Abstract
This study aims at modifying dual-cure composite cements by adding thio-urethane oligomers to improve mechanical properties, especially fracture toughness, and reduce polymerization stress. Thiol-functionalized oligomers were synthesized by combining 1,3-bis(1-isocyanato-1-methylethyl)benzene with trimethylol-tris-3-mercaptopropionate, at 1:2 isocyanate:thiol. Oligomer was added at 0, 10 or 20 wt% to BisGMA-UDMA-TEGDMA (5:3:2, with 25 wt% silanated inorganic fillers) or to one commercial composite cement (Relyx Ultimate, 3M Espe). Near-IR was used to measure methacrylate conversion after photoactivation (700 mW/cm2 × 60s) and after 72 h. Flexural strength and modulus, toughness, and fracture toughness were evaluated in three-point bending. Polymerization stress was measured with the Bioman. The microtensile bond strength of an indirect composite and a glass ceramic to dentin was also evaluated. Results were analyzed with analysis of variance and Tukey’s test (α = 0.05). For BisGMA-UDMA-TEGDMA cements, conversion values were not affected by the addition of thio-urethanes. Flexural strength/modulus increased significantly for both oligomer concentrations, with a 3-fold increase in toughness at 20 wt%. Fracture toughness increased over 2-fold for the thio-urethane modified groups. Contraction stress was reduced by 40% to 50% with the addition of thio-urethanes. The addition of thio-urethane to the commercial cement led to similar flexural strength, toughness, and conversion at 72h compared to the control. Flexural modulus decreased for the 20 wt% group, due to the dilution of the overall filler volume, which also led to decreased stress. However, fracture toughness increased by up to 50%. The microtensile bond strength increased for the experimental composite cement with 20 wt% thio-urethane bonding for both an indirect composite and a glass ceramic. Novel dual-cured composite cements containing thio-urethanes showed increased toughness, fracture toughness and bond strength to dentin while demonstrating reduced contraction stress. All of these benefits are derived without compromising the methacrylate conversion of the resin component. The modification does not require changing the operatory technique.
Keywords: prepolymerized additives, polymerization stress, fracture toughness, delayed gelation, kinetics of conversion, volumetric shrinkage
Introduction
Dual-cured composite cements are extensively used in restorative dentistry. Examples of their clinical applications include the bonding of ceramic fragments, crowns, bridges, and intracanal posts. Due to the tapered configuration of the tooth preparation for indirect restorations, characteristics such as resistance to dissolution, strong bond to tooth and prosthetic structures, and excellent mechanical properties are requisites for composite cements (Meyer et al., 1998; Rosenstiel et al., 1998; Manso et al., 2011). Current commercial methacrylate-based materials present some drawbacks, such as polymerization shrinkage values between 1.77% and 5.28% (Spinell et al., 2009), which increase the risk of stress concentration and gap formation at the cement margin and ultimately may lead to adhesion failure (D’Arcangelo et al., 2009).
At least for heavily filled composites, reduction in polymerization shrinkage and stress can be accomplished by replacing monomers with prepolymerized resin particles (Moraes et al., 2011). Stress reduction can also be achieved through delayed vitrification, achieved by adding thiol-modified molecules, such as thiol-ene oligomers. Chain transfer reactions of the thiol to the ene/vinyl lead to more homogeneous network formation while delaying vitrification to higher conversion levels, with the potential to reduce stress and with the added benefit of increased final conversion (Lu et al., 2005; Cramer et al., 2010; Boulden et al., 2011) but unfortunately with some loss in mechanical properties (Lu et al., 2005).
Thio-urethane oligomers present an attractive alternative to common nonfunctionalized prepolymers and even thiol-ene oligomers, due to the high toughness imparted by the thio-urethane bonds to the polymer matrix (Senyurt et al., 2007), in addition to an overall more homogeneous network formation compared to urethane counterparts. Materials involving thiol/isocyanate reactions have been used to produce 2-part systems for applications requiring high fracture toughness and impact resistance (Senyurt et al., 2007).
This study describes a novel approach, not previously attempted for dental applications, through the use of thiol-terminated thio-urethane oligomers as additives in methacrylate matrices for dental composite cements with enhanced toughness, reduced stress, and improved bond strength. The rationale behind these materials is to leverage the delayed gelation resulting from the pendant thiol functionalities to reduce stress, increase conversion, and improve network homogeneity (Pfeifer et al., 2012); reduce shrinkage through the addition of prepolymerized particles (Moraes et al., 2011); and increase toughness/fracture toughness and bond strength through the thio-urethane bonds (Senyurt et al., 2007). Finally, by improving fracture toughness, improved bond strength of indirect restorations cemented with the thio-urethane modified cements can be expected.
Therefore, the aim of this study was to synthesize prepolymerized oligomers based on thio-urethane chemistry, containing pendant thiol functionalities for improving the properties of dual-cured composite cements. The tested hypotheses were that the use of thio-urethanes will (1) increase the degree of conversion, (2) improve mechanical properties, and (3) reduce the polymerization stress of a secondary cross-linked methacrylate network. An additional hypothesis (4) was that the addition of thio-urethanes would improve the bond strength of indirect resin composites and ceramics cemented to dentin.
Materials & Methods
Oligomers were synthesized by combining 1,3-bis(1-isocyanato-1-methylethyl)benzene with trimethylol-tris-3-mercaptopropionate in solution (used as received, 97% purity), at 1:2 isocyanate:thiol, resulting in pendant thiols from the aromatic oligomer structure. The reaction was carried out in solution at room temperature using triethylamine in catalytic amounts, with no by-products. The oligomers were purified by precipitation in hexanes, and the solvent was removed under vacuum. The resulting product is a viscous liquid characterized by 1H-NMR to verify the absence of starting materials and the formation of thio-urethane bonds.
Experimental dual-cured composite cements were produced by combining BisGMA (Bis-phenol A diglycidyl dimethacrylate), UDMA (urethane dimethacrylate), and TEGDMA (tri-ethylene glycol dimethacrylate), all from Esstech (Essington, PA, USA), in a 50:30:20 mass ratio (BisGMA-UDMA-TEGDMA materials). Thio-urethane oligomers were added at 0 (control), 10, and 20 wt%. To this resin, 0.2 wt% of dl-camphoroquinone, 0.6 wt% of a tertiary amine (EDMAB [ethyl 4-dimethylaminobenzoate]), and 0.8 wt% of inhibitor (BHT [2,6-di-tert-butyl-4-methylphenol]; SigmaAldrich, St. Louis, MO, USA) were added to produce component A. A second component, B, was made by the addition of 0.5 wt% of benzoyl-peroxide (SigmaAldrich). To each component (A and B), a total of 25 wt% filler was added, composed of 15% of 0.04-µm average silane-treated silica (OX-50) and 85% of 0.7-µm silane-treated Barium glass (Esstech), with the aid of a mechanical mixer (DAC 150 Speed mixer, Flacktek, Landrum, SC, USA) operated for 5 min at 2,400 rpm. A second set of experiments included materials formulated with 65 wt% of 0.7-µm silane-treated Barium glass (Esstech).
A BisGMA/TEGDMA-based commercial dual-cured cement (lot no. 498131, translucent; RelyX Ultimate, 3M ESPE, St. Paul, USA) was used as received, as well as modified by the addition of 10 and 20 wt% of oligomer, resulting in cements with filler contents of 66, 63.6, and 60.8 wt%, respectively (based on manufacturer data). The viscosity of the experimental materials at both filler loadings, as well as that of the commercial materials, was measured with a rheometer. Film thickness was measured for the 65% filler and commercial material according to ISO 4049 (see appendix).
Materials were mixed immediately before use. Composite discs (0.8 mm thick, 10 mm in diameter, n = 3) were formed between 2 glass slides. Degree of conversion was obtained using near-infrared spectroscopy (2 scans/spectrum, 4 cm–1 resolution, >2 Hz data acquisition rate) based on the area of the methacrylate vinyl overtone at 6,165 cm–1 (Stansbury and Dickens, 2001) before and after 60 sec of direct irradiation with a LED light source (Bluephase, Ivoclar Vivadent, Lichtenstein) at an incident irradiance of 700 mW/cm2. Specimens were stored dry in dark containers for 72 hr and tested again. To simulate clinical conditions, in a second set of experiments, the specimens were irradiated through a composite or ceramic disc. This procedure is detailed in the supplemental materials section.
Bar specimens (n = 10, 2 × 2 × 25 mm) were fabricated with silicone molds between glass slides and photopolymerized as described above, then stored dry for 1 wk in dark containers at room temperature. Flexural strength of the samples was measured according to ISO 4049 (Standard I; 2009) in 3-point bending using a universal test machine (Q-test, MTS, Eden Prairie, WI, USA) at a cross-head speed of 0.5 mm/min. Elastic modulus (GPa) was determined from the slope of the initial linear portion of the stress-strain curve. Toughness (MPa) was calculated from the integration of the stress × stain curve (Origin 9.1, OriginLab Corporation, Northampton, MA, USA).
To determine the fracture toughness, single-edge notch beam specimens (n = 5) were fabricated according to ASTM Standard E399-90 (Designation A; American Society for Testing Materials, 1997) in a split steel mold (5 × 2 × 25 mm) with a razor-blade insert providing a 2.5 mm notch at the center of the specimens. The test was performed in 3-point bending at a cross-head speed of 0.5 mm·min−1 on the universal test machine (Q-test), and the fracture toughness (critical stress intensity factor, KIC) was calculated as previously described (Ferracane & Berge, 1995).
Polymerization stress development was followed in real-time for 30 min using the Bioman, as described previously (Watts & Satterthwaite, 2008). Briefly, the composite cement (n = 5) was applied between a silica slab and a roughened steel piston producing a cement of 0.5-mm thickness, corresponding to a C-factor of 4, then photoactivated through the glass during 60 sec with an incident irradiance of 670 mW/cm2 (Bluephase; Ivoclar-Vivadent).
Microtensile bond strength was tested with 36 caries-free human third molars extracted for periodontal reasons (Institutional Ethics Committee, approval 040/2013; see appendix). In summary, deep dentin was exposed by removing the cusps 2.0 mm above the CEJ, then roughened with SiC paper. An etch-and-rinse adhesive (Adper Singlebond 2; 3M ESPE) was applied according to manufacturer instructions. Indirect composite (Z250, 3M ESPE, A2) or a glass ceramic (IPS Empress, Ivoclar Vivadent, HT-A2) were used to fabricate blocks (8 × 5 × 3 mm). After specific surface treatments, the blocks were cemented with the commercial cement (control and modified groups), then light cured for 60 sec at 700 mW/cm2 on each side of the composite/ceramic block. Samples (n = 6) were stored for 1 wk in distilled water (37oC) before being sectioned into ~1-mm2 match-sticks. Specimens were tested in tension at 0.5 mm/min. Results within each cement were analyzed by 1-way analysis of variance and Tukey’s test (α = 0.05). The failure patterns were evaluated by stereomicroscopy/scanning electron microscope.
Results
For the 25 wt% filler loading, degree of conversion tended to increase with the addition of thio-urethanes to the experimental (BisGMA-UDMA-TEGDMA) cement when measured immediately after photoactivation (p = .145) or after 72 hr (p = .053), but the difference was not significant (Table 1). For the 65 wt% filler loading, conversion decreased with the addition of thio-urethanes when measured immediately after photoactivation (p = .001), but values were all statistically similar after 72 hr (p = .120; Appendix Table 2). For the commercial material, the thio-urethane-modified groups showed a significant reduction in degree of conversion when evaluated immediately (p = .002) but were similar to the control after 72 hr of storage (p = .930).
Table 1.
Percentage Degree of Conversion for 25 wt% Filler Experimental and Commercial Cements Immediately after Light Irradiation and 72 hr
| Experimental 25 wt% filler |
Commercial |
|||
|---|---|---|---|---|
| Immediate | After 72 hr | Immediate | After 72 hr | |
| Control | 70.6 ± 2.3a | 80.7 ± 2.4a | 65.8 ± 0.8a | 69.5 ± 1.7a |
| 10 wt% | 72.4 ± 1.0a | 83.5 ± 1.5a | 59.7 ± 3.2b | 69.5 ± 1.4a |
| 20 wt% | 73.7 ± 1.1a | 85.1 ± 0.8a | 54.0 ± 1.7b | 69.9 ± 0.9a |
Values in mean ± SD. Photoactivation was carried out through a mylar strip. Values followed by the same superscript within the same test are statistically similar (α = 5%).
Flexural strength and modulus significantly increased at both concentrations for the thio-urethane-modified BisGMA-UDMA-TEGDMA materials in relation to the control (p = .000; Table 2). The toughness increased by 3-fold for the 20 wt% oligomer concentration (p = .000) in BisGMA-UDMA-TEGDMA material. The addition of thio-urethanes to the commercial material did not significantly affect flexural strength (p = .345) or toughness (p = .202) but led to a reduction in the flexural modulus (p = .018) for the 20 wt% oligomer concentration. For the experimental cement formulated with 25 wt% filler, the groups with 10 and 20 wt% oligomer showed 41% and 53% reduced polymerization stress, respectively, vs. the control (p = .003; Figure 1A). For the experimental cement formulated with 65 wt% filler, stress reductions were 30% and 70% in relation to the control for the 10% and 20% oligomer concentration, respectively (p = .015; see appendix and Appendix Figure 4). A significant 66% reduction was observed when 20 wt% of thio-urethane was added to the commercial cement (p = .000).
Table 2.
Flexural Strength, Flexural Modulus, and Toughness for 25 wt% Filler Experimental and Commercial Cements
| Flexural Strength (MPa) |
Flexural Modulus (GPa) |
Toughness (MPa) |
µTBS Composite (MPa) |
µTBS Ceramic (MPa) |
||||
|---|---|---|---|---|---|---|---|---|
| EXP | COM | EXP | COM | EXP | COM | EXP | COM | |
| Control | 94.5 ± 16.5b | 134 ± 15.3a | 2.2 ± 0.25b | 5.5 ± 0.44a | 3.37 ± 1.48b | 1.95 ± 0.57a | 23.1 ± 4.47b | 15.3 ± 2.89b |
| 10 wt% | 119.8 ± 15.5a | 129.1 ± 20.5a | 2.8 ± 0.16a | 4.9 ± 0.67a,b | 3.89 ± 1.54b | 1.81 ± 0.49a | 26.95 ± 1.66a,b | 16.7 ± 3.25a,b |
| 20 wt% | 131.6 ± 6.6a | 121.5 ± 16.3a | 2.8 ± 0.24a | 4.3 ± 0.11b | 10.22 ± 2.61a | 2.36 ± 0.84a | 28.41 ± 2.05a | 21.4 ± 2.17a |
Values in mean ± SD. Also shown in the table are the microtensile bond strength (µTBS) of an indirect composite and glass ceramic to dentin for the commercial cement modified with thio-urethanes. Values followed by the same superscript within the same test are statistically similar (α = 5%).
COM, commercial; EXP, experimental.
Figure 1.
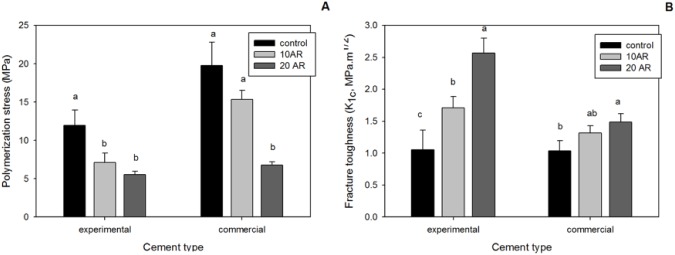
(A) Polymerization stress and (B) fracture toughness values for the 25 wt% filler BisGMA/UDMA/TEGDMA cement and for the commercial cement (RelyX Ultimate) with 0 (control), 10, and 20 wt% of thio-urethane oligomer added.
The addition of 10% and 20% thio-urethane produced significant increases of 54% and 136%, respectively, in the fracture toughness of the experimental cement formulated with 25 wt% filler (p = .000; Figure 1B). For the commercial cement, a significant increase in fracture toughness was produced at 20 wt% of oligomer (p = .002), being 50% higher than the control.
The addition of 20 wt% oligomer to the commercial cement led to a significant increase in µTBS values for both bonding substrates—indirect composite (p = .039) or glass ceramic (p = .005; Table 2). Groups with 10 wt% of oligomer presented results intermediary between the control and the 20 wt% group. The failure patterns were predominantly mixed adhesive/cohesive for all groups. Control groups presented smoother fracture surfaces in comparison to the modified groups (Figures 2 and 3).
Figure 2.
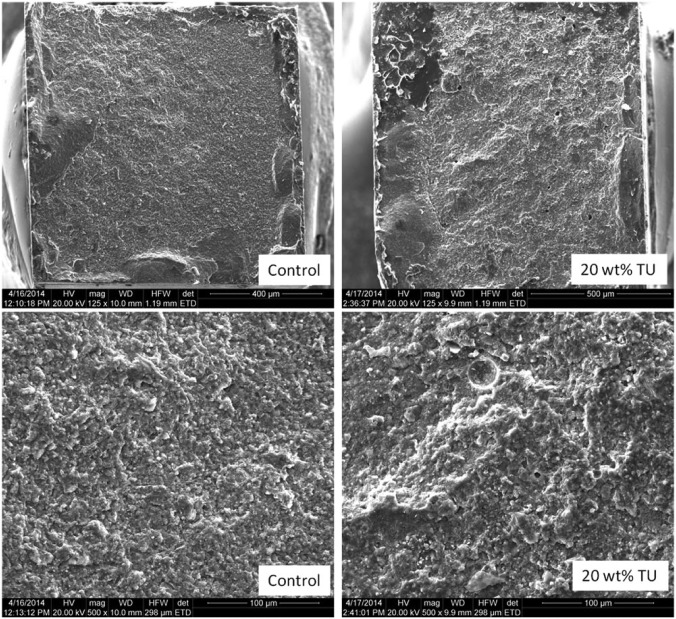
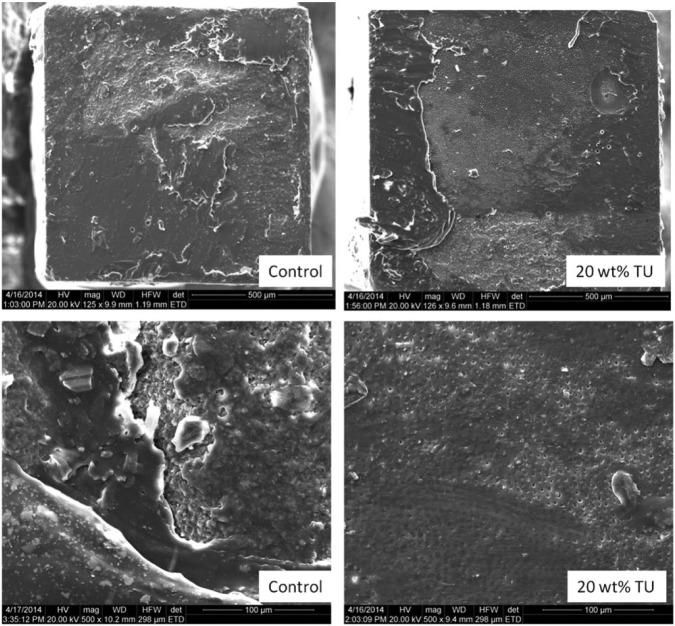
Scanning electron microscope images of fractured surfaces of microtensile bond strength ceramic specimens. In general, the modified groups presented rougher surfaces compared to the control materials. However, the majority of failures were mixed adhesive-cohesive regardless of the treatment.
Figure 3.
Scanning electron microscope images of fractured surfaces of microtensile bond strength composite specimens. Both specimens showed a mixed adhesive-cohesive failure pattern. However, exposed dentin tubules can be seen only for the modified groups, indicating that the increased toughness on the cement led the crack to propagate through the interface and not through the bulk of the material.
Discussion
The addition of thiol-ene and thio-urethane oligomers to secondary methacrylate light-cured resins improves the formation of homogeneous polymer networks with higher degree of conversion (Lu et al., 2005; Cramer et al., 2010; Pfeifer et al., 2012). Chain transfer reactions between the thiol and the vinyl group are chain breaking, which means that the polymerization progresses through a radically assisted step-growth mechanism (Cramer and Bowman, 2001; Reddy et al., 2006), delaying the point at which diffusion limitations start to hamper polymerization to much higher conversions, resulting in a higher conversion overall (Berchtold et al., 2002). In the present study, the cements were polymerized through a dual-cure mechanism, making it difficult to follow the reaction kinetics in real time. Therefore, conversion was only measured at 2 time points, immediately after polymerization and after 72 hr. For the experimental cements, immediate degree of conversion obtained in specimens photoactivated through a mylar strip was around 70% for all groups, increasing to around 83% at 72 hr. At both evaluation times, the addition of thiol-functionalized thio-urethanes did not interfere with conversion, likely due to the fact that the materials have a redox component to the polymerization, which in itself contributes to delayed vitrification (Odian, 2004), masking the influence of the additives on conversion. For the commercial cement, the immediate conversion values decreased with the addition of thio-urethanes, and it is speculated that the slower progression of the reaction was due to delayed network formation, as well as light scattering by the higher filler concentration. The values at 72 hr were all statistically similar at around 70% and increased more markedly for the thio-urethane-modified materials. The difference in degree of conversion and curing rate between the experimental and commercial cements is attributed to differences in filler loading (and potentially filler surface treatment), resin matrix, and catalyst formultion. It is also important to note that the overall conversion of the commercial materials was lower than the experimental counterparts, and those other components in the commercial formulation, outside the control of the operator, likely played a role in kinetics for the former. Indeed, the conversion presented by the same experimental materials (BisGMA-UDMA-TEGDMA) loaded with 65 wt% filler was lower than the 25 wt% filler counterparts (see Appendix and Appendix Table 2). In general, the addition of thiol-functionalized thio-urethanes did not affect conversion at 72 hr for all dual-cured materials. Therefore, the first hyphothesis must be rejected.
In spite of the similar conversion values, a significant increase in the flexural strength, modulus, and toughness was achieved via thio-urethane addition to the experimental cement with 25 wt% filler. This was expected mainly based on the known significant toughening effect of thio-urethane networks (Senyurt et al., 2007). In addition, the improvement in mechanical properties is likely an effect of the more homogeneous methacrylate network formed due to chain transfer to the thiol in the oligomer backbone, as well as on the rigidity of the aromatic rings present in this particular thio-urethane oligomer. For the commercial cement, the values of flexural strength and toughness were not affected by the addition of thio-urethane, while the flexural modulus decreased but only when 20 wt% thio-urethane was added (Table 2). Because the oligomers were added to the fully formulated commercial cement, there was an unavoidable reduction in the overall filler content for the modified materials, which accounts for the 20% reduction in flexure modulus. According to the manufacturer, the commercial material contains 66 wt% filler, which means that adding 10 and 20 wt% thio-urethane brings the filler content to 63.6% and 60.8 wt%, respectively. A previous study demonstrated a larger 30% reduction in elastic modulus when the filler content of a composite was reduced by a mere 5 vol% (Gonçalves et al., 2010), so the reduction in the present study was actually lower than expected. The inability to control filler content may explain why the significant toughening effect seen for the experimental cement with 20% thio-urethane was not replicated for the commercial cement with 20%. However, similar observations were made for the experimental material loaded with 65 wt% filler (see appendix and Appendix Figure 3), where the thio-urethane concentration was adjusted to compensate for the filler loading. A simpler hypothesis can then be drawn: for highly filled materials, flexural properties are more markedly dependent on the inorganic filler.
The most encouraging results, however, were presented by the fracture toughness tests. For the experimental cements (BisGMA-UDMA-TEGDMA) formulated with 25 wt% filler, the addition of 10 and 20 wt% oligomer led to fracture toughness values 55% and 136% higher than the control, respectively, representing more than a 2-fold increase. In the commercial cement, the addition of 20 wt% thio-urethane led to an increase of 50% in fracture toughness, in spite of the decrease in modulus described above due to the reduction in filler content. These results add to the relevance of the contribution of the flexibility conferred to the network by the incorporation of thio-urethane bonds to the resistance to crack propagation even in more highly filled materials and, ultimately, to the integrity of the network. Therefore, the second hypothesis is accepted.
The polymerization stress of the experimental cement loaded with 25 wt% filler was approximately 40% and 53% lower than the control for the groups with 10 and 20 wt% of thio-urethane oligomer, respectively. For both concentrations, this was accomplished without compromising the conversion and modulus—the latter actually increased by 27%. One explanation for the reduction in stress is the decrease in the concentration of methacrylate double bonds per unit volume of material given by the addition of prepolymerized oligomers, leading to reduction of the volumetric shrinkage (Patel et al., 1987). Second, the chain transfer reactions from the pendant thiols on the thio-urethane structure to the surrounding methacrylate matrix have been demonstrated to delay gelation/vitrification, leading to lower polymerization stress development at the later stages of polymerization (Pfeifer et al., 2011; Pfeifer et al., 2012). For the commercial material, stress for the 20 wt% oligomer also presented a significant reduction of 66%. However, in this case, on top of the expected reduction in shrinkage and delayed vitrification, the reduction in stress is also explained by the reduction in modulus observed for this group (Pfeifer et al., 2008) due to the dilution of the filler content with the addition of the thio-urethanes. For the 65 wt% filler material, even greater stress reductions were observed without compromise to the modulus values (see Appendix and Appendix Figure 4). Therefore, the third hypothesis is partially accepted.
This study also evaluated the potential of thio-urethane addition to increase the microtensile bond strength (µTBS) of an indirect composite and a glass ceramic to dentin. The improvement in fracture toughness was expected to enhance the bond strength for the thio-urethane-modified commercial cement (Fritz et al., 1996). For this analysis only the commercial material was utilized to cement blocks of indirect resin composite and Empress ceramic to midcoronal dentin. The use of 20 wt% of thio-urethane significantly increased µTBS values, being 23% higher for the composite substrate and 40% higher for ceramic compared to the control. The demonstrated increase in toughness and fracture toughness presented by this cement when modified with 20 wt% of oligomer is likely the main factor explaining the higher bond strength. Moreover, the reduced polymerization stress also likely reduced residual stresses at the interfaces and in the bulk of the cement. When the fractured surfaces were analyzed, the majority of the fracture patterns were mixed adhesive/cohesive for all groups tested. However, for the ceramic material, control groups presented smoother fracture surfaces in comparison to the modified groups, as demonstrated by scanning electron microscope images. The increased roughness on the fracture surfaces of the modified materials indicates a greater energy required for fracture of the toughened materials (Quinn, 2007), consistent with the higher results obtained in the 3-point bending test. As for the indirect composite groups, a greater incidence of exposed dentin tubules was observed for the groups modified by thio-urethane oligomers. It can be inferred that the toughening of the cement in those groups forced the crack to propagate at the dentin/adhesive interface, exposing the tubules on the surface. Therefore, the fourth hypothesis is accepted, as the increase in fracture toughness and decrease in polymerization stress contributed to higher bond strengths of dentin with indirect restorative substrates.
Conclusion
The addition of thio-urethanes to experimental and commercial dental composite cements significantly reduced the polymerization stress and increased the fracture toughness, as well as improved the bond strength of an indirect composite and a glass ceramic to dentin. This was accomplished without reducing conversion and general mechanical properties and without disrupting current operatory techniques, which greatly facilitates the translation of this novel technology from bench-top to chair-side.
Supplementary Material
Acknowledgments
We also thank Dr. Jeffrey Stansbury and Caroline Szczepanski for performing the viscosity measurements.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
We thank the National Institutes of Health and National Institute of Dental and Craniofacial Research (1R15-DE023211-01A1 and 1U01-DE02756-01), CAPES (Coordenação de Aperfeiçoamento de Pessoal em Nível Superior; grant BEX 5627-13-3), the Foundation of Meridional Faculty for financial support, Esstech for providing the dimethacrylate monomers, and 3M-ESPE for donation of the commercial material.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- American Society for Testing Materials (1997). E399-90: Standard Test Method for Plane-Strain Fracture Toughness of Metallic Materials. Philadelphia, PA. [Google Scholar]
- Berchtold KA, Lovestead TM, Bowman CN. (2002). Coupling chain length dependent and reaction diffusion controlled termination in the free radical polymerization of multivinyl (meth)acrylates. Macromolecules 35:7968-7975. [Google Scholar]
- Boulden JE, Cramer NB, Schreck KM, Couch CL, Bracho-Troconis C, Stansbury JW, et al. (2011). Thiol-ene-methacrylate composites as dental restorative materials. Dent Mater 27:267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer NB, Bowman CN. (2001). Kinetics of thiol-ene and thiol-acrylate photopolymerization with real-time Fourier transform infrared. J Polym Sci Part A: Polym Chem 39:3311-3319. [Google Scholar]
- Cramer NB, Couch CL, Schreck KM, Carioscia JA, Boulden JE, Stansbury JW, et al. (2010). Investigation of thiol-ene and thiol-ene-methacrylate based resins as dental restorative materials. Dent Mater 26:21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcangelo C, de Angelis F, D’Amario M, Zazzeroni S, Ciampoli C, Caputi S. (2009). The influence of luting systems on the microtensile bond strength of dentin to indirect resin-based composite and ceramic restorations. Oper Dent 34:328-336. [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Berge HX. (1995). Fracture toughness of experimental dental composites aged in ethanol. J Dent Res 74:1418-23. [DOI] [PubMed] [Google Scholar]
- Fritz UB, Finger WJ, Uno S. (1996). Resin-modified glass ionomer cements: bonding to enamel and dentin. Dent Mater 12:161-166. [DOI] [PubMed] [Google Scholar]
- Gonçalves F, Kawano Y, Braga RR. (2010). Contraction stress related to composite inorganic content. Dent Mater 26:704-709. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization (2000). Standard I; ISO 4049 Polymer Based Filling, Restorative and Luting Materials. Geneva, Switzerland: International Organization for Standardization, pp 1-27. [Google Scholar]
- Lu H, Carioscia JA, Stansbury JW, Bowman CN. (2005). Investigations of step-growth thiol-ene polymerizations for novel dental restoratives. Dent Mater 21:1129-1136. [DOI] [PubMed] [Google Scholar]
- Manso AP, Silva NR, Bonfante EA, Pegoraro TA, Dias RA, Carvalho RM. (2011). Cements and Adhesives for all-ceramic restorations. Dent Clin North Am 55:311-332. [DOI] [PubMed] [Google Scholar]
- Meyer JM, Cattani-Lorente MA, Dupuis V. (1998). Compomers: between glass-ionomer cements and composites. Biomaterials 19:529-539. [DOI] [PubMed] [Google Scholar]
- Moraes RR, Garcia JW, Barros MD, Lewis SH, Pfeifer CS, Liu J, et al. (2011). Control of polymerization shrinkage and stress in nanogel-modified monomer and composite material. Dent Mater 27:509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odian G. (2004). Principles of Polymerization. New York, NY: Wiley-Interscience. [Google Scholar]
- Patel MP, Braden M, Davy KW. (1987). Polymerization shrinkage of methacrylate esters. Biomaterials 8:53-56. [DOI] [PubMed] [Google Scholar]
- Pfeifer CS, Ferracane JL, Sakaguchi RL, Braga RR. (2008). Factors affecting photopolymerization stress in dental composites. J Dent Res 87:1043-1047. [DOI] [PubMed] [Google Scholar]
- Pfeifer CS, Wilson ND, Shelton ZR, Stansbury JW. (2011). Delayed gelation through chain-transfer reactions: mechanism for stress reduction in methacrylate. Polymer (Guildf) 52:3295-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer C, Lewis S, Stansbury J. (2012). Thio-urethane oligomers as low-stress, tough additives in methacrylate polymerizations. IADR General Section and Exhibition: Oral Session, 6/20/2012. URL accessed on 8/27/2014 at: https://iadr.confex.com/iadr/2012rio/webprogram/Paper163774.html.
- Quinn GB. (2007). Fractography of Ceramics and Glasses: NIST Recommended Practice Guide. Special publication 960-916. Washington, DC: National Institute of Standards and Technology. [Google Scholar]
- Reddy SK, Cramer NB, Bowman CN. (2006). Thiol-vinyl mechanisms: 1. Termination and propagation kinetics in thiol-ene photopolymerizations. Macromolecules 39:3673-3680. [Google Scholar]
- Rosenstiel SF, Land MF, Crispin BJ. (1998). Dental luting agents: a review of the current literature. J Prosthet Dent 80:280-301. [DOI] [PubMed] [Google Scholar]
- Senyurt AF, Hoyle CE. (2007). Thermal and mechanical properties of cross-linked photopolymers based on multifunctional thiol-urethane ene monomers. Macromolecules 40:3174-3182. [Google Scholar]
- Spinell T, Schedle A, Watts DC. (2009). Polymerization shrinkage kinetics of dimethacrylate resin-cements. Dent Mater 25:1058-1066. [DOI] [PubMed] [Google Scholar]
- Stansbury JW, Dickens SH. (2001). Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater 17:71-79. [DOI] [PubMed] [Google Scholar]
- Watts DC, Satterthwaite JD. (2008). Axial shrinkage-stress dependens upon both C-factor and composite mass. Dent Mater 24:1-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.