Abstract
Stemming from in vitro and in vivo pre-clinical and human models, tissue-engineering-based strategies continue to demonstrate great potential for the regeneration of the pulp-dentin complex, particularly in necrotic, immature permanent teeth. Nanofibrous scaffolds, which closely resemble the native extracellular matrix, have been successfully synthesized by various techniques, including but not limited to electrospinning. A common goal in scaffold synthesis has been the notion of promoting cell guidance through the careful design and use of a collection of biochemical and physical cues capable of governing and stimulating specific events at the cellular and tissue levels. The latest advances in processing technologies allow for the fabrication of scaffolds where selected bioactive molecules can be delivered locally, thus increasing the possibilities for clinical success. Though electrospun scaffolds have not yet been tested in vivo in either human or animal pulpless models in immature permanent teeth, recent studies have highlighted their regenerative potential both from an in vitro and in vivo (i.e., subcutaneous model) standpoint. Possible applications for these bioactive scaffolds continue to evolve, with significant prospects related to the regeneration of both dentin and pulp tissue and, more recently, to root canal disinfection. Nonetheless, no single implantable scaffold can consistently guide the coordinated growth and development of the multiple tissue types involved in the functional regeneration of the pulp-dentin complex. The purpose of this review is to provide a comprehensive perspective on the latest discoveries related to the use of scaffolds and/or stem cells in regenerative endodontics. The authors focused this review on bioactive nanofibrous scaffolds, injectable scaffolds and stem cells, and pre-clinical findings using stem-cell-based strategies. These topics are discussed in detail in an attempt to provide future direction and to shed light on their potential translation to clinical settings.
Keywords: tissue scaffolds, regeneration, nanofibers, dental pulp, dentin, stem cells
Introduction
Since the inception of tissue engineering in the early 1990s (Langer and Vacanti, 1993), numerous developments – motivated primarily by the synthesis of unique materials acting as scaffolds to support cell attachment, growth, and differentiation (Li et al., 2005), as well as the identification of novel stem cell sources (Nakashima and Iohara, 2011) and bioactive molecules (Lu and Atala, 2013) – have targeted the regeneration of tissues and organs lost due to trauma and/or diseases (Langer and Vacanti, 1993). Naturally,tissue engineering remains pivotal to the development and translational impact of scaffolds in regenerative dentistry.
The pulp tissue of immature teeth may be damaged through bacteria invasion and/or dental trauma. In these situations, pulp tissue gradually becomes inflamed, and, if such inflammation is not interrupted, pulp necrosis will occur. This, in turn, promotes the death of odontoblasts, resulting in the disruption of root development (Nagata et al., 2014), making these teeth more prone to fractures. In recent years, the field of regenerative endodontics has presented new possibilities for the treatment of necrotic immature permanent teeth through the development of new pulp tissue based on the meticulous combination and interplay of 3 key elements for tissue regeneration, namely, stem cells, bioactive molecules (e.g., growth factors), and scaffolds (Diogenes et al., 2013). Scaffolds serve as transient, three-dimensional (3D), extracellular-matrix-mimicking (ECM) porous templates used to endow mechanical support and regulate cell functions (Li et al., 2005; Bottino et al., 2012). A wide variety of polymer scaffolds – both synthetic (e.g., poly[lactic] acid) and natural (e.g., collagen), ranging from macroporous structures obtained through salt leaching/solvent casting (Cordeiro et al., 2008) and gas foaming (Huang et al., 2010), to nanofibrous scaffolds processed via electrospinning, self-assembly, and phase-separation – have been developed to support the proliferation and differentiation of dental pulp stem cells toward the functional regeneration of the pulp-dentin complex (Galler et al., 2011a; Gupte and Ma, 2012; Rosa et al., 2013). Hard dental tissue structure, such as dentin, is fairly challenging to regenerate, since it relies on the presence of odontoblasts (Huang, 2011). To that end, a recent study demonstrated the ability for odontoblast-like cells, and consequently, dentin-like tissue to be regenerated on dentinal walls in emptied human root canal space through stem cells seeding onto macroporous polymer scaffolds, followed by transplantation into an immunocompromised mouse model (Huang et al., 2010). The Table summarizes recent progress in regenerative endodontics research.
The clinical perspective for the need for a scaffold comes mostly from the formation of a blood-clot-derived fibrin-based matrix in a previously decontaminated, minimal, or non-instrumented root canal system through the intentional laceration of periapical tissues (i.e., revascularization). In brief, the blood clot acts as a natural scaffold that, together with endogenously produced growth factors and stem cells from the apical papillae (SCAPs), populates the scaffold, inducing dentinal-wall-thickening, root maturation, and, in some cases, the formation of reparative cementum-like tissue (Diogenes et al., 2013).
Traditionally, infection eradication has been achieved by the association of mechanical and chemical means. Regrettably, in immature necrotic permanent teeth, mechanical instrumentation must be avoided to prevent further weakening of already thin and fragile root dentinal walls (Banchs and Trope, 2004). Alternatively, root canal irrigation associated with antibiotic pastes composed of metronidazole, ciprofloxacin, and minocycline (Banchs and Trope, 2004; Diogenes et al., 2014) or calcium hydroxide [Ca(OH)2] (Iwaya et al., 2011) has been used. The use of Ca(OH)2 reveals no toxicity to stem cells (Ruparel et al., 2012). However, recent findings have shown that antibiotic pastes at clinically advocated concentrations affect the survivability of SCAPs (Ruparel et al., 2012). Thus, as a potentially more cell-friendly disinfection strategy, antibiotic-containing polymer nanofibers capable of acting as a drug delivery system and eliminating root canal infection have been developed (Bottino et al., 2013, 2014a; Palasuk et al., 2014).
Nevertheless, the revascularization approach quantitatively demonstrated dentinal-wall thickening, apical closure, and increased root length, based on strong radiographic evidence (Banchs and Trope, 2004; Bose et al., 2009; Jeeruphan et al., 2012; Nagy et al., 2014). Despite positive clinical evidence, limitations such as difficulty obtaining bleeding, uncertainty regarding composition of the tissue developed in the inner dentinal walls, and a lack of long-term evidence of root reinforcement may contraindicate the revascularization technique for all cases involving necrotic immature permanent teeth.
In an effort to combat the effects of endodontic infection, the steady emergence of modern tissue engineering, particularly the selection and precise design of scaffolds, and the increased understanding of the role dental stem cells play in the regenerative processes have paved the way for their successful clinical use in endodontics. Based on these facts, researchers continue to work on tissue-engineering-based strategies to obtain more convincing and predictable evidence with regard to regeneration of the pulp-dentin complex. Thus, the purpose of this review is to give a comprehensive perspective on the latest discoveries related to the use of scaffolds and/or stem cells in regenerative endodontics.
Apexification vs. Regenerative Endodontics
Treatment of necrotic immature teeth has been considered a challenge in endodontics. Therefore, the prospect for achieving regeneration of the pulp-dentin complex holds promise for prolonging the use of the natural dentition (Bottino et al., 2014b). Traditionally, these teeth have undergone apexification therapy (Damle et al., 2012; Diogenes et al., 2013). This treatment modality uses root canal instrumentation, followed by periodic changes of intracanal medication composed of Ca(OH)2 until a calcified tissue is formed in the apex. During the treatment period, intracanal medication is generally replaced every 3 months, thereby involving multiple office visits and inevitably high clinical costs. As an alternative to the classic apexification treatment, the mineral trioxide aggregate apical plug has also been used and presents the advantage of being concluded in 1 or 2 sessions (Damle et al., 2012). Nonetheless, while these apexification approaches promote only apical closure, they do not allow for root development (Damle et al., 2012).
Recently emerging as an alternative to apexification, the current clinically advocated revascularization strategy has been shown to induce root extension and radicular reinforcement (Diogenes et al., 2013). Notably, root extension has increased the survival rate of revascularized teeth (100%) when compared with teeth treated via apexification with Ca(OH)2 (77.2%) (Jeeruphan et al., 2012). Revascularization procedures account for a wide variety of clinical protocols involving an association of sodium hypochlorite (NaOCl) and intracanal medication, including a mixture of antibiotics or Ca(OH)2, and have been proposed to achieve maximum elimination of bacteria in necrotic immature teeth (Banchs and Trope, 2004). More importantly, infection should be eradicated and the microbial ecosystem disrupted when minimal root canal instrumentation is used to preserve undifferentiated cells, and when excessive root canal wall instrumentation is avoided. Hence, most pulp revascularization studies advocate root canal irrigation with antimicrobial substances and an antibiotic mixture (metronidazole, ciprofloxacin, and minocycline) (Banchs and Trope, 2004; Bose et al., 2009; Diogenes et al., 2013). Nonetheless, it has been suggested that the use of NaOCl degrades dentin-derived proteins (e.g., BMP-2), which are essential for the odontoblastic differentiation of dental pulp stem cells (Casagrande et al., 2010). From a histological standpoint, currently, only one case report has demonstrated pulp-like tissue formation after the revascularization procedure (Shimizu et al., 2012). Most studies, including findings from animal studies and case reports, show the invaginated tissue consisting of periapical tissue containing bone-like hard tissue and the thickness of root canal walls promoted by cementum-like tissue (Martin et al., 2013; Becerra et al., 2014). In summary, pulp tissue injury resulting from trauma to or caries in teeth with incomplete apical development has provided a unique opportunity for exploration of the regenerative potential in endodontics.
Nanofibrous Scaffolds for Regenerative Endodontics
The past decade has seen an exponential growth of studies involving well-known principles of tissue engineering and regenerative medicine to further advance the fairly new field of regenerative endodontics (Table). Three major components of regenerative-based strategies have been explored, both individually and in association, to achieve tissue regeneration, namely, (i) cell-based therapies, (ii) signaling molecules (e.g., growth factors), and (iii) scaffolds. Ideally, a scaffold should accurately reproduce the features of the native ECM at the nanoscale to regulate cellular responses and encourage and regulate specific events at the cellular and tissue levels (Li et al., 2005; Huang, 2009; Gupte and Ma, 2012). Furthermore, it has been well-established that the synthesis of scaffolds should involve the use of biocompatible and biodegradable material(s) to avoid immunologically mediated reactions.
Table.
Summary of Recent Findings with Tissue-engineering Strategies in Regenerative Endodontics
| Tissue-engineering Strategy |
|||||
|---|---|---|---|---|---|
| Author/Year | Study Design | Scaffold | Bioactive Molecules | Stem Cells | Most Relevant Findings |
| Dobie et al., 2002 | In vitro | Alginate HY with TGF-β1 | Yes | No | ✓ Release of TGF-β1; ✓ odontoblast-like cell differentiation |
| Galler et al., 2008 | In vitro | PA self-assembling NF - HY | No | Yes (DPSCs and SHEDs) | ✓ Easy to handle; ✓ introduced into small defects; ✓ cell proliferation |
| Cordeiro et al., 2008 | In vivo | PLLA | No | Yes (SHEDs) | ✓ Pulp-like tissue formation |
| Prescott et al., 2008 | In vivo | Col Type I with CP and DMP-1 | No | Yes (DPSCs) | ✓ New pulp-like tissue formation and organization |
| Ishimatsu et al., 2009 | In vitro | Gelatin HY incorporation of FGF-2 | Yes | No | ✓ Release of FGF-2 ✓ Induces the invasion of dental pulp cells and vessels |
| Yang et al., 2010 | In vitroIn vivo | NF-PCL/gelatin/nHA | No | Yes (DPSCs) | ✓ DPSC differentiation toward an odontoblast-like cells in vitro and in vivo |
| Feng et al., 2010 | In vitro | NF-PLGA/PLLA scaffolds with DOX | Yes | No | ✓ Release of DOX; ✓ inhibition of bacterial growth for a prolonged duration |
| Huang et al., 2010 | In vivo | poly-D,L-lactide/glycolide | No | Yes (DPSCs and SCAPs) | ✓ Pulp-like tissue formation with vascularity and dentin-like structure |
| Nakashima and Iohara, 2011 | In vivo | Col with SDF-1 | No | Yes (dog pulp CD105+, CD31- SP cells) | ✓ Complete pulp-like tissue regeneration |
| Galler et al., 2011b | In vivo | GF–laden peptide HY with VEGF, TGFβ -1, and FGF-2 | Yes | No | ✓ Release of VEGF, TGF-β1, and FGF2; ✓ odontoblast-like cell differentiation; ✓ pulp-like tissue formation |
| Galler et al., 2011a | In vivo | PEGylated fibrin gel | No | Yes (DPSCs, SHEDs, PDLSCs, and BMSSCs) | ✓ All types of dental stem cells proliferated; ✓ excellent biocompatibility; ✓ insertion into small defects |
| Wang et al., 2011 | In vitroIn vivo | NF-PLLA | No | Yes (DPSCs) | ✓ Attachment, proliferation, and differentiation of human DPSCs; |
| Iohara et al., 2011 | In vivo | Col with SDF-1 | No | Yes (dog pulp CD105+ cells) | ✓ Complete pulp-like and dentin-like tissue regeneration; ✓ orthotopic model |
| Galler et al., 2012 | In vitro | Self-assembling MDP Peptide NF-HY | Yes | Yes (DPSCs) | ✓ Pulp-like tissue formation |
| Zhang et al., 2012 | In vivo | DDM-PLLG/Co–CS–HA | No | Yes (DPSCs) | ✓ Potential as attractive scaffolds for odontogenic differentiation |
| Ishizaka et al., 2012 | In vivo | Col with SDF-1 | No | Yes (dog pulp, BM, Adipose CD31- SP cells) | ✓ Complete pulp-like tissue regeneration; ✓ orthotopic model |
| Akkouch et al., 2013 | In vitro | 3D Col/HA/PLCL | No | Yes (DPSCs) | ✓ DPSC differentiation and proliferation |
| Bottino et al., 2013 | In vitro | NF PDS II-with MET and CIP | Yes | No | ✓ Release MET or CIP; ✓ antimicrobial activity against Ef and Pg |
| Bottino et al., 2014b | In vitro | NF PDS II-HNTs | No | No | ✓ Potential in the development of a bioactive scaffold for regenerative endodontics |
| Cavalcanti et al., 2013 | In vitro | Self-assembling peptide HY (Puramatrix™) | No | Yes (DPSCs) | ✓ DPSC survival, proliferation, and differentiation |
| Coyac et al., 2013 | In vitro | 3D dense Col HY | No | Yes (SHEDs) | ✓ Odontogenic cell differentiation and mineralization |
| Iohara et al., 2013 | In vivo | Col with G-CSF | No | Yes (dog mobilized DPSCs) | ✓ Complete pulp-like and dentin-like tissue regeneration; ✓ orthotopic pre-clinical model |
| Murakami et al., 2013 | In vivo | Col | No | Yes (human mobilized DPSCs) | ✓ Ectopic model; ✓ pulp-like tissue regeneration |
| Rosa et al., 2013 | In vivo | Peptide HY (Puramatrix™) with rhCol type I | No | Yes (SHEDs) | ✓ SHED injected into full-length human root canals differentiate into functional odontoblasts |
| Yang et al., 2014 | In vivo | Porous chitosan/col scaffold | Yes | Yes (DPSCs) | ✓ Release of BMP-7 gene; ✓ DPSC differentiation into odontoblast-like cells in vitro and in vivo |
| Iohara et al., 2014 | In vivo | Col with G-CSF | No | Yes (dog mobilized DPSCs) | ✓ Orthotopic model; ✓ less volume of regenerated pulp-like tissue in aged dogs compared with that in young dogs |
| Nakashima and Iohara, 2014 | In vivo | No | No | Yes (mobilized DPSCs) | ✓ Complete pulp-like tissue regeneration with thick coronal dentin formation in pulpectomized root canals |
H, hydrogel; TGF-β1, transforming growth factor β family; PA, peptide-amphiphile; DPSCs, dental pulp stem cells; SHEDs, stem cells from human exfoliated deciduous teeth; PLLA, Poly(L-lactic acid); CP, ceramic powder; DMP-1, dentin matrix protein 1; FGF-2, fibroblast growth factor–2; PCL, poly(ϵ-caprolactone); nHA, nano-hydroxyapatite; DOX, doxycycline; PLGA, poly(lactic-co-glycolic acid); GF, growth factor; VEGF, vascular endothelial growth factor; PEG, polyethylene glycol; PDLSCs, periodontal ligament stem cells; BMSSCs, bone marrow stromal stem cells; NF, nanofibrous; MDP, multidomain peptides; DDM, demineralized dentin matrix; Co–CS–HA, collagen–chondroitin sulfate–hyaluronic acid; Col, collagen; PLCL, poly(L-lactide-co-ϵ-caprolactone); PDS-II, nanocomposite scaffold composed of polydioxanone; HNT, halloysite nanotubes; MET, metronidazole; CIP, Ciprofloxacin; Ef, Enterococcus faecalis; Pg, Porphyromonas gingivalis; CSDP, scaffold-free stem-cell-sheet-derived pellet; SCAPs, stem cells from root apical papilla; rhCol, recombinant human collagen; BMP-7, human bone morphogenetic protein-7; SDF-1, stromal-cell-derived factor-1; SP, side-population; G-CSF, granulocyte colony-stimulating factor.
Recent advances in the field of nanotechnology have greatly contributed to the synthesis of novel ECM-mimicking structures with adequate chemistry and overall 3D porous architectures (Li et al., 2005; Bottino et al., 2012; Gupte and Ma, 2012). In this way, nanofibrous polymer scaffolds are becoming increasingly popular. They can be tailored to display mechanical competence, high processing ability, and biocompatible and biodegradable characteristics (Gupte and Ma, 2012; Bottino et al., 2012). Because of several advantages, such as high surface area, interconnected porosity, and nanoscale fiber dimension, nanofibrous scaffolds are more favorable than microfibers or any other morphological arrangements. Most importantly, nanofibrous scaffolds have been known to stimulate positive cell-ECM interactions, increase cell proliferation, maintain cell phenotype, support differentiation of stem cells, and activate cell-signaling pathways by providing physical and chemical stimuli (Li et al., 2005; Yang et al., 2005; Gupte and Ma, 2012). From a materials processing perspective, several techniques have been developed, including electrospinning, molecular self-assembly, and thermally induced phase separation.
Electrospinning or electrostatic spinning is a fairly straightforward nanotechnology-based technique. It consists of the application of a high electric field to a polymer solution or melt that flows through a needle orifice to produce continuous polymer fibers with diameters in the range of nanometers to micrometers (Reneker and Chun, 1996). Polymer solutions can be incorporated not only with bioactive nanoparticles, but also with signaling molecules and therapeutic agents (Bottino et al., 2012; Gupte and Ma, 2012). Noteworthy, by adjustment of electrospinning parameters, fiber morphology can be controlled, along with fiber diameter, pore size, and fiber alignment, among other factors known to influence cell behavior and overall tissue regeneration (Li et al., 2005; Yang et al., 2005; Gupte and Ma, 2012).
Molecular self-assembly has been used to generate nanofibrous scaffolds through spontaneous molecular arrangement via non-covalent interactions, such as hydrogen bonds. This processing technique has not only allowed for the recapitulation of collagen’s supramolecule formation, but it also has demonstrated a significant ability to enhance cell adhesion similar to that of collagen type-I (Gupte and Ma, 2012). Some of the advantages of self-assembly nanofibers for regenerative endodontics are that these nanofibers are assembled in solution and result in gels that can be useful for cell encapsulation (Ishimatsu et al., 2009; Galler et al., 2012; Zhang et al., 2012; Cavalcanti et al., 2013; Coyac et al., 2013; Rosa et al., 2013). Furthermore, the solution can be applied via injection through a minimally invasive procedure, leading to the formation of a nanofibrous scaffold in situ. Nevertheless, it is important to keep in mind that molecular self-assembly has limitations in terms of controlling pore size/shape within the hydrogel scaffold (Gupte and Ma, 2012), in addition to generally insufficient mechanical properties (Zhang et al., 2012).
Thermally induced phase separation (TIPS) has also been explored to fabricate nanofibrous scaffolds. Interestingly, TIPS can be combined with other techniques to generate macro/micro pore/channel networks within the 3D nanofibrous scaffolds to optimize cell infiltration and proliferation, nutrient transport, angiogenesis, and new tissue formation/organization (Gupte and Ma, 2012). In sum, nanofibrous scaffolds have been shown to be a promising class of biomaterials for regenerative endodontics. Within this context, a summary of the recent scaffold developments, using electrospinning both as a drug delivery system for root canal disinfection and as a bioactive scaffold, as well as self-assembled polymer hydrogels, is provided.
Bioactive Scaffolds for Pulp-Dentin Complex Regeneration
Advances in the field of nanotechnology and biomaterials science have allowed researchers to obtain scaffolds that are able to serve as delivery vehicles for bioactive and instructive factors that can be released in a controlled fashion depending on the clinical application (Sundararaj et al., 2014).
Recently, electrospinning (Fig. 1A) has been used successfully to generate scaffolds that deliver uniform and greatly controlled antibiotic(s) amounts (Bottino et al., 2013, 2014a; Palasuk et al., 2014). Considering recent concerns regarding the toxic effects of highly concentrated antibiotic pastes on SCAPs survival, antibiotic-containing scaffolds (Fig. 1A) may minimize these adverse effects and yet still promote the elimination of bacteria (Bottino et al., 2013, 2014a; Palasuk et al., 2014). Analysis of high-performance liquid chromatography (HPLC) data, in addition to cell toxicity experiments, has supported the claim of a more biologically friendly strategy when compared with the use of antibiotic pastes, since the quantity of drug(s) released occurs more gradually in a lower concentration than in those used in pastes (Bottino et al., 2013, 2014a; Palasuk et al., 2014). Meanwhile, lower concentrations of antibiotics demonstrate antimicrobial action against Porphyromonas gingivalis and Enterococcus faecalis biofilms (Bottino et al., 2013).
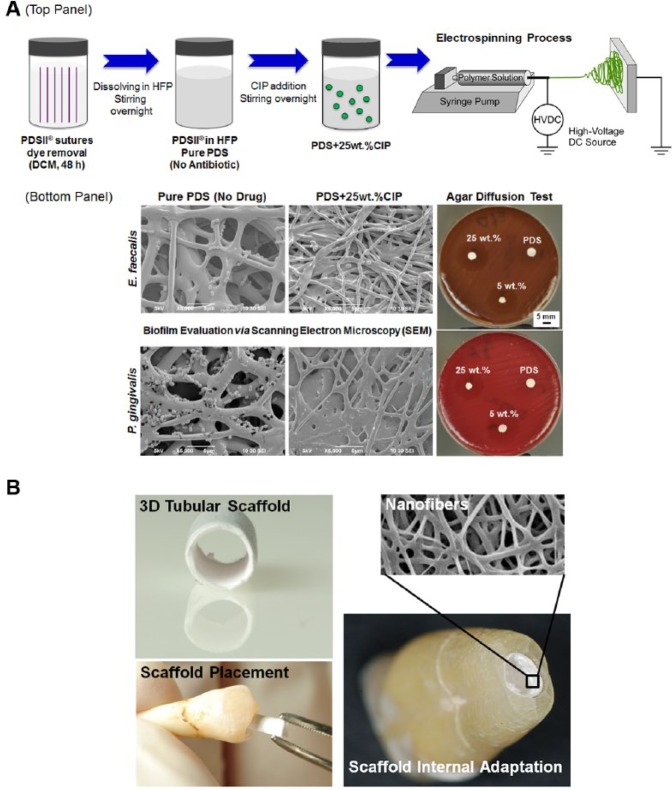
Figure 1.
Summarized schematic of nanofibrous antibiotic-containing scaffolds (e.g., ciprofloxacin [CIP]) processed via electrospinning and antimicrobial effects. (A) (top panel) FDA-approved polydioxanone suture filaments were used (PDS II®, Ethicon, Somerville, NJ, USA). First, the violet color of filament sutures is removed by immersion in dichloromethane. Then, the cleared PDS filaments are dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFP, Sigma-Aldrich, St. Louis, MO, USA) at optimized concentration under stirring conditions. CIP-containing PDS solution is prepared by the addition of CIP at a known concentration, being mixed together under vigorous stirring. (bottom panel) Representative scanning electron microscopy (SEM) micrographs showing the antimicrobial effects of antibiotic-containing PDS-based electrospun scaffolds on bacterial growth. Representative macrophotographs of the agar diffusion test show growth inhibition of E. faecalis and P. gingivalis) (adapted with permission from Bottino et al., 2013). (B) Potential clinical application of a three-dimensional (3D) tubular scaffold produced via electrospinning. Electrospun scaffolds can be fabricated in a cylindrical shape simulating the tubular and parallel format of immature root canals, making it easy to place and adapt into the root canal. Inset shows the nanofibrous structure of the 3D scaffold.
Another recently reported promising nanofibrous-based strategy pertains to the design and fabrication of 3D tubular scaffolds for pulp-dentin complex regeneration (Fig. 1B) (Bottino et al., 2014b). Briefly, biodegradable polymer nanocomposite fibrous scaffolds were synthesized with aluminosilicate clay nanotubes to serve as a delivery vehicle of bioactive agents (e.g., antimicrobial drugs and angiogenic factors, among others) for controlled release within the root canal system. Preliminary results have proven the biocompatibility of these nanocomposite scaffolds containing distinct nanotube amounts in human-pulp-derived fibroblasts (Fig. 2) (Bottino et al., 2014b).
Figure 2.
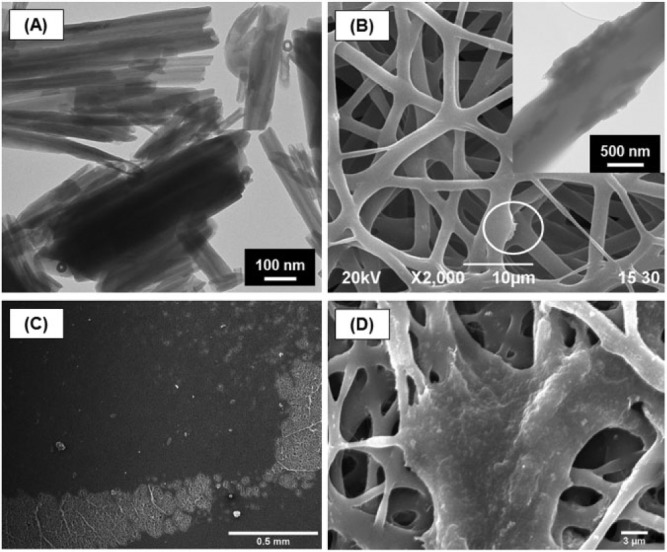
Polymer nanocomposite electrospun scaffolds synthesized with aluminosilicate clay nanotubes. (A) Representative transmission electron microscopy (TEM) micrograph of aluminosilicate clay Halloysite nanotubes (HNTs). (B) Representative scanning electron microscopy (SEM) micrograph of electrospun HNT-incorporated nanofibrous scaffolds. (inset) Representative TEM micrograph of HNTs protruding from the fiber structure. (C-D) Representative SEM micrographs showing the interaction between human-derived dental pulp fibroblast cells and PDS-HNT fibrous scaffolds (adapted with permission from Bottino et al., 2014b).
To obtain the ideal rigidity that allows for easy introduction into the root canal system without scaffold and/or cell damage, these tubular 3D electrospun scaffolds (Fig. 1B) can be developed from different polymers. Ultimately, these scaffolds can be processed in several geometries (Bottino et al., 2012). To date, the goal has been to introduce tubular 3D scaffolds into the root canal system of immature teeth that present parallel/thin dentin walls and an open apex. Nonetheless, one should note that the anatomy of immature teeth varies according to the developmental stage in which the pulp tissue incurred damage. Thus, these scaffolds can be fabricated in several diameters and lengths that can easily adapt to these anatomic variations, facilitating their insertion into the root canal with tweezers, with any excess being cut at the enamel-cementum level (Fig. 1B).
From a clinical viewpoint, one may question whether electrospinning technology could be successfully used in regenerative endodontics, since most investigators lean toward claiming that injectable scaffolds would be the most ideal strategy (Cavalcanti et al., 2013). Within this context, a recent study demonstrated the opportunity for the fabrication of injectable cell-coupled 3D nanofibrous scaffold based on electrospinning technology. An injectable osteogenic nanofibrous scaffold obtained by interspersing polycaprolactone nanofibers within pre-osteoblast cell-embedded collagen type-I revealed an intricate porous internal architecture, thus improving the proliferation and differentiation of cells (Baylan et al., 2013).
Taken together, though electrospun scaffolds have not yet been tested in vivo in either human or animal pulpless models in immature permanent teeth, recent studies (Yang et al., 2010; Kim et al., 2014) positively highlight their regenerative potential from both an in vitro and an in vivo (i.e., subcutaneous model) standpoint. Possible applications for these bioactive scaffolds are evolving, with significant prospects related to the regeneration of both dentin and pulp tissue.
Injectable Scaffolds and Stem Cells: Challenges & Perspectives
The structural support scaffolds provide to stem cells may also be achieved through the use of injectable hydrogel polymers. Notably, the non-invasive application allied to the ability of intracanal delivery has been highlighted, thus creating a niche for stem cells (Cavalcanti et al., 2013; Rosa et al., 2013). Growth factors may also be incorporated into hydrogels, targeting a gradual and localized release (Ishimatsu et al., 2009). For example, the addition of basic fibroblast growth factors (bFGF) within gelatin hydrogels led to the neovascularization and regeneration of tissues relevant to the dentin-pulp complex (Ishimatsu et al., 2009; Nagy et al., 2014).
Despite great advances in hydrogel-based bioengineering, some challenges still relate to the restricted control over new tissue formation. A very promising hydrogel-based nanofibrous scaffold refers to Puramatrix™ (Rosa et al., 2013). Puramatrix, a self-assembling peptide hydrogel, is composed of a 16-mer peptide in aqueous solution. Upon interaction with physiological conditions, it polymerizes and forms a biodegradable nanofiber hydrogel scaffold (Rosa et al., 2013). This mechanism favors clinical application that requires not only a biocompatible matrix, but also one that can be rapidly formed. It has been shown to support dental pulp stem cell survival and proliferation in vitro (Cavalcanti et al., 2013). Convincing evidence of the potential impact in the clinical setting has recently been reported. A mixture composed of stem cells from exfoliated deciduous teeth (SHED) and Puramatrix was able to generate a pulp-like tissue when injected into full-length root canals, suggesting that this strategy might aid completion of the root formation in necrotic immature permanent teeth (Rosa et al., 2013).
In recent years, the technical practicality associated with the use of injectable hydrogels that can be dispersed inside a closed, small space, such as the root canal system, has propelled studies in terms of its potential application in regenerative endodontics. However, the self-assembling design presents limitations relative to mechanical properties and structure – for example, irregular pore size, influence of the viscosity in cell proliferation, and difficulty maintaining the hydrogel throughout the whole root canal extension (Gupte and Ma, 2012; Zhang et al., 2012; Rosa et al., 2013). Meanwhile, the electrospinning technique used to generate nanofibrous scaffolds, either as sheets or in a 3D tubular form, has the advantage of controlling fiber diameter. This technique can also control pore size and interconnectivity to better support cell attachment, proliferation, and differentiation through the use of sacrificial fibers (Phipps et al., 2012). Nonetheless, taking into account the limitations and advantages of each fabrication process, recent studies have successfully demonstrated the possibility of obtaining a structurally competent injectable electrospun-based scaffold capable of improving cell retention and survival (Ravichandran et al., 2012; Baylan et al., 2013).
Stem-cell-based Regenerative Therapies: Pre-clinical Findings
No single implantable scaffold can consistently guide the coordinated growth and development of the multiple tissue types involved in the functional regeneration of the pulp-dentin complex. Therefore, in addition to the in vitro studies mentioned, which test different types of scaffolds and stem cell sources, pre-clinical research with stem cells in association with scaffolds and/or growth factors have been carried out in immunocompromised animal models (Table).
To address the clinical potential of stem cell transplantation, a well-known model, termed the “tooth slice/scaffold model,” has provided major insight into the use of scaffolds and stem cells in regenerative endodontics (Fig. 3). Briefly, scaffolds are prepared in vitro within dentin slices that are further seeded with stem cells prior to implantation into the subcutaneous tissue of immunodeficient mice (Cordeiro et al., 2008). Previous studies have used fairly stiff, macroporous scaffolds obtained through polymer casting (Sakai et al., 2011). Even though these scaffolds have provided important evidence that supports cell attachment, proliferation, and differentiation, their inherent rigidity has raised clinically relevant concerns with regard to scaffold adaptation to dentin walls over the entire length of the root canal. Self-assembly hydrogels (Cavalcanti et al., 2013) and, more recently, injectable electrospun-based scaffolds (Baylan et al., 2013) have shown important structural stability over time, with better prospects for overcoming the adaptation issue associated with initial testing of macroporous scaffolds (Cordeiro et al., 2008; Huang et al., 2010).
Figure 3.
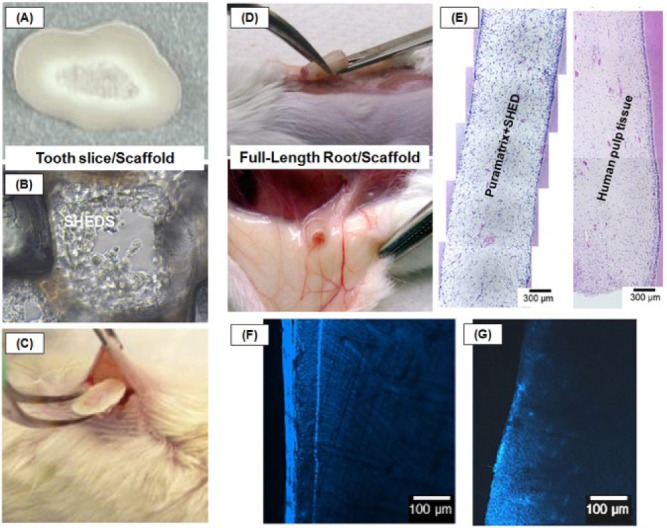
Summarized schematic of the (A-C) tooth slice and (D-G) full-length root/scaffold models. (A) Tooth slice provided from the cervical third of a human third molar with a highly porous PLLA scaffold placed within the pulp chamber. (B) SHED proliferation into the tooth slice/scaffold. (C) Insertion of a tooth slice and scaffold containing SHED into the subcutaneous space of the dorsum of an immunodeficient mouse. (D) Subcutaneous transplant of a human full-length root injected with hydrogel-based nanofibrous scaffolds containing SHEDs. (E) Photomicrographs of the engineered pulp-like tissue and human pulp tissue (control) in the root canal. (F) Layer of dentin formation after pulp tissue induction in PuraMatrix+SHEDs. (G) Dentin slice with no SHEDs (adapted with permission from Sakai et al., 2011; Casagrande et al., 2011; Rosa et al., 2013).
Full-length human roots were recently used, and injectable scaffolds (i.e., Puramatrix or recombinant human collagen) were mixed with SHED before in vivo transplantation, leading to the formation of a pulp-like tissue (Fig. 3) throughout the root canal extension (Rosa et al., 2013). Nonetheless, it is worth mentioning that the animal model involving transplantation of roots into the subcutaneous tissue of mice does not completely simulate clinical conditions, such as the presence of apical papilla that contains stem cells (SCAPs) as a source for pulp regeneration.
Despite significant progress, vasculogenesis/angiogenesis and neurogenesis/re-innervation in the regenerated pulp remain challenging. Therefore, the study of alternative sources for DPSCs, such as CD31- side-population (SP) cells and CD105+ cells with higher angiogenic and neurogenic potentials, remains paramount. Complete pulp regeneration with adequate vasculature and innervation has been observed (Figs. 4A-4C) after autologous transplantation of CD31- (SP) cells or CD105+ cells associated with stromal-cell-derived factor-1 (SDF-1) and a collagen scaffold into the pulpectomized root canals of dogs (Nakashima and Iohara, 2011; Iohara et al., 2011; Ishizaka et al., 2012). Moreover, new dentin formation along the dentinal wall was demonstrated (Fig. 4D). Transplantation of pulp CD31- (SP) cells induced higher vasculogenesis/angiogenesis, neurogenesis, and pulp regeneration in experimental models of hindlimb ischemia (Figs. 4E, 4F), brain ischemia (Figs. 4G, 4H), and ectopic tooth root transplantation (Figs. 4I, 4J), compared with that of bone marrow and adipose CD31- SP cells (Ishizaka et al., 2013), suggesting that DPSC subfractions may be superior for cell-based regenerative endodontics by higher migratory activity and trophic effects.
Figure 4.
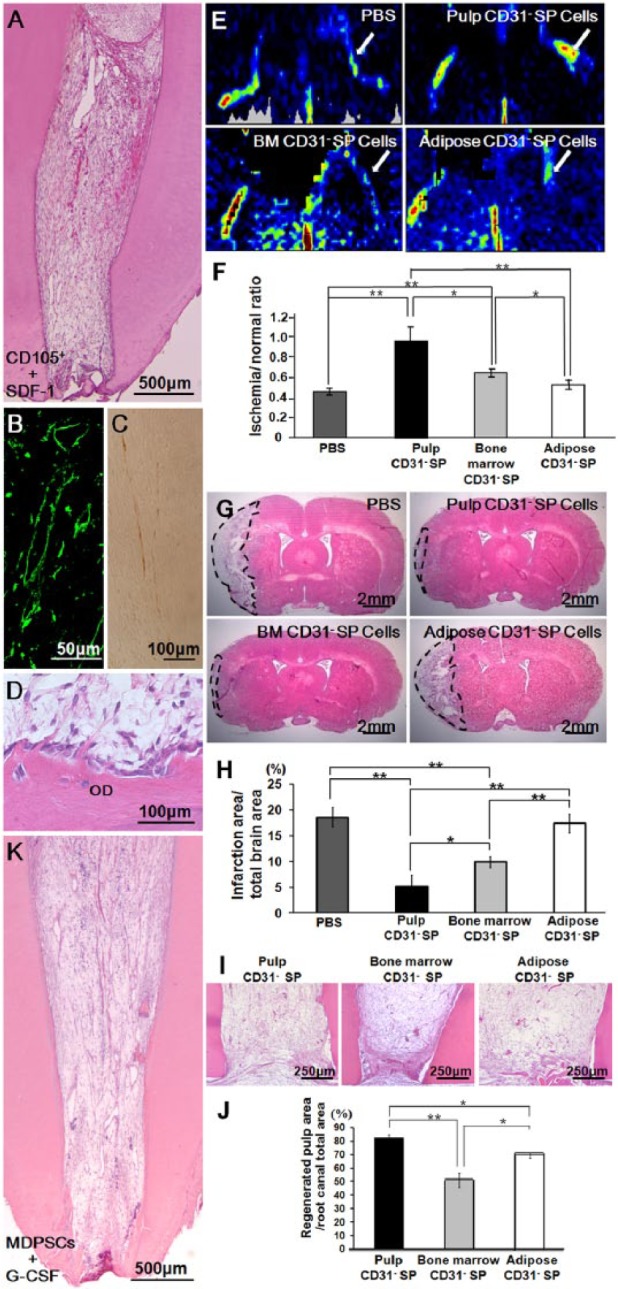
Clinical evidence of dentin-pulp complex regeneration. (A-D) Complete regeneration of pulp tissue after autologous transplantation of CD105+ cells with SDF-1 in the pulpectomized root canal in dogs. (B) Immunostaining with BS-1 lectin. (C) Immunostaining with PGP 9.5. (D) Odontoblastic cell lining to newly formed osteodentin/tubular dentin (OD), along with the dentinal wall. (E, F) Neovascularization in the ischemic hindlimb model 14 days after transplantation of pulp, bone marrow, and adipose-derived CD31- side-population (SP) cells. (E) Laser Doppler imaging. (F) Quantification of blood flow in mouse ischemic hindlimbs (n = 4 in each group). (G, H) Infarct area on day 21 after injection of PBS, pulp, bone marrow, and adipose CD31- SP cells. (H) Reduction of the infarct volume 21 days after injection (*p < .05, **p < .01). (I, J) Ectopic pulp regeneration 28 days after transplantation of pulp, bone marrow, and adipose-derived CD31- SP cells into porcine tooth root. (J) Ratio of regenerated pulp area to root canal area. Data are expressed as means ± SD of 5 determinations. *p < .05, **p < .01. (K) Complete regeneration of pulp tissue after autologous transplantation of mobilized dental pulp stem cells (MDPSCs) with G-CSF in the pulpectomized root canal in dogs (adapted with permission from Iohara et al., 2011; Ishizaka et al., 2013; Iohara et al., 2013).
Collectively, although pre-clinical animal models have provided significant evidence of the benefits associated with the utilization of tissue-engineering-based strategies, there are still challenges to making these methods and techniques applicable in humans. Above all, it is essential to manufacture clinical-grade stem cells according to good manufacturing practice (GMP) conditions. A safe technique that isolates DPSC subsets has recently been devised by the use of an optimized granulocyte-colony-stimulating factor (G-CSF)-induced mobilization (Murakami et al., 2013). The mobilized DPSCs (MDPSCs) demonstrated stem cell properties, including high proliferation rates, migratory activity, and expression of multiple trophic factors (Murakami et al., 2013). The absence of contamination, abnormalities/aberrations in karyotype, and tumorigenicity ensured excellent quality control of the MDPSCs manufactured in a GMP-compliant facility. Both pre-clinical efficacy and safety tests were performed in dogs using clinical-grade G-CSF and collagen with MDPSCs, which resulted in complete pulp regeneration (Fig. 4K) with coronal dentin formation in the pulpectomized root canal and no evidence of toxicity and adverse events (Iohara et al., 2013). G-CSF has combinatorial trophic effects with MDPSCs. Thus, based on the results of these pre-clinical trials, scientific evidence of the safety and efficacy critical for clinical applications has been presented (Nakashima and Iohara, 2014).
Conclusions
Recent clinical evidence has demonstrated potential for the currently advocated revascularization technique in regenerative endodontics. However, despite the promising observations, treatment still presents limitations, including but not limited to neural and vascular regeneration. In this way, regenerative endodontics by tissue-engineering-based strategies has gained increased attention. A variety of scaffolds, including electrospinning and self-assembly alone or associated with biomolecules, has been investigated. A promising finding toward the success of regenerative endodontics refers to the development of antibiotic-containing nanofibrous scaffolds. Future studies using infected root canals in vivo, which show development of pulp-like tissue, should be conducted to prove clinical relevance. Nevertheless, considering the advances discussed in this paper, an ideal regenerative protocol is close at hand. Such a strategy (Fig. 5) would primarily include root decontamination with antimicrobial irrigant solutions, followed by insertion of a more cell-friendly bioactive scaffold containing antimicrobial substances to be released inside the canal. Once a bacteria-free environment conducive to tissue regeneration has been established, scaffolds containing growth factors and/or stem cells would be placed to induce development of a new pulp tissue containing odontoblasts that will form dentin-like tissue. This new pulp tissue will increase the thickness of the dentin walls and successfully provide re-establishment of tooth function in the oral cavity. The authors consider, as the field progresses, that future research should be focused on the decontamination of root canals through the precise and controlled delivery of antibiotic drugs, followed by programed release of other active molecules (e.g., growth factors) to support a successful and functional regeneration of the pulp-dentin complex.
Figure 5.
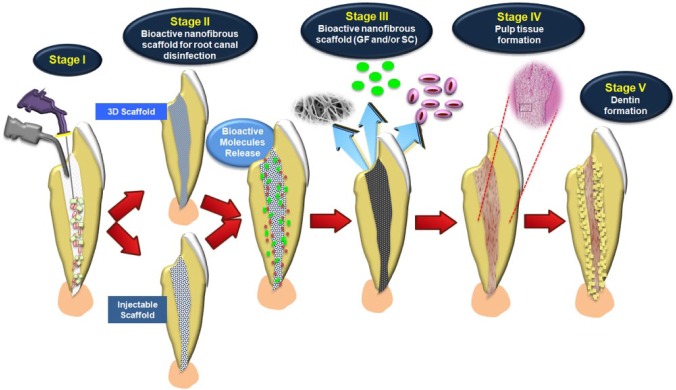
Tissue-engineering-based strategies for regenerative endodontics in immature teeth. Strategies have included the incorporation of (i) therapeutic agents, such as antimicrobial drugs to be released and promote root canal disinfection, as well as (ii) bioactive molecules that can trigger stem cell differentiation to aid in regeneration of the pulp-dentin complex. Stage I: Disinfection of the root canal using irrigant solutions. Stage II: Bioactive nanofibrous scaffold with antibiotics as intracanal medication. Stage III: Nanofibrous scaffold with growth factors and/or stem cells. Stage IV: Pulp tissue formation. Stage V: Dentin formation. (Histology of pulp-like tissue formation, adapted with permission from Rosa et al., 2013.)
Acknowledgments
The authors gratefully acknowledge the contributions of the anonymous reviewers who provided stimulating and helpful comments on a former version of this manuscript. M.C.B. thanks colleagues from the Indiana University School of Dentistry (IUSD), Drs. Krzysztof Kamocki and Richard L. Gregory, for their collaboration on the electrospun nanofibrous scaffolds research. Appreciation is expressed to Drs. Jeffrey A. Platt, Ygal Ehrlich, and Kenneth J. Spolnik (IUSD) for stimulating discussions. Thanks also go to Dr. Koichiro Iohara for his assistance in editing Figure 4. The authors apologize for not citing all relevant references due to a perceived lack of fit or space limitations.
Footnotes
This article is derived from a symposium presentation at the 92nd General Session & Exhibition of the International Association for Dental Research (IADR), which was held June 25-28, 2014, in Cape Town, South Africa.
M.C.B. also acknowledges funding from the American Association of Endodontists Foundation, start-up funds from IUSD, the National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) (Grant #DE023552), and an International Development Funds (IDF) Grant from Indiana University Purdue University (IUPUI/OVCR). J.E.N. acknowledges NIH/NIDCR Grant #R01DE21410. M.N. acknowledges the Research Grant for Longevity Sciences (23-10) from the Ministry of Health, Labour and Welfare (M.N.), and the budget for promoting science and technology in Japan.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Akkouch A, Zhang Z, Rouabhia M. (2013). Engineering bone tissue using human dental pulp stem cells and an osteogenic collagen-hydroxyapatite-poly (L-lactide-co-ϵ-caprolactone) scaffold. J Biomater Appl 28:922-936. [DOI] [PubMed] [Google Scholar]
- Banchs F, Trope M. (2004). Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod 30:196-200. [DOI] [PubMed] [Google Scholar]
- Baylan N, Bhat S, Ditto M, Lawrence JG, Lecka-Czernik B, Yildirim-Ayan E. (2013). Polycaprolactone nanofiber interspersed collagen type-I scaffold for bone regeneration: a unique injectable osteogenic scaffold. Biomed Mater 8:045011. [DOI] [PubMed] [Google Scholar]
- Becerra P, Ricucci D, Loghin S, Gibbs JL, Lin LM. (2014). Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J Endod 40:133-139. [DOI] [PubMed] [Google Scholar]
- Bose R, Nummikoski P, Hargreaves K. (2009). A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod 35:1343-1349. [DOI] [PubMed] [Google Scholar]
- Bottino MC, Thomas V, Schmidt G, Vohra YK, Chu TM, Kowolik MJ, et al. (2012). Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent Mater 28:703-721. [DOI] [PubMed] [Google Scholar]
- Bottino MC, Kamocki K, Yassen GH, Platt JA, Vail MM, Ehrlich Y, et al. (2013). Bioactive nanofibrous scaffolds for regenerative endodontics. J Dent Res 92:963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino MC, Arthur RA, Waeiss RA, Kamocki K, Gregson KS, Gregory RL. (2014a). Biodegradable nanofibrous drug delivery systems: effects of metronidazole and ciprofloxacin on periodontopathogens and commensal oral bacteria. Clin Oral Investig [Epub ahead of print 2/18/2014] (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino MC, Yassen GH, Platt JA, Labban N, Windsor LJ, Spolnik KJ, et al. (2014b). A novel three-dimensional scaffold for regenerative endodontics: materials and biological characterizations. J Tissue Eng Regen Med [Epub ahead of print 3/8/2013] (in press). [DOI] [PubMed] [Google Scholar]
- Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nör JE. (2010). Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res 89:603-608. [DOI] [PubMed] [Google Scholar]
- Casagrande L, Cordeiro MM, Nör SA, Nör JE. (2011). Dental pulp stem cells in regenerative dentistry. Odontology 99:1-7. [DOI] [PubMed] [Google Scholar]
- Cavalcanti BN, Zeitlin BD, Nör JE. (2013). A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent Mater 29:97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. (2008). Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34:962-969. [DOI] [PubMed] [Google Scholar]
- Coyac BR, Chicatun F, Hoac B, Nelea V, Chaussain C, Nazhat SN, et al. (2013). Mineralization of dense collagen hydrogel scaffolds by human pulp cells. J Dent Res 92:648-654. [DOI] [PubMed] [Google Scholar]
- Damle SG, Bhattal H, Loomba A. (2012). Apexification of anterior teeth: a comparative evaluation of mineral trioxide aggregate and calcium hydroxide paste. J Clin Pediatr Dent 36:263-268. [PubMed] [Google Scholar]
- Diogenes A, Henry MA, Teixeira FB, Hargreaves KM. (2013). An update on clinical regenerative endodontics. Endod Top 28:2-23. [Google Scholar]
- Diogenes AR, Ruparel NB, Teixeira FB, Hargreaves KM. (2014). Translational science in disinfection for regenerative endodontics. J Endod 40(4 Suppl):S52-S57. [DOI] [PubMed] [Google Scholar]
- Dobie K, Smith G, Sloan AJ, Smith AJ. (2002). Effects of alginate hydrogels and TGF-β1 on human dental pulp repair in vitro. Connective Tissue Res 43:387-390. [DOI] [PubMed] [Google Scholar]
- Feng K, Sun H, Bradley MA, Dupler EJ, Giannobile WV, Ma PX. (2010). Novel antibacterial nanofibrous PLLA scaffolds. J Control Release 146:363-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler KM, Cavender A, Yuwono V, Dong H, Shi S, Schmalz G, et al. (2008). Self-assembling peptide amphiphile nanofibers as a scaffold for dental stem cells. Tissue Eng Part A 14:2051-2058. [DOI] [PubMed] [Google Scholar]
- Galler KM, Cavender AC, Koeklue U, Suggs LJ, Schmalz G, D’Souza RN. (2011a). Bioengineering of dental stem cells in a PEGylated fibrin gel. Regen Med 6:191-200. [DOI] [PubMed] [Google Scholar]
- Galler KM, D’Souza RN, Federlin M, Cavender AC, Hartgerink JD, Hecker S, et al. (2011b). Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod 37:1536-1541. [DOI] [PubMed] [Google Scholar]
- Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN. (2012). A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A 18:176-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte MJ, Ma PX. (2012). Nanofibrous scaffolds for dental and craniofacial applications. J Dent Res 91:227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT. (2009). Pulp and dentin tissue engineering and regeneration: current progress. Regen Med 4:697-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT. (2011). Dental pulp and dentin tissue engineering and regeneration: advancement and challenge. Front Biosci (Elite Ed) 3:788-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, et al. (2010). Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A 16:605-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iohara K, Imabayashi K, Ishizaka R, Watanabe A, Nabekura J, Ito M, et al. (2011). Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A 17:1911-1920. [DOI] [PubMed] [Google Scholar]
- Iohara K, Murakami M, Takeuchi N, Osako Y, Ito M, Ishizaka R, et al. (2013). A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Transl Med 2:521-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iohara K, Murakami M, Nakata K, Nakashima M. (2014). Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp Gerontol 52:39-45. [DOI] [PubMed] [Google Scholar]
- Ishimatsu H, Kitamura C, Morotomi T, Tabata Y, Nishihara T, Chen KK, et al. (2009). Formation of dentinal bridge on surface of regenerated dental pulp in dentin defects by controlled release of fibroblast growth factor-2 from gelatin hydrogels. J Endod 35:858-865. [DOI] [PubMed] [Google Scholar]
- Ishizaka R, Iohara K, Murakami M, Fukuta O, Nakashima M. (2012). Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials 33:2109-2118. [DOI] [PubMed] [Google Scholar]
- Ishizaka R, Hayashi Y, Iohara K, Sugiyama M, Murakami M, Yamamoto T, et al. (2013). Stimulation of angiogenesis, neurogenesis and regeneration by side population cells from dental pulp. Biomaterials 34:1888-1897. [DOI] [PubMed] [Google Scholar]
- Iwaya S, Ikawa M, Kubota M. (2011). Revascularization of an immature permanent tooth with periradicular abscess after luxation. Dent Traumatol 27:55-58. [DOI] [PubMed] [Google Scholar]
- Jeeruphan T, Jantarat J, Yanpiset K, Suwannapan L, Khewsawai P, Hargreaves KM. (2012). Mahidol study 1: comparison of radiographic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods: a retrospective study. J Endod 38:1330-1336. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Bae WJ, Kim JM, Kim JJ, Lee EJ, Kim HW, et al. (2014). Mineralized polycaprolactone nanofibrous matrix for odontogenesis of human dental pulp cells. J Biomater Appl 28:1069-1078. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. (1993). Tissue engineering. Science 260:920-926. [DOI] [PubMed] [Google Scholar]
- Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, et al. (2005). A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 26:599-609. [DOI] [PubMed] [Google Scholar]
- Lu B, Atala A. (2013). Small molecules and small molecule drugs in regenerative medicine. Drug Discov Today 19:801-808. [DOI] [PubMed] [Google Scholar]
- Martin G, Ricucci D, Gibbs JL, Lin LM. (2013). Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J Endod 39:138-144. [DOI] [PubMed] [Google Scholar]
- Murakami M, Horibe H, Iohara K, Hayashi Y, Osako Y, Takei Y, et al. (2013). The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential. Biomaterials 34:9036-9047. [DOI] [PubMed] [Google Scholar]
- Nagata JY, Soares AJ, Souza-Filho FJ, Zaia AA, Ferraz CCR, Almeida JFA, Gomes BPFA. (2014). Microbial evaluation of traumatized teeth treated with triple antibiotic paste or calcium hydroxide with 2% chlorhexidine gel in pulp revascularization. J Endod 40:778–783. [DOI] [PubMed] [Google Scholar]
- Nagy MM, Tawfik HE, Hashem AA, Abu-Seida AM. (2014). Regenerative potential of immature permanent teeth with necrotic pulps after different regenerative protocols. J Endod 40:192-198. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Iohara K. (2011). Regeneration of dental pulp by stem cells. Adv Dent Res 23:313-319. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Iohara K. (2014). Mobilized dental pulp stem cells for pulp regeneration: initiation of clinical trial. J Endod 40(4 Suppl):S26-32. [DOI] [PubMed] [Google Scholar]
- Palasuk J, Kamocki K, Hippenmeyer L, Platt JA, Spolnik KJ, Gregory RL, et al. (2014). Bimix antimicrobial scaffolds for regenerative endodontics. J Endod 40:1879-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps MC, Clem WC, Grunda JM, Clines GA, Bellis SL. (2012). Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 33:524-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, et al. (2008). In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod 34:421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Sridhar R, Ramakrishna S. (2012). Minimally invasive injectable short nanofibers of poly(glycerol sebacate) for cardiac tissue engineering. Nanotechnology 23:385102. [DOI] [PubMed] [Google Scholar]
- Reneker DH, Chun I. (1996). Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 7:216-223. [Google Scholar]
- Rosa V, Zhang Z, Grande RH, Nör JE. (2013). Dental pulp tissue engineering in full-length human root canals. J Dent Res 92:970-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. (2012). Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod 38:1372-1375. [DOI] [PubMed] [Google Scholar]
- Sakai VT, Cordeiro MM, Dong Z, Zhang Z, Zeitlin BD, Nör JE. (2011). Tooth slice/scaffold model of dental pulp tissue engineering. Adv Dent Res 23:325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Jong G, Partridge N, Rosenberg PA, Lin LM. (2012). Histologic observation of a human immature permanent tooth with irreversible pulpitis after revascularization/regeneration procedure. J Endod 38:1293-1297. [DOI] [PubMed] [Google Scholar]
- Sundararaj SC, Thomas MV, Dziubla TD, Puleo DA. (2014). Bioerodible system for sequential release of multiple drugs. Acta Biomater 10:115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ma H, Jin X, Hu J, Liu X, Ni L, et al. (2011). The effect of scaffold architecture on odontogenic differentiation of human dental pulp stem cells. Biomaterials 32:7822-7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Murugan R, Wang S, Ramakrishna S. (2005). Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 26:2603-2610. [DOI] [PubMed] [Google Scholar]
- Yang X, Yang F, Walboomers XF, Bian Z, Fan M, Jansen JA. (2010). The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J Biomed Mater Res A 93:247-257. [DOI] [PubMed] [Google Scholar]
- Yang X, Han G, Pang X, Fan M. (2014). Chitosan/collagen scaffold containing bone morphogenetic protein-7 DNA supports dental pulp stem cell differentiation in vitro and in vivo. J Biomed Mater Res A [Epub ahead of print 2/18/2012] (in press). [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu S, Zhou Y, Tan J, Che H, Ning F, et al. (2012). Natural mineralized scaffolds promote the dentinogenic potential of dental pulp stem cells via the mitogen-activated protein kinase signaling pathway. Tissue Eng Part A 18:677-691. [DOI] [PubMed] [Google Scholar]