Abstract
Objective
To assess the impact of a 2008 dose-based prior authorization policy for Massachusetts Medicaid beneficiaries using buprenorphine + naloxone for opioid addiction treatment. Doses higher than 16 mg required progressively more frequent authorizations.
Data Sources
Mediciaid claims for 2007 and 2008 linked with Department of Public Health (DPH) service records.
Study Design
We conducted time series for all buprenorphine users and a longitudinal cohort analysis of 2,049 individuals who began buprenorphine treatment in 2007. Outcome measures included use of relapse-related services, health care expenditures per person, and buprenorphine expenditures.
Data Collection/Extraction Methods
We used ICD-9 codes and National Drug Codes to identify individuals with opioid dependence who filled prescriptions for buprenorphine. Medicaid and DPH data were linked with individual identifiers.
Principal Findings
Individuals using doses >24 mg decreased from 16.5 to 4.1 percent. Relapses increased temporarily for some users but returned to previous levels within 3 months. Buprenorphine expenditures decreased but total expenditures did not change significantly.
Conclusion
Prior authorization policies strategically targeted by dose level appear to successfully reduce use of higher than recommended buprenorphine doses. Savings from these policies are modest and may be accompanied by brief increases in relapse rates. Lower doses may decrease diversion of buprenorphine.
Keywords: Drug addiction treatment, prior authorization, Medicaid, buprenorphine, pharmaceutical policy
The Food and Drug Administration approved buprenorphine for medication-assisted treatment of opioid addiction in 2002. Greater flexibility in use and a better safety profile than methadone has facilitated buprenorphine's widespread adoption since then. Unlike methadone maintenance treatment, which is highly structured with most patients required to take doses at a dispensing clinic, buprenorphine patients can receive up to 30 days supply of the medication to take at home. The most widely used buprenorphine formulation in the United States combines buprenorphine with naloxone, an opioid antagonist that may reduce its abuse potential. It is sold under the brand name Suboxone. Generic equivalents of Suboxone were introduced during the first quarter of 2013, after completion of this study. Although most studies indicate that methadone maintenance therapy is somewhat more effective in preventing addiction relapse (Barnett, Zaric, and Brandeau 2001; Mattick et al. 2014; Clark et al. 2011), many providers and patients prefer buprenorphine because it is less dangerous if patients overdose and in-home administration causes fewer disruptions in employment and family life. Both forms of treatment are more effective in preventing relapses than drug free treatment alone (Mattick et al. 2009, 2014).
Medicaid, a health entitlement program jointly funded by states and the federal government, funds a significant proportion of buprenorphine treatment in the United States (Ducharme and Abraham 2008; Stein et al. 2012). The greater prevalence of substance use disorders among Medicaid beneficiaries, coupled with the relatively rapid adoption and high cost of buprenorphine + naloxone (about $325 per month for the average user) has attracted the attention of Medicaid administrators. Increasing reports of diversion have also raised concerns about buprenorphine treatment in general, and dosing levels in particular, as some reports suggest that patients prescribed high doses may use only a portion of their medication and share or sell the rest (Lofwall and Havens 2012; NYTimes article 11-17-13; Wish et al. 2012). The recommended dosage for buprenorphine + naloxone maintenance is 16 mg of buprenorphine per day with an upper limit of 24 mg (Federal Drug Administration 2010). However, dosing varies widely according to patient need and provider preference (Center for Substance Abuse Treatment 2004).
Concerns about cost and diversion have prompted many states to place limits on buprenorphine prescription. Almost all states now require some form of prior approval for buprenorphine prescriptions. Currently, 12 states limit the total amount of time that a Medicaid-eligible patient can be treated during his or her lifetime. Limitations in lifetime use range from 6 to 36 months, most limit treatment to 12 months (Rinaldo and Rinaldo 2013).
Concerns about cost and diversion led the Massachusetts Medicaid program (MassHealth) to implement a unique prior authorization policy focused on buprenorphine dose levels in January of 2008. The policy was applied to all members of the Primary Care Clinician Plan and to fee-for-service members, for whom MassHealth directly manages pharmacy benefits. Under the new policy, higher doses required more frequent prior authorization. For example, doses above 32 mg/day required prior approval with each 30-day prescription, doses less than or equal to 32 mg/day but greater than 24 mg/day required authorization every 90 days, those greater than 16 mg/day but less than or equal to 24 mg/day, every 180 days. Prescriptions of 16 mg/day or less did not require prior authorization. Unlike some other states' requirements, the Massachusetts policy did not seek to limit access to buprenorphine, but rather to lower costs and discourage diversion by reducing doses that were higher than the recommended therapeutic range.
Although prior authorization is widely used as a method for managing access to costly medications, some studies suggest that it can produce minimal savings and may actually increase costs if the added burden of the requirement leads to a break in treatment continuity or to greater treatment dropout rates (Law, Ross-Degnan, and Soumerai 2008; Abouzaid et al. 2010; Lu et al. 2011). Prior authorization policy effects can vary depending upon the type of drug to which they are applied, the population being served, and specific details of the policy. For example, a study of expensive nonsteroidal antiinflammatory drugs found significant reductions in use after implementation of prior authorization requirements in several states (Fischer et al. 2004) but prior authorization was found to have minimal effects on prescribing of oxycodone, a pain medication with high addiction potential (Morden et al. 2008). The effects of prior authorization requirements on buprenorphine use, costs, and outcomes have not previously been studied.
To understand the impact of MassHealth's policy, we examined changes in dosing, relapse rates, medication costs, and total health care costs per person before and after implementation of the 2008 policy. We also explored changes in rates of treatment discontinuation or switching.
Methods
Data
We used paid MassHealth claims and encounter data to identify members continuously enrolled in the Primary Care Clinician Plan and fee-for-service populations who filled prescriptions for buprenorphine + naloxone for addiction treatment at any time between January 1, 2007 and December 31, 2008.
Inclusion and Exclusion Criteria
Specifically, members were considered eligible for the study if they had both a diagnosis of opioid dependence and filled at least one prescription for buprenorphine + naloxone during the study period. Members enrolled in four other Medicaid managed care plans were excluded because prior authorization requirements were different from those implemented directly by MassHealth and timing of any policy changes within each plan could not be accurately determined. This represented approximately 20 percent of all MassHealth buprenorphine users during the study period. We linked claims with utilization records from the Massachusetts Department of Public Health (DPH), Bureau of Substance Abuse Services, to identify detoxifications that were paid directly by DPH.
Measures
Members were considered to be in buprenorphine treatment if they filled a prescription for buprenorphine during the current month or during the month before or after, thus allowing for no more than a 1-month break in treatment continuity within an episode. A episode began on the day that the buprenorphine prescription was first filled, provided that there had been no prescriptions filled during the 2 months preceding that month. Treatment episodes continued until the last month a prescription was filled, provided that it was followed by two consecutive months during which no buprenorphine prescriptions were filled. Total episode length was calculated by adding the days supply to the first prescription fill date.
We developed relapse indicators based on service use, defined as an emergency department visit or hospitalization with a primary diagnosis of substance abuse or dependence, or a detoxification. While this approach may have included relapses related to substances other than opioids, given the lack of precision in attributing a relapse event to one specific drug, we chose to err on the side of possibly overstating relapse rates. This did not affect our analyses measuring changes in relapse rates over time, because all relapse events are determined in the same way at each measurement point. Average buprenorphine doses, buprenorphine costs, total health care costs, and relapses were calculated for all members using buprenorphine + naloxone during the study period. The number of patients filling prescriptions for buprenorphine + naloxone increased from 3,706 in December of 2007 to 5,094 in December of 2008, with an average of 4,761 patients per month during 2008.
Because the prior authorization policy was designed to impact members differently depending on their prescribed dose, we grouped members into three categories according to their highest monthly dose in 2007: ≤16 mg/day (low dose); >16 and ≤24 mg/day (medium dose); and >24 mg/day (high dose) for some analyses.
Statistical Analyses
We conducted two different time-series analyses using different groups. The first was a population-level analysis including all individuals using buprenorphine in a given month. This resulted in different numbers of subjects in each month from January of 2007 through December of 2008. For example, an individual who began using buprenorphine in January of 2007 and ended treatment in June of 2007 would be in the sample for each of the first 6 months and not in the sample afterward. The second analysis followed a cohort of individuals who began using buprenorphine in 2007 and continued in treatment after implementation of the prior authorization requirement in 2008.
In our population-level analysis, we examined the total number of buprenorphine + naloxone users in each dose group for each of the study months. Using interrupted time-series models implemented with generalized estimating equations (GEE) with an autoregressive variance–covariance structure, we estimated the impact of prior authorization changes on (1) average monthly doses, (2) relapse rates, (3) buprenorphine costs, and (4) average monthly MassHealth expenditures for all health care used by each member. Primary analyses used July 1, 2008 as the implementation date to allow time for all dose groups to be affected by prior authorization. Sensitivity analyses were conducted using January 1, 2008, the date that implementation of the prior authorization requirement officially began, to determine if the impact of prior authorization was apparent before the policy had time to be implemented fully.
In our second analysis, we examined the effect of the new policy by following a specific cohort of buprenorphine + naloxone users over time. We identified MassHealth members who began a buprenorphine + naloxone treatment episode at any time in 2007 and had at least 1 month of treatment after initiation of the prior authorization requirement. Members were grouped into low-, medium-, or high-dose categories based on their highest dose in 2007. We followed members for up to 24 months, using a multivariable GEE model to account for potential differences in the characteristics of buprenorphine patients using various doses, adjusting for month of measurement, age, gender, race, and disease burden, as measured by the Chronic Illness and Disability Payment System or CDPS (Kronick et al. 2000), and episode length. Separate models of all members and of high-dose users included relapses, buprenorphine costs, and total health care costs as dependent measures. Cost equations were constructed using actual costs and natural log-transformed costs to adjust for skewed distributions that could have violated the underlying assumptions of the statistical models used.
To measure the total population-level impact of prior authorization on buprenorphine costs we calculated the monthly expenditures adjusting for trends in dosage shown in Figure1 that were evident during the first 11 months of 2007, which included slight decreases in high doses and increases in medium and low doses. We did not adjust for changes in dosage that occurred in December 2007, as they may have been influenced by the upcoming PA implementation. Medication costs were also adjusted for an increase in the price of buprenorphine per milligram, which occurred in 2008. We then summed the adjusted monthly expenditures to estimate the direct impact of the policy on buprenorphine expenditures. In a sensitivity analysis, we also calculated expenditures without adjusting for preexisting dose trends.
Figure 1.
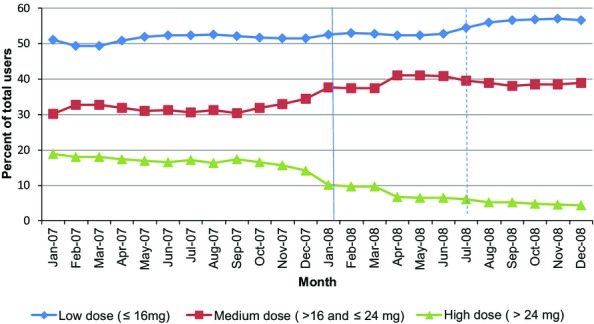
MassHealth Members with Opioid Use Disorders Who Used Buprenorphine before and after the Prior Authorization Policy (1/107 – 12/30/08) Note. Includes MassHealth members with opioid use disorders who filled a prescription for buprenorphine during a month from 1/1/07 through 12/31/08. The number of buprenorphone users ranged from 2,011 (in January 2007) to 5,094 (in December 2008). Users are allocated to a group each month based on the highest dose received that month.
This study was reviewed and approved by human subjects committees at the University of Massachusetts Medical School and at the Commonwealth of Massachusetts Department of Public Health.
Results
Changes in Dosage and Treatment Modality
In the population-level analysis more than one-fifth (21.6 percent) of members had buprenorphine + naloxone doses greater than 24 mg per day at some time during 2007. About one-third (34.1 percent) were prescribed doses between 16 and 24 mg per day, and the remainder (44.3 percent) had doses less than or equal to 16 mg per day.
The percentage of members filling doses greater than 24 mg/day began at 16.5 percent in January of 2007 and decreased by about one-quarter percent (0.28 percent) per month during the first 11 months of 2007. Figure1 shows that after implementation of prior authorization in January of 2008, the rate of decrease in the high-dose group accelerated to 0.81 percent each month, ending with 4.1 percent of all buprenorphine patients receiving doses greater than 24 mg/day by December of 2008. The medium- and low-dose groups grew proportionately after prior authorization, from 34.1 to 37.5 percent and from 44.3 to 58.4 percent, respectively. A slightly larger percentage of high- (64.6 percent) and medium- (66.3 percent) dose members discontinued buprenorphine treatment during the study period than did low-dose members (58.8 percent). Small percentages of these members switched to methadone, the remainder switched to nonmedication treatment or to no treatment. Members of the low-dose group were the most likely to switch to methadone (10.5 percent) compared to the medium- (8.7 percent) and high-dose groups (7.2 percent.) At the population level, there was no change in the overall rate of switching from buprenorphine to methadone before and after implementation of the PA.
In the cohort analysis, members of the high-dose group were somewhat older, more likely to be men, and remained in treatment longer than those in the low-dose group. Members of the low-dose group had shorter treatment episodes and higher health care costs compared to those in the medium- and high-dose groups. Among the cohort of 2007 patients, there was no evidence of disproportionate rates of switching from buprenorphine + naloxone to methadone in the higher dose groups after the PA was implemented: 9 percent of those in the low-dose group, 6.2 percent in the medium-dose group, and 6.3 percent in the high-dose group transitioned to methadone treatment in 2008 (Table1).
Table 1.
MassHealth Members with Opioid Use Disorders Who Started a Buprenorphine Treatment Episode in 2007 (N = 2,049)
|
Dose Group
‡ |
||||
|---|---|---|---|---|
| Characteristic | Total † (N = 2,049) | Low (N = 908) | Medium (N = 699) | High (N = 442) |
| Age, mean (SD) | 33.2 (9.7) | 32.9 (9.9)b | 33.1 (9.4) | 34.2 (9.7)b |
| Gender | ||||
| Female, n (%) | 797 (38.9) | 391 (43.1)a,b | 259 (37.0)a | 147 (33.3)b |
| Male, n (%) | 1,252 (61.1) | 517 (56.9)a,b | 440 (63.0)a | 295 (66.7)b |
| Race/ethnicity | ||||
| White, n (%) | 1,324 (64.6) | 583 (64.1) | 446 (63.8) | 295 (66.7) |
| Black, n (%) | 60 (2.9) | 36 (4.0)b | 17 (2.4) | 7 (1.6)b |
| Hispanic, n (%) | 111 (5.4) | 48 (5.3) | 40 (5.7) | 23 (5.2) |
| Other, n (%) | 20 (1.0) | 6 (0.7) | 6 (0.9) | 8 (1.8) |
| Unknown, n (%) | 534 (26.1) | 235 (25.9) | 190 (27.2) | 109 (24.7) |
| Health plan | ||||
| PCC, n (%) | 1,846 (90.1) | 815 (89.8) | 637 (91.1) | 394 (89.1) |
| FFS, n (%) | 203 (9.9) | 93 (10.2) | 62 (8.9) | 48 (10.9) |
| Health status | ||||
| CDPS§ score, mean (SD) | 3.3 (2.1) | 3.4 (2.4)a | 3.2 (1.8)a | 3.2 (1.8) |
| No. mental health conditions, mean (SD)¶ | 1.3 (1.4) | 1.4 (1.5)a,b | 1.3 (1.4)a | 1.2 (1.2)b |
| No. physical health conditions, mean (SD)†† | 0.7 (1.0) | 0.7 (1.0)a | 0.6 (0.9)a,c | 0.8 (1.0)c |
| Treatment episode length in months, mean (SD) | 10.4 (13.5) | 7.3 (11.6)a,b | 12.3 (14.0)a | 13.7 (14.8)b |
| Monthly outcomes in 2007 | ||||
| Buprenorphine dose (mg), mean (SD) | 16.7 (6.9) | 11.8 (3.9)a,b | 18.8 (4.2)a,c | 23.9 (7.1)b,c |
| All health care expenditures per person, mean (SD) | $1,224 (1,367) | $1,372 (1,640)a,b | $1,110 (1,025)a | $1,102 (1,185)b |
| Buprenorphine expenditures per person, mean (SD) | $248 (151) | $164 (96)a,b | $284 (128)a,c | $362 (179)b,c |
| Relapse events‡‡ per 100 users, mean (SD) | 14.8 (1.9) | 15.8 (2.9) | 14.1 (2.8) | 13.9 (2.8) |
| Arrests per 100 users, mean (SD) | 5.4 (0.8) | 5.5 (1.3) | 5.0 (1.0)b | 5.9 (1.0)b |
Includes members of the Fee-for-Service (FFS) and Primary Care Clinician (PCC) plans.
Dose groups were based on the member's highest buprenorphine dose in 2007 and were defined as follows: Low (≤16 mg), Medium (>16 mg and ≤24 mg), and High (>24 mg).
Chronic Illness and Disability Payment System (Kronick et al. 2000).
Includes schizophrenia and other psychotic disorders, bipolar, major depression, other depression, anxiety, and other mental illness.
Includes HIV, asthma, COPD, hepatitis B and C, hypertension, congestive heart failure, ischemic heart disease, diabetes I, and diabetes II.
Substance use related emergency room visits, hospital admissions, and detoxifications.
Significant differences between groups: aLow- and medium- dose groups (p < .05); bLow- and high-dose groups (p < .05); cMedium- and high-dose groups (p < .05).
Relapses
Relapse rates for all members using buprenorphine in the population-level analysis showed a general downward trend during the 24-month study period, with a significant unexplained spike among medium- and low-dose patients in the summer of 2007. When all members were combined, including those beginning treatment before 2008 and those who started after implementation of prior authorization, there was no significant change in relapse after January 2008 (results not shown). However, relapses among the cohort of 2,049 members who began treatment in 2007 rose sharply after July of 2008, when the policy change had been fully implemented (Table2). Figure2 shows that the increase in relapses was particularly pronounced in the medium- and high-dose groups compared with the low-dose group, which had previously had a higher relapse rate than medium- and high-dose groups. However, the increase among the higher dose groups was temporary and returned to the prepolicy trend by the end of 2008. Sensitivity analyses using January 2008 as the beginning of the policy period did not show a significant effect of the policy on relapse rates.
Table 2.
Multivariable Analysis of Factors Related to Relapse among MassHealth Members with Opioid Use Disorders Who Started a Buprenorphine Treatment Episode in 2007 (N = 2,049)†
| Factor | Everyone Coefficient (95% CI) | Medium/High-dose Groups‡ Coefficient (95% CI) |
|---|---|---|
| Intercept | 0.193 (0.12, 0.26)*** | 0.202 (0.12, 0.29)*** |
| Age in 2007 (10 years units) | −0.014 (−0.03, <0.01)* | −0.024 (−0.04, −0.01)** |
| Gender | ||
| Female | Reference | Reference |
| Male | 0.050 (0.03, 0.07)*** | 0.047 (0.02, 0.08)** |
| Race | ||
| Nonwhite | Reference | Reference |
| White | 0.024 (<0.01, 0.05)* | 0.024 (−0.01, 0.05) |
| MassHealth plan | ||
| FFS | Reference | Reference |
| PCC plan | −0.004 (−0.05, 0.04) | −0.024 (−0.08, 0.03) |
| Disease burden (CDPS) | 0.020 (0.01, 0.03)*** | 0.024 (0.01, 0.03)*** |
| Episode length (in 10 day units) | −0.043 (−0.05, −0.04)*** | −0.030 (−0.04, −0.02)*** |
| Study month (1–24) | −0.005 (−0.01, <0.01)*** | −0.005 (−0.01, <0.01)** |
| Policy implementation | ||
| Prior to 7/1/08 | Reference | Reference |
| After 7/1/08 | 0.046 (0.01, 0.08)** | 0.045 (0.01, 0.08)* |
Generalized estimating equations (GEE) analysis.
Includes members in the Medium- (>16 mg and ≤24 mg), and High- (>24 mg) dose groups, based on the member's highest buprenorphine dose in 2007.
Significant at p < .05;
Significant at p < .01;
Significant at p < .001.
Figure 2.
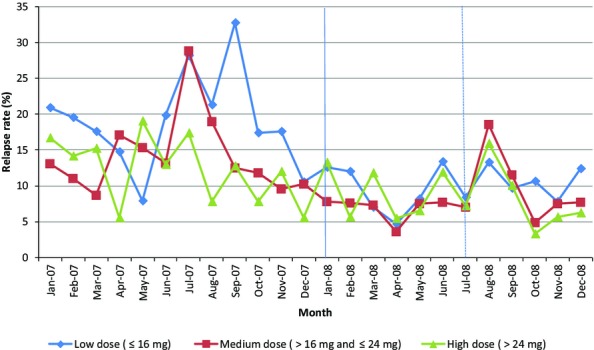
Relapse Rates among MassHealth Members with Opioid Use Disorders Who Started a Buprenorphine Treatment Episode in 2007 (N = 2,049) (1/1/07 – 12/30/08) Note. Includes 2,049 MassHealth members who started a buprenorphine treatment episode at any time during 2007. The number of members in treatment each month ranged from 188 (in January 07) to 1,051(in November 07). Members were followed from the start of treatment in 2007 through the end of the treatment episode, until 12/31/08. Dose groups were based on the member's highest buprenorphine dose in 2007 and were defined as follows: Low (≤16 mg), Medium (>16 mg and ≤24 mg), and High (>24 mg).
Changes in Cost
The price of buprenorphine + naloxone increased 12 percent during the 2 year study period from $4.68 for a single standard 8 mg dose in January of 2007 to $5.23 in December of 2008. After adjusting for price increases and the preexisting downward trend in high doses and upward trends in medium and low doses shown in Figure1, total MassHealth buprenorphine expenditures dropped by $492,641 from their expected amount during 2008, an average of $103.48 per person who used buprenorphine ($8.62 per person per month). Unadjusted savings were $131,347. Analyses of buprenorphine spending among patients who used the medication in 2007 indicate a somewhat greater reduction of $34.79 per person per month after July 1, 2008, adjusting for patient characteristics (Table3). Total health care costs per person increased significantly for all members after July 1, 2008, in both conventional (Table3) and log-transformed analyses (data not shown).
Table 3.
Multivariable Analysis of Factors Related to Monthly Expenditures for MassHealth Members with Opioid Use Disorders Who Started a Buprenorphine Treatment Episode in 2007 (N = 2,049)†
| Factor | Buprenorphine Expenditures Co-efficient (95% CI) | Total Health Care Expenditures Co-efficient (95% CI) |
|---|---|---|
| Intercept | $143.32 (113.01, 173.63)*** | $267.26 (−36.55, 571.08) |
| Age in 2007 | $0.76 (0.22, 1.31)** | $15.67 (9.60, 21.75)*** |
| Gender | ||
| Female | Reference | Reference |
| Male | $27.16 (16.06, 38.26)*** | −$123.42 (−220.48, −26.36)* |
| Race | ||
| Nonwhite | Reference | Reference |
| White | $10.44 (−0.62, 21.51) | $50.36 (−41.41, 142.14) |
| MassHealth plan | ||
| FFS | Reference | Reference |
| PCC plan | $45.69 (23.73, 67.65)*** | $163.60 (6.05, 321.15)* |
| Disease burden (CDPS) | −$3.07 (−5.55, −0.60)* | $231.81 (139.91, 323.71)*** |
| Episode length | $2.22 (1.86, 2.57)*** | −$9.43 (−12.56, −6.30)*** |
| Study month (1–24) | $4.91 (3.87, 5.95)*** | −$7.45 (−16.71, 1.81) |
| Policy implementation | ||
| Prior to 7/1/08 | Reference | Reference |
| After 7/1/08 | −$34.79 (−44.68, −24.90)*** | $79.19 (−26.36, 184.74) |
Generalized estimating equations (GEE) analysis.
Significant at p < .05;
Significant at p < .01;
Significant at p < .001.
Discussion
Implementation of a prior authorization program based on dose levels appears to have effectively reduced the number of individuals using high doses of buprenorphine + naloxone. While the policy change did not lower the total health care cost of MassHealth members using buprenorphine, decreases in the number of members using high doses appear to have lowered per person expenditures on buprenorphine by a modest amount. Price increases during the study period reduced the overall impact of the new policy on medication savings. An important consideration in assessing the potential for future savings is that generic versions of buprenorphine + naloxone were introduced during the first quarter of 2013. Generics typically offer significant price reductions compared to brand name medications and lead to lower drug spending per person. Additional savings associated with implementation of prior authorization policy changes similar to the one studied here are likely to be lower than those observed in this study.
Total MassHealth expenditures per person increased significantly for all members using buprenorphine + naloxone after July of 2008. Increases for both high dose and other groups, suggests that these costs are likely to have been driven by a general increase in treatment costs rather than the prior authorization policy change.
Significant but short-lived increases in relapses after full implementation of prior authorization requirements in July 2008 among patients who used higher doses in 2007 suggest that switching to lower doses was difficult for some patients. This finding was limited to the 2007–2008 cohort analysis. Use of relapse services did not increase significantly when new buprenorphine users were included in the total population analysis. This suggests that the relapse problem was primarily driven by patients transitioning to lower doses rather than by the dose level itself.
The small increase in the number of high-dose group members ending treatment after full implementation of the policy could be interpreted to mean that implementation of prior authorization caused some members to terminate treatment prematurely. However, lacking information about the reason for treatment ending we cannot confidently conclude that the increased termination rate was unplanned. Treatment plans typically include a schedule for tapering and eventual ending of buprenorphine maintenance. Further study is recommended to better understand the reasons for treatment discontinuation and the long-term impact of unplanned termination on patient outcomes and costs.
Limitations
The nonexperimental nature of this study presents some natural challenges for interpreting the results. Reliance on Medicaid claims data allowed us to include the entire population of MassHealth members receiving buprenorphine treatment and to measure the impact on Medicaid expenditures with reasonable accuracy. However, claims data have somewhat limited diagnostic accuracy compared with studies using structured diagnostic interviews and lack important information about the reasons for terminating treatment. While we believe that using both opioid diagnoses and prescription data allowed us to accurately identify the target population, co-occurring conditions such as mental illness may have been under-identified or, in some cases, inaccurately diagnosed. The absence of a comparison group of buprenorphine patients who were not affected by the prior authorization requirement does not allow us to completely rule out the possibility that other time-related secular effects concurrent with the prior authorization policy's implementation might have contributed to the outcomes observed. Other than small changes in the price of buprenorphine + naloxone, which are accounted for by the time variable in our multivariable statistical model, we could not identify other factors that were likely to have affected our results. Differences in the response of high-dose group members, who were most likely to be affected by the prior authorization requirement, compared to the low dose group increase our confidence that observed changes are due to the policy change rather than to more general changes over time.
Conclusions
Overall, prior authorization policies that are strategically targeted by dose level appear to be successful in reducing the percentage of patients who use higher than recommended doses of buprenorphine + naloxone. However, with the introduction of generic buprenorphine + naloxone these policies are unlikely to save a substantial amount of money. Even carefully designed dosing policy changes may come at the expense of some increase in the risk of relapse for those moving to lower doses. However, these changes appear to be short-lived and might be mitigated in future policy changes by allowing patients on high doses a longer period in which to taper to a lower dose. The question of how dose-related prior authorization policies affect patient retention in treatment should be explored in future studies.
The dose-related prior authorization policy studied in this analysis stands in stark contrast to policies in states such as Arkansas, Illinois, Maine, Mississippi, Montana, Utah, Virginia, and Washington, which impose lifetime caps on buprenorphine treatment or require rigid tapering schedules (Rinaldo and Rinaldo 2013). While our analysis suggests that any cost savings from lowering doses are relatively small, some would argue that reducing high doses may also reduce diversion of buprenorphine, which is reported to be growing rapidly (Johanson et al. 2012; Lofwall and Havens 2012). However, any potential benefits of restrictions in access to buprenorphine or any other form of opioid agonist treatment must be weighed against the potential harm and higher cost that is associated with untreated opioid addiction. Our preliminary conclusion is that dose-related prior authorization policies such as the one implemented in Massachusetts strike a reasonable balance between limiting the use of higher than recommended buprenorphine doses and maintaining access to an effective treatment.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: Supported by National Institute of Drug Abuse grant 5R01DA029741 to Robin Clark. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Data access was granted by the Office of MassHealth and the Bureau of Substance Abuse Services in the Department of Public Health both of which are part of the Massachusetts Executive Office of Health and Human Services.
Disclosures: None.
Disclaimers: None.
Supporting Information
Additional supporting information may be found in the online version of this article:
Author Matrix.
References
- Abouzaid S, Jutkowitz E, Foley KA, Pizzi LT, Kim E. Bates J. Economic Impact of Prior Authorization Policies for Atypical Antipsychotics in the Treatment of Schizophrenia. Population Health Management. 2010;13(5):247–54. doi: 10.1089/pop.2009.0063. and. “. ”. [DOI] [PubMed] [Google Scholar]
- Barnett PG, Zaric GS. Brandeau ML. The Cost–Effectiveness of Buprenorphine Maintenance Therapy for Opiate Addiction in the United States. Addiction. 2001;96(9):1267–78. doi: 10.1046/j.1360-0443.2001.96912676.x. and. “. ”. [DOI] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) Series 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. DHHS Publication No. (SMA) 04-3939. [PubMed] [Google Scholar]
- Clark RE, Samnaliev M, Baxter JD. Leung GY. The Evidence Doesn't Justify Steps by State Medicaid Programs to Restrict Opioid Addiction Treatment with Buprenorphine. Health Affairs. 2011;30(8):1425–33. doi: 10.1377/hlthaff.2010.0532. and. “. ”. [DOI] [PubMed] [Google Scholar]
- Ducharme LJ. Abraham AJ. State Policy Influence on the Early Diffusion of Buprenorphine in Community Treatment Programs. Substance Abuse Treatment, Prevention, and Policy. 2008;3(1):17. doi: 10.1186/1747-597X-3-17. and. “. ”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Drug Administration. 2010. “ NDA 22-140, Approved Risk Evaluation and Mitigation Strategies (REMS) for Suboxone ” [accessed on April 23, 2013]. Available at http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM227949.pdf.
- Fischer MA, Schneeweiss S, Avorn J. Solomon DH. Medicaid Prior-Authorization Programs and the Use of Cyclooxygenase-2 Inhibitors. New England Journal of Medicine. 2004;351(21):2187–94. doi: 10.1056/NEJMsa042770. and. “. ”. [DOI] [PubMed] [Google Scholar]
- Johanson C, Arfken CL, di Menza S. Schuster CR. Diversion and Abuse of Buprenorphine: Findings from National Surveys of Treatment Patients and Physicians. Drug and Alcohol Dependence. 2012;120(1):190–5. doi: 10.1016/j.drugalcdep.2011.07.019. and. “. ”. [DOI] [PubMed] [Google Scholar]
- Kronick R, Gilmer T, Dreyfus T. Lee L. Improving Health-Based Payment for Medicaid Beneficiaries: CDPS. Health Care Financing Review. 2000;21(3):29–64. and. “. ”. [PMC free article] [PubMed] [Google Scholar]
- Law M, Ross-Degnan D. Soumerai S. Effect of Prior Authorization of Second-Generation Antipsychotic Agents on Pharmacy Utilization and Reimbursements. Psychiatric Services. 2008;59(5):540–6. doi: 10.1176/ps.2008.59.5.540. and. “. ”. [DOI] [PubMed] [Google Scholar]
- Lofwall MR. Havens JR. Inability to Access Buprenorphine Treatment as a Risk Factor for Using Diverted Buprenorphine. Drug and Alcohol Dependence. 2012;126(3):379–83. doi: 10.1016/j.drugalcdep.2012.05.025. and. “. ”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CY, Adams AS, Ross-Degnan D, Zhang F, Zhang Y, Salzman C. Soumerai SB. Association between Prior Authorization for Psychiatric Medications and Use of Health Services among Medicaid Patients with Bipolar Disorder. Psychiatric Services. 2011;62(2):186. doi: 10.1176/appi.ps.62.2.186. and. “. ”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C. Davoli M. Buprenorphine Maintenance versus Placebo or Methadone Maintenance for Opioid Dependence. Cochrane Database Systematic Review. 2014;2:1–63. doi: 10.1002/14651858.CD002207.pub4. and. “. ”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J. Davoli M. Methadone Maintenance Therapy versus No Opioid Replacement Therapy for Opioid Dependence. Cochrane Database Systematic Review. 2009;3:1–27. doi: 10.1002/14651858.CD002209.pub2. and. “. ”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morden NE, Zerzan JT, Rue TC, Heagerty PJ, Roughead EE, Soumerai SB. Sullivan SD. Medicaid Prior Authorization and Controlled-release Oxycodone. Medical Care. 2008;46(6):573. doi: 10.1097/MLR.0b013e31816493fb. and. “. ”. [DOI] [PubMed] [Google Scholar]
- Rinaldo SG. Rinaldo DW. Availability without Accessibility? State Medicaid Coverage and Authorization Requirements for Opioid Dependence Medications. San Francisco, CA: The Avis Group; 2013. and. Report prepared for the American Society of Addiction Medicine. [Google Scholar]
- Stein BD, Gordon AJ, Sorbero M, Dick AW, Schuster J. Farmer C. The Impact of Buprenorphine on Treatment of Opioid Dependence in a Medicaid Population: Recent Service Utilization Trends in the Use of Buprenorphine and Methadone. Drug and Alcohol Dependence. 2012;123(1):72–8. doi: 10.1016/j.drugalcdep.2011.10.016. and. “. ”. [DOI] [PubMed] [Google Scholar]
- Wish ED, Artigiani E, Billing A, Hauser W, Hemberg J, Shiplet M. DuPont RL. The Emerging Buprenorphine Epidemic in the United States. Journal of Addictive Diseases. 2012;31(1):3–7. doi: 10.1080/10550887.2011.642757. and. “. ”. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Matrix.


