Abstract
Large mandibular defects are difficult to reconstruct with good functional and aesthetic outcomes because of the complex geometry of craniofacial bone. While the current gold standard is free tissue flap transfer, this treatment is limited in fidelity by the shape of the harvested tissue and can result in significant donor site morbidity. To address these problems, in vivo bioreactors have been explored as an approach to generate autologous prefabricated tissue flaps. These bioreactors are implanted in an ectopic site in the body, where ossified tissue grows into the bioreactor in predefined geometries and local vessels are recruited to vascularize the developing construct. The prefabricated flap can then be harvested with vessels and transferred to a mandibular defect for optimal reconstruction. The objective of this review article is to introduce the concept of the in vivo bioreactor, describe important preclinical models in the field, summarize the human cases that have been reported through this strategy, and offer future directions for this exciting approach.
Keywords: bioengineering, craniomaxillofacial surgery, bone graft(s), bone remodeling/regeneration, clinical studies/trials, tissue engineering
Introduction
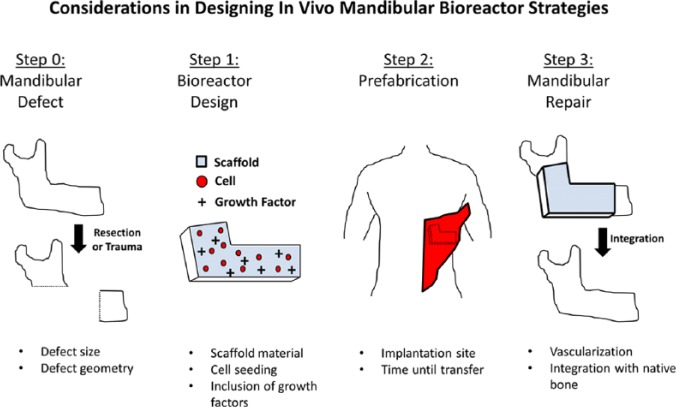
While all the components of the human skeleton serve important functional roles, the craniofacial bone plays a particularly essential part in the human psyche. Large mandibular defects result in the loss of functional capacity, such as the ability to masticate, but the accompanying loss of aesthetics can have equally devastating psychosocial complications (Macgregor, 1990; Hickey and Salter, 2006). The current techniques available to surgeons to repair these defects are limited; treatment often requires donor tissue, resulting in additional morbidity, and aesthetic restoration is limited in fidelity by the match of the donor tissue geometry (Table 1). Therefore, new treatment modalities are needed to improve patient outcome. One developing approach is the use of in vivo bioreactors to generate autologous bone tissue (prefabricated flaps) to fill mandibular defects. Bioreactors in general are chambers in which biological tissues can be grown. Classically, bioreactors are ex vivo, and different conditioning regimes have been explored to optimize tissue growth, as explored in a recent review (Salehi-Nik et al., 2013). However, it is difficult to fully recapitulate a physiological environment under ex vivo circumstances. In vivo bioreactors are those implanted inside an organism, leveraging the natural regenerative capacity of the body to generate tissue. In this work, we review both preclinical and clinical data available on the in vivo bioreactor strategy for mandibular repair (Fig.). This information is synthesized to present goals for the field in further advancing the strategy of in vivo bioreactors for the treatment of human patients.
Table 1.
Advantages and Disadvantages of Current Repair Strategies
| Reconstruction Strategy | Autologous Grafts | Autologous Free Tissue Flaps | Alloplastic Permanent Prostheses | Alloplastic Synthetic Grafts |
|---|---|---|---|---|
| Advantages | • Possess endogenous osteoinductive / osteoconductive factors • Consist of relatively simple procedure |
• Established as current gold standard • Result in vascularized tissue |
• Result in no donor site morbidity • Have capability of fully recapitulating defect geometry |
• Result in no donor site morbidity • Are available in different ceramic compositions |
| Disadvantages | • Result in donor site morbidity • Lack vasculature • Are appropriate only for small defects |
• Result in donor site morbidity • Give rise to increased operation room time • Are technically demanding |
• Demonstrated to have high risk of infection • Often result in poor soft tissue integration • Carry potential risk of extrusion |
• Lack vasculature • Prediction of resorption rates is difficult |
Figure.
Schematic of the in vivo bioreactor strategy and variables that must be considered at each step. In this example, the latissimus dorsi is used as a prefabrication site for bioreactor implantation.
Principles of In Vivo Bioreactors
More than 25 yr after Marshall R. Urist (1965) recognized and isolated what is now known as bone morphogenetic protein, Khouri et al. (1991) were the first to implant silicone molds loaded with osteogenic growth factors to generate vascularized bone tissue with specific anatomic geometries ectopically in rats. This landmark study demonstrated the feasibility of harnessing the native regenerative capacity of the body with exogenous signals to generate autologous tissue of prespecified shape. While there have been many attempts to grow large complex tissues in vitro, the vascularization and architecture of tissues are very difficult to recapitulate in artificial environments. Therefore, new strategies are being developed to utilize the body itself as a bioreactor to grow new tissue for transplantation to a defect. The in vivo bioreactor strategy has been applied for the synthesis of bone to repair musculoskeletal defects. For example, Stevens et al. (2005) created an injectable hydrogel capable of functioning as a bioreactor supporting new bone growth when injected underneath the periosteum in a rabbit model. However, geometric precision is less critical in the repair of musculoskeletal defects compared with defects of the facial bone. Therefore, strategies for the generation of craniofacial bone require more control over the shape of generated tissue than what an injectable hydrogel system would allow. While the focus of this review is the use of in vivo bioreactors for mandibular tissue, readers are directed to a previous review on the subject of in vivo tissue engineering for more musculoskeletal applications (McCullen et al., 2011) and a review with broad craniofacial focus (Torroni, 2009).
For the purposes of mandibular reconstruction, the in vivo bioreactor has been evaluated in many preclinical models and in several patient case reports. Investigators use different combinations of material scaffold, seeded cell populations, and exogenous growth factors to generate autologous prefabricated flaps. There is a large variety of biodegradable scaffold materials, including the broad classes of ceramics and polymers. Ceramics possess strong compressive properties and may be naturally derived (e.g., morcellized autograft) or synthetic (e.g., hydroxyapatite or beta-tricalcium phosphate; Betz, 2002). In addition, ceramics may degrade in the form of free calcium and phosphate ions that may promote future osteogenesis. Nonetheless, some ceramics are limited in degradability. In contrast, polymer-based scaffolds have high tunability of material properties, such as mechanical strength and degradation (Athanasiou et al., 1998). For the purposes of the mandible, however, these scaffolds may be less biomimetic, and many of the common biodegradable polymers degrade into acidic by-products that can inhibit bone formation (Athanasiou et al., 1998). Cells may or may not be seeded onto the scaffold; the most commonly used cell population is bone marrow aspirate from the iliac crest (Orringer et al., 1999; Warnke et al., 2004). Last, exogenous growth factors may or may not be added to the bioreactor before implantation. The 2 growth factors that have been most explored are bone morphogenetic protein 2 and 7 (BMP2 and BMP7; Warnke et al., 2004; Heliotis et al., 2006). However, as many patients in need of mandibular reconstruction have a history of craniofacial tumors, there is concern with the use of growth factors in this population; in fact, BMP2 is contraindicated in patients with a history of cancer (Gitelis et al., 2008; Carragee et al., 2011). Ideally, an in vivo bioreactor strategy minimizes the use of the following:
Autologous scaffold, thereby mitigating donor site morbidity
Seeded cells, as bone marrow aspiration also involves (albeit limited) donor site morbidity and increases approach complexity
Exogenous growth factors, thereby mitigating the risks accompanying therapy
Design Optimization in Preclinical Models
Model Selection
Small Animal Models
For the purposes of this review, small animal models are defined as mice, rats, and rabbits. The use of small animals is attractive, as they are inexpensive to purchase and maintain. The amount of growth factors and scaffold material needed in small animals is also significantly reduced. Not surprising, a number of studies have taken advantage of small animal models. For example, rat mesenchymal stem cells were seeded in a polymer hydrogel and implanted within the dorsum of immunodeficient mice to form mandibular condyle constructs of human proportion. These condyles had both cartilage and bony sections, demonstrating the efficacy of the in vivo bioreactor approach in generating complex tissues with multiple components (Alhadlaq and Mao, 2003). An early study in rabbits demonstrated histologically that bone generation in an in vivo bioreactor was similar to native bone in architecture and vascularization (Celik et al., 2000). The rat latissimus dorsi has been used in several studies as the site for bioreactor implantation, most often to study the effects of different amounts and types of growth factors (Kusumoto et al., 1998; Roldan et al., 2004; Holt et al., 2005). Ultimately, defects in small animals lack the diffusional challenges presented in defects of clinical relevance. This presents a significant limitation in their use as models; large animals are more appropriate for generating approaches that can be translated for human treatment.
Large Animal Models
Sheep
Regarding large animals, the most prevalent in the field of in vivo bioreactors for mandibular reconstruction are sheep, minipigs, and nonhuman primates. One of the earliest of these models was the sheep (Miller et al., 1996). In this model, tissue chambers made of polymethylmethacrylate were filled with scaffold material (typically, morcellized autograft) and placed against the cambrium layer of the rib periosteum. Up to 4 of these chambers were implanted per animal on alternating ribs. Vascularized bone tissue grew into the chamber from the periosteum and ultimately took the shape of the chamber. In this manner, tissues of complex geometry and clinically relevant size were generated (Miller et al., 1996; Cheng et al., 2009). Using this model, investigators determined optimal prefabrication time (Cheng et al., 2005) and optimal implantation site (Brey et al., 2007) and investigated the use of biodegradable polymers as scaffold to mitigate the need for autologous donor tissue (Thomson et al., 1999). This strategy was translated into the clinic in a human patient (Cheng et al., 2006). In the future, it is envisioned that this periosteal in vivo bioreactor will be translated in combination with the 2-stage mandibular reconstruction approach (Atala et al., 2012). In this strategy, a space maintainer is inserted at the time of mandibular resection. The space maintainer acts as a template for soft tissue regrowth, maintains facial contours, and prevents scarring within the defect space (Henslee et al., 2014). It is worth noting that others have also used the sheep for prefabricated mandibular flap generation, with implantation in the latissimus dorsi (Kokemueller et al., 2010; Kokemuller et al., 2013).
Minipig
Another well-established in vivo bioreactor large animal model is the minipig. In this model, titanium cages were loaded with bovine hydroxyapatite (Bio-Oss) as a scaffold with the addition of BMP7. These constructs were implanted in the latissimus dorsi of minipigs for the generation of ossified tissue (Terheyden et al., 1999). This prefabricated flap was transferred into a minipig mandibular defect with great success. In fact, when the contralateral mandible was treated with primary reconstruction via the same ceramic scaffold and growth factor regimen, the prefabricated flap resulted in bone of better quantity and quality (Terheyden et al., 1999; Terheyden et al., 2001). As evaluated by mechanical testing, these autologously generated tissues had similar compressive properties to native mandibular bone (Warnke et al., 2006a). The strategy applied in minipigs was also clinically translated in a human patient (Warnke et al., 2004; Warnke et al., 2006a).
Nonhuman primates
Nonhuman primates have also been used as a model for the development of in vivo bioreactors—specifically, to compare (1) reconstruction of a mandibular defect with tissue from a bioreactor implanted in the latissimus dorsi treated with BMP2 with (2) primary defect repair with BMP2. In this model, the in vivo bioreactor approach demonstrated better bone formation in both quantity and quality (Zhou et al., 2010). While primates most closely approximate the human condition, this research is very costly and raises complex ethical questions.
Prefabrication Site
One important consideration for the in vivo bioreactor strategy is the site of bioreactor implantation. Implantation in different environments may result in the recruitment of different types of factors, such as blood vessels, nerves, progenitor cells, and signaling proteins. An ideal ectopic site also minimizes inflammation, pain on implantation, and complications upon harvest (McCullen et al., 2011; Miller et al., 1996). For the purposes of bone tissue engineering, bioreactor implantation against the periosteum has been demonstrated as a successful environment for ossified tissue generation (Miller et al., 1996; Thomson et al., 1999; Cheng et al., 2005; Stevens et al., 2005; Brey et al., 2007; Cheng et al., 2009). In a study in which identical tissue chambers (filled with morcellized bone graft) were implanted against either the rib periosteum or the fascia of the latissimus dorsi muscle in sheep, both sites were able to generate vascularized tissue. However, the intramuscular implants had significant graft resorption and resulted in primarily fibrovascular tissue. Chambers implanted against the periosteum had active bone formation with increased calcified tissue area (Brey et al., 2007). Nevertheless, with the addition of exogenous growth factors, intramuscular implantation can result in ectopic bone formation within a bioreactor (Kusumoto et al., 1998; Roldan et al., 2004; Geuze et al., 2012).
Prefabrication Time and Geometry
For the formation of ossified tissue in particular, the time of implantation has a significant impact on the tissue quality. The sheep rib periosteum model has demonstrated that there is a specific window at which ossified tissue is at peak quantity and quality (6-9 wk), after which resorption occurs within the bioreactor (Cheng et al., 2005; Cheng et al., 2009). The shape of the bioreactor chamber, so long as it can fit within its prefabrication site, appears to be flexible and can be designed to conform to the geometry of the defect (Miller et al., 1996; Cheng et al., 2009). However, the composition of the bioreactor chamber itself may play a significant role in tissue development. In the minipig model, chambers constructed from biodegradable polymers were not able to maintain their predefined shape over the implantation period (Warnke et al., 2006a). As control over geometry is one of the key advantages to the in vivo bioreactor strategy, this suggests that chambers should be composed of nonbiodegradable materials, such as titanium or polymethylmethacrylate.
Scaffold Material, Growth Factors, and Seeded Cells
In addition to prefabrication site and implantation time, the 3 aspects of the classic tissue engineering paradigm (cells, chemical cues, and scaffolds) are also critical in the design of the in vivo bioreactor strategy. In preclinical models, a variety of combinations have been demonstrated to promote the growth of a prefabricated tissue flap. For scaffold material, investigators have experimented with morcellized autograft, decellularized bone graft, collagen, biodegradable polymer, hydroxyapatite, beta-tricalcium phosphate, and various biphasic ceramics (Kusumoto et al., 1998; Thomson et al., 1999; Holt et al., 2005; Eweida et al., 2011). In one study, in vivo bioreactors filled with different scaffold materials (morcellized autograft, decellularized autograft, and empty chambers) were implanted against sheep rib periosteum for comparison (Cheng et al., 2005). Morcellized autograft generated significantly more bone tissue than any of the other groups. Otherwise, as there have not been additional studies in which different scaffold materials have been compared within the same animal model, there is little evidence regarding which scaffold material is optimal for the generation of ossified tissue. While it has been suggested that osteogenic growth factors are required for bone tissue formation in intramuscular implants, the addition of exogenous growth factors in bioreactors implanted against the periosteum is not required for bone growth (Miller et al., 1996; Thomson et al., 1999; Cheng et al., 2005; Brey et al., 2007; Cheng et al., 2009). Last, while cell seeding is an absolute necessity for in vitro approaches, cells can be locally recruited with in vivo bioreactors (although bone marrow aspirates can also be used to seed the bioreactors upon implantation; Cheng et al., 2005; McCullen et al., 2011). The lessons learned from preclinical models have resulted in the translation of this technology to human patients.
Human Case Reports
As an exciting development in the field, 5 different in vivo bioreactor approaches in human patients for mandibular reconstruction have been reported in the literature. The first of these cases occurred in 1990 (although it was not reported until 1999). In this landmark case, a patient who had lost the mandible due to recurrent ameloblastoma was treated with a mandibular-shaped Dacron-polyurethane tray packed with autologous bone graft and exogenous growth factor (Orringer et al., 1999). This tray was implanted in the fascia above the scapula and retrieved after 4 mo (skin grafting over the retrieval site was required). The patient’s lower lip was also reconstructed with strips from the harvested tensor fasciae latae. While the patient tolerated the procedure well, the reconstruction did not permit oral feeding and was not sufficient to support dental implants. Eventually, a revision was performed to improve function as well as augment the bone with grafts for the insertion of dental implants. The patient never regained the ability to swallow solids and unfortunately passed away due to disseminated disease.
The next reported case was presented by Warnke et al., the same group that developed the minipig model (Terheyden et al., 1999; Terheyden et al., 2001; Warnke et al., 2006a). In this case, a patient with a history of oral tumor was treated with a titanium cage filled with Bio-Oss and BMP7 (Warnke et al., 2004). This bioreactor was implanted intramuscularly within the latissimus dorsi. Bone growth and remodeling within the bioreactor were confirmed before harvest by use of skeletal scintigraphy. After 7 wk, the prefabricated flap was harvested and transferred along with the accompanying titanium mesh. Scintigraphy was repeated after transfer and revealed further bone remodeling, indicating integration with native bone. The patient had restored aesthetics and function and regained the capacity to enjoy solid foods (Warnke et al., 2006b). The authors of the study particularly comment on the sociopsychological effects of this treatment on the patient, noting that the patient’s “mood turned from one previously of depression and suicidality to one of excitement and optimism.” Ultimately, after 13 mo, the titanium mesh fractured, and the soft tissue overlying the construct failed. This resulted in infection and necrosis of the transferred bone. Two subsequent revisions were performed, and eventually the patient died as a result of cardiac arrest.
Using the same strategies as developed in the sheep periosteal model, Cheng et al. (2006) augmented a mandibular reconstruction with an in vivo bioreactor approach. In brief, a patient presented with numerous reconstructions of both hard and soft tissue to restore function after removal of an oral squamous cell carcinoma. However, these reconstructions did not generate mandibular bone of sufficient height to accommodate dental implants. Thus, a polymethylmethacrylate chamber was filled with harvested autograft and implanted against the periosteum of the iliac crest. After 8 wk, the tissue was harvested, and donor periosteum was sutured to mandibular periosteum to reestablish blood supply. The patient eventually died of hepatocellular carcinoma, but the transferred tissue was functional and retained dental implants at 16 mo.
In an effort to reduce donor site morbidity, Heliotis et al. (2006) designed an in vivo bioreactor approach without the use of harvested autologous bone or bone marrow cells. BMP7 was added to hydroxyapatite blocks and implanted in the pectoralis major muscle of a patient who had suffered from oral squamous cell carcinoma. After 3.5 mo, bone scintigraphy revealed bone formation in the construct. After 6.5 mo of implantation time, the construct was harvested, along with muscle tissue, for transfer into the mandibular defect. The tissue was covered with a split skin graft. Unlike previous studies, a biopsy was taken from the tissue at the time of transfer. The transferred construct was 17% bone, 37% hydroxyapatite, and 46% fibrovascular tissue. After 5 wk, the transferred tissue became infected, and the flap was removed. This case is notable for its histologic analysis of prefabricated clinical tissue and lack of need for donor tissue in flap generation.
Finally, a recent case was reported in which cylinders of beta-tricalcium phosphate were loaded with cells and morcellized autologous bone graft from the iliac crest (Kokemueller et al., 2010). Four of these cylinders were implanted in the latissimus dorsi of a patient who had been suffering from chronic mandibular osteomyelitis requiring resection. After 6 mo, these cylinders were harvested, shaped by piezoelectric surgery, and transferred to the defect. Before transfer, vascularization of these cylinders was confirmed by angio–computed tomography. Gaps between cylinders were filled with additional iliac crest graft. At 12 mo of follow-up, the reconstructed mandible was still viable.
Table 2 summarizes the use of autologously harvested scaffold, bone marrow–derived seeded cells, and exogenous growth factors in each case. These in vivo bioreactors were implanted for a minimum of 7 wk and maximum of 6.5 mo before transfer. All approaches resulted in at least temporarily successful reconstruction (although it is possible that additional failed approaches have gone unreported). In 2 out of 5 of these cases, the reconstructed mandible failed or otherwise required significant revision. It is worth noting that in both cases, infection was involved with failure.
Table 2.
Case Report Strategies and Outcomes
| Prefabrication Site | Scaffold Material | Growth Factors | Seeded Cells | Outcome | Author |
|---|---|---|---|---|---|
| Scapular fascia, 4 mo | Dacron-polyurethane cage + autograft | BMP (undefined) | Bone marrow aspirate | N/A; died of recurrence after ~2 yr | Orringer et al., 1999 |
| Latissimus dorsi, 7 wk | Titanium cage + decellularized xenograft | BMP7 | Bone marrow aspirate | Infection and revision, 13 mo | Warnke et al., 2004; Warnke et al., 2006b |
| Iliac crest periosteum, 8 wk | Autograft | None | None | N/A; died of unrelated cancer after ~ 16 mo | Cheng et al., 2006 |
| Pectoralis major, 6.5 mo | Hydroxyapatite | BMP7 | None | Infection, 5 mo | Heliotis et al., 2006 |
| Latissimus dorsi, 6 mo | Beta-tricalcium phosphate + autograft | None | None | N/A, 13 mo | Kokemueller et al., 2010 |
Closing Remarks and Future Directions
These initial results demonstrate early promise in the use of an in vivo bioreactor strategy for the reconstruction of large mandibular defects, although significant research is still required. While mandibular reconstruction is difficult due to complex geometry, in vivo bioreactors can produce bone tissue with dimensions of high fidelity and mitigate donor site morbidity. Preclinical models, including nonhuman primates, have demonstrated that reconstruction performed with prefabricated tissue compared with primary definitive repair resulted in higher quantity and quality. Case reports in the literature have demonstrated some short-term efficacy of these therapies in human patients. However, as 2 of the 5 reported cases resulted in tissue failure, it is clear that more study needs to be done for this approach to be performed in the clinic on a regular basis.
In regard to preclinical data, small animals have limited utility in investigating the generation of tissue for reconstruction of large mandibular defects due to the lack of diffusional challenge to tissue growth. Out of the large animal models presented, the 2 most established are the minipig latissimus dorsi and sheep periosteal implant models. These 2 models represent both the intramuscular and periosteal implantation strategies that have been used in the clinic to generate tissue for mandibular repair. As these models have been used to compare factors such as implantation time, scaffold material, chamber material, chamber dimensions, and prefabrication versus primary reconstruction, it would be beneficial for future work to continue to make use of these well-established models.
For future preclinical work, it would advance the field to explore the effects and necessity of cell seeding on tissue growth. In addition, synthetic ceramic particles should be compared with morcellized autologous graft to potentially reduce the need for any harvested donor tissue. As some biphasic ceramics have been described as osteoconductive and osteoinductive, it may be possible that the use of these scaffolds may circumvent the need to use autologous morcellized bone. In addition, more preclinical studies need to demonstrate tissue transfer into mandibular defects. As the average time to failure in the human reported cases was approximately 9 mo, the long-term efficacy of prefabricated tissue needs to be examined in preclinical models as well.
Despite the available preclinical studies, the ideal implantation site for tissue generation remains unclear. Intramuscular sites facilitate relatively easy transplantation and allow for constructs of large volume to be implanted. However, intramuscular implantation commonly requires the addition of exogenous osteogenic growth factors to produce ossified tissue; this may be concerning for use in patients with a history of oral cancer and thus introduces additional regulatory hurdles in clinical translation. While the periosteum is a deeper implantation site with less available volume, ossified tissue can easily be generated without any additional growth factors. In addition, infection played a role in both reported cases of failure in the in vivo bioreactor approach. The role of antibiotic delivery, especially with new developments in local release, is an important consideration and should be emphasized while moving ahead.
Ultimately, one of the largest barriers to universal translation of the in vivo bioreactor strategy for mandibular repair is the lack of a unified approach, as illustrated by the variety of factors in Table 2. Choice of scaffold, inclusion of growth factors and seeded cells, prefabrication site, and prefabrication time are all variables in both preclinical and clinical cases. Based on these case reports, a single approach may be developed and applied in a clinical study. After this point, individual variables could be examined and tuned to further optimize outcomes. Until a single approach is rigorously applied in a clinical study, the strategy lacks the evidence needed to impact the field.
In conclusion, the in vivo bioreactor has seen limited success in a small number of case reports. In these select patients, at least temporarily, there was restoration of aesthetics and function. With the developments in areas such as growth factor delivery and synthetic scaffolds, new technologies may enable greater success in the in vivo bioreactor strategy. The face plays an essential role in the sense of self; we look forward to the advancement of therapies to restore the mandible even in the most difficult of cases.
Footnotes
This work was supported by the U.S. Army, U.S. Navy, National Institutes of Health, U.S. Air Force, VA, and Health Affairs to support the AFIRM II effort, under award W81XWH-14-2-0004. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014, is the awarding and administering acquisition office. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. In addition, A.M.T. would like to thank the Baylor College of Medicine Medical Scientist Training Program (National Institutes of Health T32 GM007330) and the Barrow Scholars Program.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alhadlaq A, Mao JJ. (2003). Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res 82:951-956. [DOI] [PubMed] [Google Scholar]
- Atala A, Kasper FK, Mikos AG. (2012). Engineering complex tissues. Sci Transl Med 4:160rv12. [DOI] [PubMed] [Google Scholar]
- Athanasiou KA, Agrawal CM, Barber FA, Burkhart SS. (1998). Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy 14:726-737. [DOI] [PubMed] [Google Scholar]
- Betz RR. (2002). Limitations of autograft and allograft: new synthetic solutions. Orthopedics 25(5):s561-s570. [DOI] [PubMed] [Google Scholar]
- Brey EM, Cheng MH, Allori A, Satterfield W, Chang DW, Patrick CW, Jr, et al. (2007). Comparison of guided bone formation from periosteum and muscle fascia. Plast Reconstr Surg 119:1216-1222. [DOI] [PubMed] [Google Scholar]
- Carragee EJ, Hurwitz EL, Weiner BK. (2011). A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 11:471-491. [DOI] [PubMed] [Google Scholar]
- Celik M, Tuncer S, Emekli U, Kesim SN. (2000). Histologic analysis of prefabricated, vascularized bone grafts: an experimental study in rabbits. J Oral Maxillofac Surg 583:292-295. [DOI] [PubMed] [Google Scholar]
- Cheng MH, Brey EM, Allori A, Satterfield WC, Chang DW, Patrick CW, Jr, et al. (2005). Ovine model for engineering bone segments. Tissue Eng 11:214-225. [DOI] [PubMed] [Google Scholar]
- Cheng MH, Brey EM, Ulusal BG, Wei FC. (2006). Mandible augmentation for osseointegrated implants using tissue engineering strategies. Plast Reconstr Surg 118:1e-4e. [DOI] [PubMed] [Google Scholar]
- Cheng MH, Brey EM, Allori AC, Gassman A, Chang DW, Patrick CW, Jr, et al. (2009). Periosteum-guided prefabrication of vascularized bone of clinical shape and volume. Plast Reconstr Surg 124:787-795. [DOI] [PubMed] [Google Scholar]
- Eweida AM, Nabawi AS, Marei MK, Khalil MR, Elhammady HA. (2011). Mandibular reconstruction using an axially vascularized tissue-engineered construct. Ann Surg Innov Res 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze RE, Theyse LF, Kempen DH, Hazewinkel HA, Kraak HY, Oner FC, et al. (2012). A differential effect of bone morphogenetic protein-2 and vascular endothelial growth factor release timing on osteogenesis at ectopic and orthotopic sites in a large-animal model. Tissue Eng Part A 18:2052-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelis S, Wilkins RM, Yasko AW. (2008). BMP’s and cancer: is the risk real? AAOS NOW 2:31. [Google Scholar]
- Heliotis M, Lavery KM, Ripamonti U, Tsiridis E, di Silvio L. (2006). Transformation of a prefabricated hydroxyapatite/osteogenic protein-1 implant into a vascularised pedicled bone flap in the human chest. Int J Oral Maxillofac Surg 35:265-269. [DOI] [PubMed] [Google Scholar]
- Henslee AM, Spicer PP, Shah SR, Tatara AM, Kasper FK, Mikos AG, et al. (2014). Use of porous space maintainers in staged mandibular reconstruction. Oral Maxillofac Surg Clin North Am 26:143-149. [DOI] [PubMed] [Google Scholar]
- Hickey AJ, Salter M. (2006). Prosthodontic and psychological factors in treating patients with congenital and craniofacial defects. J Prosthet Dent 95:392-396. [DOI] [PubMed] [Google Scholar]
- Holt GE, Halpern JL, Dovan TT, Hamming D, Schwartz HS. (2005). Evolution of an in vivo bioreactor. J Orthop Res 23:916-923. [DOI] [PubMed] [Google Scholar]
- Khouri RK, Koudsi B, Reddi H. (1991). Tissue transformation into bone in vivo: a potential practical application. JAMA 266:1953-1955. [PubMed] [Google Scholar]
- Kokemueller H, Spalthoff S, Nolff M, Tavassol F, Essig H, Stuehmer C, et al. (2010). Prefabrication of vascularized bioartificial bone grafts in vivo for segmental mandibular reconstruction: experimental pilot study in sheep and first clinical application. Int J Oral Maxillofac Surg 39:379-387. [DOI] [PubMed] [Google Scholar]
- Kokemuller H, Jehn P, Spalthoff S, Essig H, Tavassol F, Schumann P, et al. (2013). En bloc prefabrication of vascularized bioartificial bone grafts in sheep and complete workflow for custom-made transplants. Int J Oral Maxillofac Surg 43:163-172. [DOI] [PubMed] [Google Scholar]
- Kusumoto K, Bessho K, Fujimura K, Akioka J, Ogawa Y, Iizuka T. (1998). Prefabricated muscle flap including bone induced by recombinant human bone morphogenetic protein-2: an experimental study of ectopic osteoinduction in a rat latissimus dorsi muscle flap. Br J Plast Surg 51:275-280. [DOI] [PubMed] [Google Scholar]
- Macgregor FC. (1990). Facial disfigurement: problems and management of social interaction and implications for mental health. Aesthetic Plast Surg 14:249-257. [DOI] [PubMed] [Google Scholar]
- McCullen SD, Chow AG, Stevens MM. (2011). In vivo tissue engineering of musculoskeletal tissues. Curr Opin Biotechnol 22:715-720. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Goldberg DP, Yasko AW, Lemon JC, Satterfield WC, Wake MC, et al. (1996). Guided bone growth in sheep: a model for tissue-engineered bone flaps. Tissue Eng 2:51-59. [DOI] [PubMed] [Google Scholar]
- Orringer JS, Shaw WW, Borud LJ, Freymiller EG, Wang SA, Markowitz BL. (1999). Total mandibular and lower lip reconstruction with a prefabricated osteocutaneous free flap. Plast Reconstr Surg 104:793-797. [DOI] [PubMed] [Google Scholar]
- Roldan JC, Jepsen S, Miller J, Freitag S, Rueger DC, Acil Y, et al. (2004). Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone 34:80-90. [DOI] [PubMed] [Google Scholar]
- Salehi-Nik N, Amoabediny G, Pouran B, Tabesh H, Shokrgozar MA, Haghighipour N, et al. (2013). Engineering parameters in bioreactor’s design: a critical aspect in tissue engineering. Biomed Res Int 2013:762132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MM, Marini RP, Schaefer D, Aronson J, Langer R, Shastri VP. (2005). In vivo engineering of organs: the bone bioreactor. Proc Natl Acad Sci U S A 102:11450-11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terheyden H, Jepsen S, Rueger DR. (1999). Mandibular reconstruction in miniature pigs with prefabricated vascularized bone grafts using recombinant human osteogenic protein-1: a preliminary study. Int J Oral Maxillofac Surg 28:461-463. [PubMed] [Google Scholar]
- Terheyden H, Warnke P, Dunsche A, Jepsen S, Brenner W, Palmie S, et al. (2001). Mandibular reconstruction with prefabricated vascularized bone grafts using recombinant human osteogenic protein-1: an experimental study in miniature pigs: part II. Transplantation. Int J Oral Maxillofac Surg 30:469-478. [DOI] [PubMed] [Google Scholar]
- Thomson RC, Mikos AG, Beahm E, Lemon JC, Satterfield WC, Aufdemorte TB, et al. (1999). Guided tissue fabrication from periosteum using preformed biodegradable polymer scaffolds. Biomaterials 20:2007-2018. [DOI] [PubMed] [Google Scholar]
- Torroni A. (2009). Engineered bone grafts and bone flaps for maxillofacial defects: state of the art. J Oral Maxillofac Surg 67:1121-1127. [DOI] [PubMed] [Google Scholar]
- Urist MR. (1965). Bone: formation by autoinduction. Science 150:893-899. [DOI] [PubMed] [Google Scholar]
- Warnke PH, Springer IN, Wiltfang J, Acil Y, Eufinger H, Wehmoller M, et al. (2004). Growth and transplantation of a custom vascularised bone graft in a man. Lancet 364:766-770. [DOI] [PubMed] [Google Scholar]
- Warnke PH, Springer IN, Acil Y, Julga G, Wiltfang J, Ludwig K, et al. (2006a). The mechanical integrity of in vivo engineered heterotopic bone. Biomaterials 27:1081-1087. [DOI] [PubMed] [Google Scholar]
- Warnke PH, Wiltfang J, Springer I, Acil Y, Bolte H, Kosmahl M, et al. (2006b). Man as living bioreactor: fate of an exogenously prepared customized tissue-engineered mandible. Biomaterials 27:3163-3167. [DOI] [PubMed] [Google Scholar]
- Zhou M, Peng X, Mao C, Xu F, Hu M, Yu GY. (2010). Primate mandibular reconstruction with prefabricated, vascularized tissue-engineered bone flaps and recombinant human bone morphogenetic protein-2 implanted in situ. Biomaterials 31:4935-4943. [DOI] [PubMed] [Google Scholar]