Abstract
Conditions such as congenital anomalies, cancers, and trauma can all result in devastating deficits of bone in the craniofacial skeleton. This can lead to significant alteration in function and appearance that may have significant implications for patients. In addition, large bone defects in this area can pose serious clinical dilemmas, which prove difficult to remedy, even with current gold standard surgical treatments. The craniofacial skeleton is complex and serves important functional demands. The necessity to develop new approaches for craniofacial reconstruction arises from the fact that traditional therapeutic modalities, such as autologous bone grafting, present myriad limitations and carry with them the potential for significant complications. While the optimal bone construct for tissue regeneration remains to be elucidated, much progress has been made in the past decade. Advances in tissue engineering have led to innovative scaffold design, complemented by progress in the understanding of stem cell–based therapy and growth factor enhancement of the healing cascade. This review focuses on the role of biomaterials for craniofacial bone engineering, highlighting key advances in scaffold design and development.
Keywords: scaffolds, bioengineering, biocompatibility, biomimetism, skeleton, regeneration
Introduction
The repair of complex craniofacial bone defects is challenging, and a successful result depends on the defect size, the quality of the soft tissue covering the defect, and the choice of reconstructive method. The complex craniofacial skeleton is involved with various specific functions, such as protection of the brain and optic tracts, breathing, mastication, speech, and hearing. In addition to these functional requirements, the craniofacial unit is important for social acceptance and self-esteem (Adams, 1981). Hostile surgical environments (e.g., due to irradiation and infection) can further challenge craniofacial reconstruction.
Traditional surgical techniques for reconstruction have implemented autogenous, allogeneic, and prosthetic materials to achieve bone reconstruction. Autologous bone—the best option—is in limited supply, and available bone may neither be the type nor the complex shape required. Autologous bone grafting also mandates an additional surgical procedure, with associated donor site morbidity and risk of significant resorption (Neovius and Engstrand, 2010). Furthermore, traditional techniques struggle to fully replicate normal form and function.
Biomaterials are defined as natural or synthetic materials used to replace part of a living system or function in intimate contact with living tissue. Biomaterials serve as matrices for tissue formation, and surface properties promoting cell adhesion, proliferation, and differentiation, as well as desirable mechanical strength and osteoconductivity, are all essential (Eldesoqi et al., 2013).
Tissue engineering approaches to regeneration utilize 3-dimensional (3D) biomaterial matrices that interact favorably with cells. The potential benefits of using a tissue engineering approach include reduced donor site morbidity, shortened operative time, decreased technical difficulty of the repair, and, most important, ability to closely mimic the in vivo microenvironment in an attempt to recapitulate normal craniofacial development (Ward et al., 2010). Recent advances in computer-aided design and computer-aided manufacturing have begun to revolutionize craniofacial surgery, which is frequently confronted with the reconstruction of challenging 3D anatomic structures. Rapid prototyping technology, with widespread availability of high-resolution medical imaging, has allowed for the generation of contoured 3D prostheses for craniofacial reconstruction.
The purpose of this review is to highlight the evolving role of biomaterial incorporation into craniofacial bone reconstruction. Discussion focuses on biomaterial scaffold design and development, highlighting innovation and recent advances in fabrication. While human clinical applications are limited to date, great promise exists as our understanding of this dynamic area develops abreast of technical advances.
Role of Scaffolds
Scaffolds are mechanical constructs that can act as carriers for cells and growth factors (GFs). Ideally, the role of a biomaterial in reconstructive surgery is not to simply replace the missing section of bone but to also provide an osteoconductive environment by acting as a scaffold for bone regrowth (Hyun et al., 2013b). The main function of a scaffold is to simulate the extracellular matrix, which, as an active biological tissue, affects cellular adhesion, migration, proliferation, and differentiation (Petrovic et al., 2012). Mechanical properties of cortical and cancellous bone differ greatly; thus, the task of designing the aforementioned ideal bone scaffold is fraught with difficulty. Scaffolds must meet some essential biomechanical criteria, such as biocompatibility, adequate mechanical strength, bioresorbability, and sufficient porosity and transport properties (Bose et al., 2012; Petrovic et al., 2012; Table).
Table.
The Elusive “Ideal” Scaffold
| Biocompatibility |
| The manner in which a mutually acceptable coexistence of biomaterials and tissues is developed and sustained has been the focus of attention in biomaterials science for many years and forms the foundation of the subject of biocompatibility (Williams, 2008). Biocompatibility of a scaffold is defined as its ability to support normal cellular activity, including molecular signaling systems, without any local and systemic toxic effects to the host tissue (Williams, 2008; Bose et al., 2012). Properties of osteoinduction, osteoconduction, and vascular invasion are of great importance in biomaterial engineering. Osteoinduction is the ability to induce new bone formation through biomolecular signaling and recruitment of progenitor cells. Osteoconduction, however, occurs when the scaffold allows bone cells to adhere, proliferate, and form extracellular matrix on its pores and surface. Lastly, scaffolds need to encourage new blood vessel formation following placement to actively support the cells by providing nutrients, waste transport, and oxygen (Bose et al., 2012). |
| Bioresorbability |
| Once implanted, an ideal scaffold should be able to degrade with time at a controlled and well-defined rate, allowing space for ingrowth of new bone. The degradation pattern of a scaffold should occur at an appropriate pace specific to the host tissue; for example, degradation occurring within 3 to 6 mo would be acceptable in the craniofacial skeleton, where there is lower mechanical demand, but inappropriate following spinal fusion (Bose et al., 2012). The ability of the scaffold to fully resorb and remodel completely is especially important for pediatric craniofacial applications, where the skull must be able to develop and mature during normal growth of the immature pediatric skeleton (Patel and Fisher, 2008; Ricci et al., 2012). |
| Porosity |
| Interconnected porosity is another key factor in scaffold design. To facilitate diffusion of essential nutrients and oxygen for cell survival, pores should be interconnected and at least 100 µm in diameter (Bose et al., 2012). O’Brien and colleagues investigated the effect of mean pore size on osteoblast adhesion and early proliferation in collagen-glycosaminoglycan scaffolds and reported that pore sizes in the range of 200 to 350 µm were optimum for bone tissue ingrowth (Murphy et al., 2010). Interestingly, multiscale porosity—for example, both micropores (<8 µm) and macropores (250-350 µm) present in one construct—was shown to be beneficial to osteoconduction and mechanical strength in a study of bone morphogenetic protein microsphere incorporation in hydroxyapatite scaffolds (Woodard et al., 2007). |
| It is well understood that porosity reduces compressive strength of the scaffold and results in increased difficulty in reproducible scaffold fabrication (Bose et al., 2012). However, recent advances in 3-dimensional printing techniques, such as robocasting, have enabled scaffold manufacture with highly controlled pore architecture and morphology (Ricci et al., 2012). A study from the National Institute of Dental and Craniofacial Research demonstrated that the combination of titanium dioxide with a hydroxyapatite-gelatin macroporous scaffold had comparable strength relative to natural calvarial bone in a study of rat critical-sized calvarial defects (Ferreira et al., 2013). |
| Mechanical strength |
| As a mechanical support, scaffolds must possess adequate mechanical stability to withstand the implantation procedure and the mechanical forces that are typically experienced at the scaffold-tissue interface, as well as resist collapse during a patient’s normal activities. The scaffold strength should ideally match that of the host bone. Note that dense bioceramic scaffold has a mechanical strength profile similar to that of cortical bone, while polymers are, in the majority, similar to cancellous bone. Ceramic-polymer scaffolds are typically weaker than cancellous bone but can confer advantages of increased biodegradability and flexibility, ameliorating the brittleness associated with ceramic scaffolds (Wahl and Czernuszka, 2006; Bose et al., 2012; Zhang et al., 2014). |
Types of Scaffolds
Biomaterials utilized in scaffolds can be divided into natural and synthetic polymers, bioactive ceramics and glass, hydrogels, and metals. Composite scaffolds are those made up of 2 or more materials—for example, a scaffold composed of both ceramic and polymer.
Natural and Synthetic Polymers
Biodegradable polymers are widely used for scaffold development due to controllable degradation, biocompatibility, and ease of processing (Yusop et al., 2012). These scaffolds are degraded by hydrolysis and gradually resorbed, allowing the supported tissue to gradually recover functionality. Biodegradable polymers can be divided into 2 categories: natural—including polysaccharides (e.g., chitosan) and proteins (e.g., collagen)—and synthetic, such as poly(lactic acid).
Synthetic biodegradable polymers can be produced under controlled conditions and therefore exhibit reproducible mechanical and physical properties. The use of synthetic biodegradable polymers in craniofacial surgery, especially regarding pediatric intervention, is well described (Ahmad et al., 2008). Biodegradable polymers confer advantages in pediatric applications, as they do not promote neighboring bone resorption; they may also be clinically beneficial during subsequent radiography, as, once resorbed, they do not obscure subsequent computed tomography scans (Ahmad et al., 2008).
Bioactive Ceramics and Glass
Calcium phosphate bioceramics—including hydroxyapatite (HA), β-tricalcium phosphate, and biphasic calcium phosphate—are promising candidates in bone engineering, as these chemicals reflect the chemistry and structure of the native mineral components of bone extracellular matrix, 85% of which is composed of calcium phosphate. Importantly, HA has been shown to have a slower degradation profile than that of biphasic calcium phosphate, whereas β-tricalcium phosphate degrades too quickly when placed in vivo (Petrovic et al., 2012).
Porous calcium phosphate bioceramics are not without fault, however, as they are brittle and difficult to process and, in general, have a slow degradation rate. Thus, Liu et al. (1998) studied the effect of combining the osteoconductivity of HA with the biodegradability of polymer and found significant improvements in composite mechanical properties. By introducing chemical linkage between HA and polymer matrix, they were able to significantly enhance mechanical properties compared to other composite types. Indeed, the incorporation of HA nanoparticles into polymer scaffolds has been shown to increase protein adsorption, cell attachment and migration, and osteogenesis (Kim et al., 2007). Building on this, Engstrand et al. (2014) described an eloquent computer-aided design method of using a mosaic of calcium phosphate bioceramic tiles supported by titanium wires in the repair of complex cranial defects.
Recent results detailing the effect of trace impurity elements (e.g., SiO2, ZnO, Fe) added to calcium phosphate bioceramics indicate an enhanced ability to control dissolution rates as well as increase density, mechanical strength, and biocompatibility of bioceramics (Petrovic et al., 2012). Both Zn and Si doping can promote type 1 collagen gene expression and extracellular signal-regulated kinase secretion that positively regulates angiogenesis, osteoblast differentiation, and morphogenesis (Ricci et al., 2012; Shie et al., 2012).
Glass-based scaffolds can be classified into 2 groups: glass/glass-ceramic porous scaffolds and glass-polymer porous composites (Mantripragada et al., 2013). Silicon found in glass has been shown to enhance angiogenesis and gene expression in osteoblasts that regulate osteogenesis and GF production. Silicon-substituted HA has greater bone ingrowth compared to HA alone. However, a disadvantage of bioglass is low fracture toughness and strength. Therefore, limitations exist in load-bearing regions.
Hydrogels
Hydrogels—formed by the cross-linking of hydrophilic polymers with a bridging agent, called a cross-linker—are capable of absorbing a large amount of water. The amount of water absorbed, though, depends on the type and concentration of the specific cross-linker used (Lee and Shin, 2007). Hydrogels not only serve as matrices for tissue engineering but are also capable of mimicking extracellular matrix topography and delivering bioactive agents that promote tissue regeneration (Lee and Shin, 2007). For example, Cao et al. (2014) developed a composite polymerizable hydrogel incorporating recombinant human bone morphogenetic protein (BMP) and chitosan nanoparticles as a bone substitute.
Metals
Metals currently in use clinically include gold, stainless steel, cobalt-chromium, and titanium. Metals are inert alloplasts: they neither integrate with adjacent tissues nor induce new bone formation, which is ultimately needed for long-term success. However, metals that degrade in the physiologic environment have been proposed as promising candidates for scaffold engineering. These biodegradable metals may have superior mechanical properties in comparison to biodegradable polymers (Yusop et al., 2012). In particular, magnesium alloys possess mechanical properties that are very similar to those of bone yet retain the ability to naturally degrade when placed within an aqueous type of environment (Staiger et al., 2006). Efforts to design polymer-magnesium composites are ongoing. Theoretically, the polymer matrix benefits from magnesium incorporation, as magnesium may confer higher mechanical strength and fracture toughness while the polymer may prevent premature degradation (Mantripragada et al., 2013). Furthermore, 3D fabrication techniques, as discussed below, can be applied to metals in the quest for a porous scaffold with significant strength for use in load-demanding regions of the craniofacial skeleton. Metallic nanoparticles have been incorporated into scaffolds with evidence of increased mechanical strength, increased cellular adhesion of osteoblasts and chondrocytes, and increased long-term osteoblast function, with notable improvements in collagen synthesis, alkaline phosphatase activity, and calcium deposition (Kim and Fisher, 2007; Tran and Webster, 2011).
Composite Scaffolds
Composite materials are composed of 2 or more biomaterials, in the form of copolymers, polymer-polymer blends, or polymer-ceramic composites (Hyun et al., 2013a; Figure 1). Copolymers are defined as being derived from 2 or more monomeric species. By developing a copolymer system, one can potentially offer the best qualities of each material—balancing glass transition temperature (temperature region where the polymer transitions from a hard, glassy material to a soft, rubbery material) and degradation potential (Amini et al., 2012). Furthermore, composite biomaterials can capitalize on the advantages of each component when properly balanced; for example, integration with poly(lactic-co-glycolic acid) can ameliorate brittleness associated with bioceramic materials alone (Zhang et al., 2014).
Figure 1.
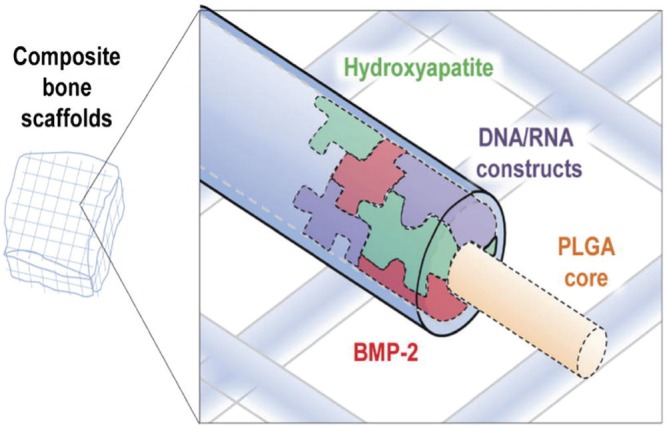
Diagram of a highly osteogenic niche in a composite poly(lactic-co-glycolic acid)–hydroxyapatite scaffold. The potential exists to enhance bone differentiation by incorporation of gene therapy, DNA/RNA construct, and growth factors. Reproduced with permission (Hyun et al., 2013a).
A Successful Biomaterial: The Importance of Angiogenesis
Despite the variety of available scaffolds for bone reconstruction, bone-healing capacity is still dependent on 2 key attributes: the ability to recruit progenitor cells to the injury site and the presence of healthy vasculature near the injury site—both of which are attenuated in the face of traumatic injury and critical-sized bone defects (He et al., 2013). Thus, the development of an efficient neovascularization method to sustain engineered implants is clinically important. This process requires cross talk between bone and the vasculature. As demonstrated by Kusumbe et al. (2014), osteoprogenitor cells and osteoblasts preferentially associate themselves with a subset of endothelial cells, which are a rich source of several GFs relevant for the survival and proliferation of osteoprogenitors. Furthermore, Kaigler et al. (2010) demonstrated that the addition of bone repair cells (BMSCs enriched for mesenchymal and endothelial phenotypes) resulted in the formation of highly vascularized bone at 6 wk after transplantation into a mandibular defect. In addition, scaffold microarchitecture plays a significant role in vascularization. A pore size of 150 to 500 µm is recommended for scaffolds to support vascularization and blood vessel invasion (Muschler et al., 2004). GF- and cell-based therapy can be further exploited to enhance vascularization, as detailed below.
Fabrication of Scaffolds
Recent Advances in Complex 3D Scaffold Development
Craniofacial scaffolds must fit complex 3D anatomic defects, be porous enough to effectively deliver bioactive agents (e.g., recombinant proteins), and be dense and strong enough for a long-enough period to bear forces until the regenerate can assume this responsibility. An engineering process that can generate scaffolds fulfilling these 3 requirements must therefore be able to rigorously control both scaffold exterior shape and interior porous architecture (Kim, Shin, et al., 2012). Thus, a fabrication approach should include design techniques that can utilize patient imaging data to create complex 3D shapes and, at the same time, optimize porous scaffold architecture to provide the right balance between load-bearing strength and delivery of biomolecules (Hollister et al., 2005). Cutting et al. (1986) first described the use of 3D computed tomography images in virtual surgical planning, and these principles have since been expanded to facilitate development of custom 3D scaffolds for craniofacial reconstruction.
Computer-aided design and computer-aided manufacturing can manipulate 3D computed tomography images of bone via a virtual reality force feedback device. Rapid prototyping is an engineering innovation that has been applied to regenerative medicine to build models that provide visual and tactile information. Collectively, these technologies allow generation of scaffolds that are custom-made to fit unique bone defects (Figure 2). As craniofacial bone is irregular with a subtle 3D structure, consideration of an individualized bone defect is very important. Computer-aided design, computer-aided manufacturing, laser scanning, and rapid prototyping technologies have therefore been applied with increasing frequency in craniomaxillofacial surgery (Xu et al., 2010).
Figure 2.
Anatomically shaped scaffolds. Left: Isolated 3-dimensional geometries of the maxilla (top) and mandible (bottom). Right: 3-dimensionally printed porous polycaprolactone scaffolds. Reproduced with permission (Temple et al., 2014).
Controlled 3D structures can be fabricated through additive manufacturing techniques, also known as 3D printing. Additive manufacturing techniques can be broadly classified as follows: extrusion (deformation + solidification), polymerization, laser-assisted sintering, and direct writing-based processes. For example, with extrusion 3D printing, a biomaterial (e.g., polycaprolactone) can be melted and extruded in a computer-controlled pattern to construct scaffolds, laying down layer on top of layer to create patient-specific customized scaffolds (Hollister, 2005). In addition, 3D printing can be used to regulate the internal architecture of the scaffold and its gross geometry. Three-dimensional models of desired bone can be extracted from patient computed tomography scans, providing a blueprint for a personalized scaffold that interfaces with the defect site and re-creates the complex anatomical features (Hollister, 2005; Figure 3).
Figure 3.
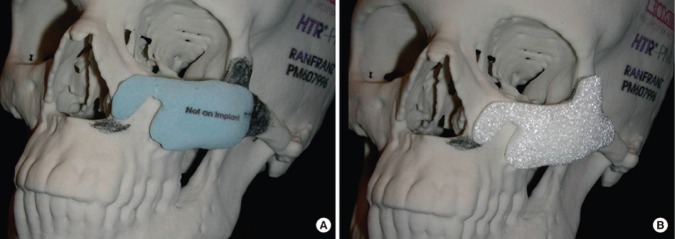
Virtual surgical planning: implant and skull model fabricated through rapid prototyping technology. (A) The plaster piece in light blue is the initial design for the surgeon to review. The surgeon’s modification is marked on the skull model with a pencil. (B) The final design of the custom implant, which was fabricated with porous PMMA material (Biomed Microfixation). Reproduced with permission (Zhao et al., 2012).
Inkjet-based 3D printing has been employed to fabricate calcium phosphate scaffolds. This technique, however, frequently requires high temperatures that preclude the incorporation of bioactive molecules and drugs during the 3D printing process that could stimulate bone regeneration or combat infection. Thus, efforts are underway to produce ceramic-polymer composites utilizing low-temperature 3D printing, where calcium phosphate powder is bound by aqueous binder solutions delivered from the inkjets through a dissolution-precipitation reaction (Butscher et al., 2012).
Electrospinning: Emulating the Native Extracellular Matrix
Electrospinning is an innovative technique for obtaining nanofibers from polymeric solutions (Petrovic et al., 2012; Li et al., 2014). The electrospinning process creates polymer nanofibers by applying a high voltage to a syringe filled with a polymer solution (Figure 4). Electrospun porous, nanofibrous scaffolds have supported various stem cells and differentiated cells to regenerate many hard and soft tissues (Li et al., 2014). Nanofibrous scaffolds provide a more favorable environment for cellular ingrowth and subsequent bone regeneration, as they have architectural, functional, and morphologic similarities to collagen fibrils. This is due to their nanometer-order diameter, high porosity, and high surface-to-volume ratio (Kim and Fisher, 2007). Nanofibrous scaffolds also induce a more favorable environment for bone tissue formation due to enhanced cellular attachment and proliferation (Bhattarai et al., 2004).
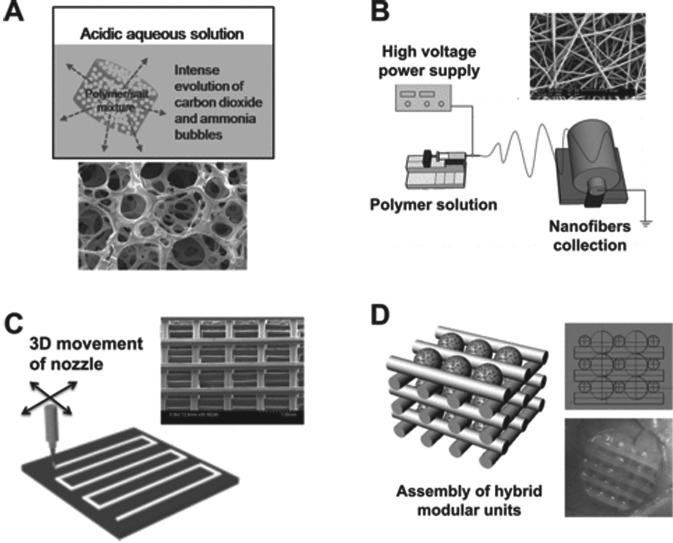
Figure 4.
Methods of scaffold fabrication: examples of different technological approaches used to produce polymeric scaffolds for tissue engineering. (A) Highly porous scaffolds fabricated by gas foaming and salt leaching. (B) Electrospinning is an ultrathin fiber-forming technique that uses an electrostatic force. The applied voltage creates an electric field, which causes a jet stream of polymer solution by creating a force greater than the surface tension of the solution. The jet then bends and elongates due to electrical instability, causing a spiraling motion and smaller-diameter stream. The solvent then evaporates, leaving only a charged polymer nanofiber. The nanofiber is attracted to a grounded collector, where it solidifies into a nonwoven mat. The collector can also be rotated to produce a desired fiber orientation (Kim, Shin, et al., 2012a). (C) Rapid prototyping allows production of 3-dimensional porous scaffolds with defined and regular pore structures. (D) A tissue construct is prepared by modular assembly via multicellular spheroids with the aid of polymeric templates. Reproduced with permission (Kim et al., 2012a).
Biomimetic Scaffolds: Innovative Geometric Cues for Bone Formation
Biomimetism is the creative initiation of specific biological systems gaining inspiration from nature (Ripamonti et al., 2011). Recently, biomimetism has been recognized for its importance in scaffold design simply because an optimal approach to achieve tissue regeneration should be one that follows the natural tissue regenerative process. One must reflect on the shape of the basic multicellular unit of corticocancellous bone when designing a construct for bone tissue regeneration (Ripamonti et al., 2011). The basic multicellular unit is a long cylindrical structure that burrows through the bone in a direction generally aligned with the long axis of the bone, with a leading cutting cone of osteoclasts and a closing cone lined by osteoblasts filling the cavity. An exciting and novel strategy to initiate the induction of bone formation is to create “smart” self-inducing geometric cues within biomimetic scaffolds. Ripamonti et al. (2011) hypothesized that the induction of bone formation can be initiated without the exogenous application of osteogenic soluble molecular signals, by assembling repetitive concavities mimicking the basic multicellular unit within biomimetic constructs. Ultimately, induction of bone formation thus occurs without the exogenous application of soluble osteogenic-promoting molecular signals (Wu et al., 2009; Ripamonti et al., 2011; Appendix Figure).
Enhancing Regenerative Capacity of Biomaterials: Growth Factors
While biomaterials are the focus of this review, it is prudent to briefly emphasize the importance of GF incorporation into biomaterials. Several GFs are known to enhance bone regeneration, including transforming GF beta family members, fibroblast GFs, vascular endothelial GF, and platelet-derived GF (Cillo et al., 2000). BMP-2 and BMP-7 have been described in clinical use for induction of bone formation in alveolar ridge and sinus augmentation and the repair of critical-sized defects of craniofacial bones (Dickinson et al., 2008; Yuan et al., 2012; Hong et al., 2013). Two main collagen-based products containing recombinant BMP have been approved by the Food and Drug Administration for regenerative applications: Infuse Bone Graft (Medtronik and Wyeth, Watford, UK) and Osigraft (Stryker Biotech, Ontario, Canada), containing rhBMP-2 and rh-BMP-7, respectively.
GFs may be incorporated into constructs in a number of ways: by simply soaking the scaffold in a solution of GF for fast release, by incorporation and encapsulation into scaffolds, and by covalent immobilization for controlled and extended release. Conversely, GFs may be incorporated into seeded cells via molecular and genetic modification (Amini et al., 2012). The release profile for GFs added to scaffolds, however, is often not in tune with the process of healing and cellular demands, as the GF is most frequently released by passive diffusion or coupled to the rate of biomaterial degradation (Amini et al., 2012). GFs covalently linked to scaffolds may be released according to cellular demands, in a closely controlled method (Zisch et al., 2003). Importantly, controlled release of VEGF from scaffold leads to a more organized vasculature in comparison to the vasculature that arises from uncontrolled VEGF release (Ehrbar et al., 2004). Furthermore, addition of multiple GFs, which complement one other temporally and spatially, should be considered (Jain, 2005; Rouwkema et al., 2008).
Enhancing Regenerative Capacity of Biomaterials: Stem and Progenitor Cells
Over the past decade, craniofacial bone tissue engineering modalities that couple stem cell–based therapy with biomaterials have been extensively studied, and this strategy has become one of the most promising methods in both animal and human studies (Kørbling and Estrov, 2003; Figure 5). For this purpose, multiple progenitor cells have been studied for craniofacial bone tissue engineering.
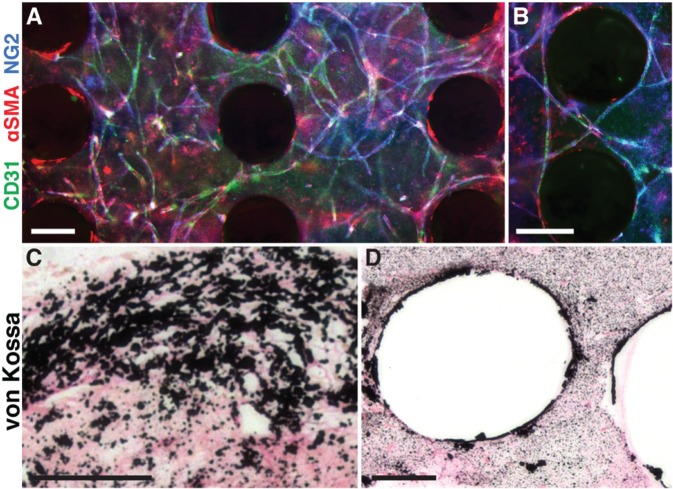
Figure 5.
In vitro vascularization and mineralization of a polycaprolactone (PCL) scaffold. Adipose-derived stem cell aggregates were seeded with fibrin gel into PCL scaffolds and cultured in vascular or osteogenic medium for 14 d. (A) In vascular conditions, extensive vascular networks form within the pore spaces of the scaffold. (B) Vessels wrap closely around PCL fibers. Colors: CD31 (green, endothelial), aSMA (red, perivascular), NG2 (blue, perivascular). (C, D) In osteogenic conditions, mineral (black) is deposited throughout the pore spaces and along PCL fibers. Scale bars: 5,250 lm (A, B), 100 lm (C, D). Reproduced with permission (Temple et al., 2014).
Mesenchymal stem cells (MSCs) are increasingly applied in craniofacial tissue engineering (Ueda et al., 2005), and the concept of critical-sized defect reconstruction using MSCs harvested from bone marrow has been validated in numerous animal models (Levi et al., 2010; Chung et al., 2013). Several limitations have made bone marrow–derived MSCs less attractive for craniofacial implementation—including donor site morbidity, the low frequency of MSCs within the bone marrow fraction, and concerns related to donor age–associated changes in cellular biology. Adipose-derived stem cells are more accessible and represent a readily available, expandable building block for osseous regeneration (Kim, Ji, et al., 2012; Carvalho et al., 2014; Chung et al., 2013). Recently, our laboratory showed that it is possible to isolate a subpopulation of adipose-derived stem cells with enhanced osteogenic potential (Chung et al., 2013). In addition, gene delivery into adipose-derived stem cells has shown excellent results in enhancing their osteogenic differentiation (Liao et al., 2014).
Endothelial progenitor cells (EPCs), first characterized by Asahara et al. (1997), have been shown to initiate and facilitate neovascularization and collateral vessel growth in ischemic tissues (Eldesoqi et al., 2013; He et al., 2013). The combination of MSCs and EPCs in an injectable porous nano–calcium sulfate/alginate scaffold was superior to MSCs alone when blood vessel density was examined in rat critical-sized calvarial defects (He et al., 2013). More recently, Seebach et al. (2012) demonstrated that early vascularization is improved in murine critical-sized bone defects by transplanted EPCs loaded onto β-tricalcium phosphate scaffolds, which not only formed new vessels directly but also acted indirectly to promote vascularization in the bone defect by release of chemotactic factors (e.g., VEGF) to recruit host EPCs.
As multiple types of stem cells have been recognized in the craniofacial region, it has been hypothesized that induced pluripotent stem cells may be the optimal building block for reconstruction of complex defects (Takahashi et al., 2007; Jin et al., 2013; Fu et al., 2014; Tang et al., 2014). Our laboratory group evaluated the ability of an osteogenic microniche consisting of an HA-coated, BMP-2-releasing poly-L-lactic acid scaffold placed into a skeletal defect to guide in vivo differentiation of induced-pluripotent stem cells. In this setting, we found de novo bone formation and participation by implanted cells in skeletal regeneration, suggesting that local cues from the implanted scaffold/cell microniche and surrounding macroniche may act synergistically to promote cellular survival and in vivo acquisition of a terminal cell fate, thereby further suggesting a role for functional engraftment of induced pluripotent stem cells into regenerating tissue (Levi et al., 2012).
Critical Clinical Challenges of Craniofacial Bone Reconstruction
Numerous conditions can compromise the function and architecture of the craniofacial skeleton: trauma, cancer, congenital malformations, and progressive skeletal disease. When shifting rehabilitation strategies from prosthetic to regenerative, one must deal with the uniqueness of the craniofacial structures in their development and function. Indeed, craniofacial bone reconstruction is frequently complicated by hostile surgical environments, secondary to scarring, previous irradiation, osteomyelitis, and osteonecrosis. When one is contemplating surgery involving the oral cavity, one easily understands these complications when considering the impact of immunosuppressive therapy and the universal presence of myriad microorganisms that together provide a perfect environment for chronic infections such as osteomyelitis. As detailed throughout this review, there is a plethora of animal studies examining biomaterial use in calvarial bone regeneration; however, the majority of these studies fail to address the complexity associated with craniofacial reconstruction in the setting of infection, irradiation, or osteonecrosis. Animal models simulating osteonecrosis of the jaw and infected/irradiated calvarial defects have been recently reported; thus, we hope that these advances will serve as an impetus for further exploration of bone regeneration in this complex clinical setting (Kinsella et al., 2012a; Kinsella et al., 2012b; Kuroshima and Yamashita, 2013).
As radiation remains a common postoperative treatment for head and neck cancers, it is critical to determine whether new bone regenerative approaches are effective for healing craniofacial bone defects challenged by therapeutic radiation. Krebsbach explored the effect of BMP-7 gene therapy for skeletal regeneration in cranial defects compromised by postoperative radiotherapy and reported that BMP-7 ex vivo gene therapy was capable of successfully regenerating bone in rat calvarial defects even after therapeutic radiation (Nussenbaum et al., 2003). BMP-2 therapy delivered via an absorbable collagen sponge has also been shown to be effective in reconstructing calvarial defects in the unfavorable irradiated calvarial wound (Yuhasz et al., 2014) and the challenging infected unfavorable wound (Yuhasz et al., 2014) but not in a calvarial wound complicated by dural compromise (Yuhasz et al., 2014). Further studies are required to identify additional biomaterials, cell-, and GF therapy that can be translated to the clinic to address these challenging clinical dilemmas.
When planning craniofacial reconstruction, surgeons must also address the regional environmental differences found in the craniofacial skeleton. As highlighted above, the many studies investigating bone regeneration in the noninfectious, clean environment of the critical-sized calvarial defect do not address the full complement of challenges encountered. The oral cavity is a particularly challenging environment that is colonized by an impressive array of microorganisms, many of which can colonize the implants often used in regenerative procedures, posing significant problems for the patient and the clinician. Periodontal bone regeneration, while complex and beyond the scope of this review, requires the combination of biomaterial scaffolds, and/or GF- and/or cell-based therapy to achieve successful regeneration of tooth-supporting structures, including cementum, periodontal ligament, and alveolar bone. Even though modified microsurgical approaches are shown to improve the outcome of soft and hard tissue in periodontal bone regeneration, the clinical success for periodontal regeneration remains limited in many cases (Ramseier et al., 2012).
Conclusion
Regeneration of tissue defects in the craniofacial skeleton requires a sound understanding of complex developmental processes, molecular pathways, physiology, and remodeling characteristics. Although it is difficult to mimic nature, recent scientific and technological findings show great potential to achieve bone scaffolds that encourage local and systemic biological functions, drawing us closer to the goal of repairing or reshaping bone to predetermined specifications, with precision not previously considered possible. Proper selection of scaffold material, geometry, porosity, and ability to release biomolecules at a desired rate will play critical roles in the future development of bone scaffolds. In the past 10 yr, craniofacial bone tissue engineering modalities that couple GF- or stem cell–based therapy with biomaterials have been extensively studied and have become one of the most promising methods in both animal and human studies. In the ongoing quest to improve bone tissue regeneration, symbiosis between surgeons and bioengineers is of the upmost importance. Much progress has already been accomplished by interdisciplinary collaboration in the past decade, and great promise lies just around the corner.
Acknowledgments
We acknowledge the kind assistance of Dr. Michael S. Hu, MD, for his help in planning and proofreading the manuscript.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
We acknowledge the following ongoing support for this work: grants R01DE02183, R21DE02423001, R01DE019434, and U01HL099776 from the National Institutes of Health (to M.T.L.); the Oak Foundation, the Hagey Laboratory for Pediatric Regenerative Medicine, the ACS Franklin Martin Faculty Research Fellowship (to D.C.W.); the Stanford University Child Health Research Institute Faculty Scholar Award (to D.C.W.); and the Plastic Surgery Foundation / Plastic Surgery Research Council Pilot Grant and the Stanford University Transplant and Tissue Engineering Center of Excellence Fellowship (to R.T.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Adams GR. (1981). The effect of physical attractiveness on the socialization process. In: Psychological aspects of the facial form craniofacial monograph no 11. Lucker GW, Ribbens KA, McNamara J, editors. Ann Arbor, MI: University of Michigan Press, pp. 25-47. [Google Scholar]
- Ahmad N, Lyles J, Panchal J, Deschamps-Braly J. (2008). Outcomes and complications based on experience with resorbable plates in pediatric craniosynostosis patients. J Craniofac Surg 19:855-860. [DOI] [PubMed] [Google Scholar]
- Amini AR, Laurencin CT, Nukavarapu SP. (2012). Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40:363-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. (1997). Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964-967. [DOI] [PubMed] [Google Scholar]
- Bhattarai SR, Bhattarai N, Yi HK, Hwang PH, Cha DI, Kim HY. (2004). Novel biodegradable electrospun membrane: scaffold for tissue engineering. Biomaterials 25:2595-2602. [DOI] [PubMed] [Google Scholar]
- Bose S, Roy M, Bandyopadhyay A. (2012). Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 30:546-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butscher A, Bohner M, Roth C, Ernstberger A, Heuberger R, Doebelin N, et al. (2012). Printability of calcium phosphate powders for three-dimensional printing of tissue engineering scaffolds. Acta Biomater 8:373-385. [DOI] [PubMed] [Google Scholar]
- Cao L, Wang J, Hou J, Xing W, Liu C. (2014). Vascularization and bone regeneration in a critical sized defect using 2-N,6-O-sulfated chitosan nanoparticles incorporating BMP-2. Biomaterials 35:684-698. [DOI] [PubMed] [Google Scholar]
- Carvalho PP, Leonor IB, Smith BJ, Dias IR, Reis RL, Gimble JM, et al. (2014). Undifferentiated human adipose-derived stromal/stem cells loaded onto wet-spun starch-polycaprolactone scaffolds enhance bone regeneration: nude mice calvarial defect in vivo study. J Biomed Mater Res A 102:3102-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MT, Liu C, Hyun JS, Lo DD, Montoro DT, Hasegawa M, et al. (2013). CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A 19:989-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillo JE, Jr, Gassner R, Koepsel RR, Buckley MJ. (2000). Growth factor and cytokine gene expression in mechanically strained human osteoblast-like cells: implications for distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 90:147-154. [DOI] [PubMed] [Google Scholar]
- Cutting C, Bookstein FL, Grayson B, Fellingham L, McCarthy JG. (1986). Three-dimensional computer-assisted design of craniofacial surgical procedures: optimization and interaction with cephalometric and CT-based models. Plast Reconstr Surg 77:877-887. [DOI] [PubMed] [Google Scholar]
- Dickinson BP, Ashley RK, Wasson KL, O’Hara C, Gabbay J, Heller JB, et al. (2008). Reduced morbidity and improved healing with bone morphogenic protein-2 in older patients with alveolar cleft defects. Plast Reconstr Surg 121:209-217. [DOI] [PubMed] [Google Scholar]
- Ehrbar M, Djonov VG, Schnell C, Tschanz SA, Martiny-Baron G, Schenk U, et al. (2004). Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res 94:1124-1132. [DOI] [PubMed] [Google Scholar]
- Eldesoqi K, Seebach C, Nguyen Ngoc C, Meier S, Nau C, Schaible A, et al. (2013). High calcium bioglass enhances differentiation and survival of endothelial progenitor cells, inducing early vascularization in critical size bone defects. PloS One 8:e79058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrand T, Kihlstrom L, Neovius E, Skogh AC, Lundgren TK, Jacobsson H, et al. (2014). Development of a bioactive implant for repair and potential healing of cranial defects. J Neurosurg 120:273-277. [DOI] [PubMed] [Google Scholar]
- Ferreira JR, Padilla R, Urkasemsin G, Yoon K, Goeckner K, Hu WS, et al. (2013). Titanium-enriched hydroxyapatite-gelatin scaffolds with osteogenically differentiated progenitor cell aggregates for calvaria bone regeneration. Tissue Eng Part A 19:1803-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Deng S, Wang J, Chen Z, Zhang S, Wu S, et al. (2014). Potential replication of induced pluripotent stem cells for craniofacial reconstruction. Curr Stem Cell Res Ther 9:205-214. [DOI] [PubMed] [Google Scholar]
- He X, Dziak R, Yuan X, Mao K, Genco R, Swihart M, et al. (2013). BMP2 genetically engineered MSCs and EPCs promote vascularized bone regeneration in rat critical-sized calvarial bone defects. PloS One 8:e60473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister SJ. (2005). Porous scaffold design for tissue engineering. Nat Mater 4:518-524. (Published erratum in: Nat Mater 5:590, 2006) [DOI] [PubMed] [Google Scholar]
- Hollister SJ, Lin CY, Saito E, Lin CY, Schek RD, Taboas JM, et al. (2005). Engineering craniofacial scaffolds. Orthod Craniofac Res 8:162-173. [DOI] [PubMed] [Google Scholar]
- Hong P, Boyd D, Beyea SD, Bezuhly M. (2013). Enhancement of bone consolidation in mandibular distraction osteogenesis: a contemporary review of experimental studies involving adjuvant therapies. J Plast Reconstr Aesthet Surg 66:883-895. [DOI] [PubMed] [Google Scholar]
- Hyun JS, Montoro DT, Lo DD, Flynn RA, Wong V, Chung MT, et al. (2013a). The seed and the soil: optimizing stem cells and their environment for tissue regeneration. Ann Plast Surg 70:235-239. [DOI] [PubMed] [Google Scholar]
- Hyun JS, Tran MC, Wong VW, Chung MT, Lo DD, Montoro DT, et al. (2013b). Enhancing stem cell survival in vivo for tissue repair. Biotechnol Adv 31:736-743. [DOI] [PubMed] [Google Scholar]
- Jain RK. (2005). Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58-62. [DOI] [PubMed] [Google Scholar]
- Jin GZ, Kim TH, Kim JH, Won JE, Yoo SY, Choi SJ, et al. (2013). Bone tissue engineering of induced pluripotent stem cells cultured with macrochanneled polymer scaffold. J Biomed Mater Res A 101:1283-1291. [DOI] [PubMed] [Google Scholar]
- Kaigler D, Pagni G, Park CH, Tarle SA, Bartel RL, Giannobile WV. (2010). Angiogenic and osteogenic potential of bone repair cells for craniofacial regeneration. Tissue Eng Part A 16:2809-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Shin T, Lim D. (2012). Biomimetic scaffolds for tissue engineering. Adv Funct Mater 22:2446-2468. [Google Scholar]
- Kim HP, Ji YH, Rhee SC, Dhong ES, Park SH, Yoon ES. (2012). Enhancement of bone regeneration using osteogenic-induced adipose-derived stem cells combined with demineralized bone matrix in a rat critically-sized calvarial defect model. Curr Stem Cell Res Ther 7:165-172. [DOI] [PubMed] [Google Scholar]
- Kim K, Fisher JP. (2007). Nanoparticle technology in bone tissue engineering. J Drug Target 15:241-252. [DOI] [PubMed] [Google Scholar]
- Kim SS, Ahn KM, Park MS, Lee JH, Choi CY, Kim BS. (2007). A poly(lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. J Biomed Mater Res A 80:206-215. [DOI] [PubMed] [Google Scholar]
- Kinsella CR, Jr, Cray JJ, Smith DM, Rottgers SA, Mooney MP, Cooper GM, et al. (2012a). Novel model of calvarial defect in an infected unfavorable wound: reconstruction with rhBMP-2. Part II. J Craniofac Surg 23:410-414. [DOI] [PubMed] [Google Scholar]
- Kinsella CR, Jr, Macisaac ZM, Cray JJ, Smith DM, Rottgers SA, Mooney MP, et al. (2012b). Novel animal model of calvarial defect: part III. Reconstruction of an irradiated wound with rhBMP-2. Plast Reconstr Surg 130:643e-650e. [DOI] [PubMed] [Google Scholar]
- Kørbling M, Estrov Z. (2003). Adult stem cells for tissue repair: a new therapeutic concept? N Engl J Med 349:570-582. [DOI] [PubMed] [Google Scholar]
- Kuroshima S, Yamashita J. (2013). Chemotherapeutic and antiresorptive combination therapy suppressed lymphangiogenesis and induced osteonecrosis of the jaw-like lesions in mice. Bone 56:101-109. [DOI] [PubMed] [Google Scholar]
- Kusumbe AP, Ramasamy SK, Adams RH. (2014). Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507:323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Shin H. (2007). Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev 59:339-359. [DOI] [PubMed] [Google Scholar]
- Levi B, James AW, Nelson ER, Vistnes D, Wu B, Lee M, et al. (2010). Human adipose derived stromal cells heal critical size mouse calvarial defects. PloS One 5:e11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi B, Hyun JS, Montoro DT, Lo DD, Chan CK, Hu S, et al. (2012). In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proc Natl Acad Sci U S A 109:20379-20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang T, Li M, Fu N, Fu Y, Ba K, et al. (2014). Electrospun fibers for dental and craniofacial applications. Curr Stem Cell Res Ther 9:187-195. [DOI] [PubMed] [Google Scholar]
- Liao YH, Chang YH, Sung LY, Li KC, Yeh CL, Yen TC, et al. (2014). Osteogenic differentiation of adipose-derived stem cells and calvarial defect repair using baculovirus-mediated co-expression of BMP-2 and miR-148b. Biomaterials 35:4901-4910. [DOI] [PubMed] [Google Scholar]
- Liu Q, de Wijn JR, van Blitterswijk CA. (1998). Composite biomaterials with chemical bonding between hydroxyapatite filler particles and PEG/PBT copolymer matrix. J Biomed Mater Res 40:490-497. [DOI] [PubMed] [Google Scholar]
- Mantripragada VP, Lecka-Czernik B, Ebraheim NA, Jayasuriya AC. (2013). An overview of recent advances in designing orthopedic and craniofacial implants. J Biomed Mater Res A 101:3349-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CM, Haugh MG, O’Brien FJ. (2010). The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 31:461-466. [DOI] [PubMed] [Google Scholar]
- Muschler GF, Nakamoto C, Griffith LG. (2004). Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am 86-A:1541-1558. [DOI] [PubMed] [Google Scholar]
- Neovius E, Engstrand T. (2010). Craniofacial reconstruction with bone and biomaterials: review over the last 11 years. J Plast Reconstr Aesthet Surg 63:1615-1623. [DOI] [PubMed] [Google Scholar]
- Nussenbaum B, Rutherford RB, Teknos TN, Dornfeld KJ, Krebsbach PH. (2003). Ex vivo gene therapy for skeletal regeneration in cranial defects compromised by postoperative radiotherapy. Hum Gene Ther 14:1107-1115. [DOI] [PubMed] [Google Scholar]
- Patel M, Fisher JP. (2008). Biomaterial scaffolds in pediatric tissue engineering. Pediatr Res 63:497-501. [DOI] [PubMed] [Google Scholar]
- Petrovic V, Zivkovic P, Petrovic D, Stefanovic V. (2012). Craniofacial bone tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol 114:e1-e9. [DOI] [PubMed] [Google Scholar]
- Ramseier CA, Rasperini G, Batia S, Giannobile WV. (2012). Advanced reconstructive technologies for periodontal tissue repair. Periodontol 2000 59: 185-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci JL, Clark EA, Murriky A, Smay JE. (2012). Three-dimensional printing of bone repair and replacement materials: impact on craniofacial surgery. J Craniofac Surg 23:304-308. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Roden LC, Ferretti C, Klar RM. (2011). Biomimetic matrices self-initiating the induction of bone formation. J Craniofac Surg 22:1859-1870. [DOI] [PubMed] [Google Scholar]
- Rouwkema J, Rivron NC, van Blitterswijk CA. (2008). Vascularization in tissue engineering. Trends Biotechnol 26:434-441. [DOI] [PubMed] [Google Scholar]
- Seebach C, Henrich D, Wilhelm K, Barker JH, Marzi I. (2012). Endothelial progenitor cells improve directly and indirectly early vascularization of mesenchymal stem cell-driven bone regeneration in a critical bone defect in rats. Cell Transplant 21:1667-1677. [DOI] [PubMed] [Google Scholar]
- Shie MY, Chang HC, Ding SJ. (2012). Effects of altering the Si/Ca molar ratio of a calcium silicate cement on in vitro cell attachment. Int Endod J 45:337-345. [DOI] [PubMed] [Google Scholar]
- Staiger MP, Pietak AM, Huadmai J, Dias G. (2006). Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials 27:1728-1734. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861-872. [DOI] [PubMed] [Google Scholar]
- Tang M, Chen W, Liu J, Weir MD, Cheng L, Xu HH. (2014). Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration. Tissue Eng Part A 20:1295-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JP, Hutton DL, Hung BP, Huri PY, Cook CA, Kondragunta R, et al. (2014). Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res A [Epub ahead of print 2/8/2014]. [DOI] [PubMed] [Google Scholar]
- Tran N, Webster TJ. (2011). Increased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticles. Acta Biomater 7:1298-1306. [DOI] [PubMed] [Google Scholar]
- Ueda M, Yamada Y, Ozawa R, Okazaki Y. (2005). Clinical case reports of injectable tissue-engineered bone for alveolar augmentation with simultaneous implant placement. Int J Periodontics Restorative Dent 25:129-137. [PubMed] [Google Scholar]
- Wahl DA, Czernuszka JT. (2006). Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater 11:43-56. [DOI] [PubMed] [Google Scholar]
- Ward BB, Brown SE, Krebsbach PH. (2010). Bioengineering strategies for regeneration of craniofacial bone: a review of emerging technologies. Oral Dis 16:709-716. [DOI] [PubMed] [Google Scholar]
- Williams DF. (2008). On the mechanisms of biocompatibility. Biomaterials 29:2941-2953. [DOI] [PubMed] [Google Scholar]
- Woodard JR, Hilldore AJ, Lan SK, Park CJ, Morgan AW, Eurell JA, et al. (2007). The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 28:45-54. [DOI] [PubMed] [Google Scholar]
- Wu Q, Shao H, Darwin ED, Li J, Li J, Yang B, et al. (2009). Extracellular calcium increases CXCR4 expression on bone marrow-derived cells and enhances pro-angiogenesis therapy. J Cell Mol Med 13:3764-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Han D, Dong JS, Shen GX, Chai G, Yu ZY, et al. (2010). Rapid prototyped PGA/PLA scaffolds in the reconstruction of mandibular condyle bone defects. Int J Med Robot 6:66-72. [DOI] [PubMed] [Google Scholar]
- Yuan J, Cao Y, Liu W. (2012). Biomimetic scaffolds: implications for craniofacial regeneration. J Craniofac Surg 23:294-297. [DOI] [PubMed] [Google Scholar]
- Yuhasz MM, Koch FP, Kwiatkowski A, Young C, Clune J, Travieso R, et al. (2014). Comparing calvarial transport distraction with and without radiation and fat grafting. J Craniomaxillofac Surg [Epub ahead of print 4/23/2014]. [DOI] [PubMed] [Google Scholar]
- Yusop AH, Bakir AA, Shaharom NA, Abdul Kadir MR, Hermawan H. (2012). Porous biodegradable metals for hard tissue scaffolds: a review. Int J Biomater 2012:641430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chang W, Lee P, Wang Y, Yang M, Li J, et al. (2014). Polymer-ceramic spiral structured scaffolds for bone tissue engineering: effect of hydroxyapatite composition on human fetal osteoblasts. PloS One 9:e85871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Patel PK, Cohen M. (2012). Application of virtual surgical planning with computer assisted design and manufacturing technology to cranio-maxillofacial surgery. Arch Plast Surg 39:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, et al. (2003). Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J 17:2260-2262. [DOI] [PubMed] [Google Scholar]