Abstract
Objectives
Biomaterials able to mimic the mechanical properties of vocal fold tissue may be particularly useful for furnishing three dimensional microenvironment allowing for in vitro investigation of cell and molecular responses to vibration. Motivated by the dearth of biomaterials available to be used in an in vitro model for vocal fold tissue, we investigated polyether polyurethane (PEU) matrices which are porous, mechanically tuneable biomaterials that are inexpensive and require only standard laboratory equipment for fabrication.
Methods
Rheology, dynamic mechanical analysis and scanning electron microscopy were performed on PEU matrices at 5%, 10% and 20% w/v mass concentrations.
Results
For 5%, 10%, and 20% w/v concentrations, shear storage modulus were 2 kPa, 3.4 kPa, and 6 kPa, respectively with shear loss modulus being 0.2 kPa, 0.38 kPa and 0.62 kPa, respectively. Storage modulus responded to applied frequency as a linear function. Mercury intrusion porosimetry revealed that all three mass concentrations of PEU have similar overall percent porosity, but differ in pore architecture.
Conclusions
20 µm diameter pores are ideal for cell seeding, and range of mechanical properties indicates that the higher mass concentration PEU formulations are best suited for mimicking the viscoelastic properties of vocal fold tissue for in vitro research.
Keywords: cellular therapy, vocal folds, mechanomimetic, Tecoflex, elasticity, rheology, polyether polyurethane
Introduction
Matrix stiffness is a tissue engineering parameter that can be exploited to direct tissue development, cell differentiation or encourage regeneration[1]. Cells are physically engaged in bi-directional communication with their mechanoenvironment via transcellular adhesions and acto-myosin contractility[2, 3, 4]. As such, it is critical that the mechanical and morphological properties of biomaterials used to examine in vitro behavior be characterized and reported. Polyether polyurethanes (PEUs) are commercially available medical-grade polyurethanes which have demonstrated promise as synthetic matrices for tissue engineering of elastic tissue for both in vitro an in vivo needs[5–8]. The objective of this work is to characterize the morphological and mechanical properties of a commercially available PEU of varying porosities to determine the best match to vocal fold mechanical properties. This would allow for a 3D cellular environment that could be utilized for in vitro characterization of vocal fold cell therapeutics. Presently, such characterization and matching does not exist, limiting exploitation of this material for in vitro tissue engineering research specific to the human vocal fold. It should be highlighted that the ideal PEU would not be implanted or used in vivo for vocal fold tissue regeneration but rather provide a 3D environment that candidate therapeutics would be seeded and vibrated in a bioreactor to investigate the effects of stress, strain and vibration[9].
Biomaterial mechanical properties, such as elasticity and yield strength, help determine candidacy for tissue mimics. Naturally, substrates with a higher elastic modulus are typically used in bioreactors for tissues with a stiffer extracellular matrix (ECM), such as bone or cartilage, while substrates with a lower elastic modulus are used in more flexible tissues such as the vocal fold. The protein content of the ECM, especially collagen and elastin, as well as it’s glycosaminoglycan content, dictate the ECM mechanical properties[10, 11]. Tissues with high elastin concentration have a lower elastic modulus, while tissues with high collagen content are stiffer. ECM viscosity is heavily influenced by the degree of glycosaminoglycans present. In order to achieve the desired mechanical properties, synthetic substrates are typically formed using tunable polymers. The mechanical properties of vocal fold tissue have been characterized, as well as the ECM content.[12, 13] Synthetic polymer matrices for stiff tissues typically contain a carbon backbone of similar repeating side chains, and have been well characterized in the literature[14]. For elastic tissues, such as the vocal fold, polyurethane block copolymers have been used[15]. In addition to polyurethanes, polyethylene glycol (PEG) microparticles have also been investigated as space filling hydrogels for soft tissue applications[16]. One formulation of the PEG microparticles was injected into canine vocal folds, with promising results[17]. However, the PEG microparticles are not a continuous scaffold, and inherently lack the elastomeric properties of polyurethanes.
Polyurethanes (PEU) have several unique properties due to the alternating segments comprising their copolymer backbone. All PEU contain a soft segment and a hard segment, which alternate down the length of the polymer chain. Hard segments often crystallize, creating a rigid section, while the soft segments usually remain amorphous. This alternating phase separation imparts elastomeric properties to PEU that are typically high tensile and tear strength, and long elongation capability. Many types of PEU also have excellent biocompatibility profiles[7, 18, 19], and as such several are commercially available for medical grade applications[20]. The elastomeric properties inherent to PEU makes them an ideal candidate for reproducing the natural viscoelastic state of the vocal fold lamina propria for in vitro study. Additionally, the pliable copolymer backbone readily lends itself to the formation of an interconnected matrix for cell seeding.
In this study, parallel plate rheology, dynamic mechanical analysis (DMA), and mercury intrusion porosimetry were used to report the physical and mechanical properties of porous PEU matrices of various mass concentrations in order to optimize a material with properties similar to that of the human vocal fold ECM. Further, the lowest and highest mass concentration limits allowing for adequate formulation necessary for the material to be structurally viable were determined. Finally, scanning electron microscopy was used to examine whether the matrices would be porous enough to allow cell engraftment and chemical diffusion. Taken together this data allows for the formulation of a preferred biomaterial for utilization in an in vitro bioreactor for the study of cellular therapeutic candidates for vocal fold tissue engineering.
1. Materials and Methods
1.1 Porous scaffold synthesis
Three different mass concentrations (5% w/v, 10% w/v, and 20% w/v) of Tecoflex® SG-80A (Thermedics, Wickliffe, OH) beads were desiccated using lyophilization for 24 hours, after which they were dissolved in DMAC(39.1 ml, Fisher Scientific, Pittsburgh, PA) for 24 hours. Temperature of the solution was increased to 60°C and added to one side of a 2:1 dual component adhesive cartridge (MixPAC, Germantown, WI). Pluronic 10R5 (18.95ml, Sigma-Aldrich, St Louis, MO) was added to the other side of the adhesive cartridge. Adhesive cartridge was then allowed to remain heated for roughly 5 minutes. A helical static mixer was attached to the dispensing end of the cartridge and a handheld dual component dispensing gun (MixPAC, Germantown, WI) was used to dispense the solution into custom Delrin molds (94 mm long×80mm wide×3.175mm deep). Use of the helical mixer and dual component cartridge thoroughly mixed the dissolved polyurethane and the Pluronic 10R5 in a 2:1 vol/vol ratio. Polymer-containing molds were placed in a 70% ethanol/dry ice bath at −40°C for approximately 20 minutes. Molds were then placed in de-ionized water for 48 hours with three deionized water changes. Design of the molds was such that the all sides of the polymer sheet were soaked equally. For DMA and SEM analysis, each PEU sheet was then cut into 25 mm long×10 mm wide×2 mm deep strips and dried by a 24-hour lyophilization. For rheological analysis, each PEU sheet was cut into a 30 mm radius×2 mm deep disc and dried by a 24-hour lyophilization.
PEU concentration limits were established by attempting to formulate the polymers ranging from 1 gram Tecoflex® SG-80A beads/39 mL DMAC (2.5% w/v) to 12 grams Tecoflex® SG-80A beads/39 mL DMAC (30% w/v). On three attempts, the material would not consistently form a porous structure at the 2.5% w/v concentration so the lower limit was established at the 5% w/v concentration. On three attempts, the Pluronic 10R5 would not adequately mix with the Tecoflex/DMAC solution at concentrations higher than 20% w/v, so the upper limit was established at 20% w/v. Therefore, for this investigation porous Tecoflex® (PEU) strips were formulated at 3 different mass concentrations: 5% w/v, 10% w/v, and 20% w/v.
1.2 SEM microscopy
Five% w/v, 10% w/v, and 20% w/v PEU formulation samples were ion beam coated with platinum at a thickness of 2.5 nm. Strips were trimmed down to a 4mm×8mm dimension and affixed to a copper planchet with the use of double sided carbon adhesive tabs. Strips were imaged with a Hitachi S900 field emission scanning electron microscope at an accelerating voltage of 10kV and visualized with scanning electron microscopy to characterize the substrate morphology.
1.3 Mercury intrusion porosimetry
Mercury intrusion porosimetry was used to evaluate three samples of each PEU concentration (Particle Technology Labs, Downers Grove, IL). Briefly, the sample is placed into a penetrometer, which consists of a capillary stem with a glass bulb at the end. The penetrometer, containing the sample in the glass bulb, is filled with mercury at 0 psi. Pressure is then increased, causing the mercury to intrude into the pores within the sample. The volume of mercury that intrudes into the sample is recorded at each increasing pressure point. Pore size can be calculated from the pressure needed to force that volume of mercury into a pore. Data for each condition (5% w/v, 10% w/v, and 20% w/v) was compared using ANOVA and Tukey’s HSD for post-hoc analysis. Findings were considered significant at P < .05.
1.4 Dynamic mechanical testing
An RSA III Dynamic Mechanical Analyzer was used to obtain storage and loss moduli for all PEU samples. Briefly, each sample was placed within a rectangular tension clamp at room temperature and pressure. Individual sample dimensions were input to the system, and a frequency sweep analysis was performed in order to determine the linear elastic domain. Strain sweep analysis was then performed at 1 Hz, 10 Hz, and 35 Hz frequencies. Three samples were tested for each PEU concentration, and each sample was tested three times. A two way ANOVA was performed comparing storage modulus of all three concentrations, as well as the response of all concentrations to frequency change. P values < .05 were considered significant.
1.5 Rheological analysis
A Gemini stress-control rheometer (Malvern Instruments, UK) was used for all rheologic testing of PEU samples. A total of 3 samples were evaluated at each PEU formulation. All samples were immersed in cell culture medium and stored at 4°C for 3 – 5 days. At time of testing, each wetted sample was placed between stainless steel stationary base and upper parallel plate. Complete sample contact and optimal sample adhesion was achieved by using 220-grit wet-dry sandpaper on the stainless steel components and by compressing each sample. Final sample compressions were 7–15% with distance from base to upper plate ranging from 1.15 – 3.22 mm. As per standard rheology protocol, a 1 Hz amplitude sweep (20 stress values 1 – 100 Pa administered to each sample) was performed. Using the specific linear strain value (.0014 – 0.0185) for a given sample, viscoelastic measurements were then made at 27 frequencies logarithmically spaced between 1 – 231Hz. A two way ANOVA was performed comparing shear storage modulus and shear loss modulus of all three concentrations, as well as the response of all concentrations to frequency change. P values less than .05 were considered significant.
2. Results
2.1 SEM microscopy
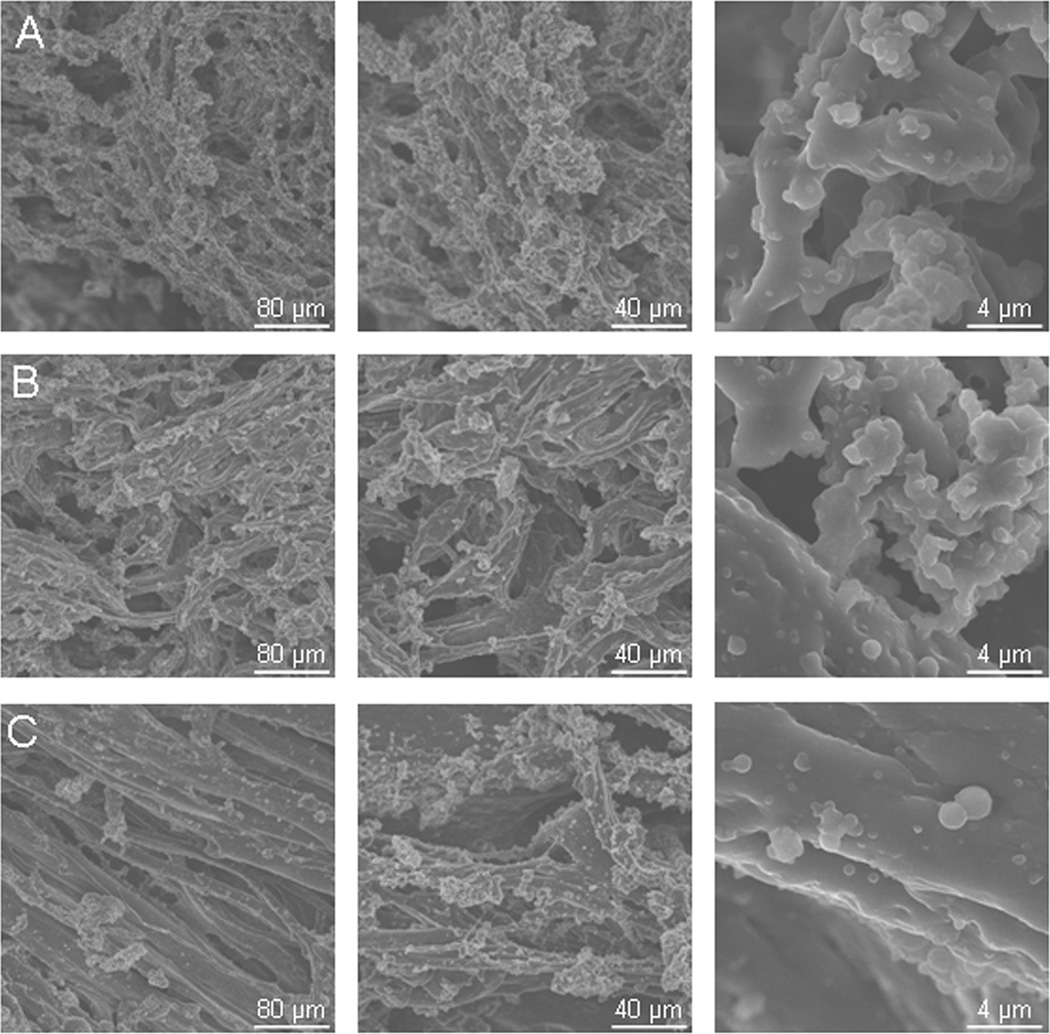
SEM microscopy revealed that all three PEU concentrations are porous substrates, with ample room for cell attachment. Two basic pore sizes are present in each formulation; 2–20 µm micropores and larger, 40+ µm pores. The 5% w/v substrates appeared to be the most porous and have the highest void volume, while the 20% w/v substrates appeared the least porous (Figure 1). In addition, the size of the PEU fibers appeared to increase with increasing concentration. This is especially evident in the 20% w/v substrate image, where randomly oriented fibers can be observed.
Figure 1.
Scanning electron microscopy images. Magnification is 250x in the first column, 500x in the second column, and 5000x in the third column. Representative images of A. 5% w/v PEU formulation. B. 10% w/v PEU formulation. C. 20% w/v PEU formulation
2.2 Porosimetry
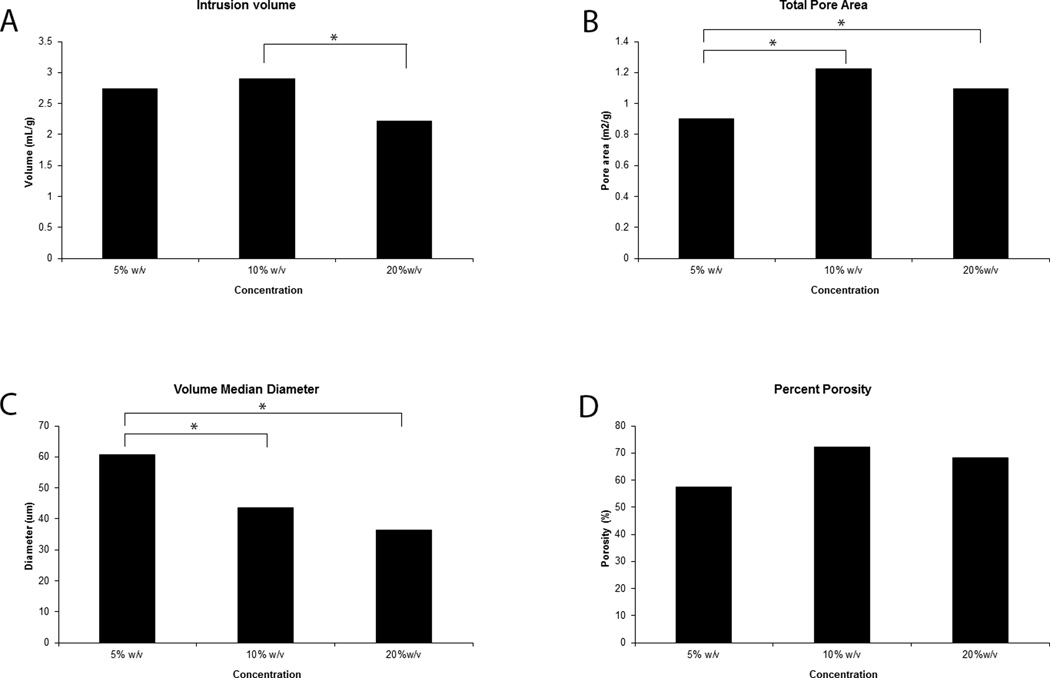
Porosimetry analysis performed on samples of the 5% w/v, 10 % w/v, and 20% w/v concentrations provided details regarding the extent of the porous network of the scaffolds. The 5% w/v samples had an average intrusions volume of 2.74 mL/g, whereas the 10% w/v and 20% w/v samples had average volumes of 2.9 mL/g and 2.22 mL/g, respectively (Figure 2). A significant difference was only observed between the 10% w/v and 20% w/v samples (p = .0254).
Figure 2.
5% w/v to 20% w/v increase in PEU mass concentration generated matrices with differing intrusion volumes (A), total pore area (B), pore diameter (C), and overall porosity (D). The 5% w/v sample had the smallest total pore area, and largest median pore diameter, but the overall porosity did not differ between samples
The 5% w/v samples had the smallest total pore area, with an average value of 0.903 m2/g. The 10% w/v and 20% w/v samples had a total pore area of 1.22 m2/g and 1.1 m2/g. Total pore area of the 5% w/v samples was smaller than either the 10% w/v sample (p = .0007) or the 20% w/v sample (p = .0091). No significant difference was observed between the 10% w/v and 20% w/v samples (p = .0554).
In addition to total pore area, pore median diameter was also measured for each sample. The 5% w/v, 10% w/v, and 20% w/v samples had 60.69 µm, 43.57 µm, and 36.25 µm diameter pores, respectively (Figure 2). Significant difference in pores diameter was observed between the 5% w/v and 10% w/v samples (p =.0368), as well as the 5% w/v and 10% w/v samples (p = .0076). Median pore diameter was not significantly different between the 10% w/v and 20% w/v samples.
Bulk and apparent densities were obtained for each sample, and used to compute the porosity of the material. The average porosities for the 5% w/v, 10% w/v, and 20% w/v samples were 57.43%, 72.07%, and 68.27%, respectively (Figure 2). Statistical analysis shows no significant difference between 5% w/v and 10% w/v PEU (p = .0916), 5% w/v and 20% w/v PEU (p = .2149), or 10% w/v and 20% w/v PEU(p = .7878).
2.3 DMA analysis
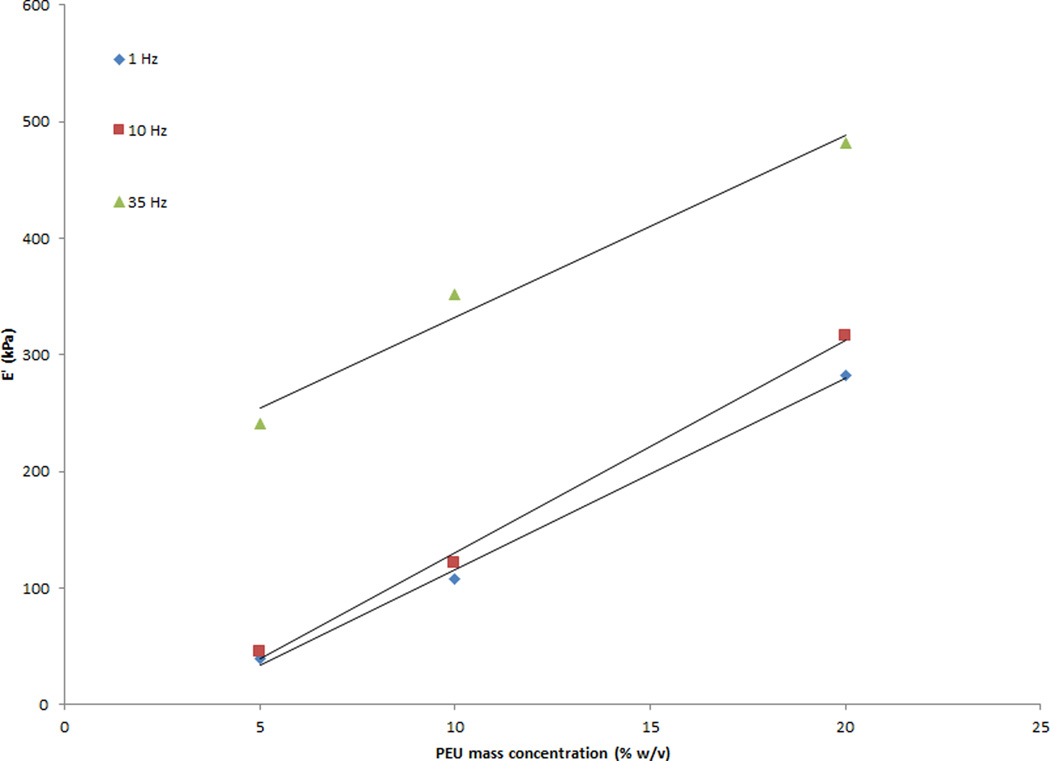
DMA data indicated a linear relationship between PEU mass concentration and the storage modulus. A 5% w/v to 20% w/v increase in mass concentration correlated with a roughly 6 fold increase in storage modulus (E’) values (Figure 3). E’ values for the 5% w/v concentration ranged from 39.1 kPa to 241.4 kPa over a 35 Hz frequency range. Testing at frequencies higher than 35 Hz was attempted, but limitations due to the PEU dimensions and the Dynamic Mechanical Analyzer resulted in unusable data. 10% w/v and 20% w/v concentrations ranged from 107–352 kPa and 312–526.5 kPa, respectively, over the same frequency range. Variability between samples was small. Coefficients of variation for E’ were 1–16% for the 5% w/v samples, 1.1–4.6% for the 10% w/v samples, and 1–2.6% for the 20% w/v samples. Statistical differences were found for storage moduli between 5% w/v and 10% w/v concentrations (p = .02), 5% w/v and 20% w/v concentrations (p < .0001), and 10% w/v and 20% w/v concentrations (p < .0001). In addition, all three concentrations responded to increased frequency in the same manner, as indicated by the slopes of the best fit line. No significant differences were found for the slopes of the 5% w/v and 10% w/v concentrations (p = .3531), the 5% w/v and 20% w/v concentrations (p = .9189), or the 10% w/v and 20% w/v gram concentrations (p = .4028).
Figure 3.
5% w/v to 20% w/v increase in PEU mass concentration generated a roughly 6 fold increase in storage modulus values. Three samples were tested for each concentration at 1 to 50 Hz sinusoidal frequency
2.4 Rheology
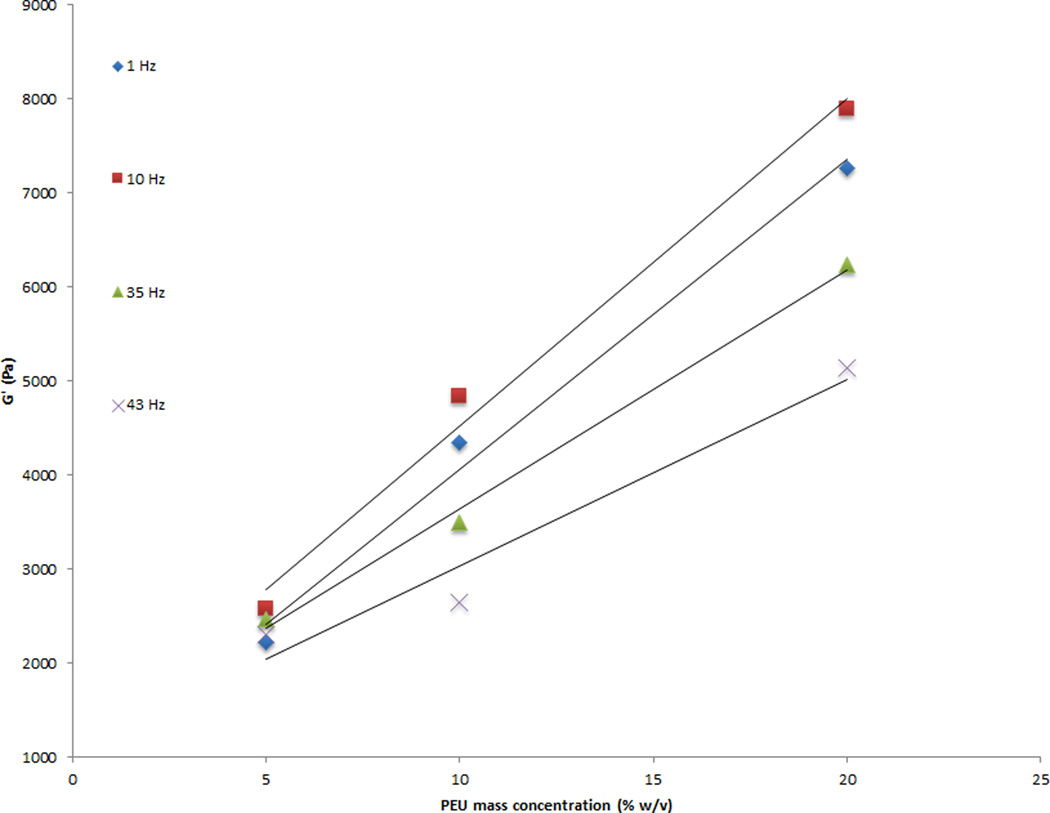
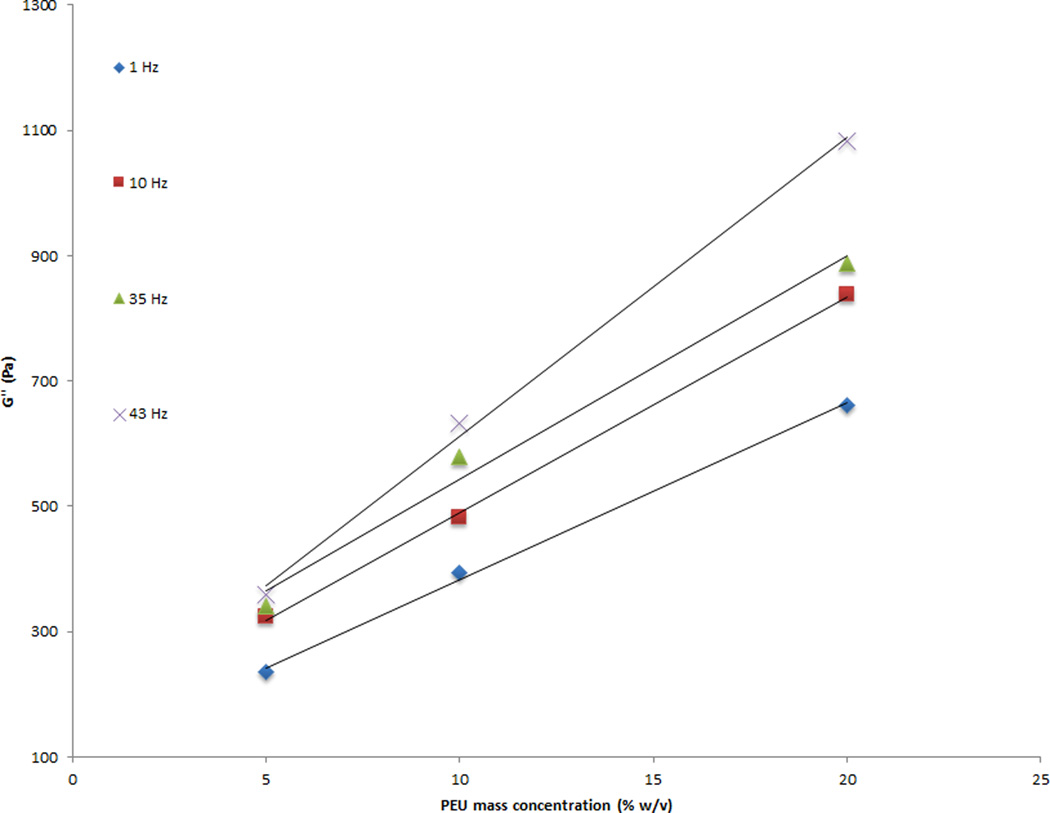
Rheology data indicated a linear relationship between the PEU concentration and the shear moduli. A four fold increase in PEU concentration, 5% w/v to 20% w/v, generated a 2.5-fold increase in shear storage modulus (G') values (Figure 4). The 5%, 10%, and 20% w/v samples generated G' values of 2 kPa, 3.4 kPa, and 5 kPa, respectively. A 3 fold increase in shear loss modulus (G”) was observed between the 5% w/v and 20% w/v samples (Figure 5). The 5%, 10%, and 20% w/v samples generated G” values of .2 kPa, .38 kPa, and .62 kPa, respectively. The loss tangent was unaffected. Variability between samples was small. Coefficients of variation for G' were 2–7% for the 5% w/v samples, 9 – 78% for the 10% w/v samples, and 41–76% for the 20% w/v samples. Coefficients of variation for G'' were 4–23% for the 5% w/v samples, 4–24% for the 10% w/v samples, and 17–37% for the 20% w/v samples. No significant differences were found between 5% and 10% w/v concentrations (p = .1496) and 10% and 20% w/v concentrations (p < .0838), but 5% and 20% w/v concentrations were statistically different. (p = .0098). In addition, the elastic shear moduli of the three concentrations differed in their response to increased frequency, as indicated by the slopes of the best fit line. A significant difference was found in the slopes of the 5% w/v and 10% w/v concentrations (p = .0058) and 5% w/v and 20% w/v concentrations (p = .0021), whereas no difference was observed for the 10% w/v and 20% w/v concentrations (p = .3641). ANOVA demonstrated that the shear loss modulus was not different between 5% and 10% w/v concentrations (p = .2578), but there were significant differences measured between the 5% and 20% w/v concentrations (p < .0095) and 10% and 20% w/v concentrations (p = .0465). In addition, the shear loss moduli of all three concentrations differed in their response to increased frequency, as indicated by the slopes of the best fit line. A significant difference was observed in the slopes of the 5% and 10% w/v concentrations (p = .0042), the 5% and 20% w/v concentrations (p = .0001), and the 10% and 20% w/v concentrations (p = .0056).
Figure 4.
A four fold increase in PEU concentration, 5% w/v to 20% w/v, generated a 2.5-fold increase in shear storage modulus values. Three samples were tested for each concentration at 1 to 65 Hz sinusoidal frequency
Figure 5.
A three fold increase in shear loss modulus was observed between 5% w/v and 20% w/v samples. Three samples were tested for each concentration at 1 to 65 Hz sinusoidal frequency
3. Discussion
In order to create a PEU polymer foam for in vitro use, a variation of the hydrocarbon templating technique[6] was used in combination with polymer phase separation. In hydrocarbon templating, a water-soluble porogen is used to create a macroporous polymer foam[21]. Traditionally, this has been performed with hydrocarbon chains, giving the technique its name. For our purposes, the copolymer poly(propylene glycol)-block-poly(ethylene glycol)-block-poly(propylene glycol), also known as Pluronic 10R5, was used as the porogen. At temperatures ranging from 40–50°C, this trublock polymer forms micelles, with the propylene glycol blocks at the center. At lower temperatures, the micelle collapses, and all blocks (PPG and PEO) become water soluble. SEM images show that the pores formed within the PEU varied in size. Porosimetry data confirmed this finding, with pores ranging from .3 µm to 218 µm in diameter. Smaller pores, up to 20 µm in diameter, were most likely formed when the polymer solution was quenched in the −40 °C dry ice/ethanol bath[22]. Larger pores, typically greater than 20 µm in diameter, were irregularly shaped and most likely formed from the change in Pluronic 10R5 micelle organization due to change in temperature[22]. Pores of this size allow for cell seeding with fibroblasts, the primary cell type responsible for vocal fold ECM architecture, as these are roughly 20 µm in diameter.
Mercury intrusion porosimtery data indicated no difference in overall percent porosity between mass concentrations, but did identify a difference in pore structure. Five percent w/v concentration had a significantly lower total pore area than either the 10% or 20% w/v samples. This finding, combined with the lack of difference in overall percent porosity between mass concentrations, may indicate that 5% w/v samples have a porous network with low surface area to volume ratio. This conclusion is also supported by the difference observed in median pore diameter across concentrations. Median pore diameter was greatest in the 5% w/v concentration, with statistically significant higher measurements than both the 10% and 20% w/v concentrations. The porous network of the 5% w/v samples most likely consists of large diameter pores with either limited interconnectivity or shallow depth compared to the 10% or 20% w/v samples. The porous network of the 10% and 20% w/v samples is most likely very similar, as there was no significant difference between total pore area, median pore diameter, or percent porosity across these concentrations. Pore diameter and morphology could be further regulated through the choice of polymer porogen.
As with most biomaterials used for tissue engineering investigation, cell attachment and cell interaction must be considered. Chemical groups present in the PEU polymer are not typically associated with cell attachment, and as such attachment will have to be mediated through other means. Wettability studies indicate that materials made with Tecoflex® have a contact angle of roughly 95.2°[25], making them slightly hydrophobic. This hydrophobicity will readily lend itself to adsorbing proteins such as fibronectin[26], which cells bind with great affinity. Adsorption of poly-L-lysine can also be used to facilitate non-specific cell attachment, or as an intermediate step for the addition of synthetic ligands such as RGD.[25] Similar methods can be used to add mono- or polysaccharides to the polymer surface, facilitating non-specific cell attachment in vitro.[28] Other methods, such as air plasma treatment or radio frequency glow discharge, have also been shown to facilitate cell attachment to polyurethane surfaces[29, 30]. While it may be possible to chemically add cell attachment motifs into the backbone of the PEU, such an addition may alter the bulk mechanical properties of the material.
DMA analysis demonstrates a linear relationship between the concentration of PEU and the observed storage modulus for all tested frequencies. The storage modulus measures the stored energy in a viscoelastic material, and is analogous to the elastic modulus of linearly elastic materials. Data indicates that the storage modulus can be calculated by the equation given in Table 1. This equation can be used to tailor the PEU storage modulus to reflect the mechanical properties of various vocal fold disease states[31]. It should be noted that high frequency axial strain causes PEU to become less flexible. In addition, the frequency ranged tested using DMA (1–35 Hz) is suitable for axial stress in vocal fold bioreactors, as shear stress is more important in human vocal fold models. Rheology data are extremely useful when designing biomaterials to be used for vocal fold application. The biomaterial must have the correct rheological properties in order to accurately withstand and deform under the shear and mechanical forces seen in human vocal. Our data indicate that the G’ has a wide range, from 2 kPa to 8 kPa depending on the PEU concentration and frequency. This range of values is also reflected in the G’’, which ranges from 200 Pa to 1 kPa. The wide range of values is particularly relevant for vocal fold scar research, as scarred lamina propria is know to have significantly higher elastic shear modulus and viscosity measurements[32]. Human vocal fold lamina propria G' can range from roughly 10 Pa to 1 kPa, and G" values typically range from 10 to 150 Pa[33, 34].
Table 1.
| Storage modulus = m* (PEU concentration) + b | ||
|---|---|---|
| Frequency | m | b |
| 1 | 16.41 | −48.41 |
| 10 | 18.27 | −52.29 |
| 35 | 15.62 | 176.35 |
One significant drawback of using a porogen is that the porosity and mechanical properties cannot be independently altered. DMA and rheology results indicate that as the porosity increases, the structural integrity decreases. This trend has also been noted for similar polymer foams[35], and occurs due to the decreased structural stability associated with an increase in porosity. Another limitation of our results pertains to the thickness of the PEU strips and discs. Using the described fabrication protocol, 2mm thickness was the smallest dimension that could be reproducibly made. Thinner substrates may have allowed for a higher upper frequency for DMA and rheology measurements. Finally, one of the 20% w/v samples used for the rheological analysis produced shear storage and shear loss moduli data below that observed for similar 20% w/v samples. This one sample most likely skewed the 20% w/v rheology data set, leading to the lack of significant difference observed between the slopes of the shear storage modulus between the 10% and 20% w/v samples. The lower values observed with this sample could be due to increased batch variability with the 20% w/v samples. As the 20% w/v concentration is the upper limit of PEU attainable with this experimental setup, the matrix structure may be less predictable than the other samples. The pore architecture, including percent porosity and pore diameter, may vary considerably between samples. This would have implications not only for mechanical properties, but also for cell seeding applications.
Conclusions
We have developed a simple, low cost method for controlling the mechanical properties and porosity of polyether polyurethane foams for use in vocal fold tissue regeneration. Scanning electron microscopy reveals the polyurethane foams have both micropores, for diffusion of nutrients, and macropores, for cell seeding. We have also outlined the relationship between PEU concentration and storage modulus, shear storage modulus, and shear loss modulus. Finally, DMA and rheology data both indicate that the chemical preparation of samples is highly reproducible. Using these reference data and fabrication parameters, future researchers will be able to tailor these properties to make elastic scaffolds for in vitro vocal fold tissue engineering research. Further the range of mechanical properties displayed by the PEU may be conducive for modeling various vocal fold diseases, such as scarred tissue or different states of mucosal hydration.
Acknowledgements
The authors would like to acknowledge the National Institute of Deafness and Other Communication Disorders – R01 DC009600 and the David Bradley Fund for supporting this project. We thank Glen E. Leverson, PhD for assistance with statistical analysis and Tim Osswald, PhD for his knowledge and insight concerning polymer processing. We also extend our gratitude to Joseph Heintz, BS and Ralph M. Albrecht, PhD at the Biological & Bio-materials Preparation, Imaging, and Characterization Laboratory at UW-Madison for their expertise and assistance with the Hitachi S900 field emission scanning electron microscope.
References
- 1.Discher D, Janmey P, Wang Y. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 2.Even-Ram S, Artym V, Yamada KM. Matrix control of stem cell fate. Cell. 2006;126:645–647. doi: 10.1016/j.cell.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Pelham R, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JC, Brokopp C, Underwood CJ, Tresco PA. The effect of bioreactor induced vibrational stimulation on extracellular matrix production from human derived fibroblasts. Biomaterials. 2009;30:327–335. doi: 10.1016/j.biomaterials.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Titze IR, Hitchcock RW, Broadhead K, et al. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. J Biomech. 2004;37:1521–1529. doi: 10.1016/j.jbiomech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Sakas D, Charnvises K, Borges L, Zervas N. Biologically inert synthetic dural substitutes - appraisal of a medical-grade aliphatic polyurethane and a polysiloxane-carbonate block copolymer. J Neurosurg. 1990;73:936–941. doi: 10.3171/jns.1990.73.6.0936. [DOI] [PubMed] [Google Scholar]
- 8.Mulder M, Hitchcock R, Tresco P. Skeletal myogenesis on elastomeric substrates: implications for tissue engineering. J Biomater Sci Polym Ed. 1998;9:731–748. doi: 10.1163/156856298x00118. [DOI] [PubMed] [Google Scholar]
- 9.Gaston J, Rios BQ, Bartlett R, Berchtold C, Thibeault SL. The Response of Vocal Fold Fibroblasts and Mesenchymal Stromal Cells to Vibration. Plos One. 2012:7. doi: 10.1371/journal.pone.0030965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 11.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular biology of the cell. New York: Garland; 1994. pp. 971–995. [Google Scholar]
- 12.Hahn MS, Kobler JB, Starcher BC, Zeitels SM, Langer R. Quantitative and comparative studies of the vocal fold extracellular matrix - I: Elastic fibers and hyaluronic acid. Ann Otol Rhinol Laryngol. 2006;115:156–164. doi: 10.1177/000348940611500213. [DOI] [PubMed] [Google Scholar]
- 13.Hahn MS, Kobler JB, Zeitels SM, Langer R. Quantitative and comparative studies of the vocal fold extracellular matrix II: Collagen. Ann Otol Rhinol Laryngol. 2006;115:225–232. doi: 10.1177/000348940611500311. [DOI] [PubMed] [Google Scholar]
- 14.Han D, Hubbell J. Synthesis of polymer network scaffolds from L-lactide and poly(ethylene glycol) and their interaction with cells RID A-9266-2008. Macromolecules. 1997;30:6077–6083. [Google Scholar]
- 15.Xue L, Greisler H. Biomaterials in the development and future of vascular grafts. J Vasc Surg. 2003;37:472–480. doi: 10.1067/mva.2003.88. [DOI] [PubMed] [Google Scholar]
- 16.Chan KM, Li RH, Chapman JW, Trac EM, Kobler JB, Zeitels SM, et al. Functionalizable hydrogel microparticles of tunable size and stiffness for soft-tissue filler applications. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karajanagi SS, Lopez-Guerra G, Park H, Kobler JB, Galindo M, Aanestad J, et al. Assessment of Canine Vocal Fold Function After Injection of a New Biomaterial Designed to Treat Phonatory Mucosal Scarring. Annals of Otology Rhinology and Laryngology. 2011;120:175–184. doi: 10.1177/000348941112000306. [DOI] [PubMed] [Google Scholar]
- 18.Laschke MW, Strohe A, Scheuer C, et al. In vivo biocompatibility and vascularization of biodegradable porous polyurethane scaffolds for tissue engineering. Acta Biomater. 2009;5:1991–2001. doi: 10.1016/j.actbio.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Thomas V, Jayabalan M. A new generation of high flex life polyurethane urea for polymer heart valve-Studies on in vivo biocompatibility and biodurability. J Biomed Mater Res A. 2009;89A:192–205. doi: 10.1002/jbm.a.31937. [DOI] [PubMed] [Google Scholar]
- 20.Mishkin GJ. Compatibility of electrolytically produced sodium hypochlorite solutions on long-term implanted dialysis catheters. Contrib Nephrol. 2007;154:72–83. doi: 10.1159/000096815. [DOI] [PubMed] [Google Scholar]
- 21.Shastri V, Martin I, Langer R. Macroporous polymer foams by hydrocarbon templating. Proc Natl Acad Sci U S A. 2000;97:1970–1975. doi: 10.1073/pnas.97.5.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nam Y, Park T. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J Biomed Mater Res. 1999;47:8–17. doi: 10.1002/(sici)1097-4636(199910)47:1<8::aid-jbm2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Riess G. Micellization of block copolymers. Prog Polym Sci. 2003;28:1107–1170. [Google Scholar]
- 24.Li W, Laurencin C, Caterson E, Tuan R, Ko F. Electrospun nanofibrous structure: A novel scaffold for tissue engineering RID A-7002-2008. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 25.Vance R, Miller D, Thapa A, Haberstroh K, Webster T. Decreased fibroblast cell density on chemically degraded poly-lactic-co-glycolic acid, polyurethane, and polycaprolactone. Biomaterials. 2004;25:2095–2103. doi: 10.1016/j.biomaterials.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 26.Sigal G, Mrksich M, Whitesides G. Effect of surface wettability on the adsorption of proteins and detergents RID G-2469-2011. J Am Chem Soc. 1998;120:3464–3473. [Google Scholar]
- 27.Walluscheck KP, Steinhoff G, Kelm S, Haverich A. Improved endothelial cell attachment on ePTFE vascular grafts pretreated with synthetic RGD-containing peptides. Eur J Vasc Endovasc Surg. 1996;12:321–330. doi: 10.1016/s1078-5884(96)80251-6. [DOI] [PubMed] [Google Scholar]
- 28.Elbert DL, Hubbell JA. Surface treatments of polymers for biocompatibility. Annu Rev Mater Res. 1996;26:365–394. [Google Scholar]
- 29.Williams R, Krishna Y, Dixon S, Haridas A, Grierson I, Sheridan C. Polyurethanes as potential substrates for sub-retinal retinal pigment epithelial cell transplantation. J Mater Sci Mater Med. 2005;16:1087–1092. doi: 10.1007/s10856-005-4710-y. [DOI] [PubMed] [Google Scholar]
- 30.Guan JJ, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61:493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 31.Rousseau B, Hirano S, Scheidt TD, Welham NV, Thibeault SL, Chan RW, et al. Characterization of vocal fold scarring in a canine model. Laryngoscope. 2003;113:620–627. doi: 10.1097/00005537-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96–104. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 33.Klemuk SA, Titze IR. Viscoelastic properties of three vocal-fold injectable biomaterials at low audio frequencies. Laryngoscope. 2004;114:1597–1603. doi: 10.1097/00005537-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Chan RW, Rodriguez ML. A simple-shear rheometer for linear viscoelastic characterization of vocal fold tissues at phonatory frequencies. Journal of the Acoustical Society of America. 2008;124:1207–1219. doi: 10.1121/1.2946715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function RID F-4707-2011. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]