Abstract
In the United States, composites accounted for nearly 70% of the 173.2 million composite and amalgam restorations placed in 2006 (Kingman et al., 2012), and it is likely that the use of composite will continue to increase as dentists phase out dental amalgam. This trend is not, however, without consequences. The failure rate of composite restorations is double that of amalgam (Ferracane, 2013). Composite restorations accumulate more biofilm, experience more secondary decay, and require more frequent replacement. In vivo biodegradation of the adhesive bond at the composite-tooth interface is a major contributor to the cascade of events leading to restoration failure. Binding by proteins, particularly gp340, from the salivary pellicle leads to biofilm attachment, which accelerates degradation of the interfacial bond and demineralization of the tooth by recruiting the pioneer bacterium Streptococcus mutans to the surface. Bacterial production of lactic acid lowers the pH of the oral microenvironment, erodes hydroxyapatite in enamel and dentin, and promotes hydrolysis of the adhesive. Secreted esterases further hydrolyze the adhesive polymer, exposing the soft underlying collagenous dentinal matrix and allowing further infiltration by the pathogenic biofilm. Manifold approaches are being pursued to increase the longevity of composite dental restorations based on the major contributing factors responsible for degradation. The key material and biological components and the interactions involved in the destructive processes, including recent advances in understanding the structural and molecular basis of biofilm recruitment, are described in this review. Innovative strategies to mitigate these pathogenic effects and slow deterioration are discussed.
Keywords: dentin bonding agents, methacrylate, gp340, Streptococcus mutans, esterases, biofilm
Introduction
In 2006, 173.2 million composite and amalgam restorations were placed in the United States (Kingman et al., 2012), and the results from clinical studies suggest that more than half were replacements for failed restorations (Murray et al., 2002). Replacement of failed restorations consumes 60% of the average dentist’s practice time (National Institute of Dental and Craniofacial Research, 2009). This emphasis on replacement therapy will increase as dentists discontinue their use of dental amalgam (Krifka et al., 2013). Dental amalgam is being discontinued in response to global concerns about mercury in the environment.
Resin composite is the most common alternative to dental amalgam, but moderate to large composite restorations have higher failure rates, more recurrent decay, and increased frequency of replacement as compared with amalgam (Simecek et al., 2009). For example, the need for additional restorative care was 50% greater in children treated with composite as compared with amalgam (DeRouen et al., 2006). In comparison to amalgam, caries is the most frequent reason for failure of composite (Opdam et al., 2010). In vivo biodegradation of the bond between the composite and tooth (i.e., the composite restoration’s adhesive bond layer) is considered a particularly critical contributor to secondary loss of adhesion, microleakage, and decay (Donmez et al., 2005).
This review article focuses on key biological and physicochemical interactions involved in the failure of composite restorations. While it is likely that degradation of the restoration starts with the tooth surface, the interface between the synthetic material and biological tissue plays a vital role in shifting the microbial ecology from a state of health to a disease-associated state. Recent advances in biofilm recruitment and innovative strategies to mitigate its pathogenic effects at the composite-tooth interface are discussed.
Recurrent Decay
Clinically, 80% to 90% of recurrent decay is located at the gingival margin of class II and V restorations (Figure 1; Li et al., 2014). Recurrent decay is linked to failure of the bond between the tooth and composite and increased levels of the cariogenic bacterium, Streptococcus mutans, localized around the perimeter of these materials (Leinfelder, 2000). The composite is too viscous to bond directly to the tooth—a low viscosity adhesive must be used to bond the composite to the tooth. Clinicians frequently find very little enamel available for bonding at the gingival margin; thus, the bond at this margin depends on the integrity of the adhesive seal formed with dentin. At the vulnerable gingival margin, the adhesive is the barrier between the prepared tooth and the surrounding environment.
Figure 1.
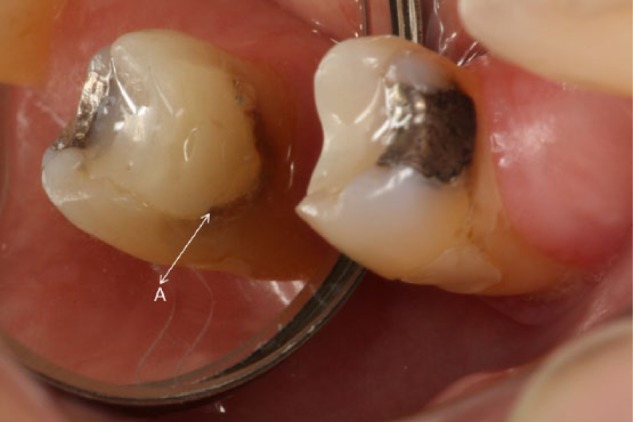
Clinical photo of a composite restoration showing decay along the gingival margin. The letter A marks the gingival margin.
A failed adhesive means that there are gaps between the tooth and composite: gaps that can be penetrated by oral fluids, bacterial enzymes, and bacteria. The infiltration of these noxious agents into the gaps between the tooth and composite will lead to recurrent decay, hypersensitivity, pulpal inflammation, and restoration failure (Murray et al., 2002). The lack of durable and effective dentin adhesives is considered one of the major problems with the use of composites in direct restorative dentistry (Spencer et al., 2010).
Adhesive Bond
The fundamental processes involved in bonding total-etch (etch-and-rinse) adhesives to dentin include removal of the mineral phase from the dentin substrate without altering the collagen matrix and filling the voids left by the mineral with adhesive that undergoes complete in situ polymerization (i.e., the hybrid layer). In self-etch adhesive systems, the etching and priming/bonding are combined in 1 step (Liu et al., 2011). The adhesive formulations include hydrophilic and acidic monomers to achieve the task of etching and priming simultaneously (Moszner et al., 2005).
The ideal hybrid layer is characterized as a collagen network infused and reinforced by polymer (Singh et al., 2014). Ideally, this polymer-collagen composite will provide a durable and continuous link between the bulk adhesive and dentin. Numerous studies indicate that this ideal is not achieved, and the major mechanisms involved in deterioration of the hybrid layer are degradation of water-rich, resin-sparse collagen fibrils and hydrolysis of the adhesive (Hashimoto, 2010; Perdigão et al., 2013).
Discrepancy between the depth of dentin demineralization and adhesive infiltration means that there is exposed collagen within the hybrid layer (Spencer and Swafford, 1999; Kermanshahi et al., 2010). Degradation of the exposed collagen matrix follows a cascade of events that begins with acid etching of the dentin. Acid etching disrupts the tooth structure—creating the porosity that is critical to adhesive infiltration, but acid etching also stimulates proteolytic enzymes (e.g., matrix metalloproteinases [MMPs]), which can degrade the exposed collagen (Pashley et al., 2004). It is hypothesized that this type of degradation is most important acutely in the period following adhesive application. The predominant methods to address this acute attack on the hybrid layer have been MMP inhibitors (e.g., chlorhexidine) or MMP inhibitor–conjugated resin monomers (Brackett et al., 2011). These methods have shown promise in terms of slowing collagen degradation and shifting the major site of failure elsewhere (Tjäderhane et al., 2013). The method is not, however, without its limitations. For example, the addition of 2 wt% chlorhexidine to a 70/30 mol% 2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy) phenyl]-propane (BisGMA)/ triethylene glycol dimethacrylate (TEGDMA) resin system led to a statistically significant decrease in degree of conversion (Pallan et al., 2012). The degree of polymerization of urethane dimethacrylate (UDMA)/TEGDMA resin system was reduced by the addition of chlorhexidine (Anusavice et al., 2006). Biomimetic remineralization (Tay and Pashley, 2008) impedes MMP attack of the exposed collagen layer, but this technique does not prevent water sorption and hydrolysis of the adhesive components (Brackett et al., 2011).
Chronic deterioration of the hybrid layer involves hydrolysis and leaching of the adhesive that has infiltrated the collagen matrix. Leaching is facilitated by water ingress into the loosely cross-linked or hydrophilic domains of the adhesive (Parthasarathy et al., 2012). Water may also be trapped within the adhesive during photopolymerization. Water promotes the chemical hydrolysis of ester bonds in methacrylate materials, and although this reaction is expected to be relatively slow at neutral pH, excursions in pH may lead to transient acid or base catalysis. With time, local domains of the methacrylate network become sufficiently degraded to permit access by esterases (Finer et al., 2004; Bourbia et al., 2013), which greatly accelerate ester bond hydrolysis. Esterase activities of S. mutans are also at levels sufficient to hydrolyze the ester bonds in methacrylate adhesives (Bourbia et al., 2013).
Molecular and mechanical modeling in conjunction with synthesis of new methacrylate monomers and multiscale adhesive/dentin (a/d) interfacial characterization has been used as a path to the development of new water-compatible, esterase-resistant adhesives (Spencer et al., 2010). Methacrylate side chains are selectively modified so that they are both water compatible and esterase resistant (Park et al., 2008; Park et al., 2009a; Park et al., 2009b). For example, adhesive formulations containing BisGMA, 2-hydroxyethyl methacrylate (HEMA), and a new multifunctional methacrylate with a branched side chain (i.e., trimethylolpropane mono allyl ether dimethacrylate) showed significantly greater esterase resistance than BisGMA/HEMA adhesives when both formulations are photopolymerized in the presence of water (Park et al., 2009a). Adhesive formulations containing BisGMA, HEMA, and a new trimethacrylate monomer with urethane-linked groups—that is, 1,1,1-tri-[4-(methacryloxyethylamino-carbonyloxy)-phenyl]ethane (MPE)—showed greater esterase resistance than BisGMA/HEMA formulations when both are photopolymerized in 16 wt% water (Park et al., 2008).
Composite Failure
After nearly 6 decades of research, dental composites continue to show limited clinical service as a result of decay or fracture (Drummond, 2008; Opdam et al., 2010). Recurrent decay at the composite-tooth interface has consistently been the primary reason for replacement of composite restorations (Ferracane, 2013), and S. mutans is a primary pathogenic bacterium responsible for recurrent decay (Nyvad and Kilian, 1987). Adhesion of S. mutans to the tooth surface changes the local environment to support the subsequent colonization of the surface by other bacterial species, ultimately forming a microecosystem known as a biofilm. In addition to its role as a “pioneer” organism in biofilm formation, S. mutans increases the acidity of the environment by producing lactic acid, which damages the tooth, adhesive, and composite.
Composites accumulate more biofilm than other restorative materials (Beyth et al., 2008). Cavity preparations are infected with residual S. mutans (Li et al., 2009). Degradation of methacrylate ester groups in adhesives and composites produces carboxylic acids—the same functional group that is the culprit in lactic acid–induced demineralization. The breaking of covalent bonds by addition of water to ester bonds is considered one of the main reasons for resin degradation within the hybrid layer (Brackett et al., 2011). The failure of the adhesive bond layer provides crevices that are readily penetrated by pathogens. Adhesive failure in the presence of bacteria, esterases (Bourbia et al., 2013), and dental plaque biofilm (Busscher et al., 2010) provokes a cascade of events leading to deterioration of the interfacial bond and failure of the composite restoration.
Biofilms, Dental Plaque, and Composite
The direct cause of decay at the margins of composite restorations is cariogenic plaque (Filoche et al., 2010). The cariogenic plaques result when the low populations of acidogenic and aciduric bacteria increase following high-frequency sugars and other fermentable carbohydrate exposure (Marsh, 2003). The extended acidification of plaque (pH < 5) is a result of the metabolic activity of the microbiota. The acid demineralizes the tooth and causes severe damage to the surface of the composite restoration, prompting further biofilm-microbial attachment and deterioration of the restoration (Beyth et al., 2008).
While there is extensive evidence supporting the role of pathogenic bacteria in the destruction of dental restorations, the extent to which the restoration itself provokes a microenvironment that contributes to the progression of pathogen-based destruction of the tooth is still being defined, particularly at the molecular level. Composite restorative systems permit attachment of salivary proteins and pioneer microorganisms involved in decay. The metabolism of these bacteria is altered in the presence of the composite restorative system, enabling promotion of the pathogenic effects (Delaviz et al., 2014). As discussed above, the interface between the restoration and tooth (i.e., the composite restoration’s adhesive bond layer) is the most vulnerable site (Donmez et al., 2005). This interface has greater surface roughness, and the polymer is more heterogeneous and porous than enamel, providing increased opportunity for attachment of proteins, primarily gp340, and microbes that lead to subsequent degradation of the repaired tooth (Beyth et al., 2008). This degradation is particularly evident at the gingival margin where it is difficult to clean (Spencer et al., 2010).
Binding studies to dental materials and methacrylate polymers demonstrate that modulating the chemical properties of the polymer affects protein adhesion and microbial attachment (Weerkamp et al., 1988; Olsson et al., 1992; Murata et al., 2007), but insufficient data are available to define the molecular basis of attachment. These studies have examined resin systems in which the chemical composition has been modified by substituting functional groups on the monomers, altering the cross-linker and/or changing the formulation components. While studies have reported that, in the oral cavity, less biofilm is recovered from supragingival hydrophobic surfaces (Busscher et al., 2010), it has been very difficult to provide unequivocal evidence of systematic differences in salivary protein binding as a function of material composition and surface charge. Chemical changes to individual monomers not only introduce new moieties onto the surface of the polymer (Weerkamp et al., 1988; Murata et al., 2007) but also affect its physical properties, including surface roughness and porosity, which are known to influence adhesion to surfaces and infiltration (Busscher et al., 2010). Charged moieties promote hydration of the polymer, which can increase swelling and encourage degradation (Delaviz et al., 2014). As such, balancing the various advantageous and destructive processes that affect a formulation is required to optimize longevity. While the individual studies are certainly correct, the limited data set includes seemingly contradictory results and emphasizes the need for a thorough, systematic characterization of all relevant parameters.
Modification of the resin composition has been examined in an effort to prevent attachment of bacteria to the polymer surface. Quaternary ammonium compounds have demonstrated antibacterial properties and have been incorporated into methacrylate polymers for this purpose (Li et al., 2014), where it does appear that the antibacterial properties of an individual monomer depend on the surface density of that modified monomer (Murata et al., 2007). The free monomers, however, have proven to be significantly more potent at preventing growth of pathogenic microbes in solution than through contact killing at the polymer surface once incorporated (Murata et al., 2007). In addition, monomers specifically containing quaternary amines have been shown to inhibit MMP activity (Tezvergil-Mutluay et al., 2011). An interesting adaptation used to improve surface properties is the subsequent physical or chemical modification of the polymer. Physical modification includes polishing the surface of the polymer to reduce surface roughness. Methacrylate polymer surfaces may be chemically modified to make them zwitterionic to prevent fouling (Xiang et al., 2014). Our group recently applied this approach to dentin adhesive formulations and demonstrated that grafting the metal abstraction peptide, a novel inhibitor of MMP-8, via a synthetic linker to the surface of an amine-containing methacrylate polymer completely abrogated catalysis by this enzyme (Dixit et al., 2014).
Composition of the Pathogenic Biofilm
Dental plaque is a complex biofilm composed of proteins and microorganisms. The main players identified in cariogenic pathogenicity are a salivary agglutinin (SAG) glycoprotein (gp340) and the pioneer bacterium S. mutans, although others may contribute. The normal function of gp340, a component of the salivary pellicle, is to bind to and clear microbes from the oral cavity by inducing their aggregation. In the presence of a composite restoration, gp340 binds bacteria and adheres to the nonnative surface to support the attachment of the pathogenic biofilm at the vulnerable composite-adhesive-tooth interfaces (Gibbons and Hay, 1989). The gp340 protein binds to the partner protein AgI/II on the surface of S. mutans and its homologues on other bacteria, thereby promoting aggregation of the microbes and enabling their recruitment and retention at the restoration-tooth interface. Following adhesion, when bacteria become exposed to sugars that fuel the metabolic production and extracellular release of lactic acid, the tooth is exposed to low pH, which promotes demineralization. Because the acid is produced in the immediate proximity of residual healthy tooth, loss of exposed enamel and dentin occurs.
gp340 also adheres to the crystalline hydroxylapatite matrix of the tooth’s enamel and supports subsequent attachment of S. mutans and other microbes to this surface. Based on investigations with sintered hydroxylapatite, which provides surface characteristics similar to human enamel, attachment to enamel is inhibited by the small salivary phosphoproteins statherin and histatin 1 (Shimotoyodome et al., 2006). It has been demonstrated that soluble, synthetic phospho-containing compounds also inhibit binding to enamel. Methacryloyloxydecyl phosphate–PEG competes effectively for binding to the enamel surface and successfully prevents adherence by salivary proteins and microbes to this highly regular surface (Shimotoyodome et al., 2007). Reversal by methacryloyloxydecyl phosphate–PEG after salivary protein adhesion is less effective and does not prevent subsequent microbial attachment (Shimotoyodome et al., 2007). This result suggests that it is more difficult to displace microbes than to prevent attachment of the proteins responsible for adhesion of the biofilm. The development of strategies to prevent adhesion to the tooth and polymeric restorative materials is important to preserving the integrity of both the healthy tooth and the repair. Under in vivo conditions, successful application of these strategies may be confounded by variables such as wear and shear forces.
gp340 Structure and Interactions
gp340 is a ~360-kDa multidomain glycosylated protein. The protein consists of 17 folded domains: 14 scavenger receptor cysteine-rich (SRCR) repeat domains, the last of which is sandwiched between 2 CUB (C1r/C1s Uef Bmp1) domains, followed by a C-terminal zona pellucida domain to cap the polypeptide chain. All gp340 domains have architectures that typically function in binding interactions, especially SRCR. Each SRCR domain, named for the order in which it appears beginning with SRCR1, is 100-110 residues, and there is a short flexible intervening segment between each, except the fourth and fifth domains (Purushotham and Deivanayagam, 2013). These connector segments are referred to as SIDs, and this is where the covalent attachment of O-glycans, which contribute to bacterial binding, occurs (Purushotham and Deivanayagam, 2014). The SRCR domains in gp340 interact with microorganisms and can either facilitate clearance or mediate infection (Stoddard et al., 2007). The first repeat domain (SRCR1) binds to the capsid proteins of HIV, whereas SRCR2 aggregates bacteria (Chu et al., 2013). These interactions are specific, but individual domains can interact with multiple partners (Loimaranta et al., 2005). The sequence motif VEVLXXXXW within the SRCR domains was identified as a critical feature involved in aggregation of bacteria (Bikker et al., 2004). Importantly, calcium binding to the SRCR domains of gp340 was shown to greatly enhance binding to AgI/II (Purushotham and Deivanayagam, 2014).
No high-resolution structure information is available for any of the domains of gp340, but a crystal structure of the SRCR fold was solved for a homologous protein domain derived from a macrophage receptor called MARCO (Ojala et al., 2007), which also can aggregate bacteria. This structure shows a clear patch of negative charge on the surface, for which calcium ion binding was observed. A region of positive charge is also apparent, and this feature was shown to participate in binding to the ligands found on pathogens. Coordination of calcium to the acidic residues appears to enhance aggregation by promoting ligand binding in a cooperative manner. The SRCR domain from MARCO is not embedded within a string of like domains, as is the case for gp340, and it is interesting that this protein had to form a trimer for ligand binding to occur. This parallels the increased aggregation observed for the first 3 SRCR domains of gp340 over SRCR1 (Purushotham and Deivanayagam, 2014). Structure comparison suggests that similar electrostatic features exist on gp340 SRCR domains, which likely accomplish calcium binding (Figures 2, 3). This interaction is particularly interesting because calcium is required under flow conditions for high-affinity binding between gp340 and AgI/II (Hajishengallis et al., 1994). These findings indicate that calcium plays an important role in modulating adhesion and aggregation via SRCR domains.
Figure 2.
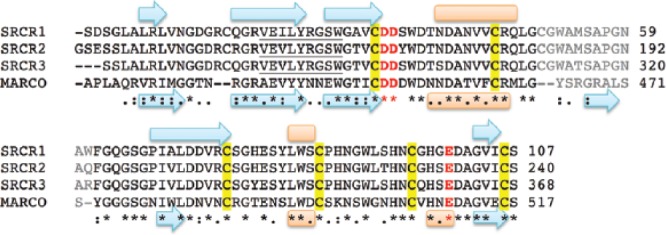
Sequence alignment of scavenger receptor cysteine-rich (SRCR) domains from gp340 and MARCO performed with ClustalW. Secondary structure elements shown below the sequence reflect the crystal structure of MARCO (PDB: oy1a), and those above were predicted via Protein Predict for SRCR1. Beta strands are shown as cyan arrows and alpha helices as orange cylinders. The acidic residues involved in calcium binding are shown in red. The region with low similarity is shown in gray. The sequence identified by Bikker et al. (2004) involved in aggregation is underlined.
Figure 3.
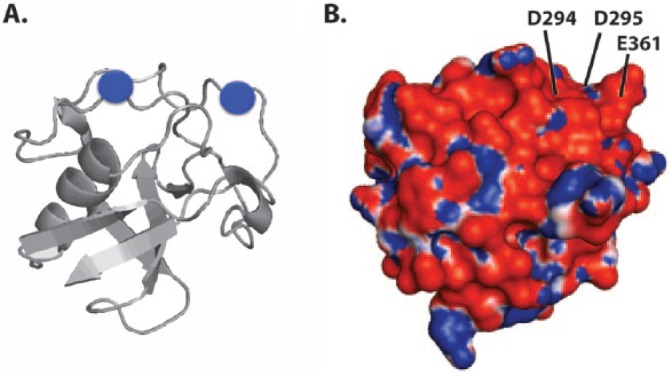
Homology model of SRCR3 domain from gp340 based on the crystal structure of the scavenger receptor cysteine-rich (SRCR) domain from MARCO (PDB: o1y3). (A) Ribbon diagram of the backbone fold is shown in gray with the 2 Ca2+ ions (blue circles) positioned in the same acidic regions as in MARCO. (B) Electrostatic surface map of gp340 SRCR domain (same orientation), in which red coloration reflects acidic and blue indicates basic regions. The acidic residues corresponding to the analogous positions in MARCO involved in calcium binding are labeled.
A high-resolution crystal structure of a portion of the AgI/II protein from S. mutans (A3VP1) was solved independently (Larson et al., 2010). The bacterial antigen has 3 regions (P, V, and A; named for the proline-rich, variable, and alanine-rich sequences, respectively), which arrange to form a fibrillar structure (Figure 4) based on a unique interaction between α-helices in the A region and polyproline type II helices in the P region. Here, only 1 P and A unit each are present, but 3 repeats exist in the full-length protein, which also has a C-terminal domain that anchors AgI/II to the bacterial cell wall. The P and A regions associate to form a long stalk that positions the intervening globular V domain at the far end, approximately 50 nm away from the bacterial surface. A stable interaction is achieved through 2 chemical means: van der Waals contact between the side chains of proline (within PXXP motifs from the P region) and tyrosine (from the A region) and also a repeating pattern of hydrogen bonds between the backbone of the P region residues and the side chain moieties from asparagine residues in the helical A region. Binding to SAG was also evaluated in this study, and 2 distinct regions of the bacterial antigen were shown to participate. SAG is a complex that contains gp340, sIgA, and a yet unidentified 80-kDa protein. The isolated V domain bound to SAG but had reduced affinity compared to the larger A3VP1 fragment, which was comparable to full-length AgI/II. Despite being located at the opposite end of the fibrillar structure, the C-terminal domain also bound to SAG. Based on these combined data sets, a model of the direct interaction between AgI/II and SRCR was proposed in which both ends of the 50-nm long filbrillar structure interact with SAG to accomplish aggregation (Figure 4). Some conformational flexibility was observed in the AgI/II bacterial antigen protein (Larson et al., 2010), and as such, it is possible that the structure folds up on itself to bind gp340 at a single high-affinity site. It also is highly feasible that different domains of the long gp340 protein (and/or other components of the SAG complex) bind selectively to each of these 2 distinct regions and tether the bacteria to assemble the larger aggregate.
Figure 4.
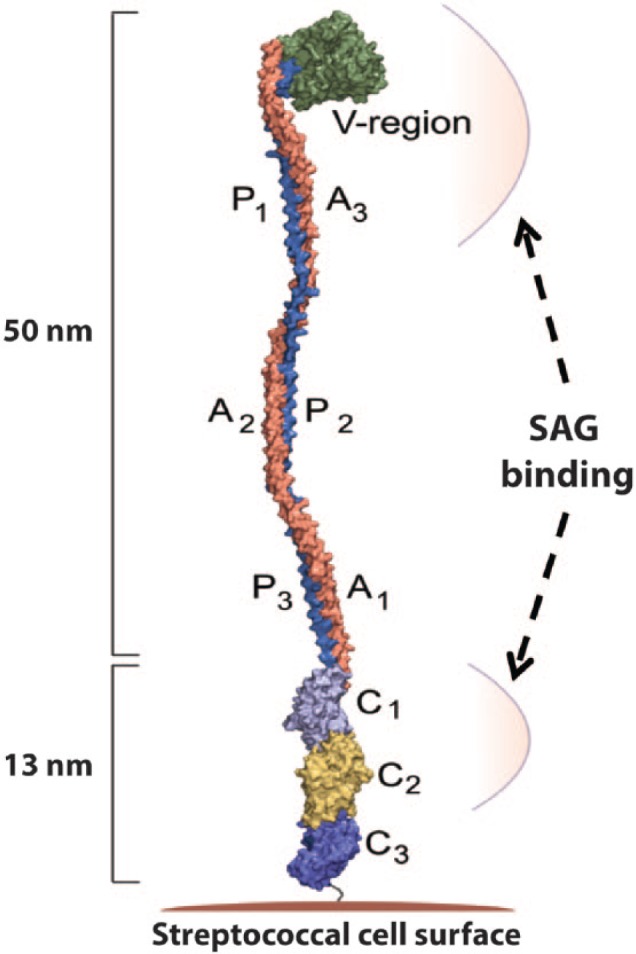
Space-filling model of the structure of the A3VP1 fragment from the AgI/II protein. The V- and C-terminal regions of AgI/II involved in attachment of Streptococcus mutans to salivary agglutinin (SAG) are indicated by arrows. Figure is provided by C. Deivanayagam at University of Alabama at Birmingham.
The recent availability of this high-resolution structure information is already enabling better understanding of how native components may facilitate pathogenic outcomes, and it has provided a basis for further hypothesis development. Careful structural analysis of the AgI/II structure shows parallels to the collagen triple helix and suggests that the similarities may support direct binding interactions with the collagen matrix in the dentin (Larson et al., 2010). Erosion of the protective enamel and demineralization of the underlying dentin adjacent to a restoration exposes the collagenous substrate and permits infiltration of proteins and microbes. This creates opportunity for microbial attachment via the A and P regions of AgI/II on S. mutans to the softer tooth structure. Once this type of extended interaction occurs, competition for binding by SAG would become less effective and would inhibit the ability of SAG to clear pathogenic bacteria. This activity leads to a negative spiral of events that degrades the composite restoration and culminates in secondary caries and pulp pathology (Beyth et al., 2008; Busscher et al., 2010). This negative spiral of events is apparent at the gingival margin of the composite restoration (Spencer et al., 2010).
There are frequently gaps between the tooth and composite at the gingival margin of the composite restoration (Spencer et al., 2010). Oral fluids, salivary enzymes, and bacteria infiltrate the marginal gaps and crevices between the tooth and composite. Once S. mutans is bound to the collagenous dentin matrix, the metabolic production of acid would accelerate demineralization because of close proximity and persistence of a low pH environment (Delaviz et al., 2014). Because the substrate is in a crevice that is more protected from fluid flow, it is also largely isolated from saliva and the neutralization and pH equilibration provided by saliva (Marsh, 2003; Beyth et al., 2008). In light of these pathogenic mechanisms, effective approaches to increasing longevity of composite restorations would be to prevent gp340-based attachment of microbes, particularly S. mutans, and to slow acid erosion of the adjacent mineralized native tooth structure by introducing a buffering agent into the adhesive (Laurence et al., 2013).
Summary
Acidification of the oral microenvironment caused by metabolic activity of the microbiota promotes demineralization of tooth structure at the margin of composite restorations. Demineralization creates additional opportunity for adhesion by the biofilm—for example, salivary protein gp340 and the pioneer pathogenic bacterium S. mutans—thereby accelerating the degradation process of the composite restoration.
With a failure rate nearly double that of amalgam (Ferracane, 2013), the increasing trend to replace amalgam with composite could be detrimental for patients. Removal of these restorations leads to loss of sound tooth structure with concomitant weakening of the tooth and possible pulpal injury (Hunter et al., 1995). Over the lifetime of the patient, the repeated loss of sound tooth structure will translate to the need for more complex restorations and, eventually, total tooth loss. The higher failure rate and need for frequent replacement could translate to a significant reduction in the quality of life for the nearly 32% of the U.S. population with natural dentition who do not receive regular dental treatment (Health US, 2007). Similarly, the higher total colony-forming units of S. mutans at the margins of composite as compared to comparable amalgam restorations could translate to an increase in untreated dental disease and a significant reduction in the quality of life for the 4 million U.S. children who do not receive regular dental care (Palmer, 2013).
It is unlikely that dental plaque biofilm can be eliminated. It may, however, be possible to reduce the pathogenic impact of the biofilm by engineering novel anticariogenic dentin adhesives or by engineering resin matrices that shift the microbial ecology at the composite-tooth interface from a disease-associated state to a “healthy” state.
Footnotes
This investigation was supported by research grants R01DE143 92, R01DE14392-08S1, and R01DE022054 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, Maryland.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Anusavice KJ, Zhang NZ, Shen C. (2006). Controlled release of chlorhexidine from UDMA-TEGDMA resin. J Dent Res 85:950-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyth N, Bahir R, Matalon S, Domb AJ, Weiss EI. (2008). Streptococcus mutans biofilm changes surface-topography of resin composites. Dent Mater 24:732-736. [DOI] [PubMed] [Google Scholar]
- Bikker FJ, Ligtenberg AJ, End C, Renner M, Blaich S, Lyer S, et al. (2004). Bacteria binding by DMBT1/SAG/gp-340 is confined to the VEVLXXXXW motif in its scavenger receptor cysteine-rich domains. J Biol Chem 279:47699-47703. [DOI] [PubMed] [Google Scholar]
- Bourbia M, Ma D, Cvitkovitch DG, Santerre JP, Finer Y. (2013). Cariogenic bacteria degrade dental resin composites and adhesives. J Dent Res 92:989-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackett MG, Li N, Brackett WW, Sword RJ, Qi YP, Niu LN, et al. (2011). The critical barrier to progress in dentine bonding with the etch-and-rinse technique. J Dent 39:238-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. (2010). Biofilm formation on dental restorative and implant materials. J Dent Res 89:657-665. [DOI] [PubMed] [Google Scholar]
- Chu Y, Li J, Wu X, Hua Z, Wu Z. (2013). Identification of human immunodeficiency virus type 1 (HIV-1) gp120-binding sites on scavenger receptor cysteine rich 1 (SRCR1) domain of gp340. J Biomed Sci 20:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaviz Y, Finer Y, Santerre JP. (2014). Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater 30:16-32. [DOI] [PubMed] [Google Scholar]
- DeRouen TA, Martin MD, Leroux BG, Townes BD, Woods JS, Leitao J, et al. (2006). Neurobehavioral effects of dental amalgam in children: a randomized clinical trial. JAMA 295:1784-1792. [DOI] [PubMed] [Google Scholar]
- Dixit N, Settle JK, Ye Q, Berrie CL, Spencer P, Laurence JS. (2014). Grafting MAP peptide to dental polymer inhibits MMP-8 activity. J Biomed Mater Res B Appl Biomater [Epub ahead of print 5/29/2014] in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez N, Belli S, Pashley DH, Tay FR. (2005). Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. J Dent Res 84:355-359. [DOI] [PubMed] [Google Scholar]
- Drummond JL. (2008). Degradation, fatigue, and failure of resin dental composite materials. J Dent Res 87:710-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferracane JL. (2013). Resin-based composite performance: are there some things we can’t predict? Dent Mater 29:51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoche S, Wong L, Sissons CH. (2010). Oral biofilms: emerging concepts in microbial ecology. J Dent Res 89:8-18. [DOI] [PubMed] [Google Scholar]
- Finer Y, Jaffer F, Santerre JP. (2004). Mutual influence of cholesterol esterase and pseudocholinesterase on the biodegradation of dental composites. Biomaterials 25:1787-1793. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, Hay DI. (1989). Adsorbed salivary acidic proline-rich proteins contribute to the adhesion of Streptococcus mutans JBP to apatitic surfaces. J Dent Res 68:1303-1307. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Koga T, Russell MW. (1994). Affinity and specificity of the interactions between Streptococcus mutans antigen I/II and salivary components. J Dent Res 73:1493-1502. [DOI] [PubMed] [Google Scholar]
- Hashimoto M. (2010). A review: micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. J Biomed Mater Res B Appl Biomater 92:268-280. [DOI] [PubMed] [Google Scholar]
- Health US. (2007). Health, United States, 2007 Chartbook on Trends in the Health of Americans. Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- Hunter AR, Treasure ET, Hunter AJ. (1995). Increases in cavity volume associated with the removal of class 2 amalgam and composite restorations. Oper Dent 20:2-6. [PubMed] [Google Scholar]
- Kermanshahi S, Santerre JP, Cvitkovitch DG, Finer Y. (2010). Biodegradation of resin-dentin interfaces increases bacterial microleakage. J Dent Res 89:996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingman A, Hyman J, Masten SA, Jayaram B, Smith C, Eichmiller F, et al. (2012). Bisphenol A and other compounds in human saliva and urine associated with the placement of composite restorations. J Am Dent Assoc 143:1292-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krifka S, Spagnuolo G, Schmalz G, Schweikl H. (2013). A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials 34:4555-4563. [DOI] [PubMed] [Google Scholar]
- Larson MR, Rajashankar KR, Patel MH, Robinette RA, Crowley PJ, Michalek S, et al. (2010). Elongated fibrillar structure of a streptococcal adhesin assembled by the high-affinity association of alpha- and PPII-helices. Proc Natl Acad Sci U S A 107:5983-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence JS, Nelson B, Ye Q, Park J, Spencer P. (2014). Characterization of acid-neutralizing basic monomers in co-solvent systems by NMR. Int J Polym Mater 63:361-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinfelder KF. (2000). Do restorations made of amalgam outlast those made of resin-based composite? J Am Dent Assoc 131:1186-1187. [DOI] [PubMed] [Google Scholar]
- Li F, Chai ZG, Sun MN, Wang F, Ma S, Zhang L, et al. (2009). Anti-biofilm effect of dental adhesive with cationic monomer. J Dent Res 88:372-376. [DOI] [PubMed] [Google Scholar]
- Li F, Weir MD, Fouad AF, Xu HH. (2014). Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent Mater 30:182-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tjaderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. (2011). Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res 90:953-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loimaranta V, Jakubovics NS, Hytonen J, Finne J, Jenkinson HF, Stromberg N. (2005). Fluid- or surface-phase human salivary scavenger protein gp340 exposes different bacterial recognition properties. Infect Immun 73:2245-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PD. (2003). Are dental diseases examples of ecological catastrophes? Microbiology 149(Pt 2):279-294. [DOI] [PubMed] [Google Scholar]
- Moszner N, Salz U, Zimmermann J. (2005). Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent Mater 21:895-910. [DOI] [PubMed] [Google Scholar]
- Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. (2007). Permanent, non-leaching antibacterial surface—2: how high density cationic surfaces kill bacterial cells. Biomaterials 28:4870-4879. [DOI] [PubMed] [Google Scholar]
- Murray PE, Windsor LJ, Smyth TW, Hafez AA, Cox CF. (2002). Analysis of pulpal reactions to restorative procedures, materials, pulp capping, and future therapies. Crit Rev Oral Biol Med 13:509-520. [DOI] [PubMed] [Google Scholar]
- National Institute of Dental and Craniofacial Research (2009). Dental resin composites and caries [announcement 13-DE-102]. Bethesda, MD: National Institute of Dental and Craniofacial Research. [Google Scholar]
- Nyvad B, Kilian M. (1987). Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res 95:369-380. [DOI] [PubMed] [Google Scholar]
- Ojala JR, Pikkarainen T, Tuuttila A, Sandalova T, Tryggvason K. (2007). Crystal structure of the cysteine-rich domain of scavenger receptor MARCO reveals the presence of a basic and an acidic cluster that both contribute to ligand recognition. J Biol Chem 282:16654-16666. [DOI] [PubMed] [Google Scholar]
- Olsson J, van der Heijde Y, Holmberg K. (1992). Plaque formation in vivo and bacterial attachment in vitro on permanently hydrophobic and hydrophilic surfaces. Caries Res 26:428-433. [DOI] [PubMed] [Google Scholar]
- Opdam NJ, Bronkhorst EM, Loomans BA, Huysmans M. (2010). 12-year survival of composite vs. amalgam restorations. J Dent Res 89:1063-1067. [DOI] [PubMed] [Google Scholar]
- Pallan S, Furtado Araujo MV, Cilli R, Prakki A. (2012). Mechanical properties and characteristics of developmental copolymers incorporating catechin or chlorhexidine. Dent Mater 28:687-694. [DOI] [PubMed] [Google Scholar]
- Palmer C. (2013). Census Bureau targets unmet need. ADA News. URL accessed on 8/13-2014 at: http://editiondigital.net/article/Census_Bureau_Targets_Unmet_Need/1359106/153106/article.html.
- Park JG, Ye Q, Topp EM, Kostoryz EL, Wang Y, Kieweg SL, et al. (2008). Preparation and properties of novel dentin adhesives with esterase resistance. J Appl Polym Sci Symp 107:3588-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JG, Ye Q, Topp EM, Lee CH, Kostoryz EL, Misra A, et al. (2009a). Dynamic mechanical analysis and esterase degradation of dentin adhesives containing a branched methacrylate. J Biomed Mater Res B Appl Biomater 91:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JG, Ye Q, Topp EM, Misra A, Spencer P. (2009b). Water sorption and dynamic mechanical properties of dentin adhesives with a urethane-based multifunctional methacrylate monomer. Dent Mater 25:1569-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy R, Misra A, Park J, Ye Q, Spencer P. (2012). Diffusion coefficients of water and leachables in methacrylate-based crosslinked polymers using absorption experiments. J Mater Sci Mater Med 23:1157-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. (2004). Collagen degradation by host-derived enzymes during aging. J Dent Res 83:216-221. [DOI] [PubMed] [Google Scholar]
- Perdigão J, Reis A, Loguercio AD. (2013). Dentin adhesion and MMPs: a comprehensive review. J Esthet Restor Dent 25:219-241. [DOI] [PubMed] [Google Scholar]
- Purushotham S, Deivanayagam C. (2013). Cloning, expression and purification of the SRCR domains of glycoprotein 340. Protein Expr Purif 90:67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham S, Deivanayagam C. (2014). The calcium induced conformation and glycosylation of Gp340’s SRCR domains influences the high affinity interaction with antigen I/II homologs. J Biol Chem 289:21877-21887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotoyodome A, Kobayashi H, Tokimitsu I, Matsukubo T, Takaesu Y. (2006). Statherin and histatin 1 reduce parotid saliva-promoted Streptococcus mutans strain MT8148 adhesion to hydroxyapatite surfaces. Caries Res 40:403-411. [DOI] [PubMed] [Google Scholar]
- Shimotoyodome A, Koudate T, Kobayashi H, Nakamura J, Tokimitsu I, Hase T, et al. (2007). Reduction of Streptococcus mutans adherence and dental biofilm formation by surface treatment with phosphorylated polyethylene glycol. Antimicrob Agents Chemother 51:3634-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simecek JW, Diefenderfer KE, Cohen ME. (2009). An evaluation of replacement rates for posterior resin-based composite and amalgam restorations in US Navy and Marine Corps recruits. J Am Dent Assoc 140:200-209. [DOI] [PubMed] [Google Scholar]
- Singh V, Misra A, Parthasarathy R, Ye Q, Spencer P. (2014). Viscoelastic properties of collagen-adhesive composites under water saturated and dry conditions. J Biomed Mater Res A [Epub ahead of print 4/20/2014] in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer P, Swafford JR. (1999). Unprotected protein at the dentin-adhesive interface. Quintessence Int 30:501-507. [PubMed] [Google Scholar]
- Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, et al. (2010). Adhesive/dentin interface: the weak link in the composite restoration. Ann Biomed Eng 38:1989-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard E, Cannon G, Ni H, Kariko K, Capodici J, Malamud D, et al. (2007). gp340 expressed on human genital epithelia binds HIV-1 envelope protein and facilitates viral transmission. J Immunol 179:3126-3132. [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2008). Guided tissue remineralisation of partially demineralised human dentine. Biomaterials 29:1127-1137. [DOI] [PubMed] [Google Scholar]
- Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, et al. (2011). The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res 90:535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, et al. (2013). Strategies to prevent hydrolytic degradation of the hybrid layer-A review. Dent Mater 29:999-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp AH, Uyen HM, Busscher HJ. (1988). Effect of zeta potential and surface energy on bacterial adhesion to uncoated and saliva-coated human enamel and dentin. J Dent Res 67:1483-1487. [DOI] [PubMed] [Google Scholar]
- Xiang T, Wang R, Zhao W, Sun S, Zhao C. (2014). Covalent deposition of zwitterionic polymer and citric acid by click chemistry-enabled layer-by-layer assembly for improving the blood compatibility of polysulfone membrane. Langmuir 30:5115-5125. [DOI] [PubMed] [Google Scholar]


