Abstract
IMPORTANCE
Surgical skin markers are used off-label to mark human saphenous veins (HSVs) to maintain orientation before implantation as aortocoronary or peripheral arterial bypass grafts. These surgical skin markers impair functional responses of the HSV tissue.
OBJECTIVES
To investigate the effect of brilliant blue dye 1 (brilliant blue FCF [for food coloring]; hereinafter, FCF) as a nontoxic alternative marking dye and to determine whether FCF has pharmacological properties.
DESIGN, SETTING, AND PARTICIPANTS
Segments of HSVs were collected in university hospitals from patients undergoing coronary artery bypass grafting procedures immediately after harvest (unmanipulated) or after typical intraoperative surgical graft preparation (after manipulation). Rat inferior venae cavae were used to determine the pharmacological properties and cellular targets of FCF. Endothelial and smooth muscle functional responses were determined in a muscle bath, and intimal thickening in HSVs was determined after 14 days in organ culture.
MAIN OUTCOMES AND MEASURES
Contractile responses were measured in force and converted to stress. Smooth muscle function was expressed as maximal responses to potassium chloride depolarization contractions. Endothelial function was defined as the percentage of relaxation of maximal agonist-induced contraction. Neointimal thickness was measured by histomorphometric analysis.
RESULTS
Human saphenous veins stored in the presence of FCF had no loss of endothelial or smooth muscle function. Unmanipulated HSVs preserved in the presence of FCF demonstrated a significant increase in endothelial-dependent relaxation (mean [SEM], 25.2% [6.4%] vs 30.2% [6.7%]; P = .02). Application of FCF to functionally nonviable tissue significantly enhanced the smooth muscle responses (mean [SEM], 0.018 [0.004] × 105N/m2 vs 0.057 [0.016] × 105 N/m2; P = .05). Treatment with FCF reduced intimal thickness in organ culture (mean [SEM], −17.5% [2.1%] for unmanipulated HSVs vs −27.9% [3.7%] for HSVs after manipulation; P < .001). In rat inferior venae cavae, FCF inhibited the contraction induced by the P2X7 receptor agonist 2′(3′)-O-(4-benzoyl)benzoyl-adenosine-5′-triphosphate (mean [SEM], 14.8% [2.2%] vs 6.5% [1.8%]; P = .02) to an extent similar to the P2X7 receptor antagonist oxidized adenosine triphosphate (mean [SEM], 5.0% [0.9%]; P < .02 vs control) or the pannexin hemichannel inhibitor probenecid (mean [SEM], 7.3% [1.6%] and 4.7% [0.9%] for 0.5mM and 2mM, respectively; P < .05).
CONCLUSIONS AND RELEVANCE
Treatment with FCF did not impair endothelial or smooth muscle function in HSVs. Brilliant blue FCF enhanced endothelial-dependent relaxation, restored smooth muscle function, and prevented intimal hyperplasia in HSVs in organ culture. These pharmacological properties of FCF may be due to P2X7 receptor or pannexin channel inhibition. Brilliant blue FCF is an alternative, nontoxic marking dye that may improve HSV conduit function and decrease intimal hyperplasia.
The leading cause of failure of saphenous vein grafts used in arterial bypass procedures is intimal hyperplasia.1 While incompletely understood, intimal hyperplasia results from a cascade of molecular and cellular events that are triggered by injury. This process leads to pathologic narrowing of the vessel lumen, graft stenosis, and ultimately graft failure.2 Despite the many recent technological advances in cardiovascular interventions, intimal hyperplasia remains an expensive, morbid, and unsolved problem.
It has long been recognized that technical elements have a significant role in determining vein graft patency.3 Surgical harvest of the vein results in mechanical injury, endothelial damage, and vasospasm.2 After harvest, vein grafts are prepared on the back table via a series of manipulations before implantation. This process involves distending the veins to identify and repair unligated tributaries or graft injuries, marking with a surgical skin marker to preserve orientation, preventing twisting and kinking on implantation, and storing in solution. Manual vein graft distension can lead to intraluminal pressures in excess of 800 mm Hg, endothelial denudation, and ultimately acceleration of intimal hyperplasia.4 Surgical skin markers contain isopropyl alcohol as a solvent, which is injurious to the graft.5 Heparinized normal saline (0.9% sodium chloride solution) is commonly used to store grafts before implantation. This solution is nonbuffered and has a pH of approximately 6, which may cause further harm to the conduit (C.B. and J.C.-F., unpublished observations, 2014). Given that saphenous vein is an autologous transplanted organ, harvest and preparation manipulations should be designed to minimize injury, which may lead to reduced intimal hyperplasia and improved clinical outcomes.6,7
In this investigation, a water-soluble dye was examined as a potential intervention to mitigate preparation-induced vein graft injury. Brilliant blue dye 1 ([brilliant blue FCF [for food coloring]; hereinafter, FCF) is a food dye that is structurally related to brilliant blue G, which is an antagonist of the purinergic P2X7 receptor8 and the pannexin hemichannel (Panx1).9 We determined the effect of FCF on endothelial and smooth muscle function in human saphenous veins (HSVs). Brilliant blue FCF enhanced endothelial function and restored smooth muscle function, suggesting that it had pharmacological properties via inhibition of vascular P2X7 receptors and of Panx1.10,11
Methods
Procurement of HSVs
After written informed consent and approval by the institutional review boards of Vanderbilt University and the Veterans Affairs Tennessee Valley Healthcare System, 42 HSV segments were collected from patients undergoing coronary artery bypass grafting procedures. Vein segments were collected immediately following surgical harvest (unmanipulated [UM]) and after typical intraoperative manipulations, including hydrostatic distension with a handheld syringe, marking with a surgical skin marker, and storage in room temperature heparinized saline (10 U/mL) (HS) or heparinized PlasmaLyte (HP), both from Baxter, at the surgeon's discretion, until implantation (after manipulation [AM]). Segments were transported to the laboratory for immediate testing.
Procurement of Rat Inferior Venae Cavae
All chemicals were purchased from Sigma-Aldrich unless otherwise specified. Inferior venae cavae (IVCs) were collected from 7 euthanized Sprague-Dawley rats. Animal procedures followed study protocols approved by the Vanderbilt University Institutional Animal Care and Use Committee and adhered to National Institutes of Health guidelines for care and use of laboratory animals. Immediately after euthanasia, the abdominal IVC was isolated via an incision along the midabdomen, placed in cold transplant harvest buffer (100mM potassium lactobionate, 25mM monopotassium phosphate, 5mM magnesium sulfate, 30mM raffinose, 5mM adenosine, 3mM glutathione, 1mM allopurinol, 50 g/L of hydroxyethyl starch, and pH 7.4), and transported to the laboratory for immediate testing.
Physiologic Responses of HSVs
Rings approximately 1.0 mm in width were cut from HSV segments after dissection free from connective tissue and fat. Rings from UM-HSV segments were suspended in a muscle bath immediately (control) or stored in HP with or without 50μM FCF. Fifty micromolar is the approximate concentration of FCF in the cellular microenvironment of a 1-mm ring marked topically with a 2.6mM FCF solution and placed in 4 mL of HP for 2 hours (as determined by a calorimetric assay). In other experiments, AM-HSV segments were left untreated or marked with a 2.6mM solution of FCF to approximate clinically applicable conditions. Tissues were suspended in the muscle bath containing a bicarbonate buffer (120mM sodium chloride, 4.7mM potassium chloride, 1.0mM magnesium sulfate, 1.0mM monosodium phosphate, 10mM glucose, 1.5mM calcium chloride, 25mM sodium bicarbonate, and pH 7.4) and equilibrated with 95% oxygen–5% carbon dioxide at 37°C. Tissues were sequentially stretched to the optimal resting tension (approximately 1g), stretched 3 to 4 times the resting tension to determine the passive length-tension relationship,12,13 and followed by maintenance at 1g for an additional 1 hour. Force measurements were obtained using a force transducer (model 159901A; Radnoti) interfaced with a data acquisition system (16/30; PowerLab) and a software program (Chart; AD Instruments).
The maximal response to 110mM potassium chloride (with equimolar replacement of sodium chloride in bicarbonate buffer) was determined, which was equivalent to an initial tension of 4g. Smooth muscle functional viability was determined by repeatedly contracting the tissues with 110mM potassium chloride until consistent maximal force was generated. The UM-HSV rings generating stress of 0.025 × 105 N/m2 or less were considered nonviable and were not used for further measurements of endothelial-dependent relaxation. To determine endothelial-dependent relaxation, viable UM-HSV tissue was then contracted with phenylephrine (1–5μM) and relaxed with 0.5μM carbachol, an acetylcholine analogue. Relaxation was reported as percentage decrease of maximal phenylephrine-induced contraction.14
Physiologic Responses of Rat IVCs
Rat IVC rings approximately 2.0 mm in width were cut, suspended in the muscle bath, and equilibrated as described above for HSVs. The rings were then contracted with 110mM potassium chloride to determine maximal contractile response. Next, functionally viable rat IVC rings were left untreated or treated with 50μM FCF, 50μM periodate-oxidized adenosine triphosphate (oATP) (an irreversible P2X7 antagonist), or probenecid (a Panx1 inhibitor, 0.5mM and 2mM; Life Technologies) for 30 minutes before contraction with 100μM 2′(3′)-O-(4-benzoyl) benzoyl-adenosine-5′-triphosphate (BzATP) (a prototypical P2X7 receptor agonist). The BzATP-induced contraction was expressed as the percentage of maximal potassium chloride–induced contraction.
Organ Culture
Rings (1–2 mm in width) were cut from HSV segments (UM and AM). Two rings from each sample were placed in 10% neutral buffered formalin (Fisher Scientific) to measure basal (preculture) intimal thickness. Additional rings were placed in organ culture as described previously15 in the absence or the presence of 50μM FCF. This method of vein culture has been validated as an ex vivo model system of the changes occurring in vivo and has been used previously in our laboratory.4,15,16 After 14 days, rings were fixed in 10% formalin and sent for histological preparation and Verhoeff–van Gieson staining at the Pathology Histochemistry Core at Vanderbilt University. Measurements of intimal and medial thickness were made on transverse sections of each vessel using a microscope (Axiovert 200M; Carl Zeiss) with a computerized image analysis system (AxioVision 4.8; Carl Zeiss and Photoshop; Adobe Systems) as described previously.17
Statistical Analysis
Contractile responses were defined by stress. The stress was calculated using force generated by tissues as follows:
where Area = [Wet Weight (mg)/Maximal Length (mm)]/1.055.
Data were reported as mean (SEM) responses. Paired t tests or 1-way analysis of variance (ANOVA) with Tukey posttests between treatment groups were conducted to determine the significance (P value) of experiments. P < .05 was considered statistically significant. The P value for 1-way ANOVA analysis was computed from the F ratio (Fn,n′, where F represents the ratio between-column to within-column mean squares, and n and n′ represent the respective degrees of freedom used to calculate the mean squares).
Results
Endothelial and Smooth Muscle Function of HSVs Treated With FCF
Storage of UM-HSVs (n = 6–11) in HP, both with and without 50μM FCF, did not impair functional viability of tissue (mean, 0.180 [0.018] × 105 N/m2 for control vs 0.133 [0.010] × 105 N/m2 for HP alone vs 0.174 [0.023] × 105 N/m2 for HP + FCF) (Figure 1A). No significant difference in the response to potassium chloride was observed among the 3 conditions in a 1-way ANOVA (F2,25 = 1.081, P = .35 [n = 6]). Furthermore, storage of UM-HSVs in HP preserved endothelial-dependent relaxation (mean, 25.2% [6.4%] for control vs 24.3% [6.3%] for HP; P = .84 [n = 6]) (Figure 1B). The presence of FCF in the HP storage solution led to a significant increase in endothelial-dependent relaxation (mean, 30.2% [6.7%]; P = .02 vs HP alone [n = 8]). When 12 HSV segments that had minimal to no smooth muscle functional viability were treated with FCF, smooth muscle functional responses to 110mM potassium chloride were restored in 42% (5 of 12) of the specimens examined (mean, 0.018 [0.004] × 105 N/m2 vs 0.057 [0.016] × 105 N/m2; P = .05 [n = 5]) (Figure 2).
Figure 1.
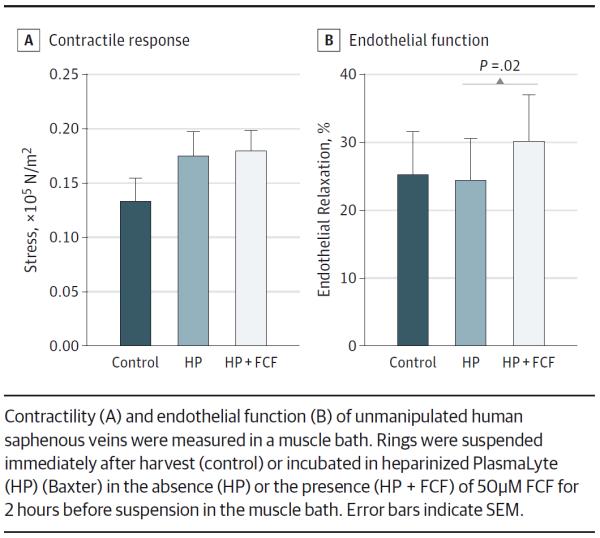
Brilliant Blue FCF (FCF) Is a Nontoxic Alternative for Vein Graft Marking That Enhances Endothelial Function
Figure 2.
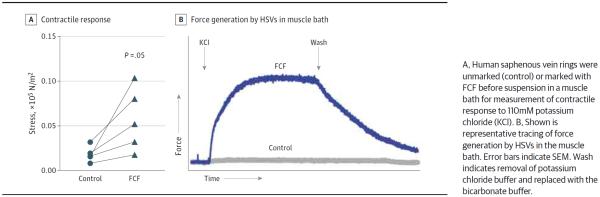
Brilliant Blue FCF (FCF) Restores Contractility of Functionally Nonviable Human Saphenous Veins (HSVs)
BzATP-Induced Contraction of Rat IVCs Treated With FCF and Probenecid
The BzATP induced contractions in rat IVCs (mean, 14.8% [2.2%] of maximal potassium chloride response) (Figure 3A). Pretreatment with 50μM FCF for 30 minutes significantly inhibited BzATP-induced contraction in rat IVCs to an extent similar to inhibition by 50μM oATP treatment (mean, 14.8% [2.2%] for control vs 6.5% [1.8%] for FCF; P = .02 [n = 4]; vs 5.0% [0.9%] for oATP; P < .02 [n = 4–5]). Probenecid pretreatment (0.5mM and 2mM) significantly inhibited BzATP-induced contraction (mean, 14.8% [2.2%] for control vs 7.3% [1.6%] for 0.5mM and 4.7% [0.9%] for 2mM; P < .05 [n = 3–4]) (Figure 3B).
Figure 3.
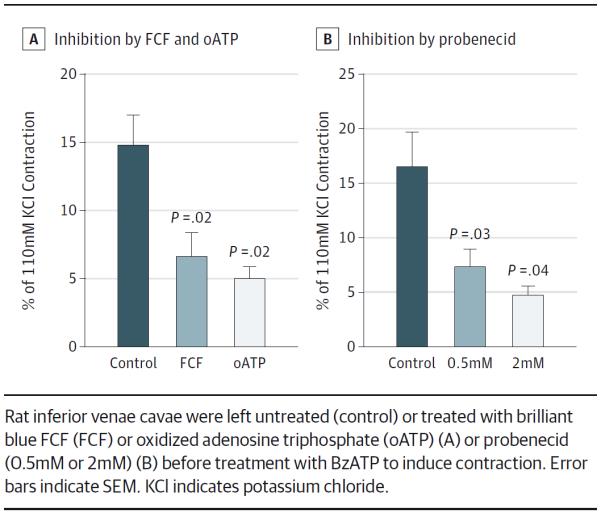
Inhibition of 2′(3′)-O-(4-Benzoyl)Benzoyl-Adenosine-5′-Triphosphate (BzATP)-Induced Contraction of Rat Inferior Venae Cavae
Intimal Thickening of HSVs Treated With FCF
After 2 weeks in organ culture, the increase in intimal thickness of AM-HSVs was significantly greater than that of UM-HSVs (mean, 67.0% [11.4%] vs 37.4% [11.8%]; P = .05 [n = 12]) (Figure 4). Treatment with FCF significantly mitigated the increase in intimal thickness in UM-HSVs and in AM-HSVs (mean, −17.5% [2.1%] for UM-HSVs and −27.9% [3.7%] for AM-HSVs; P < .001 [n = 12–19]).
Figure 4.
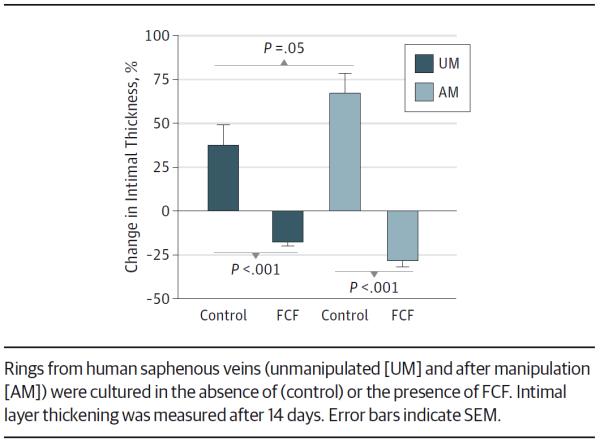
Brilliant Blue FCF (FCF) Reduces Intimal Thickness in Human Saphenous Veins in Organ Culture
Discussion
Back-table manipulation of HSVs, including handheld syringe distension, marking with a surgical skin marker for orientation, and storage in HS led to significantly impaired endothelial and smooth muscle function compared with UM segments.18 Our group previously showed that the isopropyl alcohol in surgical skin markers is injurious to the conduit and may contribute to vein graft failure.5 In this study, we investigated the use of a water-soluble dye, brilliant blue FCF, to mark HSV segments. This dye was not only nontoxic (Figure 1A) but also fully enhanced endothelial function present in UM-HSVs (Figure 1B). Moreover, 42% (5 of 12) of HSV segments that had almost no smooth muscle functional responses and would otherwise be considered nonviable showed significant improvement in contractile responses after treatment with FCF (Figure 2). Taken together, these data suggest that the FCF dye has pharmacological properties that may be beneficial to the conduit.
Brilliant blue FCF is structurally similar to brilliant blue G, a dye shown to have pharmacological properties by restoring function and diminishing cell death after stretch injury in rat spinal cord.19 Brilliant blue G antagonizes the P2X7 receptor, a member of the P2X purinergic receptor family. These receptors are transmembrane, ligand-gated ion channels that are activated by extracellular ATP and its analogues.20 In the presence of agonists, P2X7 receptor activation led to formation of large-pore complexes contributing to ion fluxes and cellular cytolysis.21 In addition, P2X7 receptors have been implicated in the regulation of vascular tone, modulation of inflammatory mediators within the vessel wall, and vascular smooth muscle and endothelial cell injury.22 von Albertini and colleagues23 demonstrated the association of purinergic signaling via P2X7 receptors with nuclear factor κB activation, inflammation, and endothelial cell apoptosis. It is conceivable that vein injury during intraoperative manipulation leads to local release of extracellular ATP,24 thereby activating P2X7 receptors and contributing to tissue dysfunction. Therefore, it is likely that FCF treatment enhanced endothelial function in HSV grafts via inhibition of P2X7 receptors. In addition, inhibition of purinergic signaling by FCF may have prevented P2X7 receptor activation–induced smooth muscle cell lysis, restoring smooth muscle function.25
Activation of the P2X7 receptor by ATP or its analogues induces a contraction of small magnitude in vascular tissues, and blockade of the receptor reduces the magnitude of this contraction.25 In this study, FCF inhibited BzATP-induced contraction in rat IVCs to an extent similar to the P2X7 receptor antagonist oATP (Figure 3A), suggesting that the P2X7 receptor serves as a cellular target of FCF.
Pannexin 1 receptor (Panx1) is associated with P2X7 receptor activation.21,26 Probenecid, a Panx1 inhibitor, reduced BzATP-induced contraction in rat IVCs (Figure 3B), suggesting that blockade of Panx1 interferes with P2X7 receptor activation. Ligands to the P2X7 receptor also inhibit Panx19; FCF has also been shown to selectively inhibit Panx1.27 Given that elevated extracellular ATP is a hallmark of tissue damage,22,28,29 it is plausible that FCF modulates ATP release and ameliorates graft injury through inhibition of P2X7 receptor–induced Panx1 activation.
In blood vessels, the fragile endothelial monolayer is essential in preventing exposure of the medial layer to serum growth factors, producing nitric oxide that inhibits platelet activation and smooth muscle cell proliferation, and promoting decreased thrombosis and inflammation.30 Intimal hyperplasia has been associated with decreased endothelial-dependent relaxation in HSVs.17 Smooth muscle injury leads to migration, proliferation, and production of extracellular matrix proteins, which contribute to intimal hyperplasia.31 Our group has previously shown that intraoperative manipulation of the grafts promotes intimal thickening in an organ culture model.18 In this study, we showed that FCF prevents intimal thickening in HSVs, both UM and AM, in the organ culture model (Figure 4).
Taken together, these data suggest that impaired endothelial and smooth muscle functions are associated with increased intimal thickening ex vivo. These data also suggest that ATP may be released during preparation-induced injury and activate the P2X7 and Panx1 channels (Figure 5). These receptors may be implicated in endothelial and smooth muscle injury and the development of intimal hyperplasia in saphenous vein grafts via downstream signaling of the P2X7 receptor. Therefore, the use of FCF during vein graft preparation may attenuate activation of these purinergic signaling pathways, vascular injury, and the development of intimal hyperplasia.
Figure 5.
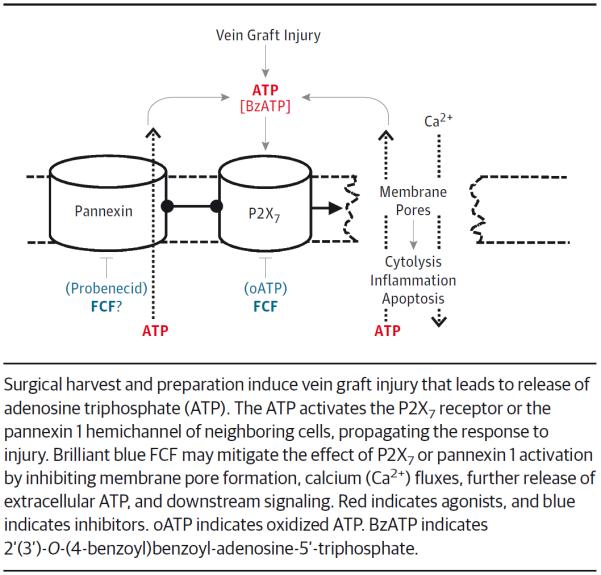
Proposed Model of Brilliant Blue FCF (FCF) Shows the Mechanism of Action in the Prevention of Vein Graft Injury
A limitation of this investigation is that it relied primarily on studies of vascular function. However, functional studies of intact tissues have advantages over cultured cells in that they represent the complex biology associated with the interactions of the components of the tissues. In addition, while the organ culture model contains serum, it lacks in vivo elements (pressure, flow, and exposure to blood components) that are associated with arterialization. Further studies are needed to determine whether FCF prevents intimal hyperplasia and vein graft failure in grafts implanted in vivo.
Conclusions
Because HSV is an autologous transplanted organ, it is likely that addressing and preventing injury during the time that the graft is ex vivo would improve vein graft patency. The period of ex vivo manipulation is an optimal time for targeting therapeutics to prevent vein graft failure because the therapeutic can be delivered directly to the conduit, limiting systemic administration and toxic effects. Moreover, to enhance evaluation of new clinical therapeutics that may prevent intimal hyperplasia, standardizing graft preparation with careful techniques to preserve conduit viability and function is essential. In this investigation, we showed that FCF not only serves as an alternative to the current off-label use of surgical skin markers but also enhances physiologic function. We also demonstrated that current vein graft preparation techniques promote intimal hyperplasia, which can be reduced by FCF treatment. Further investigation is warranted to determine whether antagonism of the P2X7 receptor by FCF during graft preparation will improve graft patency.
Acknowledgments
Funding/Support: This study was supported in part by resources and materials from the Veterans Affairs Tennessee Valley Healthcare System and by a Biomedical Laboratory Research and Development grant from the Department of Veterans Affairs to Dr Brophy. Dr Voskresensky was supported by National Research Service Award F32HL110588 from the National Institutes of Health. Dr Osgood was supported by National Research Service Award F32HL104965 from the National Institutes of Health. Dr Brophy was supported by grant R01HL70715-09 from the National Institutes of Health. Dr Cheung-Flynn was supported by grant R01HL105731-01A1 from the National Institutes of Health.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Voskresensky and Wise and Mr Hocking contributed equally to this article. Dr Cheung-Flynn had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Voskresensky, Wise, Hocking, Osgood, Komalavilas, Brophy, Cheung-Flynn.
Acquisition, analysis, or interpretation of data: Voskresensky, Wise, Li, Komalavilas, Brophy, Cheung-Flynn.
Drafting of the manuscript: Voskresensky, Wise, Osgood, Brophy, Cheung-Flynn.
Critical revision of the manuscript for important intellectual content: Voskresensky, Hocking, Li, Osgood, Komalavilas, Brophy, Cheung-Flynn.
Statistical analysis: Voskresensky, Wise, Hocking, Osgood, Cheung-Flynn.
Obtained funding: Voskresensky, Osgood, Brophy, Cheung-Flynn.
Administrative, technical, or material support: Hocking, Brophy.
Study supervision: Komalavilas, Brophy, Cheung-Flynn.
Conflict of Interest Disclosures: Drs Brophy and Cheung-Flynn reported having a financial relationship with VasoPrep Surgical. No other disclosures were reported.
Previous Presentation: This study was presented at the 38th Annual Surgical Symposium of the Association of VA Surgeons; April 8, 2014; New Haven, Connecticut.
Additional Contributions: The cardiac surgical teams at the Veterans Affairs Tennessee Valley Healthcare System and Vanderbilt University Medical Center provided human specimens for this study.
REFERENCES
- 1.Clowes AW, Reidy MA. Prevention of stenosis after vascular reconstruction: pharmacologic control of intimal hyperplasia: a review. J Vasc Surg. 1991;13(6):885–891. doi: 10.1067/mva.1991.27929. [DOI] [PubMed] [Google Scholar]
- 2.LoGerfo FW, Quist WC, Cantelmo NL, Haudenschild CC. Integrity of vein grafts as a function of initial intimal and medial preservation. Circulation. 1983;68(3, pt 2):II117–II124. [PubMed] [Google Scholar]
- 3.Conte MS. Technical factors in lower-extremity vein bypass surgery: how can we improve outcomes? Semin Vasc Surg. 2009;22(4):227–233. doi: 10.1053/j.semvascsurg.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Li FD, Eagle S, Brophy C, et al. Pressure control during preparation of saphenous veins. JAMA Surg. 2014;149(7):655–662. doi: 10.1001/jamasurg.2013.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eagle S, Brophy CM, Komalavilas P, et al. Surgical skin markers impair human saphenous vein graft smooth muscle and endothelial function. Am Surg. 2011;77(7):922–928. [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson BL, Souza DS, Bodin L, Filbey D, Bojö L. No touch vein harvesting technique for CABG improves the long-term clinical outcome. Scand Cardiovasc J. 2009;43(1):63–68. doi: 10.1080/14017430802140104. [DOI] [PubMed] [Google Scholar]
- 7.Johansson BL, Souza DS, Bodin L, et al. Slower progression of atherosclerosis in vein grafts harvested with “no touch” technique compared with conventional harvesting technique in coronary artery bypass grafting: an angiographic and intravascular ultrasound study. Eur J Cardiothorac Surg. 2010;38(4):414–419. doi: 10.1016/j.ejcts.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X7 receptors. Mol Pharmacol. 2000;58(1):82–88. [PubMed] [Google Scholar]
- 9.Qiu F, Dahl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2009;296(2):C250–C255. doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahl G, Keane RW. Pannexin: from discovery to bedside in 11±4 years? Brain Res. 2012;1487:150–159. doi: 10.1016/j.brainres.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 2010;24(2):337–345. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- 12.Bai TR, Bates JH, Brusasco V, et al. On the terminology for describing the length-force relationship and its changes in airway smooth muscle. J Appl Physiol (1985) 2004;97(6):2029–2034. doi: 10.1152/japplphysiol.00884.2004. [DOI] [PubMed] [Google Scholar]
- 13.Herlihy JT, Murphy RA. Length-tension relationship of smooth muscle of the hog carotid artery. Circ Res. 1973;33(3):275–283. doi: 10.1161/01.res.33.3.275. [DOI] [PubMed] [Google Scholar]
- 14.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 15.Tessier DJ, Komalavilas P, Liu B, et al. Transduction of peptide analogs of the small heat shock-related protein HSP20 inhibits intimal hyperplasia. J Vasc Surg. 2004;40(1):106–114. doi: 10.1016/j.jvs.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Lopes LB, Brophy CM, Flynn CR, et al. A novel cell permeant peptide inhibitor of MAPKAP kinase II inhibits intimal hyperplasia in a human saphenous vein organ culture model. J Vasc Surg. 2010;52(6):1596–1607. doi: 10.1016/j.jvs.2010.06.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li FD, Sexton KW, Hocking KM, et al. Intimal thickness associated with endothelial dysfunction in human vein grafts. J Surg Res. 2013;180(1):e55–e62. doi: 10.1016/j.jss.2012.06.017. doi:10.1016/j.jss.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osgood MJ, Hocking KM, Voskresensky IV, et al. Surgical vein graft preparation promotes cellular dysfunction, oxidative stress, and intimal hyperplasia in human saphenous vein. J Vasc Surg. 2014;60(1):202–211. doi: 10.1016/j.jvs.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Arcuino G, Takano T, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10(8):821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 20.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 21.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006;25(21):5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volonté C, Apolloni S, Skaper SD, Burnstock G. P2X7 receptors: channels, pores and more. CNS Neurol Disord Drug Targets. 2012;11(6):705–721. doi: 10.2174/187152712803581137. [DOI] [PubMed] [Google Scholar]
- 23.von Albertini M, Palmetshofer A, Kaczmarek E, et al. Extracellular ATP and ADP activate transcription factor NF-κB and induce endothelial cell apoptosis. Biochem Biophys Res Commun. 1998;248(3):822–829. doi: 10.1006/bbrc.1998.9055. [DOI] [PubMed] [Google Scholar]
- 24.Angelini GD, Passani SL, Breckenridge IM, Newby AC. Nature and pressure dependence of damage induced by distension of human saphenous vein coronary artery bypass grafts. Cardiovasc Res. 1987;21(12):902–907. doi: 10.1093/cvr/21.12.902. [DOI] [PubMed] [Google Scholar]
- 25.Cario-Toumaniantz C, Loirand G, Ladoux A, Pacaud P. P2X7 receptor activation-induced contraction and lysis in human saphenous vein smooth muscle. Circ Res. 1998;83(2):196–203. doi: 10.1161/01.res.83.2.196. [DOI] [PubMed] [Google Scholar]
- 26.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett. 2007;581(3):483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Jackson DG, Dahl G. The food dye FD&C Blue No. 1 is a selective inhibitor of the ATP release channel Panx1. J Gen Physiol. 2013;141(5):649–656. doi: 10.1085/jgp.201310966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dale N. A classic review on extracellular ATP and its signalling functions that helped to define the field's agenda for many years. Biochem J. 2012;2012(1):1–6. [Google Scholar]
- 29.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3(7):e2599. doi: 10.1371/journal.pone.0002599. doi:10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reidy MA. Factors controlling smooth-muscle cell proliferation. Arch Pathol Lab Med. 1992;116(12):1276–1280. [PubMed] [Google Scholar]
- 31.Johnson JL, van Eys GJ, Angelini GD, George SJ. Injury induces dedifferentiation of smooth muscle cells and increased matrix-degrading metalloproteinase activity in human saphenous vein. Arterioscler Thromb Vasc Biol. 2001;21(7):1146–1151. doi: 10.1161/hq0701.092106. [DOI] [PubMed] [Google Scholar]


