Abstract
One of the leading causes for the failure of dental composite restorations is secondary caries. Effectively inhibiting cariogenic biofilms and reducing secondary caries could extend the service life of composite restorations. Dental composites releasing antibacterial agents such as chlorhexidine (CHX) have shown biofilm-inhibitory efficacy, but they usually have poor physical and mechanical properties. Herein, we present a study of a new method to encapsulate and release CHX from dental composite using mesoporous silica nanoparticles (MSNs). SBA-15 MSNs were synthesized according to a reported procedure. CHX (62.9 wt%) was encapsulated into dried MSN from 0.3 M CHX ethanol solution. The dental composites containing 0% (control), 3%, 5%, and 6.3% CHX or the same amounts of CHX entrapped in MSN (denoted as CHX@MSN) were fabricated with methacrylate monomers and silanized glass fillers (CHX or CHX@MSN + glass filler particle = 70 wt%). The monomer mixture consisted of bisphenol A glycidyl methacrylate (BisGMA), hexanediol dimethacrylate (HDDMA), ethoxylated bisphenol A dimethacrylate (EBPADMA), and urethane dimethacrylates (UEDMA) at a weight ratio of 40:30:20:10. The composites were tested for CHX release and recharge, flexural strength and modulus (at 24 hr and 1 mo), surface roughness, in vitro wear, and antibacterial activity against Streptococcus mutans and Lactobacillus casei (in both planktonic growth and biofilm formation). The results showed that the composites with CHX@MSN largely retained mechanical properties and smooth surfaces and showed controlled release of CHX over a long time. In contrast, the composites with directly mixed CHX showed reduced mechanical properties, rough surfaces, and burst release of CHX in a short time. The composites with CHX either directly mixed or in MSN showed strong inhibition to S. mutans and L. casei. This research has demonstrated the successful application of MSNs as a novel nanotechnology in dental materials to inhibit oral biofilm without sacrificing materials’ mechanical properties and surface integrity.
Keywords: controlled release, surface roughness, bacteria, biofilm, mechanical properties, Streptococcus mutans
Introduction
Resin-based dental composites have been widely used in dentistry to restore decayed teeth. These composites have sufficient flexural strength (from 80 to 140 MPa) and outstanding esthetics. However, dental composite restorations have limited service life (average, 7 yrs) due to fracture of the restoration itself or to secondary caries developed at the restoration margins caused by accumulation of cariogenic biofilms (Mjör et al., 2000; Deligeorgi et al., 2001; Leung et al., 2005). The subsequent replacement of restorations by drilling and filling in the United States alone costs more than $5 billion annually (Jokstad et al., 2001). Strategies are needed to extend the service life of the dental composite restorations by effectively inhibiting cariogenic biofilms and thus reducing secondary caries, especially in high-caries-risk populations (Imazato, 2009; Bekhuis, 2011). Dental composites that can inhibit cariogenic biofilms while maintaining their mechanical properties are highly desirable.
Chlorhexidine (CHX), with its broad-spectrum antibacterial activity and low cytotoxicity, has been widely used in oral infection control as a mouthrinse, dental coating (Prevora®), and denture wash (Achong et al., 1999; Slot et al., 2011) and is considered a “gold standard” for the evaluation of antimicrobial agents. CHX-containing dental composites have been studied (Jedrychowski et al., 1983; Riggs et al., 2000; Leung et al., 2005; Anusavice et al., 2006; Cheng et al., 2012), and the CHX release rate has been controlled by the degree of cross-linking, which also contributed to a balance between swelling induced by water sorption of hydrophilic composition and polymerization shrinkage (Riggs et al., 2000; Leung et al., 2005). The presence of CHX, however, incurred detrimental mechanical or physical properties of the composites, including decreased strength (Jedrychowski et al., 1983; Slot et al., 2011), porous surface (Anusavice et al., 2006), and increased water sorption (Deligeorgi et al., 2001). The main reason is that CHX (a salt) is immiscible with dental monomers. It forms aggregates in the resin matrix of the composite, and the dissolution of CHX aggregates leads to the formation of a porous surface, which has poor wear resistance. It also increases the potential for staining and bacterial biofilm accumulation. Therefore, simply mixing CHX into a dental composite will produce an inferior material that does not meet the requirements for dental applications (Deligeorgi et al., 2001).
Mesoporous silica nanoparticles (MSNs) with high pore volume and surface area (Zhao et al., 1998) have attracted increasing attention as reservoirs to encapsulate and release disparate molecules including fenbufen (Carriazo et al., 2010), silver (Liong et al., 2009), CHX (Izquierdo-Barba et al., 2009; Raso et al., 2010), gene delivery system (Petkar et al., 2011), etc. However, studies of applications of MSN in dental materials have been rare. So far only one report (Carpenter et al., 2013) has described the release of nitric oxide as an antibacterial agent from the dental composite containing MSN modified by O2-protected N-diazeniumdiolate-based silanes. Two other reports showed that a combination of MSN with non-porous fillers seems to increase the mechanical properties of the composites (Praveen et al., 2006; Samuel et al., 2009). There are also major differences in the design and applications of MSN between dental composites and other drug-releasing materials or biomaterials. The objective of this study was to investigate the effects of MSN (SBA-15) on dental composites for the controlled release of CHX and its physical, mechanical, and antibacterial properties. The hypothesis was that the dental composite containing MSNs with entrapped CHX can provide sustainable release and recharge of CHX while maintaining its physical and mechanical properties.
Materials & Methods
Chlorhexidine diacetate salt (CHX) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Mesoporous silica nanoparticles (SBA-15) were synthesized following the reported procedure (Zhao et al., 1998). Prior to CHX loading, mesoporous SBA-15 was vacuum-dried at 75°C overnight to remove moisture residue. The dried SBA-15 (about 1 g) was dispersed into 15 mL absolute ethanol containing 0.3 M CHX and sonicated for 10 min, vacuum-infiltrated at room temperature 3 times, and vigorously stirred for 3 days. The mixture was filtered, briefly rinsed with ethanol, and vacuum-dried before use. The CHX loading capacity on SBA-15 was determined by means of a thermogravimetric analyzer (TGA) (Q600, TA Instruments, New Castle, DE, USA) at 10°C/min to 800°C.
The resin monomer mixture consisted of bisphenol A glycidyl methacrylate (BisGMA) (Sigma-Aldrich), hexanediol dimethacrylate (HDDMA), ethoxylated bisphenol A dimethacrylate (EBPADMA) (both from Esstech, Essington, PA, USA), and urethane dimethacrylate (UEDMA) (Sigma-Aldrich) at a weight ratio of 40:30:20:10. A 1% photo-initiator [camphorquinone: phenyl bis(2,4,6-trimethyl benzoyl)phosphine oxide: ethyl 4-dimethylaminobenzoate = 1:2:4 by weight] (Xu et al., 2006, 2012) was added to the mixture as well. CHX at 0%, 3%, 5%, and 6.3%, or the same amounts of CHX entrapped in MSN (denoted as CHX@MSN), was mixed as filler with resin monomers by replacing the same amount of silanized glass fillers (Dentsply/Caulk, Milford, DE, USA) with total filler load maintained at 70 wt%. All components were blended in a SpeedMixerTM (FlackTek, Inc., Landrum, SC, USA) for 3 × 30 sec to form a resin paste, which was stored in the dark at room temperature for 4 hr to allow sufficient time for the monomers to infiltrate MSN mesopores. Bar specimens for flexural strength testing (2 mm × 2 mm × 25 mm, n = 12 each group) were prepared by being light-cured (6 × 40 sec on both sides) with an Optilux 501 curing light (Kerr Corp., Orange, CA, USA) in stainless steel molds. All specimens were polished with 600-grit SiC abrasive paper and stored in deionized water at 37°C for 24 hr or 1 mo before mechanical testing. Flexural strength and modulus were measured by a three-point-bending fixture on an MTS Landmark machine (MTS Systems Corp., Eden Prairie, MN, USA) with a crosshead speed of 1 mm/min.
For CHX release and recharge, disc specimens containing either 5% CHX or 5% CHX@MSN with a diameter of 10 mm and a thickness of 1.2 mm were light-cured on both sides for 40 sec each. The disc specimens (n = 4 per group, weight 0.205 – 0.226 g) were immersed in 2 mL high-purity water (18 MΩ·cm) at 37°C. A 2-mL quantity of equilibrated water was taken at 0, 2, 4, and 8 hr, then every 24 hr up to 10 days and finally at 16 days. The concentration of CHX in the solution was analyzed by high-performance liquid chromatography (HPLC) [HP 1100 (Agilent Technologies, Santa Clara, CA, USA)] with a surfactant column and acetonitrile/water (1:1) mobile phase at 0.5 mL/min. A series of known CHX concentrations (10−6 to 10−4 M) was prepared for calibration of the HPLC. The analyzed CHX concentration was converted to weight and compared with initial CHX weight in individual discs. For CHX recharge, the disc specimens after release of CHX were further immersed in large amounts of water and sonicated to reduce the amount of CHX. The specimens were then immersed in 10 mL 0.2% CHX aqueous solution for 1 min, then rinsed with deionized water for 10 sec and placed in 5 mL high-purity water at 37°C (replaced every 24 hr); this continued for 5 days and was repeated for 3 cycles. The collected solution was analyzed by HPLC.
The antibacterial activity of the composites against Streptococcus mutans and Lactobacillus casei was tested in both planktonic growth and biofilm formation. Planktonic growth of two bacteria was tested with Bioscreen CTM (Oy Growth Curves AB Ltd, Helsinki, Finland), which automatically measures the optical density of the bacterial culture in the plates at 600nm (OD600nm). Ring specimens (inner diameter 3.1 mm, outer diameter 5.2 mm, thickness 1.1 mm, n = 3) were made by light-curing the pastes for 40 sec in a Teflon ring mold. After ultraviolet (UV) sterilization, the specimens were laid at the bottoms of 100-well honeycomb microtiter plates of Bioscreen CTM. Overnight broth cultures (S. mutans and L. casei) were transferred to fresh, pre-warmed brain heart infusion (BHI, Becton, Dickinson and Company, Sparks, MD, USA) for S. mutans or Lactobacillus MRS (deMan Rogosa, Sharpe) (Difco Laboratories, Detroit, MI, USA) for L. casei, and allowed to continue to grow in an aerobic chamber with 5% CO2 at 37°C (Wen et al., 2010). When reaching mid-exponential phase (OD600nm = 0.5), the cultures were diluted 1:100 with proper broth medium, and aliquots (250 µL) were applied to the honeycomb plates. Bacterial growth at 37°C was monitored every half-hour continuously for 24 hr with Bioscreen CTM with moderate shaking for 10 sec prior to OD measurements (Bitoun et al., 2011).
For scanning electron microscopic (SEM) analysis of biofilms, disc specimens were sterilized by UV exposure and placed in 20-mL glass vials, following the same water immersion procedure as for CHX release. Mid-exponential-phase cultures of S. mutans were diluted 1:100 into biofilm medium with glucose (18 mM) and sucrose (2 mM) (BMGS), and aliquots (1.5 mL) were added to each glass vial. Biofilms were allowed to grow in a 5% CO2 incubator at 37°C (Wen and Burne, 2002, 2004). After 24 hr, the specimens from each group were fixed with 2.5% glutaraldehyde overnight at 4°C, dehydrated in 25, 50, 75, 90, and 100% ethanol, and then critical-point-dried in a CO2 critical point dryer (Electron Microscopy Sciences, Hatfield, PA, USA) (Wen and Burne, 2004; Wen et al., 2010). After carbon coating, the biofilm morphology on disc surfaces was observed by field emission scanning electron microscopy (FE-SEM, Hitachi 4800).
Disc specimens (n = 4) of the composites were mounted on a SABRI Oral Simulating Posterior Composite Wear Test Apparatus (Downer’s Grove, IL, USA) and subjected to wear under a 2-N load for 5,000 cycles. The worn specimens were scanned by means of the 3D profilometer scanner TalyScan 150 (Taylor Hobson, West Chicago, IL, USA), and maximum wear depth was measured with the associated image-processing software.
Mechanical properties of the composites were analyzed by ANOVA and Tukey’s post hoc HSD test (Statistica 8.0, StatSoft Inc., Tulsa, OK, USA) for comparison of means, with a significance level of 95% (α = 0.05).
Results
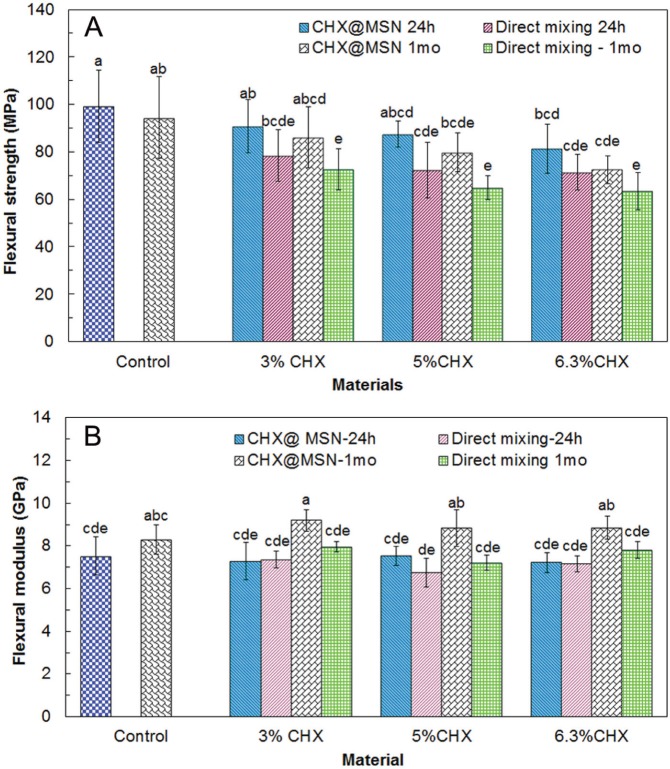
Mechanical properties of composites containing CHX alone or with CHX@MSN after 24-hour and 1-month water immersion are shown in Fig. 1. Release rates of CHX from composites over time are shown in Fig. 2A. Fig. 2B shows the cumulative CHX release over time as the percentage of the total loaded CHX. Fig. 2C elucidates the recharge and release profiles over 3 repeating cycles. Cumulative CHX release over 3 days after recharge is presented in Fig. 2D.
Figure 1.
Flexural strength (A) and flexural modulus (B) of dental composites with different amounts of CHX by direct mixing or entrapment in MSN (CHX@MSN) after 24-hour and 1-month water immersion at 37°C. Composites with 70% glass fillers without CHX or CHX@MSN served as controls. Data are shown as mean ± standard deviation (n = 12). The groups with the same label letters have no significant difference (P > .05).
Figure 2.
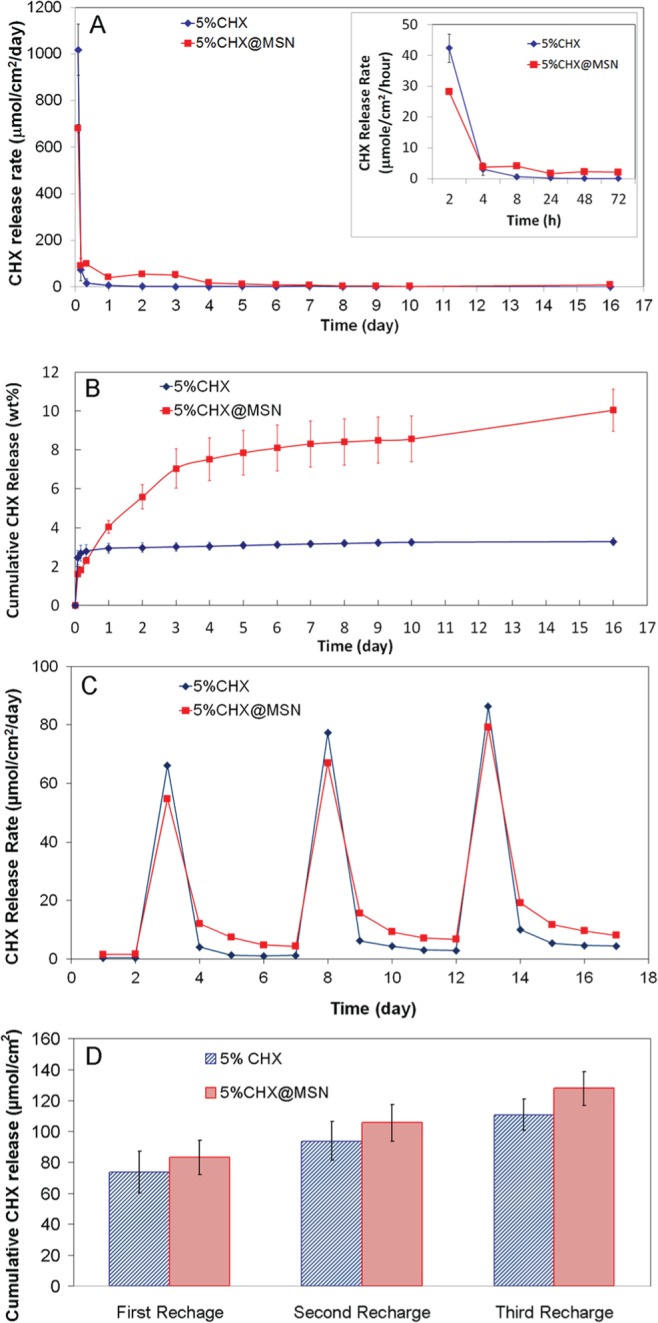
CHX release and recharge. (A) CHX release profiles from composites containing directly mixed CHX or CHX@MSN. (The insert is the release rate (µmol/cm2/hr) during the first 72 hr.) (B) Cumulative CHX release as the weight percentage of the total loaded CHX. (C) CHX recharge profile in 3 repeated recharges. (D) Cumulative CHX release over 3 days after recharge.
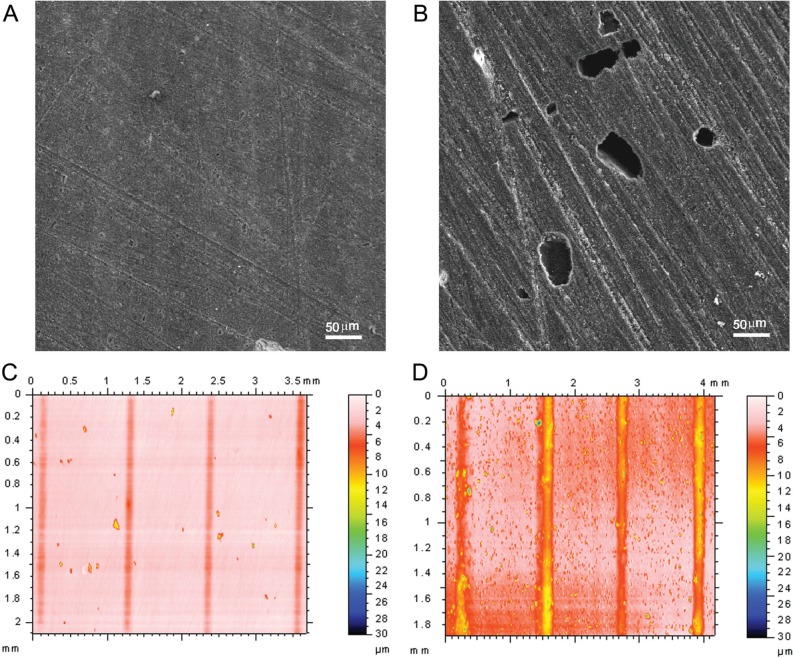
The composite containing 5% CHX@MSN has similar surface roughness and few visible voids on the surface before and after water immersion (Figs. 3A, 3C). Conversely, composites with directly mixed 5% CHX have rough surfaces and deep voids left by dissolution of CHX aggregates (Figs. 3B, 3D). The maximum depth of in vitro wear of the composite with 5% CHX@MSN (3.41 ± 1.42 µm) is significantly smaller than that of the composite with directly mixed CHX (14.47 ± 3.40) (P < .05).
Figure 3.
SEM of composites with 5% CHX @MSN (A) and 5% directly mixed CHX (B) after water immersion. Wear grooves and surface roughness on composites with 5% CHX@MSN (surface roughness: Sa = 0.344 ± 0.050a before water immersion, and 0.410 ± 0.099a after water immersion) (C), and directly mixed 5% CHX (Sa = 0.311 ± 0.060a before water immersion, and 1.113 ± 0.055b after water immersion) (D).
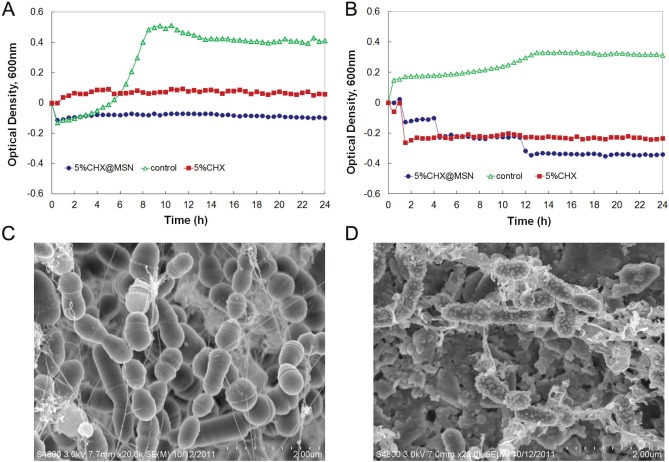
The planktonic bacterial growth of S. mutans and L. casei results is shown in Figs. 4A and 4B, respectively. The control composite had no inhibitory function, leading eventually to S. mutans biofilm formation on its surface (Fig. 4C). Conversely, only a few bacteria with deformed membranes accumulated on the surface of the composites containing CHX@MSN (Fig. 4D).
Figure 4.
Planktonic growth curves of S. mutans (A) and L. casei (B) in the presence of the composites containing the same amount of CHX. SEM images of S. mutans biofilms grown on control composite (C) and composite with 5% CHX@MSN (D).
Discussion
In this study, well-dispersed MSNs allowed resin monomers or polymer chains to penetrate mesopores forming a necklace-like physical interlocking structure for improvement of polymers’ or composites’ mechanical strength. This process may substitute hydrolytically sensitive coupling agents such as silane in the composites (Luo et al., 1998; Praveen et al., 2006; Samuel et al., 2009). Considering CHX@MSN as a whole particle, the CHX loading capacity on MSN is 62.9%, or 3.36 mmol CHX per gram of MSN, determined by thermal gravimetric analysis (TGA). The leftover mesopores may still allow for the infiltration of dental monomers (Bowen and Reed, 1976; Samuel et al., 2009). Incorporation of CHX alone as filler decreased the composites’ flexural strength and modulus, similar to previous reports (Jedrychowski et al., 1983; Slot et al., 2011). The composites containing CHX@MSN with the same CHX content had significantly higher flexural strength than those directly mixed with CHX, and even higher flexural modulus than the control containing 70% glass filler. To encapsulate the same amount of CHX, at 5%, for example, in dental composites, 7.5% CHX@MSN particles are needed. Of the composites, there are 2.5% neat MSN and 62.5% glass filler as part of the total 70% filler weight ratio. The rigid MSNs in composites impart higher modulus to the composites than the low-molecular-weight CHX (Fig. 1B). After 1-month water immersion, all experimental composites showed reduced flexural strength, which is a common phenomenon in dental composites due to sorption of water as a plasticizer, dissolution of uncured monomer, etc. The composites with CHX@MSN after 1 mo displayed better flexural strength than those with directly mixed CHX after 24 hr. This rigid inorganic characteristic of the MSN manifests an improved modulus after water immersion. The more uniform filler distribution in the composite with CHX@MSN also contributes to improved composite mechanical properties.
Light-cured dental composites containing CHX@MSN showed a more sustainable, controlled release of CHX for 16 days as compared with that of the composite with directly mixed CHX. The latter had a high release rate in the initial 2 hr, after which the release quickly diminished (Fig. 2A) and the cumulative release leveled off after one day (Fig. 2B). This may be caused by the quick dissolution and depletion of CHX aggregates on the composite surface. Except for the initial burst release, the composite with directly mixed CHX had a CHX release rate lower than that with CHX@MSN. Furthermore, the CHX aggregates embedded inside the hydrophobic resin matrix were unable to release from the composites. Therefore, the CHX release rate decreased sharply after the first few hours, even though more than 96% CHX remained in the composites. In contrast, MSN seemed to provide a better pathway and reservoir for sustainable release of CHX over a long time. Even after 16 days, about 90% CHX remained within composites. Although we have tested the release of CHX for only a short period of time, we can predict that this release of CHX from MSN-containing composites can last for an extended period of time.
Sustainable release for a prolonged period of time is beneficial to cariogenic biofilm control (Chen et al., 2010). To achieve a continuous sustained release of the antibacterial agent, it is advantageous for an antibacterial dental composite to have the capability of absorbing or recharging CHX from surrounding medium—for example, from a mouthrinse or varnish containing CHX—to re-invigorate its antimicrobial activity once the loaded antimicrobial agents have been exhausted. The specimens were recharged with 0.2% CHX aqueous solution at days 2, 7, and 12. It is clear that composites directly mixed with CHX have rapid release for the first day after each recharge, and lower releasing quantity thereafter (between days 4 and 7, days 9 and 12, and days 14 and 17) than those with CHX@MSN, suggesting that larger surface voids on directly mixed composites have shorter CHX retention time. In all 3 repeating recharges, the composites with CHX@MSN exhibited higher cumulative release of CHX after recharge, indicative of a potential prolonged antimicrobial effect. The mesopores on MSN provide a permanent “sponge” that can be recharged repeatedly with antimicrobial agents, providing a new opportunity for long-term antimicrobial dental composite applications with recharge and release on demand.
Surface integrity of the composite after CHX release is of clinical importance. To test such an effect, we scanned the same disc with CHX or CHX@MSN before and after water immersion. Surface roughness (Sa) is a measure of surface texture. Before water immersion, both composites with CHX and CHX@MSN had similar surface roughnesses. After 2-week water immersion, the surface of the composite with CHX@MSN became only slightly coarser. In contrast, numerous voids with depths of more than 5 µm appeared on the surfaces of composites with directly mixed 5% CHX, causing a dramatic change in surface roughness. A rough surface usually wears more quickly and has higher friction coefficients than a smooth surface. In this study, the composites with CHX@MSN had better wear resistance (less wear), which is in agreement with results reported previously (Luo et al., 1998).
The antibacterial efficacy of the composites was evaluated by 2 methods: planktonic bacterial growth and biofilm tests. First, the planktonic bacterial growth of S. mutans and L. casei, two major cariogenic bacteria, was monitored with Bioscreen CTM (Wen et al., 2010; Xu et al., 2012), with the composite ring specimens placed at the bottoms of honeycomb plate wells. The control composite had significant growth of both bacteria [increasing in optical density (OD)], while little or no growth was measured after 24 hr for the cured composites containing 5% CHX or CHX@MSN for both bacteria.
Second, the bacterial biofilms were grown on the experimental and control composites. Relative to the control, S. mutans on the composite containing CHX@MSN also had an altered surface structure, a likely result of cell membrane damage or cell death caused by CHX. One critical factor contributing to biofilm inhibition is the retention time that antimicrobial agents can stay on the restoration or teeth. The traditional mouthrinse or varnish raises a concern of time interval between applications, which results in reduced concentration of antimicrobial agents over time between doses. Inconsistent and insufficient application of the antimicrobial agents may allow cariogenic bacteria to recolonize/survive and accumulate on the tooth or restoration surface. Our results suggest that the sustainable release of CHX from the CHX@MSN-loaded composites can provide a consistent amount of CHX—for example, at or above the minimum inhibitory concentration of 0.25-1.0 µg/mL for S. mutans (Järvinen et al., 1993)—over a prolonged period of time, which may enhance inhibitory effects against bacterial attachment and biofilm formation and minimize potential side-effects.
In summary, we have presented a type of new dental composite consisting of MSN to encapsulate or recharge and sustainably release antimicrobial agents, such as CHX, without compromising mechanical strength, surface esthetics, and surface integrity of the material. This technique is particularly useful for those antimicrobial agents or drugs that are poorly dispersed in polymers or composites, and for those hydrophobic polymers or composites containing antimicrobial agents or drugs for release. It has opened up opportunity for the application of nanotechnology in developing new and antimicrobial dental composites. We expect this method to be a general approach for antimicrobial agent release in dental materials and other biomaterials where antimicrobial function is required. Furthermore, we must acknowledge that the oral environment is complicated, since temperature, pH values, oral bacteria, and the volume and compositions of fluids fluctuate frequently. The release and antibacterial activity of these materials need to be further optimized under more clinically relevant conditions.
Acknowledgments
The authors also thank Dr. S. Costin for editing the manuscript. The donation of filler particles from Dentsply/Caulk and monomers from Esstech is much appreciated.
Footnotes
The authors thank the National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) for financial support from grant R01DE19203 (to X. Xu) and NIH/NIDCR grant R01DE19452 (to Z.T. Wen).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Achong RA, Briskie DM, Hildebrandt GH, Feigal RJ, Loesche WJ. (1999). Effect of chlorhexidine varnish mouthguards on the levels of selected oral microorganisms in pediatric patients. Pediatr Dent 21:169-175. [PubMed] [Google Scholar]
- Anusavice KJ, Zhang NZ, Shen C. (2006). Controlled release of chlorhexidine from UDMA-TEGDMA resin. J Dent Res 85:950-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhuis T. (2011). Chlorhexidine varnish May prevent dental caries in children and adolescents. J Evid Based Dent Pract 11:84-86. [DOI] [PubMed] [Google Scholar]
- Bitoun JP, Nguyen AH, Fan Y, Burne RA, Wen ZT. (2011). Transcriptional repressor Rex is involved in regulation of oxidative stress response and biofilm formation by Streptococcus mutans. FEMS Microbiol Lett 320:110-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen RL, Reed LE. (1976). Semiporous reinforcing fillers for composite resins: I. Preparation of provisional glass formulations. J Dent Res 55:738-747. [DOI] [PubMed] [Google Scholar]
- Carpenter AW, Reighard KP, Saavedra JE, Schoenfisch MH. (2013). O2-Protected diazeniumdiolate-modified silica nanoparticles for extended nitric oxide release from dental composites. Biomater Sci 1:456-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriazo D, Arco MD, Fernandez A, Martin C, Rives V. (2010). Inclusion and release of fenbufen in mesoporous silica. J Pharm Sci 99:3372-3380. [DOI] [PubMed] [Google Scholar]
- Chen F, Rice KC, Liu XM, Reinhardt RA, Bayles KW, Wang D. (2010). Triclosan-loaded tooth-binding micelles for prevention and treatment of dental biofilm. Pharm Res 27:2356-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Weir MD, Xu HH, Kraigsley AM, Lin NJ, Lin-Gibson S, et al. (2012). Antibacterial and physical properties of calcium-phosphate and calcium-fluoride nanocomposites with chlorhexidine. Dent Mater 28:573-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligeorgi V, Mjör IA, Wilson NH. (2001). An overview of reasons for the placement and replacement of restorations. Prim Dent Care 8:5-11. [DOI] [PubMed] [Google Scholar]
- Imazato S. (2009). Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent Mater J 28:11-19. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Barba I, Vallet-Regi M, Kupferschmidt N, Terasaki O, Schmidtchen A, Malmsten M. (2009). Incorporation of antimicrobial compounds in mesoporous silica film monolith. Biomaterials 30:5729-5736. [DOI] [PubMed] [Google Scholar]
- Järvinen H, Tenovuo J, Huovinen P. (1993). In vitro susceptibility of Streptococcus mutans to chlorhexidine and six other antimicrobial agents. Antimicrob Agents Chemother 37:1158-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski JR, Caputo AA, Kerper A. (1983). Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. J Oral Rehabil 10:373-381. [DOI] [PubMed] [Google Scholar]
- Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. (2001). Quality of dental restorations - FDI Commission Project 2–95. Int Dent J 51:117-158. [DOI] [PubMed] [Google Scholar]
- Leung D, Spratt DA, Pratten J, Gulabivala K, Mordan NJ, Young AM. (2005). Chlorhexidine-releasing methacrylate dental composite materials. Biomaterials 26:7145-7153. [DOI] [PubMed] [Google Scholar]
- Liong M, France B, Bradley KA, Zink JI. (2009). Antimicrobial activity of silver nanocrystals encapsulated in mesoporous silica nanoparticles. Adv Mater 21:1684-1689. [Google Scholar]
- Luo JH, Lannutti JJ, Seghi RR. (1998). Effect of filler porosity on the abrasion resistance of nanoporous silica gel/polymer composites. Dent Mater 14:29-36. [DOI] [PubMed] [Google Scholar]
- Mjör IA, Moorhead JE, Dahl JE. (2000). Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J 50:361-366. [DOI] [PubMed] [Google Scholar]
- Petkar KC, Chavhan SS, Agatonovik-Kustrin S, Sawant KK. (2011). Nanostructured materials in drug and gene delivery: a review of the state of the art. Crit Rev Ther Drug Carrier Syst 28:101-164. [DOI] [PubMed] [Google Scholar]
- Praveen S, Sun Z, Xu J, Patel A, Wei Y, Ranade R, et al. (2006). Compression and aging properties of experimental dental composites containing mesoporous silica as fillers. Mol Cryst Liq Cryst 448:223-231. [Google Scholar]
- Raso EM, Cortes ME, Teixeira KL, Franco MB, Mohallem ND, Sinisterra RD. (2010). A new controlled release system of chlorhexidine and chlorhexidine:βcd inclusion compounds based on porous silica. J Incl Phenom Macro 67:159-168. [Google Scholar]
- Riggs PD, Braden M, Patel M. (2000). Chlorhexidine release from room temperature polymerising methacrylate systems. Biomaterials 21:345-351. [DOI] [PubMed] [Google Scholar]
- Samuel SP, Li S, Mukherjee I, Guo Y, Patel AC, Baran G, et al. (2009). Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent Mater 25:296-301. [DOI] [PubMed] [Google Scholar]
- Slot DE, Vaandrager NC, Van Loveren C, Van Palenstein Helderman WH, Van der Weijden GA. (2011). The effect of chlorhexidine varnish on root caries: a systematic review. Caries Res 45:162-173. [DOI] [PubMed] [Google Scholar]
- Wen ZT, Burne RA. (2002). Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl Environ Microbiol 68:1196-1203; published erratum in Appl Environ Microbiol 69:722 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZT, Burne RA. (2004). LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J Bacteriol 186:2682-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZT, Yates D, Ahn SJ, Burne RA. (2010). Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol 10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ling L, Wang R, Burgess JO. (2006). Formulation and characterization of a novel fluoride-releasing dental composite. Dent Mater 22:1014-1023. [DOI] [PubMed] [Google Scholar]
- Xu X, Wang Y, Liao S, Wen Z, Fan Y. (2012). Synthesis and characterization of antibacterial dental monomers and composites. J Biomed Mater Res B Appl Biomater 100:1151-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, et al. (1998). Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 Ångström pores. Science 279:548-552. [DOI] [PubMed] [Google Scholar]