade-Bender J, Ivy P, Grier HE, et al. Phase I and pharmacokinetic study of sunitinib in pediatric patients with refractory solid tumors: a children's oncology group study. Clin Cancer Res. 2011;17(15):5113–5122. doi: 10.1158/1078-0432.CCR-11-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender JG, Blaney SM, Borinstein S, Reid JM, Baruchel S, Ahern C, Ingle AM, Yamashiro DJ, Chen A, Weigel B, et al. A Phase I Trial and Pharmacokinetic Study of Aflibercept (VEGF Trap) in Children with Refractory Solid Tumors: A Children's Oncology Group Phase I Consortium Report. Clinical cancer research. 2012;18(18):5081–5089. doi: 10.1158/1078-0432.CCR-12-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leary S, Park JR, Reid JM, Ralya AT, Baruchel S, Wu B, Ingle AM, Ahern CH, Weigel B, Blaney SM. A phase I trial and pharmacokinetic study of trebananib (AMG386) in children with recurrent or refractory solid tumors: A Children’s Oncology Group Phase 1 Consortium report. 2013;31(15_suppl):2538. [Google Scholar]
- 29.Laor T, Jaramillo D. MR imaging insights into skeletal maturation: what is normal? Radiology. 2009;250(1):28–38. doi: 10.1148/radiol.2501071322. [DOI] [PubMed] [Google Scholar]
- 30.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 31.Modak S, Cheung NK, Abramson SJ, Price AP. Lack of early bevacizumab-related skeletal radiographic changes in children with neuroblastoma. Pediatr Blood Cancer. 2009;52(2):304–305. doi: 10.1002/pbc.21776. author reply 306. [DOI] [PubMed] [Google Scholar]
- 32.Fox E, Aplenc R, Bagatell R, Chuk MK, Dombi E, Goodspeed W, Goodwin A, Kromplewski M, Jayaprakash N, Marotti M, et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol. 2010;28(35):5174–5181. doi: 10.1200/JCO.2010.30.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim A, Dombi E, Tepas K, Fox E, Martin S, Wolters P, Balis FM, Jayaprakash N, Turkbey B, Muradyan N, et al. Phase I trial and pharmacokinetic study of sorafenib in children with neurofibromatosis type I and plexiform neurofibromas. Pediatr Blood Cancer. 2013;60(3):396–401. doi: 10.1002/pbc.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith AR, Hennessy JM, Heisel-Kurth MA, Nelson SC. Lack of early bevacizumab-related skeletal radiographic changes in children with neuroblastoma—response. Pediatr Blood Cancer. 2009;52(2):306. doi: 10.1002/pbc.21776. [DOI] [PubMed] [Google Scholar]
- 35.Tsai A, Connolly S, Nedder A, Shapiro F. Visualization and analysis of the deforming piglet femur and hip following experimentally induced avascular necrosis of the femoral head. IEEE transactions on bio-medical engineering. 2013;60(6):1742–1750. doi: 10.1109/TBME.2012.2228860. [DOI] [PubMed] [Google Scholar]
- 36.Kaste SC, Kaufman RA, Gajjar A, Broniscer A. Magnetic resonance imaging is the preferred method to assess treatment-related skeletal changes in children with brain tumors. Pediatr Blood Cancer. 2013;60(9):1552–1556. doi: 10.1002/pbc.24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim A, Dombi E, Solomon J, Fox E, Balis FM, Widemann BC. Automated volumetric growth plate measurement using magnetic resonance imaging for monitoring skeletal toxicity in children treated on investigational drug trials. Clin Cancer Res. 2011;17(18):5982–5990. doi: 10.1158/1078-0432.CCR-10-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glade Bender J, Yamashiro DJ, Fox E. Clinical development of VEGF signaling pathway inhibitors in childhood solid tumors. The oncologist. 2011;16(11):1614–1625. doi: 10.1634/theoncologist.2011-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robison NJ, Campigotto F, Chi SN, Manley PE, Turner CD, Zimmerman MA, Chordas CA, Werger AM, Allen JC, Goldman S, et al. A phase II trial of a multi-agent oral antiangiogenic (metronomic) regimen in children with recurrent or progressive cancer. Pediatr Blood Cancer. 2014;61(4):636–642. doi: 10.1002/pbc.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]