Abstract
Background
In 2011, the FDA approved telaprevir (TVR) and boceprevir (BOC) for use with pegylated-interferon and ribavirin to treat hepatitis C virus (HCV) genotype 1. We aimed to evaluate the real-world application, tolerability, and effectiveness of TVR and BOC-based HCV treatment in a large integrated care setting.
Methods
We utilized Northern California Kaiser Permanente Medical Care Program (KPNC) electronic databases and medical records to study the experience of all KPNC patients who initiated TVR or BOC from June 2011-March 2012.
Results
Compared to the pool of 5,194 treatment-eligible patients, the 352 treatment initiators were more likely to be cirrhotic (24% vs 10%, p<0.001) and treatment-experienced (44% vs 22%, p<0.001). Among the treatment initiators, 211 received TVR and 141 BOC. Overall, 31% discontinued treatment prematurely; 16% of patients stopped treatment early because of side effects. One patient with cirrhosis died of sepsis during treatment. Premature discontinuation was highest among TVR-treated cirrhotic patients (58%). Sustained virologic response (SVR) was achieved in 55% overall and was similar comparing the TVR- (56%) and BOC- (53%) treated groups. The only independent predictors of treatment failure were cirrhosis at baseline [odds ratio (OR) for SVR 0.44, p=0.004] and prior partial or null response (OR for SVR 0.57, p=0.02).
Conclusions
In the initial application of TVR and BOC, patients with cirrhosis and prior treatment failure were prioritized for treatment. In this real-world experience, most patients successfully completed a full treatment course. However, side effect-related premature discontinuations were common, and SVR rates were lower than reported in clinical trials.
Keywords: HCV, DAA, antiviral therapy, telaprevir, boceprevir
INTRODUCTION
Chronic hepatitis C virus (HCV) infection affects over 150 million individuals [1]. HCV is a leading cause of end stage liver disease and hepatocellular carcinoma, with over 350,000 annual deaths globally, and is the most common indication for liver transplantation in the United States (US) [1,2]. The goal of HCV treatment is to achieve a sustained virologic response (SVR), which represents a cure. In 2011, the FDA approved the protease inhibitors telaprevir (TVR) or boceprevir (BOC) for use with pegylated-interferon (PEG) and ribavirin (RBV) (“triple therapy”) for HCV genotype 1.
Clinical trials demonstrated superior SVR rates with TVR-based treatment compared to PEG/RBV among treatment naïve (75% vs 41%) and treatment experienced patients (64% vs 14%) [3,4]. Similarly, BOC-based regimens yielded higher SVR rates compared to PEG/RBV among treatment naïve (63–68% vs 38%) and treatment experienced subjects (59–66% vs 21%) [5,6]. However, these higher cure rates come at the cost of increased adverse events and complexity[7,8].
Clinical trial patients are selected using strict criteria and are monitored closely throughout treatment; experiences in routine practice settings often differ. Indeed, HCV treatment with PEG/RBV yields lower SVR rates in routine practice than in clinical trials[9,10]. Given the lower tolerability and increased complexity of triple therapy, the “real-world” experience may also differ substantially from registration trials. The Northern California Kaiser Permanente Medical Care Program (KPNC) is a large health care delivery system with comprehensive electronic records and thus, an ideal community-based population to evaluate HCV triple therapy [11]. Our objective was to evaluate the application, tolerability, and effectiveness of TVR- and BOC-based HCV treatment in this diverse, integrated care population.
METHODS
Base population
We studied KPNC members with chronic HCV. KPNC serves over 3.2 million members in the San Francisco and Sacramento Greater Metropolitan areas. Membership includes over 25% of the area’s insured population and is representative except at extremes in income[12,13]. KPNC first offered HCV treatment with TVR and BOC in June 2011. This study was reviewed and approved by the Institutional Review Board of the Kaiser Foundation Research Institute; informed consent was waived.
Treatment eligible cohort
To define the characteristics of the pool of patients potentially eligible to receive triple therapy, we created a cross-sectional cohort of health plan members in December 2010 who were theoretically eligible to receive TVR or BOC in June 2011. All had chronic HCV genotype 1 infection, no evidence of successful treatment for HCV, no evidence of human immunodeficiency virus (HIV) or chronic hepatitis B virus (HBV) infections, and no history of prior liver transplant, decompensated cirrhosis, or hepatoma.
Treatment initiation cohort
The treatment initiation cohort was assembled to explore how the drugs were being used, including side effects and treatment response. This cohort included all KPNC patients who began a TVR or BOC treatment course during the first 10 months of availability (June 2011–March 2012). We excluded patients with HIV or HBV infections (n=0) and prior liver transplant or on the transplant waiting list (n=3). Choice of protease inhibitor was made by the individual provider (n=25), and both drugs were equally available from the pharmacy formulary.
Data sources
We utilized the KPNC Viral Hepatitis Registry (VHR) database and other electronic health plan data to assemble the cohorts of HCV patients and retrieve demographic and clinical information. The treatment eligible cohort and characteristics were derived from the KPNC VHR. The treatment initiation cohort (dispensed either TVR or BOC) was identified directly from the pharmacy information management system (PIMS). Baseline and treatment-related information was obtained in aggregate from the VHR and KPNC databases and individually from the KPNC electronic medical record (Epic-based “HealthConnect”). Cirrhosis was classified by histologic evidence or a clinical diagnosis within 24 months prior to treatment.
All laboratory results were electronically derived, including HCV test results from outside vendors. For accuracy, prior treatment failure categories were based on laboratory data only. The PIMS database provided all pharmacy information, including supplemental growth factor use. The first drug dispense date (TVR, BOC, PEG or RBV) defined the start date. However, treatment start and stop dates were adjusted as needed based upon provider notes. Side effects during the first 24 weeks of treatment were assessed from provider notes, diagnoses, and hospitalization records. Transfusions were identified via diagnostic and procedure codes in the EMR. Reasons for premature discontinuation were obtained from provider notes.
HCV RNA tests varied during the study term and by provider. Tests to define undetectable HCV RNA included the TaqMan PCR test [lower limit of detection (LLOD) of <15 IU/ml for HCV genotype 1] and the TMA test (LLOD of 10 IU/ml). Quantitative tests included the TaqMan PCR test [lower limit of quantification (LLOQ) of 43 IU/ml] and the bDNA test (LLOQ of 615 IU/ml). Beginning in May 2012, only Roche TaqMan PCR testing at the KPNC Regional Laboratory was used. On-treatment response, end-of-treatment response, and SVR were defined as undetectable HCV RNA while on treatment, at completion of a full treatment course, and at least 12 weeks after treatment discontinuation, respectively[14]. Relapse was assigned to patients completing a full course of treatment with detectable HCV RNA after an undetectable end-of-treatment test.
Data analysis
Characteristics of the treatment eligible and initiation cohorts were compared using Pearson’s chi-square test, as were comparisons of the TVR- and BOC-treated groups. We used logistic regression to define factors associated with SVR. Prior treatment categories were dichotomized with one group including naïve patients plus those with prior relapse or breakthrough; the other included prior null, partial responders, and those with undetermined prior response. Variables that were significant on univariate analysis or determined a priori were included in multivariable logistic regression analyses. The final model included protease inhibitor type, cirrhosis at baseline, sex, race, prior treatment experience, and elevated baseline HCV RNA (≥800,000 IU/mL). A sensitivity analysis included all patients who did not discontinue treatment due to side effects. SAS version 9.1.3 and STATA version 12 were used for analyses.
RESULTS
Patients selected for treatment
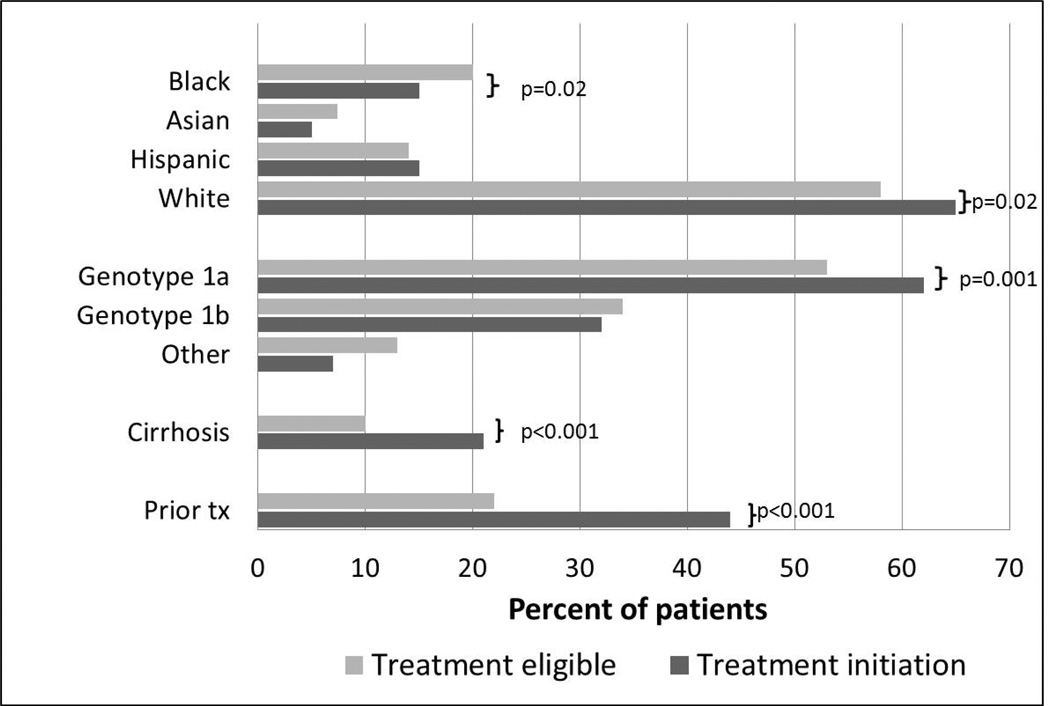
To understand which patients were being selected for treatment, we compared the cohort of patients who initiated treatment with TVR or BOC during the study period with a cross-sectional cohort of treatment eligible health plan members. The eligible (5,194) and initiation (352) cohorts were both predominantly male (60%), and had similar proportions of Asians and Hispanics (Figure 1). The initiation cohort had a higher proportion of non-Hispanic whites (65% vs 58% p=0.02) and a lower proportion of non-Hispanic Blacks (15% vs 20% p=0.02). The initiation cohort had a higher proportion of genotype 1a (66% vs 53% p=0.001) but fewer patients with undetermined subgenotype (6.5% vs 13%, p<0.001), perhaps reflecting the groups’ different racial compositions [15]. Patients with cirrhosis (21% vs 10% p<0.001) and prior treatment experience (44% vs 22% p<0.001) were more highly represented in the initiation cohort.
Figure 1.
Characteristics of the treatment eligible (5,194) and treatment initiation (352) cohorts. Race/ethnicity data was available in 91% of the treatment eligible cohort and 98% of the treatment initiation cohort. Tx denotes HCV treatment.
Characteristics of treated patients
Of the 352 treated patients, 211 received TVR and 141 BOC. As shown in Table 1, the majority was male (61%) and non-Hispanic white (63%), with a median age of 56 [interquartile range (IQR) 21, 70]. Less than half (44%) were treatment experienced; among this group, 27% had relapse or breakthrough, 16% partial response, 25% null response, and 32% had unspecified prior treatment failure. Pre-treatment liver biopsy was performed in 126 patients (36%). Cirrhosis, diagnosed either clinically or by histology, was present in 21%.
Table 1.
Baseline characteristics of treated cohort, by treatment group
| Total (N=352) | Telaprevir (n=211) | Boceprevir (n=141) | |
|---|---|---|---|
| Age, median (range) | 56 (21, 70) | 57 (25, 70) | 56 (21, 69) |
| Male | 213 (61%) | 125 (59%) | 88 (62%) |
| Race/ethnicity | |||
| Black | 52 (15%) | 34 (16%) | 18 (13%) |
| Asian | 18 (5%) | 8 (4%) | 10 (7%) |
| Hispanic | 52 (15%) | 31 (15%) | 21 (15%) |
| White non-Hispanic | 223 (63%) | 135 (64%) | 88 (62%) |
| Unknown/other | 7 (2%) | 3 (1%) | 4 (3%) |
| Health plan membership | |||
| <12 months | 14 (4%) | 8 (4%) | 6 (4%) |
| 12–59 months | 122 (35%) | 74 (35%) | 48 (34%) |
| ≥60 months | 216 (61%) | 129 (61%) | 87 (62%) |
| Diabetes | 58 (16%) | 34 (16%) | 24 (17%) |
| Body mass index | |||
| <25 kg/m2 | 87 (25%) | 52 (25%) | 35 (25%) |
| 25–30 kg/m2 | 138 (39%) | 83 (39%) | 55 (39%) |
| ≥30 kg/m2 | 127 (36%) | 76 (36%) | 51 (36%) |
| HCV treatment history | |||
| Naïve | 198 (56%) | 113 (54%) | 85 (60%) |
| Relapse/breakthrough | 41 (12%) | 27 (13%) | 14 (10%) |
| Partial responder | 25 (7%) | 18 (9%) | 7 (5%) |
| Null responder | 38 (11%) | 27 (13%) | 11 (8%) |
| Treatment experienced, response unknown | 50 (14%) | 26 (12%) | 24 (17%) |
| Fibrosis stage+ | |||
| F0 | 7 (6%) | 6 (8%) | 1 (2%) |
| F1–F2 | 60 (48%) | 29 (38%) | 31 (62%) |
| F3 | 45 (36%) | 30 (39%) | 15 (30%) |
| F4 | 14 (11%) | 11 (14%) | 3 (6%) |
| Cirrhosis* | 73 (21%) | 48 (23%) | 25 (18%) |
| HCV subtype | |||
| 1a | 218 (62%) | 132 (63%) | 86 (61%) |
| 1b | 111 (32%) | 67 (32%) | 44 (31%) |
| 1 other/unknown | 23 (7%) | 12 (6%) | 11 (8%) |
| HCV viral load | |||
| <400,000 IU/mL | 92 (26%) | 57 (27%) | 35 (25%) |
| 400,000–799,999 IU/mL | 60 (17%) | 36 (17%) | 24 (17%) |
| ≥800,000 IU/mL | 189 (54%) | 114 (54%) | 75 (53%) |
| Missing | 11 (3%) | 4 (2%) | 7 (5%) |
| Platelet count (×109/L) | |||
| <150 | 110 (31%) | 68 (32%) | 42 (30%) |
| ≥150 | 229 (65%) | 139 (66%) | 90 (64%) |
| Missing | 13 (4%) | 4 (2%) | 9 (6%) |
HCV= hepatitis C virus;
Fibrosis measured using the Batts-Ludwig scale among subjects with liver biopsy performed (total=126, telaprevir=76, boceprevir=50);
Cirrhosis by clinical diagnosis or liver biopsy
Adverse events and tolerability
Overall, 57 patients (16%) discontinued treatment prematurely due to side effects, with 40% by patient choice (versus provider-directed). Most (68%) side-effect-related discontinuations occurred within the first 12 weeks of treatment; only 6 (9%) were beyond 24 weeks. Table 2 details the factors contributing to these discontinuations. Gastrointestinal symptoms were the most common problems contributing to discontinuation (35%), followed by dermatologic (21%) and hematologic (18%) effects. While on TVR, one patient experienced acute pancreatitis, one developed homicidal ideation, and one developed aplastic anemia. Three cirrhotic patients experienced hepatic decompensation. The single death was a TVR-treated cirrhotic patient with sepsis at treatment week 14. There was no statistically significant difference found in discontinuation rate due to side effects between TVR versus BOC therapy.
Table 2.
Treatment tolerability
| Telaprevir | Boceprevir | ||||
|---|---|---|---|---|---|
| Non- cirrhotic (n=163) |
Cirrhotic (n=48) |
Non- cirrhotic (n=116) |
Cirrhotic (n=25) |
||
| Premature discontinuation due to side effects+ | 25 (15%) | 12 (25%) | 16 (14%) | 4 (16%) | |
| Gastrointestinal | 10 | 3 | 7 | 0 | |
| Dermatologic | 7 | 1 | 4 | 0 | |
| DRESS | 2 | 0 | 0 | 0 | |
| Fatigue | 3 | 3 | 2 | 1 | |
| Mental health | 3 | 0 | 3 | 0 | |
| Hematologic | 4* | 4 | 2 | 0 | |
| Sepsis | 0 | 2† | 1 | 0 | |
| Hepatic decompensation | 0 | 2 | 0 | 1 | |
| Other | 3 | 1 | 3 | 2 | |
| Hematologic side effects and related care‡ | |||||
| Anemia | |||||
| Hgb 8.5–10 g/dL | 37 (23%) | 3 (6%) | 41 (35%) | 10 (40%) | |
| Hgb <8.5 g/dL | 30 (18%) | 11 (23%) | 11 (9%) | 4 (16%) | |
| Thrombocytopenia | |||||
| 50,000–75,000 /mm3 | 30 (18%) | 9 (19%) | 17 (15%) | 5 (20%) | |
| 25,000–49,000 /mm3 | 5 (3%) | 17 (35%) | 8 (7%) | 15 (60%) | |
| <25,000 /mm3 | 1 (1%) | 7 (15%) | 1 (1%) | 0 | |
| Leukopenia | |||||
| 1,000–1,500 /mm3 | 17 (10%) | 11 (23%) | 23 (20%) | 8 (32%) | |
| <1,000 /mm3 | 1 (1%) | 2 (4%) | 2 (2%) | 1 (4%) | |
| Ribavirin dose reduction | 70 (43%) | 20 (42%) | 38 (33%) | 17 (68%) | |
| Erythropoietin use | 72 (44%) | 19 (40%) | 54 (47%) | 20 (80%) | |
| Granulocyte colony-stimulating factor use | 19 (12%) | 9 (19%) | 22 (19%) | 10 (40%) | |
| Transfusion | 14 (9%) | 7 (15%) | 4 (3%) | 6 (24%) | |
DRESS: drug reaction with eosinophilia and systemic symptoms; Hgb: hemoglobin
Side effect categories not mutually exclusive, 15 patients had >1 contributing issue
1 patient developed aplastic anemia
1 patient died due to sepsis
In the first 24 weeks of treatment
The most common adverse hematologic side effect was anemia: 147 patients (42%) had at least one hemoglobin value less than 10 g/dL during the first 24 weeks of treatment (Table 2). While 47% of all patients received erythropoietin, 9% of patients received a blood transfusion. Thrombocytopenia was also common in the first 24 weeks of treatment; one third of patients experienced a platelet count under 75,000 per mm3. Notably, this abnormality was not limited to cirrhotics and affected almost one quarter of patients without cirrhosis. One patient without cirrhosis developed severe leukopenia (white blood cell count <500/mm3). None of the cohort used Eltrombopag during treatment.
Treatment Outcomes
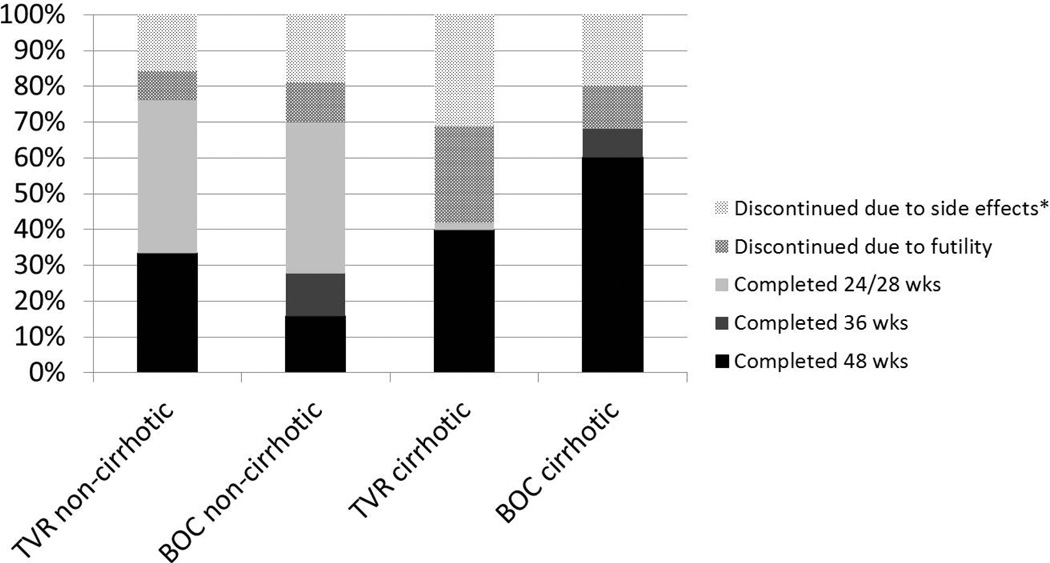
Overall, 110 patients (31%) did not complete a full treatment course; this was highest among the TVR-treated cirrhotic patients (58%), followed by the BOC-treated cirrhotics (32%), BOC-treated non-cirrhotics (30%), and least common among TVR-treated non-cirrhotics (24%) (Figure 2). In addition to patients stopping early due to side effects, 42 patients stopped due to futility: 27% of TVR-treated cirrhotics, 12% of BOC-treated cirrhotics, 11% of BOC-treated non-cirrhotics, and 8% of TVR-treated non-cirrhotics. An additional 2 patients (both TVRtreated) experienced virologic breakthrough on treatment. Discontinuations for other reasons (all after treatment week 12) included 4 treatment-unrelated medical problems, 3 insurance loss, 1 financial, and 1 non-adherence. Several patients discontinued protease inhibitor early but continued on PEG/RBV dual therapy: 6 stopped TVR early, and 2 stopped BOC early.
Figure 2.
Treatment duration, by cirrhosis and protease inhibitor
*Includes sides effects (n=56) and other reasons (n=9)
TVR: telaprevir; BOC: boceprevir
Non-cirrhotic patients may be eligible for response-guided therapy (RGT), depending on prior treatment experience and on-treatment response. More than half (56%) of non-cirrhotic TVR-treated patients who completed a full course of treatment were treated for 24 weeks rather than 48 weeks (43% of all non-cirrhotic TVR patients). Among the non-cirrhotic BOC-treated patients who completed a full course of treatment, 78% were treated for a shortened (28 or 26 weeks) duration (54% of all non-cirrhotic BOC patients). Although RGT is not recommended for patients with cirrhosis, 1 TVR-treated and 2 BOC-treated cirrhotic patients received shortened courses.
Two-thirds of the cohort achieved end-of-treatment response: 136 TVR-treated patients (71% of non-cirrhotic, 42% of cirrhotic) and 95 BOC-treated patients (68% of non-cirrhotic, 64% of cirrhotic) (Table 3). Relapse occurred in 26 patients (7.4%) and was more frequent in the BOC-treated patients (10% vs. 4%, p=0.006). Among the 17 BOC-treated patients who relapsed, 8 (one cirrhotic) received a shorter treatment duration than recommended by RGT guidelines.
Table 3.
Treatment outcomes, by treatment group and cirrhosis status
| Telaprevir | Boceprevir | |||||
|---|---|---|---|---|---|---|
| Total (n=211) |
Non- cirrhotic (n=163) |
Cirrhotic (n=48) |
Total (n=141) |
Non- cirrhotic (n=116) |
Cirrhotic (n=25) |
|
| On treatment failure | ||||||
| Virologic breakthrough | 2 (9%) | 1 (1%) | 1 (2%) | 0 | 0 | 0 |
| Futility | 26 (12%) | 13 (8%) | 13 (27%) | 16 (11%) | 13 (11%) | 3 (12%) |
| On treatment responses | ||||||
| Week 4 (TVR) or week 8 (BOC) response + | 87 (41%) | 76 (47%) | 11 (23%) | 76 (54%) | 67 (58%) | 9 (36%) |
| Week 12 response | 160 (76%) | 131 (80%) | 29 (60%) | 90 (64%) | 79 (68%) | 11 (44%) |
| End of treatment response¥, n (%) | 136 (64%) | 116 (71%) | 20 (42%) | 95 (67%) | 79 (68%) | 16 (64%) |
| Relapse†, n (%) | 9 (4%) | 6 (4%) | 3 (6%) | 17 (10%) | 13 (11%) | 4 (16%) |
| Sustained virologic response‡, n (%) | 118 (56%) | 109 (67%) | 15 (31%) | 75 (53%) | 63 (54%) | 12 (48%) |
Response was defined as undetectable HCV RNA level at a sensitivity of <15 IU/ml
End of treatment response was defined as undetectable HCV RNA level at the end of the treatment period. 4 patients (3 TVR, 1 BOC) were lost to follow-up during treatment
Relapse was defined as detectable HCV RNA level after treatment completion in a subject with an undetectable HCV RNA level at end of treatment
Sustained virologic response (SVR) was defined as undetectable HCV RNA level at least 12 weeks after treatment discontinuation. 4 TVR-treated and 3 BOC-treated patients stopped treatment prematurely but achieved SVR. 19 patients with end of treatment response were lost to follow-up after treatment ended (13 TVR, 6 BOC)
Treatment Response and Associated Factors
Overall, the crude SVR rate was 55%, with 67% in the non-cirrhotic TVR group, followed by the non-cirrhotic BOC group (53%), cirrhotic BOC group (48%), and cirrhotic TVR group (31%) (Table 3). Table 4 includes crude SVR rates stratified by various factors.
Table 4.
Baseline factors associated with sustained virologic response
| SVR | Unadjusted | Adjusted* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | N=352 | n=193 | % | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Protease inhibitor | |||||||||
| Telaprevir | 211 | 118 | 56% | 1.0 | 1.0 | ||||
| Boceprevir | 141 | 75 | 53% | 0.9 | (0.58 – 1.37) | 0.61 | 0.82 | (0.52 – 1.28) | 0.38 |
| Cirrhosis | |||||||||
| No | 279 | 166 | 59% | 1.0 | 1.0 | ||||
| Yes | 73 | 27 | 37% | 0.4 | (0.23 – 0.68) | 0.001 | 0.44 | (0.25 – 0.77) | 0.004 |
| Sex | |||||||||
| Male | 213 | 120 | 56% | 1.0 | 1.0 | ||||
| Female | 139 | 73 | 53% | 0.86 | (0.56 – 1.32) | 0.48 | 0.77 | (0.49 – 1.21) | 0.26 |
| Race | |||||||||
| Non-Black | 300 | 169 | 56% | 1.0 | 1.0 | ||||
| Black | 52 | 24 | 46% | 0.66 | (0.37 – 1.20) | 0.18 | 0.61 | (0.33 – 1.14) | 0.12 |
| Prior treatment | |||||||||
| Naïve, relapsers | 239 | 145 | 61% | 1.0 | 1.0 | ||||
| Other previously treated | 113 | 48 | 42% | 0.48 | (0.30 – 0.75) | 0.002 | 0.57 | (0.35 – 0.91) | 0.02 |
| HCV RNA | |||||||||
| <800,000 IU/mL | 152 | 89 | 59% | 1.0 | 1.0 | ||||
| ≥800,000 IU/mL | 200 | 104 | 52% | 0.77 | (0.50 – 1.17) | 0.22 | 0.78 | (0.50 – 1.21) | 0.27 |
| HCV subtype | |||||||||
| 1b | 111 | 63 | 57% | 1.0 | |||||
| 1a and other | 241 | 130 | 54% | 0.89 | (0.57 – 1.40) | 0.62 | |||
| Age | |||||||||
| <60 years | 244 | 135 | 55% | 1.0 | |||||
| ≥60 years | 108 | 58 | 54% | 0.94 | (0.59 – 1.48) | 0.78 | |||
| BMI | |||||||||
| Non-obese (<30 kg/m2) | 225 | 128 | 57% | 1.0 | |||||
| Obese (≥ 30 kg/m2) | 127 | 65 | 51% | 0.79 | (0.51 – 1.23) | 0.30 | |||
| Platelet count (×109/L) | |||||||||
| ≥150 | 242 | 132 | 55% | 1.0 | |||||
| <150 | 110 | 61 | 55% | 1.04 | (0.66 – 1.63) | 0.87 | |||
| Diabetes | |||||||||
| No | 294 | 163 | 55% | 1.0 | |||||
| Yes | 58 | 30 | 52% | 0.86 | (0.49 – 1.51) | 0.60 | |||
SVR: sustained virologic response; BMI: body mass index.
Model includes protease inhibitor, cirrhosis, sex, race, prior treatment, and HCV RNA.
We calculated crude odds ratios (OR) for SVR to compare treatment success rates between different subgroups (Table 4). Only cirrhosis and prior treatment response were significantly associated with treatment response. Similarly, adjusting in a multivariable model for protease inhibitor, gender, race, and baseline HCV RNA, patients with cirrhosis had a lower odds of SVR [odds ratio (OR) 0.44, 95% confidence interval (CI) 0.25–0.77, p=0.004)], as did treatment-experienced non-relapsers (OR 0.57, 95% CI 0.35–0.91, p=0.02). Of note, protease inhibitor type was not associated with SVR in unadjusted or adjusted analysis.
Because TVR and BOC may have different side effect profiles, we performed a sensitivity analysis limited to patients who did not discontinue treatment early due to side effects. The results of the multivariable analysis among these 299 patients (177 TVR-treated and 122 BOC-treated) were the same: only prior treatment failure and cirrhosis were significantly associated with SVR.
DISCUSSION
In this large integrated care population, treatment-eligible HCV genotype 1 patients with cirrhosis and prior treatment failure were targeted for triple therapy. Among these initial 352 treated patients, the majority completed a full course of treatment (69%), although early discontinuation due to side effects was common (16%), and SVR was lower than reported in clinical trials (55%).
Of treated patients, 21% had a clinical or histologic diagnosis of cirrhosis, versus 10% of the treatment eligible cohort. This suggests treatment prioritization was based, in part, on illness severity. This not unexpected finding is important for several reasons. First, as demonstrated in trials and as we have confirmed, cirrhotic patients have lower SVR rates with triple therapy compared to non-cirrhotics [16,17]. Second, cirrhotics are at a high risk of serious adverse events with TVR and BOC triple therapy, as demonstrated by the French Compassionate Use of Protease Inhibitors in Viral C Cirrhosis study and by a multicenter study including selected patients from our cohort[18,19]. Third, cirrhotic patients were under-represented in most TVR and BOC clinical trials[3,5,6]. Our findings underscore the importance of studying patients with cirrhosis in HCV treatment trials, particularly since this group will continue to be prioritized for novel therapies.
Side effects led to premature discontinuation in 16% of our cohort; this was similar regardless of protease inhibitor used. Discontinuation due to side effects was not substantially higher with TVR than reported rates in clinical trials (10–13%)[3,4]. In contrast, only 2% of BOC-treated patients in the SPRINT-2 trial stopped due to side effects [5]. The side effects we observed were consistent with those described in clinical trials, and the majority were those known to be associated with PEG/RBV. Many discontinuations were due to treatment intolerance, primarily gastrointestinal and dermatologic, and mental health issues. As expected, anemia was the most common hematologic side effect and was treated aggressively: nearly half of patients received erythropoietin, and 9% received a blood transfusion. This highlights the complexity of treatment management, even in patients without cirrhosis. Our study’s modest sample size precluded a meaningful investigation of the predictors of premature discontinuation due to side effects.
The TVR and BOC RGT regimens offer the advantage of potentially shortening treatment duration among qualifying patients[20,21]. In our cohort, all patients eligible for a shortened course by RGT guidelines received one. Consequently, a substantial proportion received a <48 week treatment course: 43% of TVR-treated and 54% of BOC-treated non-cirrhotics. This was consistent with the proportion in SPRINT-2 who would have been eligible for a shortened course of BOC[5]. Although a higher proportion were eligible for shortened therapy in the TVR ILLUMINATE trial (65%), our cohort included prior partial/null responders who accordingly were ineligible for RGT[22]. In some contrast to our findings, a recent Veterans Affair healthcare system study reported that among eligible patients, only 28% of TVR-treated and 37% of BOC-treated patients successfully received the short duration therapy[23]. Notably, BOC appeared to have a higher rate of relapse than did TVR in our study. However, nearly half of BOC-treated relapsers did not receive the appropriate treatment duration (i.e. they received a 24 or 36 week course that they were not eligible for by general or RGT guidelines), presumably due to provider misunderstanding. This may explain, at least in part, the discrepancy in relapse rates. Our observation demonstrates a practical real-world implication of highly complex treatment guidelines and RGT protocols.
Overall, 55% of patients achieved SVR. This is lower than Phase III study results (59–75% SVR), but is similar to the Veterans Affairs “real-world” observations of 50–52%, as well as other, smaller “real-world” HCV cohorts [3–6,23–26]. It is encouraging that our findings are similar to these studies, as our cohort reflects a different population (regularly insured, nonveterans, and a higher proportion of women) than the other large observational treatment cohorts. Of note, while our observed rates of ribavirin dose reduction were similar to those reported in a large VA population taking TVR or BOC, our rates of erythropoietin use were much higher than the less 26% found in that population[27]. Nevertheless, our SVR rate was lower than those reported in the clinical trials. Several factors may explain this. Compared to clinical trials, our population was older, had a relatively high proportion of Hispanics (15%) and Blacks (15%), and included substantial numbers of cirrhotics (21%). Additionally, the proportion of patients with baseline thrombocytopenia (31%) suggests that cirrhosis may have been more prevalent than the 21% we defined by a clinical or histologic diagnosis. Furthermore, our cirrhotic patients were more likely to be treatment-experienced, particularly null responders. That subgroup (cirrhotic, prior null response) was not included in the BOC trials and responded most poorly to TVR in the REALIZE trial (14% SVR)[4]. Finally, although side effects were managed aggressively, 40% of patients who stopped treatment early because of side effects did so by choice, rather than by provider direction. Presumably, this proportion was lower in the clinical trials.
As in the clinical trials, cirrhosis and prior partial/null response were independently associated with treatment failure in our cohort. Both of these factors are also reported as important predictors of response to dual therapy with PEG/RBV[10]. We found no difference in SVR between TVR- and BOC-treated patients, a finding that is consistent with one Veterans Affairs study[23]. In contrast, unlike our study, another Veterans Affairs study demonstrated higher odds of SVR with TVR-based triple therapy on multivariate analysis[24]. However, our study population may not have been of sufficient size to detect a small difference in SVR rate between the two groups.
Our observational study has several limitations. First, we cannot exclude an effect of provider bias in choice of protease inhibitor used. Second, although we captured all premature discontinuations due to side effects, our detailed assessment of side effects was limited to the first 24 treatment weeks, perhaps underestimating the frequency of hematologic complications. However, since the majority of captured severe side effects were manifest within the first 12 weeks of treatment, underestimation is likely minimal. Third, KPNC members are not fully representative of the US population, somewhat limiting the generalizability of our findings. However, the base population is inherently diverse, we utilized complete records of the treatment experience, and the patients were in unperturbed, community-based clinical practice settings – all important strengths of our investigation. Furthermore, our access to complete medical records, including notes, allowed us to assess in detail the reasons for treatment discontinuation, including whether discontinuation was patient- or provider-initiated.
Newer direct acting antiviral agents offer hopes of significantly improved tolerability and treatment response [28–30]. Given the difficulties using TVR- or BOC-based triple therapy among cirrhotic patients, most providers will choose newer regimens for patients with end stage liver disease. However, the current high costs of the newest antivirals preclude widespread use. Thus, TVR and BOC will still be used to treat non-cirrhotic chronic HCV patients in many settings in the US and elsewhere [31]. In particular, resource poor settings outside the US may choose first-generation protease inhibitors as their cost decreases. As more HCV treatment options become available, our findings will remain important in comparing treatment response rates, complications, and cost-effectiveness of the various regimens. In addition, our findings may speak to the challenges of the initial roll-out of any new regimen, particularly those with complex decision algorithms.
In summary, in this large integrated care setting in Northern California, TVR and BOC-based triple therapy for HCV genotype 1 infection was frequently associated with side effects which required aggressive management. A substantial proportion of patients were able to receive a shortened duration of therapy. Just 55% of treated patients achieved SVR. This highlights the importance of evaluating the real-world treatment effectiveness of all novel HCV direct-acting antiviral regimens.
ACKNOWLEDGEMENTS
We thank Dr. Suk Seo for helpful comments on the manuscript.
This work was supported by The Permanente Medical Group and was partially funded by Vertex Pharmaceuticals Incorporated. JP discloses serving as an advisor to Gilead and has ownership interest in Bristol-Myers Squibb, Johnson and Johnson, and Abbvie. MPP discloses clinical trial research support from Roche and Merck. MMM discloses research support from Merck, Gilead, and Vertex.
Abbreviations
- TVR
telaprevir
- BOC
boceprevir
- HCV
hepatitis C virus
- US
United States
- KPNC
Northern California Kaiser Permanente Medical Care Program
- SVR
sustained virologic response
- OR
odds ratio
- PEG
pegylated-interferon
- RBV
ribavirin
- HIV
human immunodeficiency virus
- HBV
hepatitis B virus
- VHR
Viral Hepatitis Registry
- PIMS
pharmacy information management system
- LLOD
lower limit of detection
- LLOQ
lower limit of quantification
- RGT
response-guided therapy
- DRESS
drug reaction with eosinophilia and systemic symptoms
- Hgb
hemoglobin
- BMI
body mass index
- IQR
interquartile range
Footnotes
Disclosures
RCM and VAS have no conflicts to declare.
REFERENCES
- 1. [Accessed January 17, 2014]; http://www.who.int/mediacentre/factsheets/fs164/en/index.html.
- 2.Kim WR, Stock PG, Smith JM, et al. OPTN/SRTR 2011 Annual Data Report: liver. Am J Transplant. 2013;13(Suppl 1):73–102. doi: 10.1111/ajt.12021. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 4.Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 5.Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson IM, Pawlotsky JM, Afdhal NH, et al. A practical guide for the use of boceprevir and telaprevir for the treatment of hepatitis C. J Viral Hepat. 2012;19(Suppl 2):1–26. doi: 10.1111/j.1365-2893.2012.01590.x. [DOI] [PubMed] [Google Scholar]
- 8.Maasoumy B, Port K, Markova AA, et al. Eligibility and safety of triple therapy for hepatitis C: lessons learned from the first experience in a real world setting. PLoS One. 2013;8:e55285. doi: 10.1371/journal.pone.0055285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridruejo E, Adrover R, Cocozzella D, Reggiardo MV, Fernandez N. Effectiveness of hepatitis C treatment with pegylated interferon and ribavirin in urban minority patients. Hepatology. 2010;51(2231) doi: 10.1002/hep.23714. author reply 2231–2232. [DOI] [PubMed] [Google Scholar]
- 10.Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46:37–47. doi: 10.1002/hep.21662. [DOI] [PubMed] [Google Scholar]
- 11.Manos MM, Darbinian J, Rubin J, et al. The effect of hepatitis C treatment response on medical costs: a longitudinal analysis in an integrated care setting. J Manag Care Pharm. 2013;19:438–447. doi: 10.18553/jmcp.2013.19.6.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon N. Similarity of the adult Kaiser Permanente membership in northern California to the insured and general population in northern California: statistics from the 2007 California Health Interview Survey. Internal Report: Kaiser Permanente Division of Research. 2012 [Google Scholar]
- 13.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Florian J, Carter W, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology. 2013;144:1450–1455. e1452. doi: 10.1053/j.gastro.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 15.Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84:1744–1750. doi: 10.1002/jmv.23399. [DOI] [PubMed] [Google Scholar]
- 16.Kwo PY. Phase III results in Genotype 1 naive patients: predictors of response with boceprevir and telaprevir combined with pegylated interferon and ribavirin. Liver Int. 2012;32(Suppl 1):39–43. doi: 10.1111/j.1478-3231.2011.02709.x. [DOI] [PubMed] [Google Scholar]
- 17.Poordad F, Bronowicki JP, Gordon SC, et al. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012;143:608–618. e601–e605. doi: 10.1053/j.gastro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Hezode C, Fontaine H, Dorival C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Saxena V, Manos MM, Yee H, et al. Telaprevir or Boceprevir Triple Therapy in Patients with Chronic Hepatitis C and Varying Severity of Cirrhosis. Aliment Pharmacol Ther. doi: 10.1111/apt.12718. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.INCIVEK [package insert] Cambridge, MA: Vertex Pharmaceuticals Incorporated; 2011. [Google Scholar]
- 21.VICTRELIS [package insert] Whitehouse Station, NJ: Schering Corporation, a subsidiary of Merck & Co, INC; 2011. [Google Scholar]
- 22.Sherman KE, Flamm SL, Afdhal NH, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014–1024. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ioannou GN, Beste LA, Green PK. Similar Effectiveness of Boceprevir and Telaprevir Treatment Regimens for Hepatitis C Virus Infection, Based on a Nationwide Study of Veterans. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39:93–103. doi: 10.1111/apt.12546. [DOI] [PubMed] [Google Scholar]
- 25.Wehmeyer MH, Eissing F, Jordan S, et al. Safety and efficacy of protease inhibitor based combination therapy in a single-center "real-life" cohort of 110 patients with chronic hepatitis C genotype 1 infection. BMC Gastroenterol. 2014;14:87. doi: 10.1186/1471-230X-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maasoumy B, Port K, Deterding K, et al. Limited effectiveness and safety profile of protease inhibitor-based triple therapy against chronic hepatitis C in a real-world cohort with a high proportion of advanced liver disease. Eur J Gastroenterol Hepatol. 2014;26:836–845. doi: 10.1097/MEG.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 27.Belperio PS, Backus LI, Ross D, Neuhauser MM, Mole LA. A population approach to disease management: hepatitis C direct-acting antiviral use in a large health care system. J Manag Care Pharm. 2014;20:533–540. doi: 10.18553/jmcp.2014.20.6.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 29.Zeuzem S, Berg T, Gane E, et al. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology. 2014;146:430–441. e436. doi: 10.1053/j.gastro.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 30.Manns M, Marcellin P, Poordad F, et al. Simeprevir (TMC435) with pegylated interferon/ribavirin for the treatment of chronic HCV genotype-1 infection in treatment-naive patients: results from QUEST-2, a phase 3 trial. J Hepatol. 58 abstract 1413. [Google Scholar]
- 31.Shiffman ML, Benhamou Y. HCV F1/F2 patients: treat now or continue to wait. Liver Int. 2014;34(Suppl 1):79–84. doi: 10.1111/liv.12408. [DOI] [PubMed] [Google Scholar]