Abstract
Background
To investigate the incidence of second malignant neoplasms (SMN) for patients with neuroblastoma, we analyzed patients from the SEER database according to three treatment eras (1: 1973–1989, 2: 1990–1996, 3: 1997–2006) corresponding to the introduction of multi-agent chemotherapy, risk-based treatment, and stem cell transplant.
Procedure
The SEER database was mined for all patients with neuroblastoma or ganglioneuroblastoma. Cumulative incidence of SMN was calculated with death as a competing risk. A poisson regression model was used to estimate incidence rate ratios and 95% confidence intervals to compare the rates of SMN between patients in different Eras.
Results
The analytic cohort included 2,801 patients. Thirty-four patients developed a SMN, accounting for 1.2% of all patients. Of the patients who developed a SMN, 47.1% received radiation for their primary neuroblastoma. Fourteen of the SMN were carcinomas, and 10 were hematologic malignancies, with 6 cases of acute myelogenous leukemia. There was no difference in the incidence of SMN in Era 1 compared to Era 3 (p=0.48). The cumulative incidence of SMN at 30 years for high-risk patients was 10.44% (95% CI 3.98–20.52%) compared to 3.57% (95% CI 1.87–6.12%) for non-high-risk patients (p<0.001).
Conclusions
This study showed no increase in the incidence of SMNs for children treated in the most recent treatment era as compared to earlier Eras. However, as the risk for developing SMN does not plateau, the number of SMNs will likely continue to rise in the cohort of patients treated after 1996. Comprehensive follow-up care for these survivors will be important.
Keywords: Neuroblastoma, SEER, second malignancies, late effects
Introduction
Treatment advances have led to significantly improved outcomes for children with neuroblastoma and consequently, there are an increased number of survivors [1]. Neuroblastoma therapy is tailored according to risk classification and based on a combination of clinical and tumor biological biomarkers [2]. During the past 4 decades, high-risk patients have been treated with increasingly intensive, multi-modality approaches, whereas patients classified as non-high-risk have received reduced therapy [3]. Although it is well established that neuroblastoma survivors are at increased risk of developing second malignant neoplasms (SMN) compared to the general population [4–6], the impact of the changes in risk-based treatment approaches on SMN rates remains unclear.
We hypothesized that the reduction in treatment for low- and intermediate-risk patients during the past 4 decades would be associated with decreased rates of SMN. We also expected high-risk patients treated with modern strategies to be at increased risk for developing SMN. High-risk patients currently receive agents that are known to increase risk of myelodysplastic syndrome and treatment-related acute myelogenous leukemia, including high-dose alkylating agents, topoisomerase II inhibitors, and platinum-based drugs [7–10]. Furthermore, radiation therapy is an integral component of modern high-risk treatment, and a dose-response relationship between therapeutic levels of radiation and second cancers in adult survivors of childhood cancers is well established [11–14]. To investigate if the rates of SMN have changed over time in conjunction with changes in therapy, we analyzed data from neuroblastoma patients in the US National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database according to three treatment eras (Era 1: 1973–1989, Era 2: 1990–1996, Era 3: 1997–2006), corresponding to the introduction of multi-agent chemotherapy, risk-based treatment, and myeloablative therapy with stem cell transplant [3].
Methods
Patients and Variables
We mined patient information from the Incidence – SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases Nov 2013 Sub (1973–2011) database, which included data from 1973–2010. This SEER database includes data from regions that represent approximately 28% of the US population. The SEER program provides data on cancer incidence, patient demographics, tumor morphology, stage at diagnosis, limited treatment data, and survival data.
We identified all patients with histologically confirmed, non-CNS neuroblastoma (ICD-O-3 9500) or ganglioneuroblastoma (ICD-O-3 9490) in the database. While the peak incidence of neuroblastoma is in early childhood, we chose to evaluate all patients less than 30 years old at the time of diagnosis in order to obtain as many cases of SMN as possible. Only patients diagnosed between 1973 and 2006 were included in the analytic cohort to ensure follow-up time of at least 4 years. Patients were evaluated according three treatment Eras (1: 1973–1989, 2: 1990–1996, 3: 1997–2006), corresponding to the introduction of multi-agent chemotherapy, risk-based treatment, and myeloablative therapy with stem cell transplant, respectively. These cutoffs had been previously used in an analysis using data collected by the International Neuroblastoma Risk Group Task Force and shown to be a meaningful surrogate for changes in treatment modality [3].
The primary site of neuroblastoma was determined with the primary site ICD-O-3 field in SEER. Classification of SMN was determined using the sequence number field and histology ICD-O-3 fields in SEER. Tumor sequence was verified for all secondary tumors using the record number and sequence number recode fields.
Patient characteristics and clinical presentation were evaluated according to the development or not of a SMN. Predictor variables of interest included: sex; extent of disease (metastatic vs. localized); age; year of diagnosis (by treatment era); race; ethnicity; histology (neuroblastoma vs. ganglioneuroblastoma) and risk group (non-high-risk vs. high-risk). Patients were considered non-high-risk if they were less than one year old at diagnosis or had localized disease. Patients older than one year at the time of diagnosis with distant spread of their disease were defined as high-risk.
The available limited data on treatment received were also collected. Radiation therapy (including radioactive implants and radioisotopes) was dichotomized as given or not given if delivered to any tumor site (primary and/or metastatic) at any time point during treatment for neuroblastoma. For analysis related to use of radiation, patients were excluded if they had missing data for radiation or if they developed, hematologic malignancies including leukemia and lymphoma as their SMN. A patient was determined to have a secondary malignancy at the site of their primary neuroblastoma if it arose from the same anatomic location as their primary tumor according to ICD-O-3 anatomy codes. Laterality was not considered in this analysis as tumors were often classified as retroperitoneal or abdomen NOS.
Statistical Methods
Fisher’s exact tests and chi-square tests were used to compare characteristics of patients and second malignancies between eras. A two-sample t-test was used to compare patient age between groups.
The cumulative incidence method was used to generate curves and to estimate the cumulative incidence rates of SMN at all time points. The significance of the difference among comparison groups (i.e., treatment eras and risk group) was assessed with Gray’s test [15]. A poisson regression model was used to estimate incidence rate ratios and 95% confidence intervals to compare the rates of SMN between patients in different Eras. We determined the risk of SMN as compared to the general population by calculating the standardized incidence ratio (SIR). This ratio is the number of observed SMN in our cohort compared to the number of primary malignancies that would be expected in the general Unites States population using age and sex matched comparison rates from the SEER database. Confidence intervals for the SIRs were calculated using the Wilson and Hilferty approximation [16].
Overall survival from the time of diagnosis of neuroblastoma was estimated by Kaplan-Meier methods and potential differences between patients with high-risk and non-high-risk disease were evaluated using the log-rank test [17]. Cox proportional hazard models [18] were used to evaluate the effect of risk group and treatment era on overall survival. We built a multivariate model including era, risk group and the interaction between them adjusted for histology, age, gender and race and the final model was determined using a backward elimination (retention threshold, p < 0.05).
The SEER database was accessed using SEER*Stat version 8.1.2. All statistical analyses were performed using R software, version 3.0.1.
Results
Patient Characteristics
From 1973–2006, the SEER database included 2,823 cases of neuroblastoma or ganglioneuroblastoma in locations other than the CNS. Five patients were diagnosed with neuroblastoma as their second cancers and were excluded from the analysis as these were considered to represent relapsed disease. Patients were excluded if no record of their SMN was available in the database despite the sequence number field indicating this event occurred after a diagnosis of neuroblastoma (n=17). The remaining 2,801 patients comprised the analytic cohort for this study. The median follow-up time for the analyzed cohort was 74 months. The median and maximum follow up times was 90 and 455 months for Era 1, 166 and 251 months for Era 2, and 67 and 155 months for Era 3. There were 11,571 person-years of follow up for patients treated in Era 1, 6,667 for those in Era 2, and 7,560 for those in Era 3.
Treatment Era and SMN
Thirty-four (1.2%) of the 2801 patients with neuroblastoma developed a second malignancy. Clinical characteristics of patients who developed SMN are shown in Table I. Few differences in clinical presentation were noted for patients who did versus did not develop a SMN (Table II). Specifically, age at the time of diagnosis, gender, race, ethnicity, histology and metastatic status were not significantly different. Histology of the SMN included renal cell carcinoma (7), thyroid carcinoma (5), acute myelogenous leukemia (AML) (6), sarcoma (7), lymphoma or other hematologic malignancy (4), meningioma (1), melanoma (1) tongue carcinoma (1), breast carcinoma (1), and ovarian carcinoma (1). The median latency time to SMN was 38 months for all hematologic malignancies and 158 months for all solid tumors.
Table I.
Characteristics of 34 patients with neuroblastoma who developed a second malignancy.
| Characteristic | n = 34 |
|---|---|
|
| |
| Median Age at Second Cancer (Range) | 15 years (1–46 years) |
|
| |
| Median Latency from Primary Cancer (Range) | 139 months (10–432 months) |
|
| |
| Stage of Second Cancer | |
| Distant Metastasis | 7 (20.6%) |
| No Distant Metastasis | 26 (76.5%) |
| Unknown | 1 (2.9%) |
|
| |
| Histology of Second Cancer | |
| Carcinoma | 14 (41.8%)a |
| Hematologic Malignancy | 10 (29.4%) |
| Sarcoma | 7 (20.1%) |
| Melanoma | 1 (2.9%) |
| Brain Tumor | 1 (2.9%) |
| Other/Unknown | 1 (2.9%) |
|
| |
| Radiation for Neuroblastoma | |
| Not Received | 18 (52.9%) |
| Received | 16 (47.1%) |
|
| |
| Prior Radiation at Site of Second Cancer | |
| No Radiation | 12 (50.0%)b |
| Radiation to Site of Secondary Malignancy | 5 (20.8%) |
| Radiation to Other Site | 5 (20.8%) |
| Unknown | 2 (8.4%) |
Of the carcinomas, 7 were from the kidney, 5 from the thyroid, 1 from breast and 1 of the tongue
Patients who developed a hematologic malignancy were not included in this analysis
Table II.
Characteristics of 34 patients with neuroblastoma who developed a second malignant neoplasm compared with 2767 patients with neuroblastoma who did not develop second malignancies.
| Characteristic | Neuroblastoma with second malignancies n = 34 (1.2%) |
Neuroblastoma n = 2767 (98.8%) |
p-valuea |
|---|---|---|---|
|
| |||
| Median age | 1.5 years | 1 year | 0.39 |
| Range | 0–21 years | 0–29 years | |
|
| |||
| Male | 18 (52.9%) | 1459 (52.7%) | 1.00 |
|
| |||
| Race | |||
| White | 27 (79.4%) | 2240 (81.0%) | 0.59 |
| Black | 3 (8.8%) | 318 (11.4%) | |
| Asian | 4 (11.8%) | 182 (6.6%) | |
| Not available | 0 (0%) | 27 (1.0%) | |
|
| |||
| Ethnicity | |||
| Hispanic | 3 (8.8%) | 398 (14.4%) | 0.47 |
| Non-Hispanic | 31 (91.2%) | 2369 (85.6%) | |
|
| |||
| Stage | |||
| Distant Metastasis | 20 (58.8%) | 1207 (43.6%) | 0.09 |
| No Distant Metastasis | 9 (26.5%) | 1312 (47.4%) | |
| Unknown | 5 (14.7%) | 248 (9.0%) | |
|
| |||
| Histology | |||
| Ganglioneuroblastoma | 3 (8.8%) | 401 (14.5%) | 0.47 |
| Neuroblastoma | 31 (91.2%) | 2366 (85.5%) | |
|
| |||
| Era of Diagnosis | |||
| 1973–1989 | 20 (58.8%)b | 803 (29.0%) | N/A |
| 1990–1996 | 4 (11.8%) | 624 (22.6%) | |
| 1997–2006 | 10 (29.4%) | 1340 (48.4%) | |
|
| |||
| Risk Group | |||
| High | 13 (38.2%) | 933 (33.7%) | 0.18 |
| Low | 17 (50.0%) | 1677 (60.6%) | |
| Not definable | 4 (11.8%) | 157 (5.7%) | |
We used t-test for age, chi-square tests for gender, and fisher’s exact tests for race, ethnicity, metastasis stage, histology.
Radiation was used for the primary neuroblastoma in 11 patients from Era 1, 1 from Era 2 and 4 from Era 3.
Six of the 10 hematologic SMN developed in high-risk patients, defined as over 1 year of age with metastatic disease, including 3 AML cases. Patients treated in Era 1 developed 18 solid tumors and 2 hematologic malignancies (median latency 213 months vs. 225 months). Those treated in Era 2 developed 3 solid tumors and 1 hematologic malignancy (median latency 182 months vs. 154 months). Patients in Era 3 developed 3 solid tumors and 7 hematologic malignancies (median latency 88 months vs. 40 months). Also, 4 of the patients who developed AML were treated in Era 3 whereas there was only one patient with this diagnosis in each of the previous Eras.
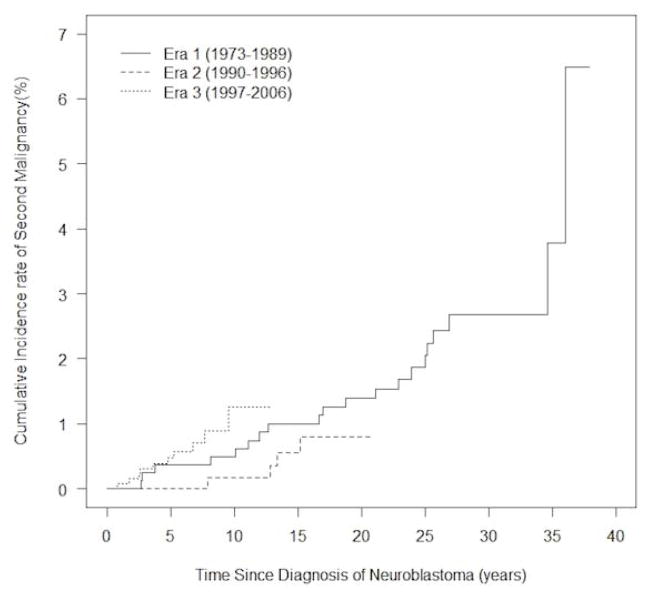
We estimated the incidence rate of SMN to be 14.94 per 11,424 person-years in Era 1, 5.14 per 6,646 person-years in Era 2, and 11.33 per 7,534 person-years in Era 3. No statistically significant differences were observed between treatment eras. Compared to patients in Era 1, the incidence rate ratios were 0.34 (95% CI 0.12–1.01; p = 0.051) for Era 2, and 0.76 (95% CI 0.35–1.62; p = 0.48) for Era 3. No difference in the cumulative incidence of SMN based on treatment era was observed (p = 0.38 for Era 2 vs. Era 1; p = 0.28 for Era 3 vs. Era 1)(Fig. 1). The cumulative incidence of SMN was higher in patients treated in Era 3 0.47% (95% CI 0.20–1.00%) compared to Era 1 0.37% (95% CI 0.11 – 1.02%) at five years from diagnosis. Ten years from diagnosis, the estimated rate of SMN reached 1.26% (95% CI 0.55–2.51%) for Era 3 compared to 0.49% (95% CI 0.17 – 1.20%) for Era 1, though these differences are not statistically significant (p = 0.28).
Figure 1.
Cumulative incidence of second malignant neoplasms from time of diagnosis in 2,801 patients diagnosed with neuroblastoma.
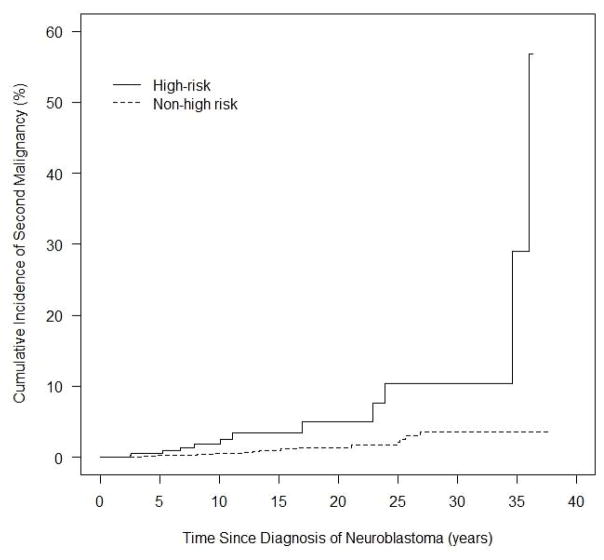
To investigate if the incidence of SMN differed in patients who received more versus less-intensive treatment, we analyzed SMN rates between the high-risk and non-high-risk cohorts. To account for the significantly higher rates of death within five years of diagnosis in the high-risk cohort (64.4%) compared to the non-high-risk patients (15.4%) (p < 0.001), we restricted the analysis to the subset of patients who remained alive five years from initial diagnosis (n = 1,848). The cumulative incidence of SMN at 30 years for high-risk patients was 10.44% (95% CI 3.98–20.52%) compared to 3.57% (95% CI 1.87–6.12%) for non-high-risk patients (p < 0.001) (Fig. 2). Recognizing that patients treated in Era 1 (n = 823) were the only Era with follow-up data 30 years from diagnosis, we reanalyzed the data restricting only to this group and showed similar estimates of 9.05% in the high-risk patients vs. 3.84% in the non-high-risk patients. This difference was not significant (p = 0.14) likely due to the smaller numbers of SMN in this subset of patients.
Figure 2.
Cumulative incidence of second malignant neoplasms in patients alive five years from initial diagnoses evaluated by risk group.
We calculated SIRs to compare rates of SMN in our cohort compared to the background rate of malignancy in the age- and sex-matched sample of the general SEER population (Table III). For all SMN, the SIR was 5.6 (95% CI 3.9–7.9), a finding that was similar between treatment eras. We repeated this analysis for the subsets of thyroid and renal carcinomas, as these were the most common SMN in our cohort and primarily adult-onset. The SIR for renal carcinomas was 128.2 (95% CI 51.3–264.0) and for thyroid carcinomas was 12.4 (95% CI 4.0–28.9). Though the confidence intervals are wide, these findings emphasize that this cohort is at high risk of developing a SMN, especially renal and thyroid carcinomas.
Table III.
Standardized incidence ratios of SMN in 2,801 patients with neuroblastoma calculated for all histologies and the subsets of renal and thyroid carcinomas.
| Characteristic | Number of patients | Pearson Years at Risk | Number of Cases
|
SIR | 95% CI | |
|---|---|---|---|---|---|---|
| Observed | Expected | |||||
|
| ||||||
| All SMN histology | ||||||
| Total cohort | 2,801 | 25,604 | 34 | 6.1 | 5.6 | 3.9–7.9 |
|
| ||||||
| Era 1 | 823 | 11,424 | 20 | 3.6 | 5.6 | 3.4–8.7 |
| Era 2 | 628 | 6,646 | 4 | 1.2 | 3.4 | 0.9–8.7 |
| Era 3 | 1350 | 7,534 | 10 | 1.3 | 7.6 | 3.6–13.9 |
|
| ||||||
| Renal Carcinomas | ||||||
| Total cohort | 2,801 | 25,604 | 7 | 0.06 | 128.2 | 51.3–264.0 |
|
| ||||||
| Era 1 | 823 | 11,424 | 6 | 0.05 | 127.30 | 46.5–277.1 |
| Era 2 | 628 | 6,646 | 0 | 0.005 | 0 | NA |
| Era 3 | 1350 | 7,534 | 1 | 0.003 | 366.7 | 4.8–2040 |
|
| ||||||
| Thyroid Carcinomas | ||||||
| Total cohort | 2,801 | 25,604 | 5 | 0.4 | 12.4 | 4.0–28.9 |
|
| ||||||
| Era 1 | 823 | 11,424 | 4 | 0.3 | 12.0 | 3.2–30.7 |
| Era 2 | 628 | 6,646 | 1 | 0.05 | 20.7 | 0.3–115.0 |
| Era 3 | 1350 | 7,534 | 0 | 0.02 | 0 | NA |
Use of Radiotherapy
Radiation therapy was used to treat neuroblastoma in 25.0% of the patients. The 48 patients with missing radiation therapy data were excluded from this analysis. Radiation was administered to a higher percentage of patients treated in Era 1 compared to either Era 2 (36.7% vs. 19.1%; p < 0.001) or Era 3 (36.7% vs. 21.5%; p < 0.001). There was no difference between Eras 2 and 3 (p = 0.25). 41.6% of patients who developed a SMN received radiation therapy for their neuroblastoma. Five patients developed a SMN in a site that received radiation for the primary neuroblastoma. Other treatment details, including radiation dose and fields as well as the use of chemotherapy, were not available.
Patient outcomes
The five-year overall survival (OS) estimate for the entire cohort was 65.4% (95% CI 63.6–67.2%). The 5-year OS for patients diagnosed during Era 1 was 52.3% (95% CI 49.0–55.9%) compared to 67.1% (95% CI 63.4–70.9%) for those diagnosed during Era 2 (p < 0.001). Patients diagnosed during Era 3 had the 5-year OS rate of 72.6% (95% CI 70.2–75.0%), significantly better than Era 2 patients (p = 0.02).
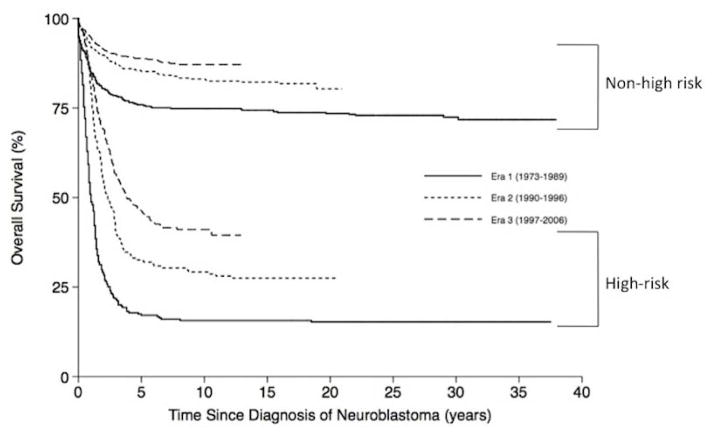
To confirm that our cohort was comprised of a representative neuroblastoma population, we analyzed survival rates by risk-group and compared the results to previous series. Five-year estimates of OS for patients diagnosed in Era 3 were 88.7% (95% CI 86.6–91.0%) in the non-high-risk patients and 46.2% (95% CI 41.9–50.9%) in the high-risk patients (p < 0.001) (Fig. 3), similar to historic controls [19,20]. The 5-year OS for high-risk patients diagnosed in Era 3 was significantly improved from Era 1 17.1% (95% CI 13.2–22.2%; p < 0.001) and Era 2 32.5% (95% CI 26.4–40.1%; p < 0.001). Similarly, the 5-year OS for non-high-risk patients diagnosed in Era 3 was improved from Era 1 75.9% (95% CI 72.2–79.9%; p < 0.001) and Era 2 85.2% (95% CI 81.8–88.8%; p = 0.004) though statistical significance was not reached between the two recent eras (p=0.06).
Figure 3.
Kaplan-Meier estimates of overall survival from time of diagnosis according to neuroblastoma risk group and treatment era.
As expected, separate Cox proportional hazards models showed that risk group was a significant predictor variable for death with the high-risk patients having a hazard ratios of 5.21 (95% CI 4.46–6.08, p < 0.001) compared to non-high-risk patients. Treatment era also was a significant predictor for death with patients diagnosed during Era 2 having a hazard ratio of 0.57 (95% CI 0.48–0.67, p < 0.001) compared to Era 1, and 0.40 (95% CI 0.35–0.47, p < 0.001) during Era 3 vs Era 1, respectively. The interaction between risk group and Era was tested but not significant (p = 0. 58 for risk and Era 2; p = 0.71 for risk and Era 3).
Discussion
In this study, we used the SEER database to investigate the rates of SMN in neuroblastoma patients diagnosed between 1973 and 2006. We show that the 1.2% of patients diagnosed with neuroblastoma will develop a SMN, and that survivors remain at risk for developing SMN many years after their primary diagnosis. Because patients diagnosed during Era 3 likely received more aggressive regimens compared to those treated during Eras 1 and 2, we hypothesized that a higher cumulative incidence of SMN would be detected in the cohort of patients diagnosed after 1996. However, we found no statistically significant difference in the cumulative incidence of SMN according to treatment eras. This result may reflect the small number of SMNs identified in the cohort and the broad latency time to develop SMN from the primary diagnosis.
During the past four decades, increasingly tailored therapy has been developed for children with neuroblastoma that is based on predicted risk of relapse. Patients classified as low- and intermediate-risk have had progressive reductions in therapy exposures in an effort to minimize the toxic effects of therapy. In contrast, high-risk patients have received increasingly intensive, multi-modality treatment strategies for in an effort to improve outcome. In the 1970s and 1980s treatment for neuroblastoma included combination chemotherapy, surgery, and radiation therapy [21–23]. Treatment was stratified largely according to stage and age [24]. With the discovery of the prognostic value of tumor MYCN status and ploidy, new risk group classification systems were developed with clinical and biologic criteria, and in the early 1990s more refined risk-based treatment strategies [25,26] were developed. While the benefit of autologous stem cell transplantation for patients with high risk disease was first reported in the 1990s [27,28], it wasn’t until the end of that decade when definitive studies were published establishing this as standard of care for high-risk patients [29].
The influence of treatment era on outcome was recently demonstrated in an analysis of more than 11,000 patients from the International Neuroblastoma Research Group (INRG) database. In that study, treatment Era 1 was defined as 1974–1989, Era 2 included patients diagnosed between 1990–1996, and Era 3 was defined as 1997–2002. [3]. Significant improvement in outcome was observed for the cohort treated after 1996 compared to those treated in earlier eras, demonstrating the efficacy of modern risk classification and stratified treatment approaches. The same treatment eras were used as a surrogate for the evolution of neuroblastoma treatment in our study, and in agreement with the report by Moroz et al, we also show that outcome for neuroblastoma patients has improved over the past 3 decades [3].
The relationship between increased exposure to chemotherapy and radiation and risk for long-term adverse events is well established [30,31]. Rates of therapy-related AML are related to treatment intensity in a wide variety of adult tumors [32]. A recent study confirmed that patients who developed therapy-related AML did so with short median latency after receiving epipodophyllin and/or an alkylating agent [6]. Of the 6 patients who developed secondary AML in our study, 3 were defined as high-risk and 3 as non-high-risk in this study based on age at diagnosis and the presence of metastatic disease. Unfortunately, information regarding tumor biology and chemotherapy are not available in the SEER data.
Radiation therapy exposure data were available for analysis, although information regarding which patients received systemic radiation exposure from radio-labeled meta-iodo-benzylguanidine (MIBG) therapy is not available [33]. Similar to other reports, we found that radiation was used to treat neuroblastoma in a high percentage of patients who developed a SMN [34,35]. Five of the 24 non-hematologic SMN in this cohort developed in a site that was radiated to treat the neuroblastoma tumor. Previous studies have demonstrated increased rates of both secondary renal and thyroid carcinomas several decades after treatment in patients who received radiation as part of therapy for their first malignancy [5,36,37]. Thus, radiation exposure may be a contributing factor for many of the SMN seen in patients treated in Era 1 in which these two tumor types represented the majority all SMN. However, because the dose and location of radiation are not available in the SEER data, the relationship between site of the SMN and radiation exposure for an individual patient cannot be confirmed.
We utilized the SEER database to capture as many SMN as possible, though our analyses were still limited by low numbers of cases. Several other limitations exist when analyzing data from a tumor registry. First, we were not able to confirm whether the information in the registry and the SEER data is representative of all patients. Further, we were unable to verify that the reported cases of neuroblastoma met current diagnostic standards for this disease. In addition, we were not able to confirm risk group assignments because tumor biological information was not available. As chemotherapy data were also lacking, we inferred treatment regimens based on the year the patient was diagnosed and published clinical trials conducted during these eras [7,20,21,29,38–42].
Our findings suggest that the risk for SMN in survivors of neuroblastoma do not plateau. Thus, the incidence of SMNs for the Era 3 cohort will likely continue to increase over time, emphasizing the need for close risk-based follow-up of these patients as recommended by the Institute of Medicine [43]. Further research focused on confirming these findings and identifying the treatment exposures, genomic variables, and gene-environment interactions that account for the second cancers is critical for the development of more effective, less toxic individualized treatment for patients with neuroblastoma.
Acknowledgments
Supported in part by the Neuroblastoma Children’s Cancer Society (SLC); the Children’s Neuroblastoma Cancer Foundation (SLC), Little Heroes Children’s Cancer Research Fund (SLC); and the Elise Anderson Neuroblastoma Research Fund (SLC), and NIH Grant Number T32GM007019 (MAA). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to disclose.
References
- 1.Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet. 2007;369(9579):2106. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27(2):289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moroz V, Machin D, Faldum A, et al. Changes over three decades in outcome and the prognostic influence of age-at-diagnosis in young patients with neuroblastoma: a report from the International Neuroblastoma Risk Group Project. Eur J Cancer. 2011;47(4):561–571. doi: 10.1016/j.ejca.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Laverdiere C, Liu Q, Yasui Y, et al. Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(16):1131–1140. doi: 10.1093/jnci/djp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubino C, Adjadj E, Guerin S, et al. Long-term risk of second malignant neoplasms after neuroblastoma in childhood: role of treatment. Int J Cancer. 2003;107(5):791–796. doi: 10.1002/ijc.11455. [DOI] [PubMed] [Google Scholar]
- 6.Federico SM, Allewelt HB, Spunt SL, et al. Subsequent Malignant Neoplasms in Pediatric Patients Initially Diagnosed With Neuroblastoma. J Pediatr Hematol Oncol. 2014 doi: 10.1097/MPH.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. 2013;14(10):999–1008. doi: 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Deley MC, Leblanc T, Shamsaldin A, et al. Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: a case-control study by the Societe Francaise d’Oncologie Pediatrique. J Clin Oncol. 2003;21(6):1074–1081. doi: 10.1200/JCO.2003.04.100. [DOI] [PubMed] [Google Scholar]
- 9.Travis LB, Holowaty EJ, Bergfeldt K, et al. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med. 1999;340(5):351–357. doi: 10.1056/NEJM199902043400504. [DOI] [PubMed] [Google Scholar]
- 10.Sandoval C, Pui CH, Bowman LC, et al. Secondary acute myeloid leukemia in children previously treated with alkylating agents, intercalating topoisomerase II inhibitors, and irradiation. J Clin Oncol. 1993;11(6):1039–1045. doi: 10.1200/JCO.1993.11.6.1039. [DOI] [PubMed] [Google Scholar]
- 11.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98(21):1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 12.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol. 2009;27(24):3901–3907. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365(9476):2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 14.Henderson TO, Rajaraman P, Stovall M, et al. Risk factors associated with secondary sarcomas in childhood cancer survivors: a report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys. 2012;84(1):224–230. doi: 10.1016/j.ijrobp.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16(3):1141–1154. [Google Scholar]
- 16.Wilson EB, Hilferty MM. The Distribution of Chi-Square. Proc Natl Acad Sci U S A. 1931;17(12):684–688. doi: 10.1073/pnas.17.12.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peto R, Peto J. Asymptotically Efficient Rank Invariant Test Procedures. Journal of the Royal Statistical Society Series A (General) 1972;135(2):185–207. [Google Scholar]
- 18.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society Series B (Methodological) 1972:187–220. [Google Scholar]
- 19.Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med. 2010;363(14):1313–1323. doi: 10.1056/NEJMoa1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finklestein JZ, Klemperer MR, Evans A, et al. Multiagent chemotherapy for children with metastatic neuroblastoma: a report from Childrens Cancer Study Group. Med Pediatr Oncol. 1979;6(2):179–188. doi: 10.1002/mpo.2950060211. [DOI] [PubMed] [Google Scholar]
- 22.Berthold F, Treuner J, Brandeis WE, et al. Neuroblastoma study NBL 79 of the German Society for Pediatric Oncology. Report after 2 years. Klin Padiatr. 1982;194(4):262–269. doi: 10.1055/s-2008-1033815. [DOI] [PubMed] [Google Scholar]
- 23.Evans AE, D’Angio GJ, Koop CE. The role of multimodal therapy in patients with local and regional neuroblastoma. J Pediatr Surg. 1984;19(1):77–80. doi: 10.1016/s0022-3468(84)80021-4. [DOI] [PubMed] [Google Scholar]
- 24.Breslow N, McCann B. Statistical estimation of prognosis for children with neuroblastoma. Cancer Res. 1971;31(12):2098–2103. [PubMed] [Google Scholar]
- 25.Haase GM, Atkinson JB, Stram DO, et al. Surgical management and outcome of locoregional neuroblastoma: comparison of the Childrens Cancer Group and the international staging systems. J Pediatr Surg. 1995;30(2):289–294. doi: 10.1016/0022-3468(95)90576-6. discussion 295. [DOI] [PubMed] [Google Scholar]
- 26.Castleberry RP. Neuroblastoma. Eur J Cancer. 1997;33(9):1430–1437. doi: 10.1016/s0959-8049(97)00308-0. discussion 1437–1438. [DOI] [PubMed] [Google Scholar]
- 27.Matthay KK, O’Leary MC, Ramsay NK, et al. Role of myeloablative therapy in improved outcome for high risk neuroblastoma: review of recent Children’s Cancer Group results. Eur J Cancer. 1995;31A(4):572–575. doi: 10.1016/0959-8049(95)00015-b. [DOI] [PubMed] [Google Scholar]
- 28.Stram DO, Matthay KK, O’Leary M, et al. Consolidation chemoradiotherapy and autologous bone marrow transplantation versus continued chemotherapy for metastatic neuroblastoma: a report of two concurrent Children’s Cancer Group studies. J Clin Oncol. 1996;14(9):2417–2426. doi: 10.1200/JCO.1996.14.9.2417. [DOI] [PubMed] [Google Scholar]
- 29.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341(16):1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 30.Travis LB, Demark Wahnefried W, Allan JM, et al. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10(5):289–301. doi: 10.1038/nrclinonc.2013.41. [DOI] [PubMed] [Google Scholar]
- 31.Robison LL, Green DM, Hudson M, et al. Long-term outcomes of adult survivors of childhood cancer. Cancer. 2005;104(11 Suppl):2557–2564. doi: 10.1002/cncr.21249. [DOI] [PubMed] [Google Scholar]
- 32.Morton LM, Dores GM, Tucker MA, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975–2008. Blood. 2013;121(15):2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jairam V, Roberts KB, Yu JB. Historical trends in the use of radiation therapy for pediatric cancers: 1973–2008. Int J Radiat Oncol Biol Phys. 2013;85(3):e151–155. doi: 10.1016/j.ijrobp.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng AK, Bernardo MV, Weller E, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long-term risks and risk factors. Blood. 2002;100(6):1989–1996. doi: 10.1182/blood-2002-02-0634. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong GT, Liu W, Leisenring W, et al. Occurrence of multiple subsequent neoplasms in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2011;29(22):3056–3064. doi: 10.1200/JCO.2011.34.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson CL, Ness KK, Neglia JP, et al. Renal carcinoma after childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2013;105(7):504–508. doi: 10.1093/jnci/djt014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatti P, Veiga LH, Ronckers CM, et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the childhood cancer survivor study. Radiat Res. 2010;174(6):741–752. doi: 10.1667/RR2240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans AR, Brand W, de Lorimier A, et al. Results in children with local and regional neuroblastoma managed with and without vincristine, cyclophosphamide, and imidazolecarboxamide. A report from the Children’s Cancer Study Group. Am J Clin Oncol. 1984;7(1):3–7. doi: 10.1097/00000421-198402000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Matthay KK, Sather HN, Seeger RC, et al. Excellent outcome of stage II neuroblastoma is independent of residual disease and radiation therapy. J Clin Oncol. 1989;7(2):236–244. doi: 10.1200/JCO.1989.7.2.236. [DOI] [PubMed] [Google Scholar]
- 40.Evans AE, Silber JH, Shpilsky A, et al. Successful management of low-stage neuroblastoma without adjuvant therapies: a comparison of two decades, 1972 through 1981 and 1982 through 1992, in a single institution. J Clin Oncol. 1996;14(9):2504–2510. doi: 10.1200/JCO.1996.14.9.2504. [DOI] [PubMed] [Google Scholar]
- 41.Matthay KK, Perez C, Seeger RC, et al. Successful treatment of stage III neuroblastoma based on prospective biologic staging: a Children’s Cancer Group study. J Clin Oncol. 1998;16(4):1256–1264. doi: 10.1200/JCO.1998.16.4.1256. [DOI] [PubMed] [Google Scholar]
- 42.Perez CA, Matthay KK, Atkinson JB, et al. Biologic variables in the outcome of stages I and II neuroblastoma treated with surgery as primary therapy: a children’s cancer group study. J Clin Oncol. 2000;18(1):18–26. doi: 10.1200/JCO.2000.18.1.18. [DOI] [PubMed] [Google Scholar]
- 43.Simone JV, Weiner SL, Hewitt M. Childhood Cancer Survivorship:: Improving Care and Quality of Life. National Academies Press; 2003. [PubMed] [Google Scholar]