Abstract
Physiologic bioengineering of the oral, dental, and craniofacial complex requires optimized geometric organizations of fibrous connective tissues. A computer-designed, fiber-guiding scaffold has been developed to promote tooth-supporting periodontal tissue regeneration and functional restoration despite limited printing resolution for the manufacture of submicron-scaled features. Here, we demonstrate the use of directional freeze-casting techniques to control pore directional angulations and create mimicked topographies to alveolar crest, horizontal, oblique, and apical fibers of natural periodontal ligaments. For the differing anatomic positions, the gelatin displayed varying patterns of ice growth, determined via internal pore architectures. Regardless of the freezing coordinates, the longitudinal pore arrangements resulted in submicron-scaled diameters (~50 µm), along with corresponding high biomaterial porosity (~90%). Furthermore, the horizontal + coronal () freezing orientation facilitated the creation of similar structures to major fibers in the periodontal ligament interface. This periodontal tissue-mimicking microenvironment is a potential tissue platform for the generation of naturally oriented ligamentous tissues consistent with periodontal ligament neogenesis.
Keywords: biomaterials, periodontal ligament, regenerative medicine, tissue engineering, freeze casting, bioengineering
Introduction
The physiologic functionalities of craniofacial and musculoskeletal complexes require spatial compartmentalization of each tissue interface, fibrous connective tissue integration to adjacent tissues, and specific fibrous organization and orientation for appropriate physical-mechanical responses (Moffat et al., 2008; Yang and Temenoff, 2009; Dormer et al., 2010). For regeneration and functioning restoration of destructed tissue complexes from injury, tumors, or trauma (Pagni et al., 2012), diverse conceptual microenvironments for growing tissues have been developed (Rehfeldt et al., 2007; Fu et al., 2010; Lee et al., 2010; Zemel et al., 2010; Bencherif et al., 2012; Guvendiren and Burdick, 2012; Mendes, 2013). However, there remains a need to better understand the 3-dimensional (3-D) orchestration of multicellular tissue formation (Burdick, 2009) as well as the relationship between engineered tissue complexes and native tissues/organs for functional restoration (Moffat et al., 2008; Park et al., 2010; Park et al., 2012).
The periodontal complex of appendicular craniofacial systems is a microscaled model system that has systematic integrations in multiphasic interfaces with varying topographies, such as alveolar bone, periodontal ligament (PDL), and cementum (Appendix Fig. 1). For effective function as tooth-supporting structures, fibrous connective tissues of teeth are spatiotemporally organized with 4 groups: alveolar crest, horizontal, oblique, and apical fiber. In this bone-ligament interface, the oblique is the largest group and has coronal anchorage to the bone surface (Appendix Fig. 1). Compared to the other groups, the oblique PDL has the important role to support tooth structures against functional loading (maximum mastication force: 500-800 N; Poiate et al., 2009) and to resist vertical and intrusive forces. Therefore, the obliquely oriented PDL is the most important key compartment for periodontal function restoration with biomechanical senses and appropriate responses (Hughes et al., 2010).
Recently, we developed a fiber-guiding scaffold for bone-ligament reconstruction under physiologic loading in periodontal defects (Park et al., 2012). This scaffold can provide high topographic adaptability to periodontal defects, perpendicular/oblique orientations of regenerated PDL, geometric organization of regenerated bone and PDL, and physiologic functioning restoration (Park et al., 2014). However, due to limited manufacturing resolutions and material-casting difficulties by physicochemical viscosities, it remains a challenge to manage complicated submicron structures, which mimic the major structure in the natural PDL interface. To create microporous architectures with oblique orientations to the root surface, we developed 3-D-patterned, periodontal mimic PDL architectures by controlling directions of ice crystal growth in a gelatin matrix, which has biocompatibility, biodegradability, and low immunogenicity currently approved for use by the U.S. Food and Drug Administration.
Freeze-casting is a simple approach to create submicron-level porous constructs via aqueous materials (Deville et al., 2006a; Ma et al., 2010; Waschkies et al., 2011). Freezing conditions can control microscopic patterns of ice crystals, and the regularity of ice growth can provide unidirectionally or radially oriented pores within the internal architectures of scaffolds (Deville et al., 2006b; Ma et al., 2010; Li et al., 2012). Here, we hypothesize that the control of freezing orientation can create submicron longitudinal pores and the angular similarities of native PDL via 3-D gelatin scaffolds. To evaluate this hypothesis, 3 sets of investigation were performed: (1) Simplified predictable computational models were developed to determine profiles of ice crystal formation using 4 freezing directions: apical (), horizontal (), horizontal + apical , and horizontal + coronal . (2) Angulations of patterned microarchitectures were evaluated. (3) Pore dimensions and porosities of each patterned scaffold were measured and compared against a paraffin tooth surface according to micro–computed tomographic (micro-CT) images.
Materials & Methods
Paraffin Tooth Preparation Model
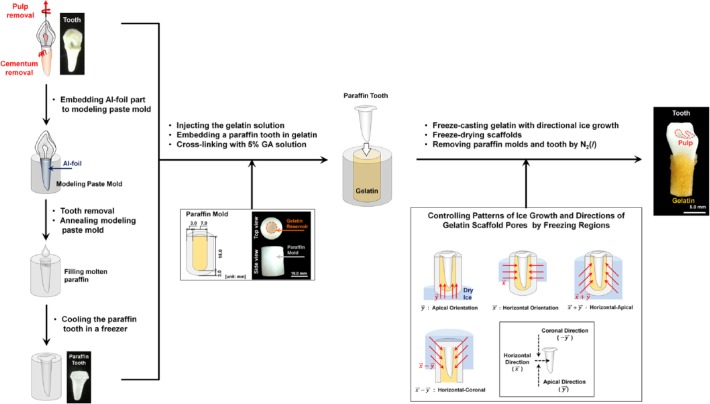
After pulp and cementum were removed from canine premolar teeth (P1), the P1 were cleansed with hydrogen peroxide (Sung Kwang Pharm. Co., Ltd., South Korea). The animal research was approved by the Institute of Laboratory Animal Resources, Seoul National University (SNU-090701-5). A paraffin tooth mold was made as follows: (1) the hydrogen peroxide–cleaned tooth was wrapped with aluminum foil; (2) the teeth were embedded in modeling paste; (3) the teeth were removed from the paste after 7 d; (4) the modeling paste mold was annealed at 60°C for 2 to 3 d; and (5) the aluminum foil was removed. Molten paraffin (Surgipath Paraplast, Leica Microsystems Ltd., Buffalo Grove, IL, USA) at approximately 65°C was injected into the mold and kept at room temperature for 1 hr. The mold with the paraffin was placed in a freezer for 30 min, and the paraffin was removed from the molds (Fig. 1).
Figure 1.
Schematic illustration of gelatin scaffold manufacturing procedures and experimental groups. After removal of periodontal ligament, the cementum layer, and pulp tissues from extracted teeth, modeling paste and paraffin were used to produce paraffin teeth (left). For the gelatin mold, cylindrical paraffin molds were manufactured with an 18.0-mm height by 13.0-mm diameter, and a reservoir was designed with a 15.0-mm height by 7.0-mm diameter (middle). Crosslinked gelatin scaffolds at 10 wt/v% were directionally frozen with dry ice, and freeze-drying was utilized for sublimation (bottom).
Gelatin Scaffold Preparation With Paraffin Mold and Teeth
A gelatin reservoir (15.0 mm in height by 7.0 mm in diameter) was prepared within a cylindrical paraffin mold (18.0 mm in height by 13.0 mm in diameter; Fig. 1). The 10% gelatin solution (Kokusan Chemical Works Ltd., Japan) was warmed at 40°C and injected into the reservoir. After cooling for 30 min at 20°C, the gelatin was crosslinked with 5% glutaraldehyde (GA; Showa Chemical Industry Co. Ltd., Japan) for 1 d. The constructs were rinsed with double-distilled water to remove GA solution approximately every 10 hr for 3 d. GA-removed gelatin in paraffin molds were frozen with 4 directions via dry ice (Fig. 1). Three samples per group were designated according to the different freezing orientations: n() = 3, n() = 3, n() = 3, and n() = 3. After paraffin molds were removed, gelatin scaffolds were freeze-dried for 4 d (Fig. 1).
Prediction of Temperature Profiles Based on 3-D Thermal Analysis
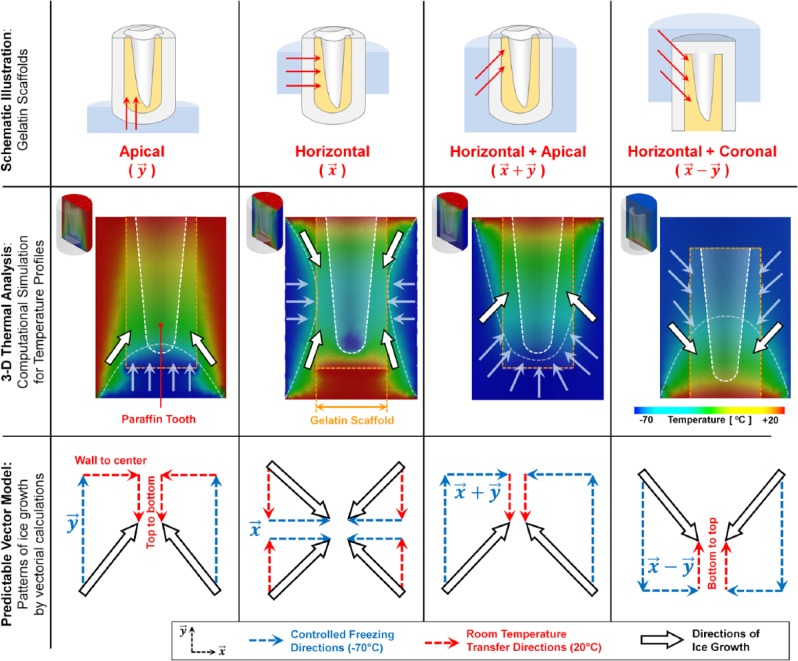
Thermal analysis based on SolidWorks 2013 (Dassault Systèmes SolidWorks Corp., Waltham, MA, USA) facilitated the prediction of thermal responses of scaffold models. Paraffin molds and gelatin containment were designed in SolidWorks 2013 (Fig. 1) with material properties (Appendix Table). The boundary conditions of each group, which had different freezing directions, were −70°C (contact regions with dry ice blocks; TCO2(s)) and 20°C (regions exposed to room temperature (Troom); Fig. 2).
Figure 2.
Three-dimensional thermal analysis for the prediction of temperature gradients for differing freezing orientations. Paraffin molds and teeth were designed with a CAD program, and the thermal analysis was performed with 4 vector orientations (, , , and ; top and middle rows). The white and orange dashed lines are the paraffin tooth surfaces and gelatin constructs, respectively. White arrows with black borders represent the freezing directions, and the semitransparent white dashed curves are borders of freezing profiles (middle). Based on temperature gradients and profiles (middle), simplified vector models were designed to predict the freezing orientations of individual methods (bottom).
Micro-CT Analysis
Gelatin scaffolds were volumetrically characterized by micro-CT, which is a nondestructive technology that allows for analyses of 3-D internal architectures. After scaffolds were freeze-dried, micro-CT (SkyScan 1172, Bruker-microCT, Kontich, Belgium) scanning was performed with an 11-µm3 voxel size, with an accelerating potential of 40 kV with a beam current of 250 µA. Scanned images were reconstructed by SkyScan NRecon 1.6.4.1 and visualized per the Hounsfield unit (HU)–based grayscale value by CT Analyser and DataViewer (SkyScan; Appendix Fig. 2).
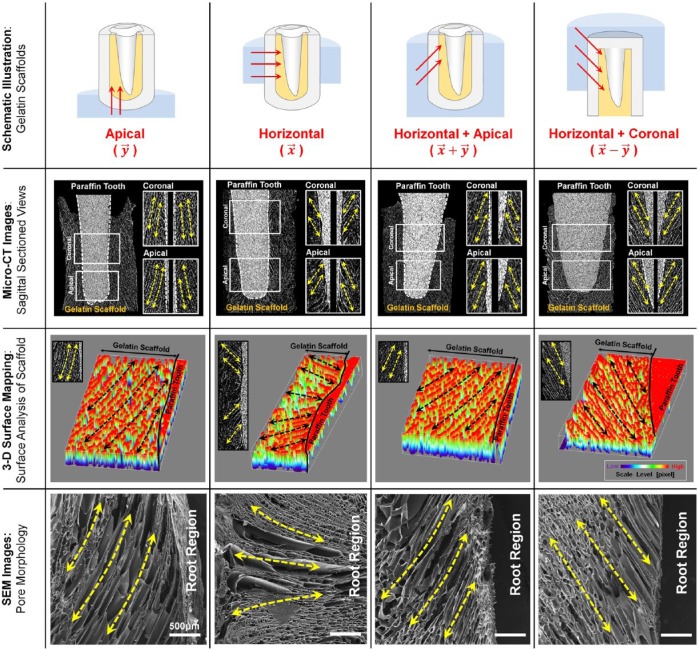
3-D Surface Mapping Pore Directionalities of Gelatin Scaffolds
Based on sectioned micro-CT images, 3-D surface mappings were generated in ImageJ 1.46r (National Institutes of Health, USA) using the function Interactive 3D Surface Plot 2.33 (Fig. 3, third row). The generated topographies visualized angulations of pores to the paraffin tooth root surface.
Figure 3.
Digitized cross-sectional views of 3-dimensional reconstructed images for qualitative analyses of directionally generated pore structures. Longitudinal cross-section images with 11-µm thickness showed the pore morphologies at the coronal and apical portions (second row). Grayscale intensity-based 3-dimensional surface mappings demonstrated pore angulations to the surface of the paraffin tooth and qualitative comparisons among groups (third row). The scanning electron microscopic images provided longitudinal pores that the freeze-casting method produced (fourth row). Yellow dashed/arrowed lines represent the pore directionalities, and black dashed/arrowed lines show the directional pore architecture. Scale bar: 500 µm.
Morphologic Characterization Based on Scanning Electron Microscope
The gelatin scaffolds by 4 freezing directions were analyzed for directional pore morphologies via scanning electron microscope at 15 kV (S-4700 FE-SEM, Hitachi, Japan).
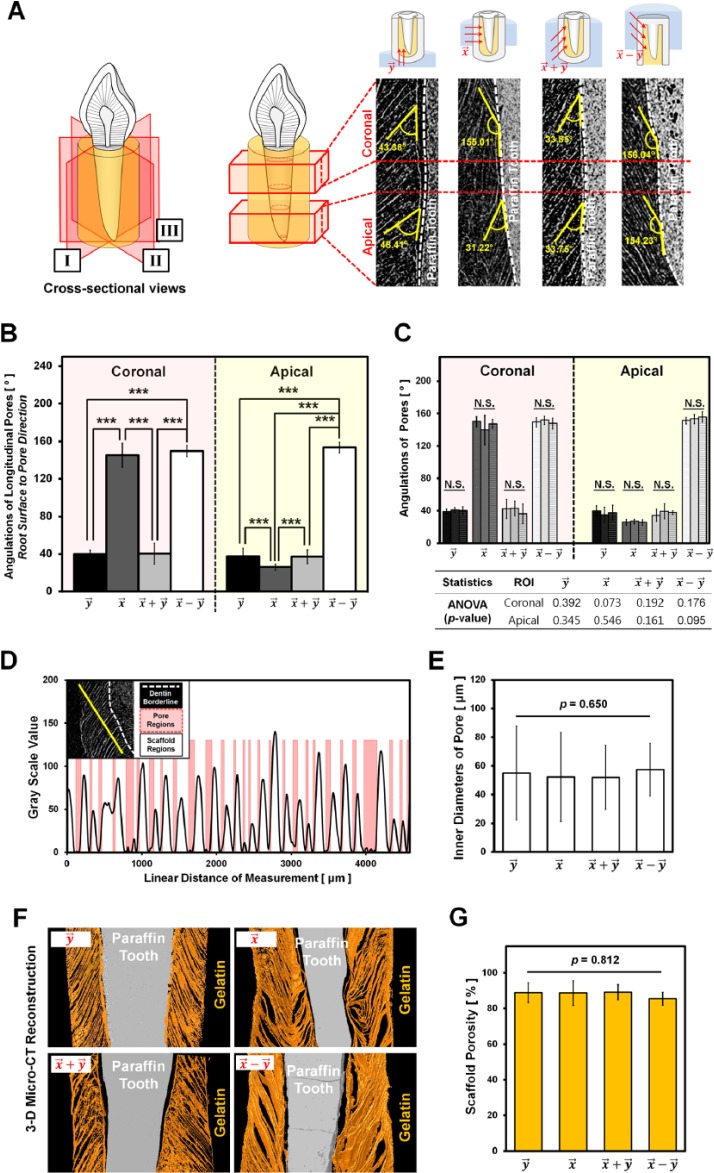
Angular Analysis of Longitudinal Pore Structures
ImageJ was used to measure pore angles of scaffolds at coronal and apical regions based on 3 cross-sectioned micro-CT images per sample (Fig. 4):
Figure 4.
Quantitative analysis of pore angulations, pore sizes, and porosities using micro–computed tomography images. (A) The schematic illustration (left) represents 3 cross sections per gelatin scaffold (I, II, and III) for spatial analyses, and each section was analyzed with the coronal and apical portions (right). The pore angles were measured via the baseline for angulation (i.e., the paraffin tooth root surface; right). (B) Quantitative analysis of pore angulations among groups showed that and had significant differences compared to the freezing groups, and (***p < .001). However, (C) the angulations of the samples in each group showed similarities according to analysis of variance p values, as shown in the table (p > .05; N.S., not significant). (D) The plot profile shows the gelatin structures and pore regions based on the grayscale values. The red-highlighted regions represent the pores, and their diameters were measured via the plot profiles. (E) There was no significant difference among the groups (, , , and ; p = .650). (F) Three-dimensional reconstructed colorized images of the groups. The gray areas represent paraffin teeth, and the orange-colored regions are the gelatin scaffolds. (G) The porosities were calculated and statistically analyzed with 3-dimensional reconstructed images. There was no significant difference in porosity (p = .812).
The pore angulations were based on formed pore directions to the paraffin tooth root surface. Three samples per group were separately analyzed in coronal and apical regions, and the mean of each group was used for statistical comparisons (Fig. 4A). In addition to the angulations, the pore sizes of 12 images per sample were measured via the plot profile function in ImageJ (Fig. 4B, 4C). Scaffold porosities were calculated per grayscale-based volumes of gelatin scaffolds and paraffin tooth structures. The volume of scaffolds was determined by subtracting the tooth volume from the whole constructs (Fig. 4D, 4E).
Ice Crystal Growth Kinetics for Directional Pore Structures
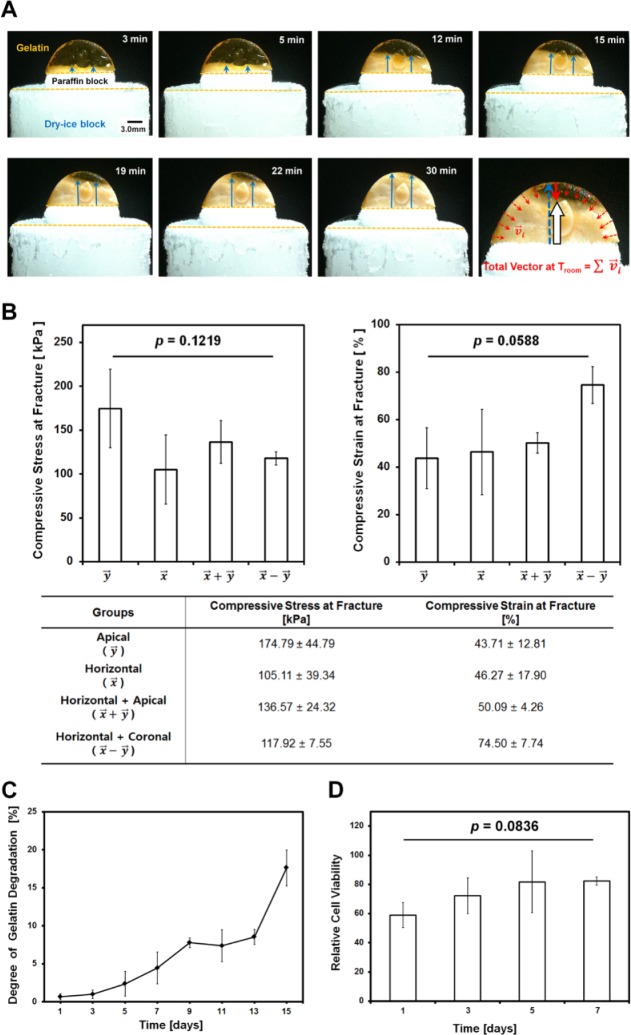
According to recent studies on controllable crystal growth kinetics and fabrications of directional porous architectures (Deville et al., 2006a; Waschkies et al., 2011), freezing temperature, freezing time, and freezing rate are significant factors that influence crystal formation and microstructure morphologies (Li et al., 2012). Because the rapid freezing process solidified different phase materials simultaneously (paraffin and gelatin at 20°C), it was difficult to control the direction of ice crystal growth with liquid nitrogen in our paraffin-gelatin system. Therefore, dry ice was used instead of liquid nitrogen to gradually grow different directional ice crystals in the crosslinked gelatin. Moreover, several thermal properties at 20°C—such as the specific heat capacity (paraffin, 4.18 J/gK; gelatin, 2.14-2.9 J/gK)—allow for slow thermal transfer (TCO2(s) < −70°C) to the gelatin (freezing rate: ~220 µm/min; Fig. 5).
Figure 5.
(A) Freezing rate analysis with 3.0-mm-thick paraffin and 10 wt/v% crosslinked gelatin construct over time. Based on the optical image results, a gelatin construct with a height of 6.6 mm is completely frozen within 30 min (freezing rate of ~220 µm/min). The red arrows represent the vectors of room-temperature transfers, and the blue arrows are growing directions of ice. The white arrow represents the calculated final vector of the direction of ice growth and regularity. (B) Compressive properties for 4 gelatin scaffolds at the fracture. After the gelatin scaffold and the paraffin tooth were assembled, compressive stress and strain were measured and the results statistically analyzed. Compressive stress and strain had no significant difference, p = .1219 and p = .0588, respectively. (C) In vitro biodegradation test for gelatin scaffolds. Sterilized gelatin scaffolds were submerged in lysozyme-containing phosphate buffered saline solution and incubated at 37°C with different time intervals. The weight loss of gelatin scaffolds can strongly correlate with progressive enzymatic degradation. The degree of gelatin weight loss was calculated with initial and final mass, and the degree of degradation continuously increased in 15 d. (D) The statistical analysis for cell viability and proliferation based on MTS assay. There were no statistically significant differences for each of the 4 d (error bar, standard deviation; mean ± SD).
Compressive Mechanical Properties
The gelatin scaffolds were characterized with compressive stress and strain at the gelatin fracture for mechanical properties. After the phosphate buffered saline (PBS)–wet gelatin scaffolds and the paraffin teeth (n = 3 per group) were assembled, compressive strength was measured through the universal testing machine (LF Plus, Lloyd Instruments, Fareham, Hampshire, UK) at a constant crosshead speed of 1.00 mm/min with 0.5-N preload.
In Vitro Biodegradation
For weight-based in vitro biodegradability evaluations, freeze-dried gelatin scaffolds were measured for their individual mass (wi; initial weight). Then they were sanitized and sterilized in 70% ethanol with ultraviolet light for 1 to 2 hr and rinsed excessively in PBS. After lysozyme (10 mg/L) was prepared from chicken egg white (Sigma-Aldrich, St. Louis, MO, USA), the gelatin scaffolds in 8-mL lysozyme-contained PBS were incubated at 37°C. At the different time intervals (1, 3, 5, 7, 9, 11, 13, and 15 d), each sample was washed in deionized water to remove buffer compounds and freeze-dried after removal of excess water. The dried gelatin scaffolds (n = 3 per time point) were weighed for the final mass (wf) and calculated for the percentage of weight loss as the degree of gelatin degradation:
Biocompatibility and Cell Proliferation for Cell Viability Evaluations
For cell viability, human PDL (hPDL) cells were cultured in gelatin scaffolds with 1 × 105 cells per scaffold. At each culture time point (1, 3, 5, and 7 d), cell viability and proliferation were determined with the MTS—3-(4,5-dimethyl-thiazol-2-yl)-5-(3-carboxymethaoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium—assay following the company instructions (Promega Corporation, Madison, WI, USA). After 3 hr of incubation for each scaffold (n = 4 per group) with MTS reagent and cell culture media, the quantification of light absorbance was performed by spectrophotometry at 490 nm in 96-well plates. The relative cell viability was calculated (Abs: absorbance):
Statistical Analysis
SPSS 20 was used, and all data were expressed as mean ± standard deviation. To calculate the statistical difference of scaffolds, the 1-way analysis of variance test with difference assessments was used according to Bonferroni post hoc multiple comparisons. All of results were significantly different, with the α-value set at a 0.05 level of significance.
Results
3-D Computational Modeling of the Thermal Response
According to dry ice contact regions—4 freezing directions with boundary temperature conditions (TCO2(s) = −70°C to Troom = 20°C)—patterns of ice crystal formation were predicted through simplified vector analysis determined per temperature profiles (Fig. 2, third row). In computational results, vectorial calculations with each freezing direction and initial contact temperatures of surfaces provided mathematical models of growth directions of ice crystals with thermal profiles (Fig. 2).
Analyses of Angulated Pore Structures
Computational simulation provided the vectorial model to predict the regularities of ice growth and angulations of longitudinal pore structures. Gelatin scaffolds contained 90% water, which was the most important factor for the formation of ice crystals with various angulations based on the freeze-casting process (Figs. 2, 5A).
For the assessment of the internal structures, morphologic similarities, such as angulated pores and directional patterns, were qualitatively provided by micro-CT and scanning electron microscope (Fig. 3). Longitudinal cross-section images demonstrated that each group was qualitatively and quantitatively identified with different spatial orientations of pores to the paraffin tooth root surfaces (Fig. 4A). Two regions of interest (ROIs) were selected in coronal and apical portions for quantitative characterizations of scaffolds, such as pore angulations, pore sizes, and porosities (Fig. 4). The pore angulations were measured to the paraffin tooth root surface at the ROIs, and (ROIcoronal = 146.86 ± 13.08; ROIapical = 27.19 ± 5.14) and () (ROIcoronal = 150.20 ± 6.10; ROIapical = 152.16 ± 5.87) were statistically different (p < .001; Fig. 4B) with (ROIcoronal = 38.88 ± 9.23; ROIapical = 38.33 ± 11.19) and () (ROIcoronal = 40.18 ± 10.94; ROIapical = 38.49 ± 8.53). However, angular analysis of samples in the same group showed no differences (p > .05; Fig. 4C), demonstrating that the directional freezing method provided topologic similarities of internal architectures. Although different freezing directions led to significantly different angulations, the pore diameters of every sample showed no significant difference among groups (p = .650; Fig. 4D, 4E). Porosities of each group, evaluated through volumetric micro-CT images, also had no differences among groups (88.03% ± 4.73%; p = .812; Fig. 4G).
Mechanical and Biological Stabilities
To provide similar conditions to in vitro culturing environment, gelatin scaffolds were soaked and stored in 36°C PBS solution prior to mechanical tests. Although individual groups had different compressive results (Fig. 5B, table), statistical results in the mechanical stability evaluations demonstrated no significant differences in compressive stress and strain at fractures (pstress = .1219; pstrain = .0588; Fig. 5B).
Biodegradability for biological stabilities was performed for gelatin scaffolds with the same GA-crosslinking conditions. The scaffolds had progressive weight loss over a period of 15 d due to the loss of gelatin components (Fig. 5C).
Biocompatibility of Gelatin Scaffolds
Although scaffolds from 4 time intervals (1, 3, 5, and 7 d) had no statistically significant difference (p = .0836), crosslinked gelatin scaffolds showed that cells were continuously proliferating and had higher relative cell viability at 7 d (Fig. 5D). In MTS results, GA-crosslinked gelatin scaffolds can have good biocompatibility and provide suitable microenvironment for cell growth and proliferation.
Discussion
Many types of microarchitectures have been developed to physically organize regenerated tissues or promote cell-substrate communications (Hollister, 2005; Rehfeldt et al., 2007; Phillips et al., 2008; Discher et al., 2009; Fu et al., 2010; Grayson et al., 2010; Lee et al., 2010; Guvendiren and Burdick, 2012, 2013; Mendes, 2013). However, it remains challenging to determine geometric influences and develop optimal microenvironments for (1) multitissue neogenesis, (2) tissue interface compartmentalization, and (3) polarization of connective tissue fibers at structural interfaces (Ho et al., 2010; Park et al., 2012; Vaquette et al., 2012; Jeon et al., 2014). Fiber-guiding scaffolds previously emphasized a topologic design of the PDL interface influences of the ligament orientation for bone-ligament complex regeneration compared to salt-leached, amorphous architectures, which facilitated limited multiple tissue regeneration without refunctionalizing (Park et al., 2012). Therefore, the determination of appropriate biomimetic scaffold geometry could be a key driver for the systematic organization and physiologic functionalization of nascent bone-ligament complexes.
Because the collagenous fiber structures at the PDL interface have differential dimensions—the cementum- and bone-associated PDL bundles have, respectively, 3- to 10-µm and 10- to 20-µm diameters (Berkovitz, 1990)—it is challenging to manufacture the submicron-interfacial PDL architectures (<50 µm) for more predictable and precise organization of PDL tissues in the 3-D printing system. Our freeze-casting approach undergoes minimal to no shrinkage and provides submicron-scaled longitudinal porous architectures (mean diameter = 54.21 ± 26.93 µm) that are controlled by directional ice crystal growth kinetics. Not only can the chemical crosslinking agent GA improve physical/mechanical properties and internal architecture stabilities of gelatin scaffolds, but it can also rapidly prevent dissolution of natural gelatin at body temperature.
The pore angulations were characterized by freezing vectors that allowed for PDL generation similar to native tooth-ligament-bone structures, but pore diameters from all the groups showed no statistically significant differences (Fig. 4E). We confirmed that the () group generated a specific topology: oblique angulation similar to natural oblique fibers. Hence, the generated gelatin scaffolds could support cell/tissue platforms and create submicron-scaled geometries for periodontal regenerative medicine applications.
Quantitative assessments based on micro-CT were performed for pore angulations, pore sizes, and scaffold porosities per group. Micro-CT is generally utilized for nondestructive 3-D quantification of bone or hydroxyapatite-like constructs (Jones et al., 2007). Based on the different x-ray attenuation levels and grayscale intensities, individual components—such as bone, solid polymeric scaffolds, pores, and surrounding tissues in a specimen—can be identified for quantitative/qualitative analyses (Jones et al., 2004a; Jones et al., 2004b). Due to no bonelike materials in this study, 2 grayscale thresholds can be considered, and phases can be clearly separated with carbon-based materials (gelatin and paraffin) and air (pores and background) even though the scaffolds had high porosity (88.03% ± 4.73%; Fig. 4G). HU-based intensity histogram provided 2 peaks: HU = −1,100 for air and HU = 270 for materials (Appendix Fig. 2). The phase identification of gelatin scaffolds was performed with the selected HU value, –250 (Appendix Fig. 2), and internal architectures can be quantitatively/qualitatively analyzed. The topographic characteristics of each group had statistically significant similarities, demonstrating that the method yielded highly reproducible structures. Various spatial freezing-directions, however, led to significantly different pore angulations among the groups (Fig. 4). Therefore, the PDL structures generated by the periodontal mimic gelatin scaffold led to the following conclusions: (1) an easy fabrication of longitudinal micro-/nanoscaled porous 3-D scaffolds and organization with oblique angulations to paraffin tooth root surfaces; (2) a manufacturing strategy for the development of predictable geometric controls and design of appropriate internal topographies of the scaffolds; and (3) a structural similarity to native oblique PDL fibers, which is the major structure against masticatory occlusal loadings at the tooth-ligament-bone interface. The other interesting finding is the gelatin scaffold surface contacted to the paraffin tooth was not completely opened. This can be the strategic approach for hPDL cells to stay on the root surface for transforming cementoblast-like cells and inducing cementum formation on the root surface.
Although multilayered scaffolds were developed with a 3-D printer for bone-ligament regeneration (Park et al., 2012; Park et al., 2014), it remains a major challenge to create 3-D submicron-scaled architectures to precisely control fibrous PDL formation with specific angulations as well as manage synthetic polymer properties, such as discouraged biological affinity and unsuitably long biodegradation. The freeze-casting methodology with various freezing directions can regulate ice crystal formations for longitudinal submicron pore structures (Fig. 3). Although gelatin scaffolds had low compressive resistance (Fig. 5B) to support tooth structures (like natural polymeric materials), the denatured form of collagen can provide biodegradability (Fig. 5C) and more significant cell affinity and growth (Fig. 5D) due to intrinsic cell interactions with adhesive RGD motifs (Hersel et al., 2003). Therefore, chemical modification of gelatin scaffolds was not required to promote cell adhesion and growth, and the directional freezing technique is expected to organize regenerated PDLs with topographic similarities to native ligament structures. To improve mechanical properties and form the engineered biomimetic periodontal complex with oblique PDL fiber arrangements, this state-of-the-art platform can be grafted onto a synthetic polymer-based fiber-guiding scaffold system (Park et al., 2012; Park et al., 2014) for multiple periodontal tissue regeneration (bone-PDL-cementum complex with 250-µm hiararchycal interfacial architectures). We believe that this periodontal mimic scaffold platform represents an important potential application for dental stem cell delivery, for periodontal regenerative medicine (Appendix Fig. 3).
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI12C0985) (Y.J.S.); by the Korea Science and Technology Foundation Nanobiotechnology Development Program entitled Regenomics (2013036438) (Y.J.S.); and by the National Institutes of Health/National Institute of Dental and Craniofacial Research (DE 13397) (W.V.G.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bencherif SA, Sands RW, Bhatta D, Arany P, Verbeke CS, Edwards DA, et al. (2012). Injectable preformed scaffolds with shape-memory properties. Proc Natl Acad Sci U S A 109:19590-19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovitz BK. (1990). The structure of the periodontal ligament: an update. Eur J Orthod 12:51-76. [DOI] [PubMed] [Google Scholar]
- Burdick JA. (2009). Bioengineering: cellular control in two clicks. Nature 460:469-470. [DOI] [PubMed] [Google Scholar]
- Deville S, Saiz E, Nalla RK, Tomsia AP. (2006a). Freezing as a path to build complex composites. Science 311:515-518. [DOI] [PubMed] [Google Scholar]
- Deville S, Saiz E, Tomsia AP. (2006b). Freeze casting of hydroxyapatite scaffolds for bone tissue engineering. Biomaterials 27:5480-5489. [DOI] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. (2009). Growth factors, matrices, and forces combine and control stem cells. Science 324:1673-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormer NH, Berkland CJ, Detamore MS. (2010). Emerging techniques in stratified designs and continuous gradients for tissue engineering of interfaces. Ann Biomed Eng 38:2121-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, et al. (2010). Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods 7:733-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson WL, Frohlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, et al. (2010). Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A 107:3299-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvendiren M, Burdick JA. (2012). Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun 3:792. [DOI] [PubMed] [Google Scholar]
- Guvendiren M, Burdick JA. (2013). Stem cell response to spatially and temporally displayed and reversible surface topography. Adv Healthc Mater 2:155-164. [DOI] [PubMed] [Google Scholar]
- Hersel U, Dahmen C, Kessler H. (2003). RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24:4385-4415. [DOI] [PubMed] [Google Scholar]
- Ho SP, Kurylo MP, Fong TK, Lee SS, Wagner HD, Ryder MI, et al. (2010). The biomechanical characteristics of the bone-periodontal ligament-cementum complex. Biomaterials 31:6635-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister SJ. (2005). Porous scaffold design for tissue engineering. Nat Mater 4:518-524. (Published erratum in Nat Mater 5:590, 2006). [DOI] [PubMed] [Google Scholar]
- Hughes FJ, Ghuman M, Talal A. (2010). Periodontal regeneration: a challenge for the tissue engineer? Proc Inst Mech Eng H 224:1345-1358. [DOI] [PubMed] [Google Scholar]
- Jeon JE, Vaquette C, Klein TJ, Hutmacher DW. (2014). Perspectives in multiphasic osteochondral tissue engineering. Anat Rec (Hoboken) 297:26-35. [DOI] [PubMed] [Google Scholar]
- Jones AC, Sakellariou A, Limaye A, Arns CH, Senden TJ, Sawkins T, et al. (2004a). Investigation of microstructural features in regenerating bone using micro computed tomography. J Mater Sci Mater Med 15:529-532. [DOI] [PubMed] [Google Scholar]
- Jones AC, Milthorpe B, Averdunk H, Limaye A, Senden TJ, Sakellariou A, et al. (2004b). Analysis of 3D bone ingrowth into polymer scaffolds via micro–computed tomography imaging. Biomaterials 25:4947-4954. [DOI] [PubMed] [Google Scholar]
- Jones AC, Arns CH, Sheppard AP, Hutmacher DW, Milthorpe BK, Knackstedt MA. (2007). Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials 28:2491-2504. [DOI] [PubMed] [Google Scholar]
- Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. (2010). Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 376:440-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WL, Lu K, Walz JY. (2012). Freeze casting of porous materials: review of critical factors in microstructure evolution. Int Mater Rev 57:37-60. [Google Scholar]
- Ma H, Hu J, Ma PX. (2010). Polymer scaffolds for small-diameter vascular tissue engineering. Adv Funct Mater 20:2833-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes PM. (2013). Cellular nanotechnology: making biological interfaces smarter. Chem Soc Rev 42:9207-9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat KL, Sun WH, Pena PE, Chahine NO, Doty SB, Ateshian GA, et al. (2008). Characterization of the structure-function relationship at the ligament-to-bone interface. Proc Natl Acad Sci U S A 105:7947-7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagni G, Kaigler D, Rasperini G, Avila-Ortiz G, Bartel R, Giannobile WV. (2012). Bone repair cells for craniofacial regeneration. Adv Drug Deliv Rev 64:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Rios HF, Jin Q, Bland ME, Flanagan CL, Hollister SJ, et al. (2010). Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials 31:5945-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Rios HF, Jin Q, Sugai JV, Padial-Molina M, Taut AD, et al. (2012). Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials 33:137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Rios HF, Taut AD, Padial-Molina M, Flanagan CL, Pilipchuk SP, et al. (2014). Image-based, fiber guiding scaffolds: a platform for regenerating tissue interfaces. Tissue Eng Part C Methods 20:533-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JE, Burns KL, Le Doux JM, Guldberg RE, Garcia AJ. (2008). Engineering graded tissue interfaces. Proc Natl Acad Sci U S A 105:12170-12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiate IA, de Vasconcellos AB, de Santana RB, Poiate E. (2009). Three-dimensional stress distribution in the human periodontal ligament in masticatory, parafunctional, and trauma loads: finite element analysis. J Periodontol 80:1859-1867. [DOI] [PubMed] [Google Scholar]
- Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. (2007). Cell responses to the mechanochemical microenvironment: implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev 59:1329-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquette C, Fan W, Xiao Y, Hamlet S, Hutmacher DW, Ivanovski S. (2012). A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 33:5560-5573. [DOI] [PubMed] [Google Scholar]
- Waschkies T, Oberacker R, Hoffmann MJ. (2011). Investigation of structure formation during freeze-casting from very slow to very fast solidification velocities. Acta Mater 59:5135-5145. [Google Scholar]
- Yang PJ, Temenoff JS. (2009). Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev 15:127-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel A, Rehfeldt F, Brown AE, Discher DE, Safran SA. (2010). Optimal matrix rigidity for stress fiber polarization in stem cells. Nat Phys 6:468-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.