Abstract
Phospholipid distribution across erythrocyte membrane bilayer is asymmetrical. In normal erythrocytes, entire phosphatidylserine (PS) and most of the phosphatidylethanolamine (PE) is present on the cytoplasmic side of membrane bilayer, whereas phosphatidylcholine (PC) and sphingomyelin (SM) are predominantly present at the outer side of membrane bilayer. The present study was undertaken to determine whether membrane lipid peroxidation has any effect on the distribution of PS, PE, and PC across erythrocyte membrane bilayer in vivo in an animal model. Erythrocyte membrane lipid peroxidation was induced in rats by administering phenylhydrazine, an oxidant drug. Membrane phospholipid organization was determined by using bee venom phospholipase-A2 and indirectly by measuring clotting time on recalcification of normal human platelet-poor plasma in the presence of Russell's viper venom. Phenylhydrazine administration to rats caused significant membrane lipid peroxidation as measured by the accumulation of malonyldialdehyde (MDA), an end product of fatty acid peroxidation, as well as externalization of a significant portion of PS and PE from the inner to the outer side of membrane bilayer in erythrocytes. There was a significant positive correlation (r) between the amount of MDA accumulated in the erythrocytes and the movement of PS (r = 0.92) and PE (r = 0.96) from inner to the outer membrane bilayer and PC (r = 0.81) from outer to the inner membrane bilayer. Erythrocytes of phenylhydrazine-treated rats also showed significantly reduced clotting time. This reduction in clotting time had a significant positive correlation with MDA accumulation (r = 0.92) and PS externalization (r = 0.90). Both the effect of phenylhydrazine on erythrocyte membrane lipid peroxidation and alterations in phospholipid organization and coagulability were blocked when rats were simultaneously administered with vitamin E or C antioxidants.
Full text
PDF
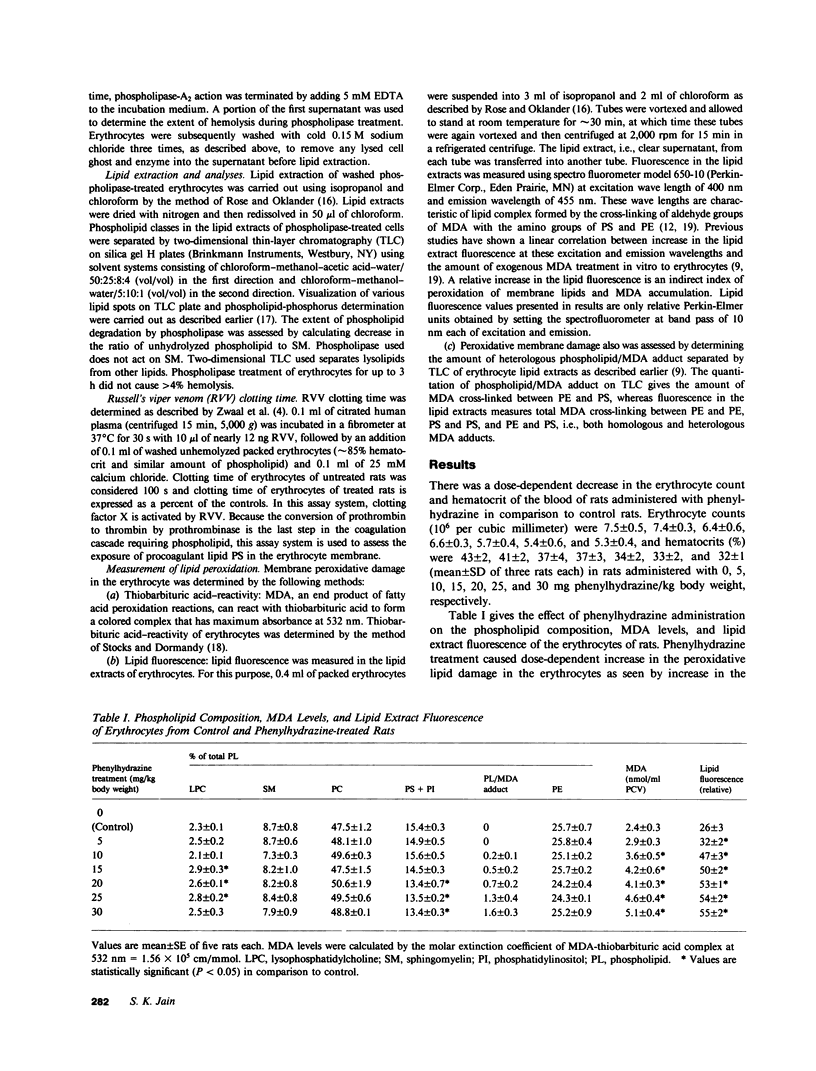
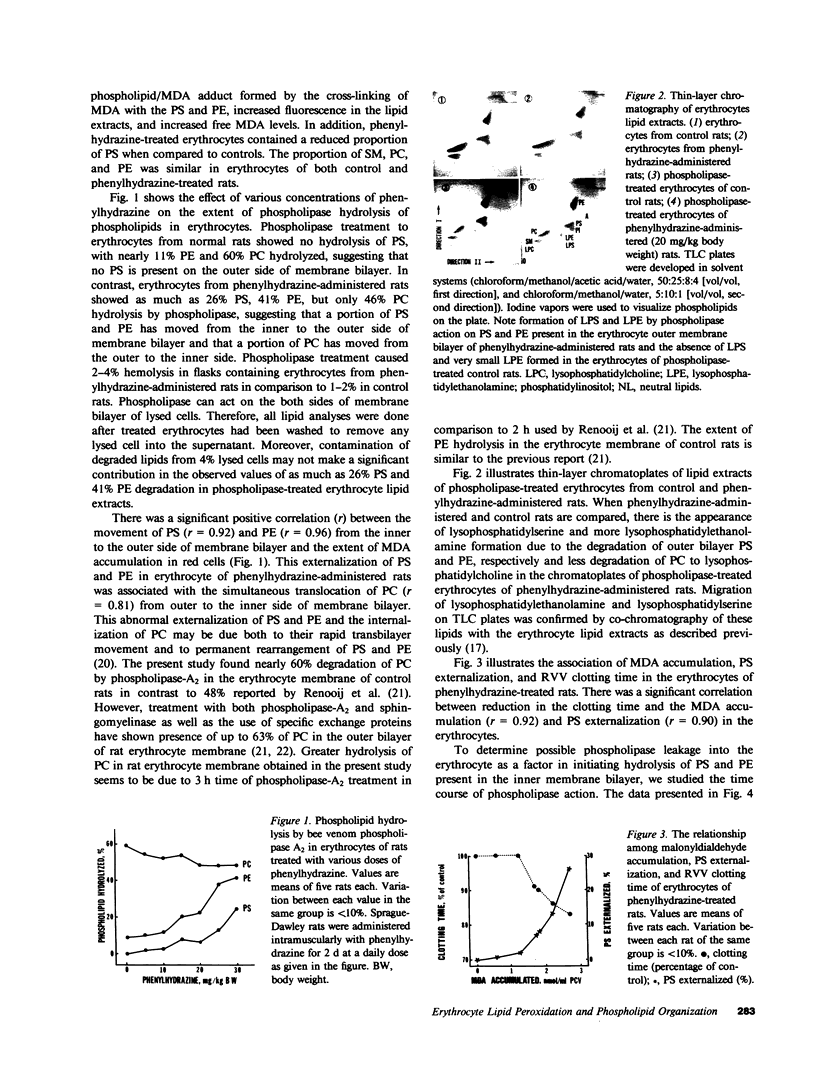

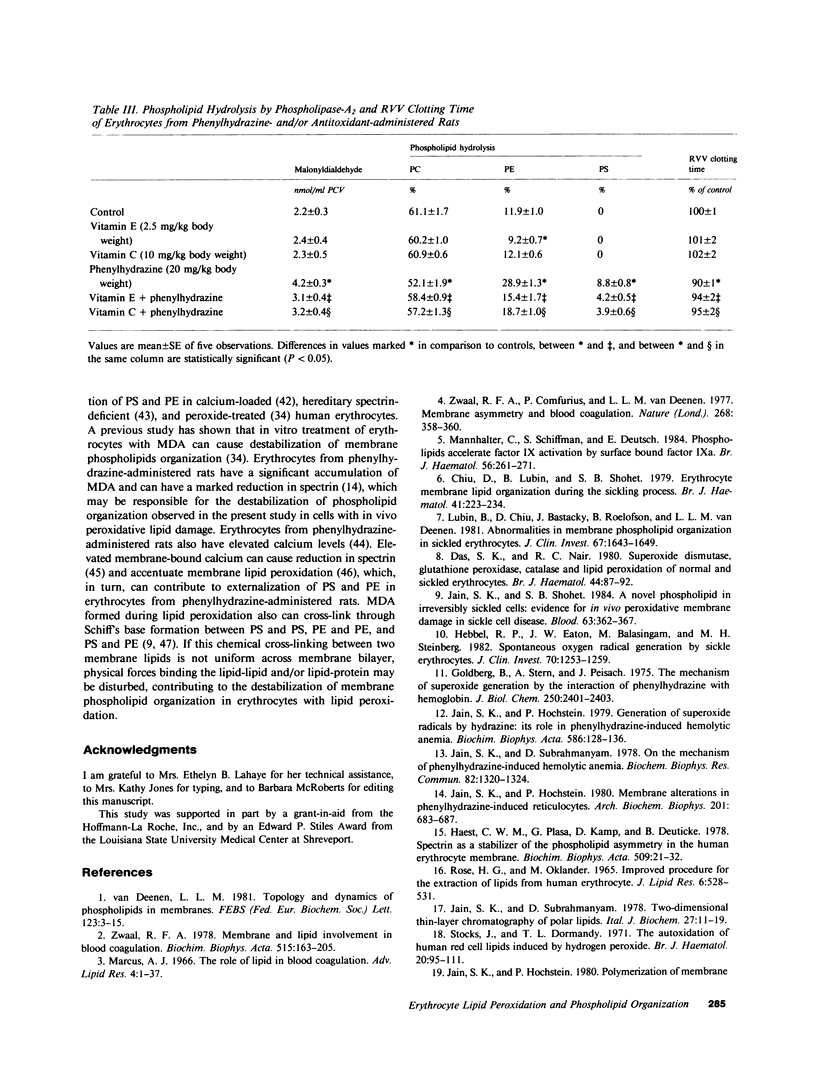

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlack W. R., Tappel A. L. Fluorescent products of phospholipids during lipid peroxidation. Lipids. 1973 Apr;8(4):203–207. doi: 10.1007/BF02544636. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Winterbourn C. C., Rachmilewitz E. A. Activated oxygen and haemolysis. Br J Haematol. 1975 Jul;30(3):259–264. doi: 10.1111/j.1365-2141.1975.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Chiu D., Lubin B., Roelofsen B., van Deenen L. L. Sickled erythrocytes accelerate clotting in vitro: an effect of abnormal membrane lipid asymmetry. Blood. 1981 Aug;58(2):398–401. [PubMed] [Google Scholar]
- Chiu D., Lubin B., Shohet S. B. Erythrocyte membrane lipid reorganization during the sickling process. Br J Haematol. 1979 Feb;41(2):223–234. doi: 10.1111/j.1365-2141.1979.tb05851.x. [DOI] [PubMed] [Google Scholar]
- Crain R. C., Zilversmit D. B. Two nonspecific phospholipid exchange proteins from beef liver. 2. Use in studying the asymmetry and transbilayer movement of phosphatidylcholine, phosphatidylethanolamine, and sphingomyelin in intact rat erythrocytes. Biochemistry. 1980 Apr 1;19(7):1440–1447. doi: 10.1021/bi00548a027. [DOI] [PubMed] [Google Scholar]
- Das S. K., Nair R. C. Superoxide dismutase, glutathione peroxidase, catalase and lipid peroxidation of normal and sickled erythrocytes. Br J Haematol. 1980 Jan;44(1):87–92. doi: 10.1111/j.1365-2141.1980.tb01186.x. [DOI] [PubMed] [Google Scholar]
- Dodge J. T., Cohen G., Kayden H. J., Phillips G. B. Peroxidative hemolysis of red blood cells from patients with abetalipoproteinemia (acanthocytosis). J Clin Invest. 1967 Mar;46(3):357–368. doi: 10.1172/JCI105537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge J. T., Phillips G. B. Composition of phospholipids and of phospholipid fatty acids and aldehydes in human red cells. J Lipid Res. 1967 Nov;8(6):667–675. [PubMed] [Google Scholar]
- Franck P. F., Chiu D. T., Op den Kamp J. A., Lubin B., van Deenen L. L., Roelofsen B. Accelerated transbilayer movement of phosphatidylcholine in sickled erythrocytes. A reversible process. J Biol Chem. 1983 Jul 10;258(13):8436–8442. [PubMed] [Google Scholar]
- Goldberg B., Stern A. The generation of O2-by the interaction of the hemolytic agent, phenylhydrazine, with human hemoglobin. J Biol Chem. 1975 Mar 25;250(6):2401–2403. [PubMed] [Google Scholar]
- Haest C. W., Deuticke B. Possible relationship between membrane proteins and phospholipid asymmetry in the human erythrocyte membrane. Biochim Biophys Acta. 1976 Jun 17;436(2):353–365. doi: 10.1016/0005-2736(76)90199-1. [DOI] [PubMed] [Google Scholar]
- Haest C. W., Plasa G., Kamp D., Deuticke B. Spectrin as a stabilizer of the phospholipid asymmetry in the human erythrocyte membrane. Biochim Biophys Acta. 1978 May 4;509(1):21–32. doi: 10.1016/0005-2736(78)90004-4. [DOI] [PubMed] [Google Scholar]
- Harm W., Fortier N. L., Lutz H. U., Fairbanks G., Snyder L. M. Increased erythrocyte lipid peroxidation in hereditary xerocytosis. Clin Chim Acta. 1979 Dec 3;99(2):121–128. doi: 10.1016/0009-8981(79)90034-2. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Eaton J. W., Balasingam M., Steinberg M. H. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest. 1982 Dec;70(6):1253–1259. doi: 10.1172/JCI110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbel R. P., Yamada O., Moldow C. F., Jacob H. S., White J. G., Eaton J. W. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980 Jan;65(1):154–160. doi: 10.1172/JCI109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R., Rubin R., Wise G., Warren R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood. 1979 Oct;54(4):872–876. [PubMed] [Google Scholar]
- Jacob H. S., Lux S. E., 4th Degradation of membrane phospholipids and thiols in peroxide hemolysis: studies in vitamin E deficiency. Blood. 1968 Oct;32(4):549–568. [PubMed] [Google Scholar]
- Jain S. K., Hochstein P. Membrane alterations in phenylhydrazine-induced reticulocytes. Arch Biochem Biophys. 1980 May;201(2):683–687. doi: 10.1016/0003-9861(80)90560-3. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Hochstein P. Polymerization of membrane components in aging red blood cells. Biochem Biophys Res Commun. 1980 Jan 15;92(1):247–254. doi: 10.1016/0006-291x(80)91545-4. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Mohandas N., Clark M. R., Shohet S. B. The effect of malonyldialdehyde, a product of lipid peroxidation, on the deformability, dehydration and 51Cr-survival of erythrocytes. Br J Haematol. 1983 Feb;53(2):247–255. doi: 10.1111/j.1365-2141.1983.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Shohet S. B. A novel phospholipid in irreversibly sickled cells: evidence for in vivo peroxidative membrane damage in sickle cell disease. Blood. 1984 Feb;63(2):362–367. [PubMed] [Google Scholar]
- Jain S. K., Shohet S. B. Calcium potentiates the peroxidation of erythrocyte membrane lipids. Biochim Biophys Acta. 1981 Mar 20;642(1):46–54. doi: 10.1016/0005-2736(81)90136-x. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Subrahmanyam D. On the mechanism of phenylhydrazine-induced hemolytic anemia. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1320–1324. doi: 10.1016/0006-291x(78)90332-7. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Subrahmanyam D. Studies on lipids of red blood cells in phenylhydrazine-treated albino rats. Indian J Biochem Biophys. 1976 Jun;13(2):155–157. [PubMed] [Google Scholar]
- Jain S. K., Subrahmanyam D. Two dimensional thin-layer chromatography of polar lipids. Ital J Biochem. 1978 Jan-Feb;27(1):11–18. [PubMed] [Google Scholar]
- Jain S. K. The accumulation of malonyldialdehyde, a product of fatty acid peroxidation, can disturb aminophospholipid organization in the membrane bilayer of human erythrocytes. J Biol Chem. 1984 Mar 25;259(6):3391–3394. [PubMed] [Google Scholar]
- Lorand L., Siefring G. E., Jr, Lowe-Krentz L. Enzymatic basis of membrane stiffening in human erythroyctes. Semin Hematol. 1979 Jan;16(1):65–74. [PubMed] [Google Scholar]
- Lubin B., Chiu D., Bastacky J., Roelofsen B., Van Deenen L. L. Abnormalities in membrane phospholipid organization in sickled erythrocytes. J Clin Invest. 1981 Jun;67(6):1643–1649. doi: 10.1172/JCI110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannhalter C., Schiffman S., Deutsch E. Phospholipids accelerate factor IX activation by surface bound factor XIa. Br J Haematol. 1984 Feb;56(2):261–271. doi: 10.1111/j.1365-2141.1984.tb03954.x. [DOI] [PubMed] [Google Scholar]
- Marcus A. J. The role of lipids in blood coagulation. Adv Lipid Res. 1966;4:1–37. [PubMed] [Google Scholar]
- Pfafferott C., Meiselman H. J., Hochstein P. The effect of malonyldialdehyde on erythrocyte deformability. Blood. 1982 Jan;59(1):12–15. [PubMed] [Google Scholar]
- Rachmilewitz E. A., Shohet S. B., Lubin B. H. Lipid membrane peroxidation in beta-thalassemia major. Blood. 1976 Mar;47(3):495–505. [PubMed] [Google Scholar]
- Renooij W., Van Golde L. M., Zwaal R. F., Van Deenen L. L. Topological asymmetry of phospholipid metabolism in rat erythrocyte membranes. Evidence for flip-flop of lecithin. Eur J Biochem. 1976 Jan 2;61(1):53–58. doi: 10.1111/j.1432-1033.1976.tb09996.x. [DOI] [PubMed] [Google Scholar]
- Shalev O., Leida M. N., Hebbel R. P., Jacob H. S., Eaton J. W. Abnormal erythrocyte calcium homeostasis in oxidant-induced hemolytic disease. Blood. 1981 Dec;58(6):1232–1235. [PubMed] [Google Scholar]
- Stocks J., Dormandy T. L. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br J Haematol. 1971 Jan;20(1):95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Papahadjopoulos D. Ca2+-induced fusion of phospholipid vesicles monitored by mixing of aqueous contents. Nature. 1979 Oct 25;281(5733):690–692. doi: 10.1038/281690a0. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Comfurius P., van Deenen L. L. Membrane asymmetry and blood coagulation. Nature. 1977 Jul 28;268(5618):358–360. doi: 10.1038/268358a0. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F. Membrane and lipid involvement in blood coagulation. Biochim Biophys Acta. 1978 Jul 31;515(2):163–205. doi: 10.1016/0304-4157(78)90003-5. [DOI] [PubMed] [Google Scholar]
- van Deenen L. L. Topology and dynamics of phospholipids in membranes. FEBS Lett. 1981 Jan 12;123(1):3–15. doi: 10.1016/0014-5793(81)80007-5. [DOI] [PubMed] [Google Scholar]