Abstract
Neuroblastoma is unique amongst common pediatric cancers for its expression of the norepinephrine transporter (NET), enabling tumor-selective imaging and therapy with radioactive analogues of norepinephrine. The majority of neuroblastoma tumors are avid for 123I-metaiodobenzaguanidine (mIBG) on imaging, yet the therapeutic response to 131I-mIBG is only 30% in clinical trials, and off-target effects cause short- and long-term morbidity. We review the contemporary understanding of the tumor-selective uptake, retention, and efflux of meta-iodobenzylguanidine (mIBG) and strategies currently in development for improving its efficacy. Combination treatment strategies aimed at enhancing NET are likely necessary to reach the full potential of 131I-mIBG therapy.
Keywords: meta-iodobenzylguanidine, neuroblastoma, norepinephrine transporter
INTRODUCTION
Neuroblastoma is the most common extra-cranial solid tumor of childhood 1. Less than 50% of patients with high-risk features survive long-term, despite multi-modal therapy with surgery, chemotherapy, antibody therapy, retinoic acid, high-dose chemotherapy, and autologous hematopoietic progenitor cell transplant 2,3. Among the novel therapeutic agents under active investigation to improve outcomes is 131I-mIBG (meta-iodobenzylguanidine).
mIBG is an analog of the catecholamine norepinephrine, which can be labeled with a radioactive isotope, 123Iodine or 131Iodine, to use clinically for imaging or treatment, respectively, of neuroendocrine tumors. Neuroblastoma and other cancers of sympathetic neuronal precursors express the norepinephrine transporter (NET), a transmembrane protein which functions to shuttle norepinephrine across the cell membrane. 90% of children with neuroblastoma have mIBG-avid tumors by imaging with 123I-mIBG, but a recent meta-analysis suggests that only 30% of children who receive 131I-mIBG radiotherapy have any clinical response, which is usually not curative 4–6. The reasons for this discrepancy are not clear but are likely multifactorial. One possibility is the lower mRNA and protein expression of NET found in children with high-risk neuroblastoma compared to those with low or intermediate-risk disease, as classified by anatomic, histologic, and genetic factors 6.
In order to improve the utility of 131I-mIBG therapy and to rationally design combination treatment strategies, it is important to understand the detailed mechanisms of tumor-selective 131I-mIBG uptake, retention, and efflux, as well as the dynamics of NET expression (Fig. 1).
Figure 1.
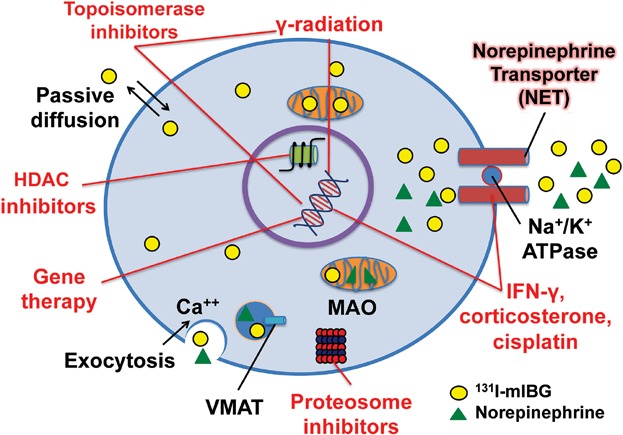
Representation of the mechanisms involved in 131I-mIBG uptake, retention, and efflux. Along with a passive diffusion phenomenon and exocytosis, 131I-mIBG may be released by the uptake carrier working in a reverse mode. The latter mechanism can be triggered either by the inversion of the sodium gradient across the cell membrane or by trans-stimulation by a ligand outside the cell membrane. Red text and lines represent the mechanisms being explored to enhance 131I-mIBG uptake, retention, and cytotoxicity. Some therapies radiosensitize neuroblastomas (HDAC inhibitors, gamma radiation, topoisomerase inhibitors, proteasome inhibitors) while others directly increase NET expression or enhance its function. Circle = 131I-mIBG, triangle = norepinephrine. HDAC = histone deacetylase; VMAT = vesicular monoamine transporter.
NOREPINEPHRINE TRANSPORTER FUNCTION
Neuroblastomas arise from the sympathetic neural precursors derived from the embryonic neural crest. As such, 90% of neuroblastomas express the NET, a 12 domain transmembrane protein encoded by the SLC6A2 gene with high affinity and specificity for norepinephrine and its analogs 5,7. NET actively transports norepinephrine primarily into adrenal chromaffin cells and pre-synaptic terminals by an ATP-dependent and specific process known as Uptake-1 8,9. Uptake-1 transportation is saturable and dependent upon serum sodium and chloride (co-transported substrates), temperature, pH, oxygen, and vascularity. Norepinephrine transportation is impaired in hypoxic, hyperthermic, and nitric oxide depleted environments 10–12. Tricyclic antidepressants, such as desmethylimipramine (DMI), specifically inhibit the norepinephrine transporter uptake of norepinephrine and its analogs 13. DMI most likely binds to the substrate recognition site but may interact with an additional sodium-dependent site of NET as DMI is more lipophilic and bulky than norepinephrine 8. Uptake-1 can also be inhibited by Na-K-ATPase inhibitors, such as ouabain and by competitive inhibition by other catecholamines/analogs 14. Several non-neuronal cells also uptake norepinephrine by a transportation system similar to Uptake-1 (adrenal medulla, locus ceruleus, lung, heart, endothelial cells of small vessels, dental polyp, myometrial cells, placenta, vas deferens, syncytiotrophoblasts, and in glial cells in the CNS) 15,16.
The majority of NET is freely mobile within the cytoplasm when it is either unoccupied or fully occupied with its substrate and co-substrates; however, once sodium binds to NET, this mobility is lost. Thus, NET localization to the cell membrane is dependent on the presence of an inward sodium gradient created by the Na+-K+-ATPase pump. Sodium and chloride binding to NET also decreases the Michaelis Constant (Km) for norepinephrine transportation, increasing the affinity of NET for norepinephrine. NET, therefore, requires sodium for both membrane localization and active transport of its substrates. NET also requires free disulfide bonds, free disulfide groups, and lipids to function. NET is recruited to the cell membrane via N-linked glycosylation in the extracellular loop between domains 3 and 4 for cell surface expression 16. This glycosylation is required for cell surface expression, but not for substrate or inhibitor recognition 17,18. Paradoxically, N-glycosylation may also be partially responsible for the efflux of norepinephrine and its analogs from cells 8.
Norepinephrine is also brought into cells by passive, non-specific diffusion (a process known as Uptake-2) that is energy-independent, unsaturable, and results in low-level norepinephrine accumulation in most tissues. After it gains entry, neuronal/adrenal cells store norepinephrine within numerous neurosecretory vesicles via the vesicular monoamine transporter (VMAT). The mechanisms of uptake and retention of mIBG are similar yet significantly differ from endogenous norepinephrine.
mIBG
In the 1980 s, Dr. Wieland and his colleagues at the University of Michigan developed mIBG, an analog of norepinephrine, as a scintigraphic agent to allow imaging of the adrenal medulla. They showed that mIBG, accumulates in the neurosecretory granules of adrenal chromaffin cells, similarly to norepinephrine 19. In 1984, mIBG was also found to accumulate in neuroblastoma 5. Numerous studies since then have provided a more detailed understanding of the mechanisms of mIBG's uptake, efflux, and retention in neural crest derived tumors.
mIBG Uptake
The majority of mIBG uptake in neuroblastoma cells is by active transport (Uptake-1), which is approximately 50 times more efficient than passive transport 8,20. DMI and other noradrenaline analogs can inhibit Uptake-1, thus patients undergoing 131I-mIBG therapy are asked to minimize concomitant medications such as tricyclic antidepressants in the recognition that these and possibly other drugs may affect Uptake-1. Any decrease in the activity of the Na/K-ATPase leads to reduced uptake and increased outward transport of norepinephrine and its analogs. Thus, if the neuroendocrine cells are located in a hypoxic and glucose-depleted microenvironment, there is decreased ATP synthesis, an increase in intracellular sodium concentration, and enhanced outward transport of norepinephrine/mIBG. These features, common to many cancers, may thus limit mIBG uptake 16. Additionally, norepinephrine and mIBG are stored in cells with large serotonin storing capacities, such as in platelets, which may contribute to the significant thrombocytopenia commonly noted in patients after high-dose mIBG therapy 21.
mIBG Retention
After 131I-mIBG gains entry, cancer cells store mIBG in neurosecretory vesicles via the VMAT, delivering and retaining targeted radiation within the cancer cells of gastrointestinal stromal cell tumors, pheochromocytomas, and other well-differentiated tumors 22. Similarly to NET, VMAT expression is thought to be an important tool to select candidates for uptake studies prior to significant radiation exposures 23,24. However, studies have shown that in neuroblastoma, the majority of mIBG remains in the cytoplasm and/or may be stored in mitochondria, presumably because neuroblastoma cells tend to have fewer storage vesicles than more mature and/or differentiated cancer cells 25–27. In neuroblastoma cells, only 7% of mIBG is stored in neurosecretory granules or vesicles as revealed by electron microscopy, while 93% is stored within the cytoplasm 28. Servidei and his colleagues confirmed this finding when they stimulated vesicular release of mIBG by a calcium-dependent process and found <15% of total mIBG efflux from cells was from the neurosecretory vesicles 29. Thus, VMAT expression likely does not play as significant a role in mIBG efficacy as NET expression for patients with neuroblastoma. Therefore, blocking VMAT is unlikely to provide a successful strategy for increasing mIBG retention. This theory is further supported by studies showing that the inhibition of catecholamine storage in neurosecretory vesicles by directly inhibiting VMAT with reserpine did not promote loss of mIBG from neuroblastoma 14,27. mIBG is not degraded by mitochondrial monoamine oxidase (like norepinephrine), but mIBG may be stored (possibly in large amounts) in the mitochondria and the cytoplasm 30.
mIBG Efflux
mIBG often follows a diffusion gradient out of the cells and requires enough NET protein expression for re-uptake of the radioactively labeled mIBG to exert its cytotoxic effects. Efflux is highly temperature-sensitive and is inducible by high extra cellular potassium concentrations or the addition of norepinephrine, unlabeled mIBG, or DMI 29. Efflux may be carrier-mediated by the same NET receptors responsible for mIBG uptake. mIBG efflux is also a saturable process, thus adding more support to the idea that NET reverses directions and transports mIBG out of cells at low intracellular mIBG concentrations. At high intracellular concentrations, mIBG efflux is due to the passive transport across the cell membrane secondary to the diffusion gradient created after mIBG uptake. NET-mediated mIBG efflux is further supported by Servidei's study, which showed that DMI likely immobilizes NET outside of the cell membrane; whereas sodium-depleted media, calcium channel blockers, cell depolarization by hyperkalemia, and additional unradiolabeled mIBG all enhanced mIBG efflux 29. Studies by Lashford et al. show 66% of the injected radioactivity remained in the cells 24 hr later. However, upon adding DMI to block NET, only 10% of injected radioactivity was detected, likely secondary to the inhibition of the reuptake of mIBG by NET. Lashford demonstrated that mIBG typically is rapidly lost from the cells and then undergoes reuptake back into the cells via NET 28. Therefore, not only is NET important for uptake, but also mIBG accumulation likely depends on a dynamic equilibrium generated by the re-uptake of the released drug 25. We can also conclude the main efflux mechanisms of mIBG are likely carrier-mediated efflux and passive diffusion. To improve the cytotoxic effect of mIBG, stimulation/enhancement of mIBG uptake and/or inhibition of mIBG release may be important 29.
Clinical Applications of mIBG
mIBG scintigraphy was first clinically utilized in adult patients with pheochromocytoma, another neuroendocrine tumor secreting catecholamines 31,32. In the early 1990's, 131I-mIBG was first found to be safe for use in children and to have a therapeutic benefit in patients with relapsed/refractory neuroblastoma 33–35. Escalating doses of 131I-mIBG showed efficacy until the maximum tolerated dose of 18 mCi/kg 35. A few small clinical trials increased the number of cycles and/or the cumulative dose of 131I-mIBG, which also demonstrated feasibility and efficacy 21,36–42. Today, mIBG remains an investigational therapy and continues to offer palliative pain relief and hope for a cure, mainly in some form of multi-modal therapy.
Current relapsed/refractory neuroblastoma protocols utilizing 131I-mIBG alongside high-dose chemotherapy and autologous stem cell rescue (ASCR) also demonstrate outcome improvement but not significantly increased long-term survival 30,43–46. Several recent European studies show efficacy of 131I-mIBG as an induction monotherapy or in combination with multiple-drug chemotherapy for newly diagnosed high-risk neuroblastoma 47–50. The Children's Oncology Group is currently evaluating 131I-mIBG during induction therapy with five cycles of multiple chemotherapy agents, surgical resection and ASCR for high-risk neuroblastoma in the pilot study ANBL-09P1. However, in spite of all of these efforts, the discrepancy between mIBG uptake and therapy response remains.
131I-mIBG uptake in tumors in children with neuroblastoma is less than 0.1% of the injected dose per gram of tumor 51. A “no-carrier added” (NCA) preparation of 131I-mIBG enhances the specific uptake and antitumor efficacy of 131I-mIBG in preclinical studies 20,52–54. However, the utility of such preparation in patients is not yet known as the increased treatment efficacy and improved imaging did not translate in the few small Phase I/II clinical trials reported to date 55,56. Further randomized clinical trials are required to evaluate the clinical utility of NCA-mIBG.
Multiple infusions of 131I-mIBG, with both higher cumulative and fractionating doses, have yielded mixed results in small Phase I/II clinical trials. While many trials show efficacy, significant improvements in long-term outcomes in children with neuroblastoma remain to be seen 35,41. Additionally, myelotoxicity, secondary malignancy, and other systemic effects occasionally occur acutely and as late-effects in patients who received mIBG radiotherapy, further demonstrating the need for a comprehensive understanding of 131I-mIBG therapy to improve its therapeutic window 57–59.
The clinical therapeutic benefit exists due to 131I-mIBG's pleomorphic effects on cells with high NET protein expression 35. Certainly some of the cytotoxic effect on cells is due to the emission of β-radiation, inducing DNA strand breaks and inducing cell death. However, the majority of cytotoxicity is thought to be due to the phenomenon known as the radiation-induced bystander effect. In this bystander effect, non-irradiated neighboring cells are affected by soluble factors transmitted from the irradiated cell through direct gap junction intercellular communications and by signaling molecules released from the irradiated cell into the immediate surrounding microenvironment 60–63. The cytotoxic effects on bystander cancer cells and/or normal tissues largely remain unclear, but studies have shown radiation-induced bystander effects to cause apoptosis and other forms of cell death, oxidative stress, proliferation, and genomic instability. Even non-radioactive mIBG exhibits cytotoxic effects, thought in part to be due to its guanidinylated side chain inhibiting neuroendocrine cellular mono ADP-ribosyl transferases 64,65 and possibly due to effects on mitochondria 64,66–69. However, the clinical significance of unlabeled mIBG's direct cytotoxicity is minimal 70.
Enhancing NET Expression
According to a recent study by the Children's Oncology Group, children with high-risk neuroblastoma frequently have negative 123I-mIBG scans and a lower mRNA and protein expression of NET in their tumors by PCR and protein analysis 6, possibly related to the undifferentiated state of high-risk neuroblastomas, since NET expression is a property of relatively mature neuroendocrine/neural cells 71,72. Low NET protein expression is likely a barrier for efficacy, though given the limited tools available; a correlation between 131I-mIBG dosimetry and 131I-mIBG efficacy in clinical trials has not been established 73,74.
It follows from these studies that one strategy to improve clinical responses in patients with neuroblastoma is to either be more selective of eligible patients or to undertake strategies to increase NET protein expression on tumor cells (Fig. 1) 7,75,76. Selecting patients for 131I-mIBG therapy eligibility is possible by testing one's tumor sample for NET mRNA by PCR analysis 7, but this approach will not help those with low or absent NET protein expression. Early studies found only a subset of neuroblastoma cell lines demonstrate specific uptake of radiolabeled mIBG while the other neuroblastoma lines show only passive diffusion of the drug into the cells 11. Interestingly, ionizing radiation itself increases mIBG uptake in SK-N-SH neuroblastoma cells in vitro, suggesting there could be a positive-feedback effect 77. Priming doses of radiation may thus show benefit in mIBG therapy, a concept not yet tested clinically.
It is also possible to pharmacologically stimulate NET expression. Neuroblastoma and adult neuroendocrine tumor cells treated with interferon-gamma and/or alpha prior to mIBG show increased mRNA NET expression, increased 131I-mIBG uptake and increased 131I-mIBG retention. This enhancement of NET expression and/or function may be due to tumor cell differentiation and maturation 70,72,78. The effects of retinoic acid are more controversial. An early study showed retinoic acid-induced terminal neuronal differentiation enhanced mIBG uptake and retention in vitro, but this was not a direct effect of retinoic acid on the neuroblastoma cells 79. Retinoic acid had no effect on mIBG uptake in another early study 80. A third study showed increased mIBG uptake in neuroblastoma cells treated with retinoids combined with gamma-interferon 72. The effect of retinoic acid on neuroblastoma mIBG uptake remains unclear due to these conflicting studies and requires further investigation.
In another neuroblastoma experiment in vitro, treatment with corticosteroids upregulated NET mRNA expression in a dose-dependent manner prior to 131I-mIBG exposure. Prolonged exposure over three weeks increased NET protein expression by almost 250% and enhanced norepinephrine uptake in neuroblastoma cells but downregulated NET mRNA expression in vitro 81. No studies have been done to date showing this same effect in vivo.
Pretreatment with cisplatin and doxorubicin, both active chemotherapy agents in treating patients with neuroblastoma, also increases NET mRNA expression and increases Uptake-1 transportation of mIBG in vitro and in vivo 82,83. Cisplatin has been studied in combination with 131I-mIBG in several small clinical trials in children in Italy and showed two patients with a complete response and 14 patients had a partial response of 21 total patients 48,84,85.
Several signaling pathways have been implicated in regulating NET expression, suggesting that targeted therapies may have utility in enhancing mIBG therapy. Protein kinase C (PKC) activation causes decreased NET protein surface expression and/or NET transport activity via PKC-dependent and PKC-independent pathways. Decreased norepinephrine uptake was noted in the presence of PKC activation and phorbol esters (which inhibit PKC) increased NET expression and norepinephrine uptake as well 86. Thus, as PKC inhibitors are developed, they may be useful in upregulating NET. Other intracellular signaling cascades involved in augmenting the activity of membrane-localized NET include mitogen activated protein kinase (MAPK), phosphatidyl inositol-3 kinase (PI3K), and calcium/calmodulin dependent protein kinase (CaMK). Brief membrane depolarization of neuroblastoma cells with potassium chloride rapidly enhances mIBG uptake via the calcium/calmodulin pathway. Potassium chloride may act through CaMKII and myosin light chain kinase to upregulate the functional capacity surface NET 87. Further exploitation of the calcium/calmodulin pathway could thus also lead to increased NET expression. Caution is required for interpreting cell line studies, however, which may not correlate with effects on cells within a tumor microenvironment.
Radiosensitizers
HDAC inhibitors, such as Vorinostat, are another category of novel anti-cancer therapeutics under clinical investigation that may play a role in enhancing 131I-mIBG therapy. HDAC inhibitors have radiosensitizing effects in a variety of cancer models 88. Vorinostat inhibits the expression of double strand DNA break repair enzyme Ku-86 and also prolongs the expression of phosphorylated γH2AX; thus, Vorinostat sensitizes cells to radiation by inhibiting repair of radiation-induced DNA damage. HDAC inhibitors also increase NET protein expression and demonstrate an additive effect with 131I-mIBG radiotherapy in neuroblastoma cell cytotoxicity 89. The use of Vorinostat prior to mIBG is being tested in a current clinical trial (www.clinicaltrials.gov, NCT02035137).
Proteasome inhibitors are currently in preclinical evaluation for use in combination with 131I-mIBG for neuroblastoma. Proteasome inhibitors downregulate NF-κB and induce cell cycle arrest in the G2/M phase, thus leading to radiosensitization of cancer cells. Proteasome inhibitors, such as Bortezomib, further enhance targeted radiotherapy by enhancing the effect of HDAC inhibitors and topoisomerase I inhibitors. Early studies suggest the combination of proteasome inhibition and 131I-mIBG radiation shows promise for enhancing radiation-induced cancer cell kill 90.
Topoisomerase I inhibitors increase mIBG uptake in neuroblastoma cells and disrupt DNA repair, thus also serving as radiosensitizers 91. Topotecan is active as monotherapy for some patients with refractory neuroblastoma 92. Irinotecan, in combination with vincristine and escalating doses of 131I-mIBG, also shows a 25% complete or partial response rate in patients with relapsed/refractory disease 93. When combined with 131I-mIBG, topoisomerase inhibitors induce supra-additive levels of cancer cytotoxicity and increased efficacy against neuroblastoma xenografts in vivo 94.
Even further efficacy was noted in vitro and in vivo when PJ34, a poly (ADP-ribose) polymerase (PARP) inhibitor, was added to mIBG and Topotecan. Disruption of PARP activity leads to further disruption in DNA repair pathways, increased formation of double-stranded DNA breaks, and increased G2/M cell cycle arrest (which thus enhances radiosensitivity). Another contribution to this combination's anti-tumor efficacy is the simultaneous inhibition of PARP-1 by PJ34 and PARP-3 by mIBG. Of note, resistance to radiation developed in cancer cells after pretreatment with the PARP-inhibitor PJ34 94. Further investigation utilizing a PARP-1/2 inhibitor (MK-4827) together with irradiation in neuroblastoma cells revealed radiosensitization by inhibition of DNA repair, enhanced cytotoxicity, and improved survival in a metastatic murine model compared to either MK-4827 or radiation monotherapy. Preclinically, a PARP inhibitor and 131I-mIBG combination holds promise for more efficacious targeted radiotherapy 95.
Gene therapy is also effective in increasing both NET expression and mIBG uptake in vitro and in vivo 96–98. Neuroblastoma cell lines can be transfected with the NET gene and induced to actively take-up mIBG when they previously only demonstrated passive mIBG diffusion 97. However, further targeting cytotoxicity to cancer cells by gene therapy is dependent upon achieving selective expression of therapeutic transgenes in tumors. Gene promoters stimulated by ionizing radiation are especially of interest in the field of radiotherapy; promoters regulating early growth response gene 1 (Egr-1), the bacterial RecA gene, GADD45a, the NF-κB binding site of the c-IAP2 gene, and the promoter WAF1 for the p21WAF1/CIP1 gene all show promise. The WAF1 promoter is of particular interest to neuroblastoma as it displays radiation-, tumor- and hypoxia-specificity in addition to increasing NET mRNA expression and increasing 131I-mIBG uptake with both external beam gamma radiation and with the radionuclide, 211astatine (At)-mABG 99. Notably, gene therapy with NET creates the potential for utilizing this targeted radiation therapy for tumors that don't normally express NET.
Further investigation into interferon gamma, HDAC inhibitors, PKC modulation, gamma irradiation, and gene therapy are also necessary to improve the clinical efficacy of 131I-mIBG radiotherapy, as these treatments demonstrate pre-clinical and/or clinical efficacy, as summarized in Table1. Utilization of other radionuclides, such as 4-methylated 131I-mIBG (able to retain mIBG within neuroblastoma cells longer), and 211At-mABG may improve the therapeutic window of radiotherapy as its shorter wavelength may cause less damage to surrounding normal tissue 60,63. It is likely that curative therapy for neuroblastoma will require combinations of such targeted and/or molecular therapies.
I.
Summary of Enhancing NET Expression and 131I-mIBG Efficacy in Pre-clinical and Clinical Trials
| Treatment | Preclinical data | Clincal data | References | |
|---|---|---|---|---|
| Giving more 131I-mIBG | Escalating doses of 131I-mIBG | n/a | + | 35 |
| Increasing cycles and/or cumulative doses of 131I-mIBG | n/a | + | 21,35–42 | |
| 131I-mIBG as first-line therapy | 131I-mIBG given in induction or consolidation + / − CT + / − ASCR | 0 | + | 47–50 |
| 131I-mIBG (relapsed/refractory) | 131I-mIBG + HDCT + / − ASCR | + | + | 30,33–35,43–46 |
| No-carrier added 131I-mIBG | + | = | 20,52–57 | |
| 211At-mABG,125I-mIBG | + | 0 | 61,64 | |
| Increasing NET expression | Cisplatin/Doxorubicin | + | + | 48,83–86 |
| Differentiating agents | Retinoic acid | + / − | 0 | 73,80,81 |
| IFN alpha | + | 0 | 73 | |
| IFN gamma | + | 0 | 71–73,79,81 | |
| Modulating intracellular cascades | Protein Kinase C, CaMK | + | 0 | 87,88 |
| HDAC inhibitors | + | + | 89,90 | |
| Corticosteroids | + | 0 | 82 | |
| Hyperthermia | − | − | 12 | |
| Radiosensitization | HDAC inhibitors | + | + | 89 |
| Proteasome inhibitors | + | 0 | 91 | |
| Topoisomerase inhibitors | + | P | 92–94 | |
| PARP inhibitors | + | 0 | 95,96 | |
| Ionizing radiation | External beam radiation | + | 0 | 78,100 |
| Gene therapy | + | 0 | 97–99 | |
| Hyperbaric O2 | + | 0 | 16 |
(+): increases NET expression/131I-mIBG efficacy, (−): ineffective, (=): no change in 131I-mIBG efficacy or NET, P: pending results, 0: no studies conducted, mIBG: meta-iodobenzylguanidine, mCi: milliCuries, kg: kilogram, ASCR: autologous stem cell rescue, CT: chemotherapy, HDCT: high-dose chemotherapy, NET: norepinephrine transporter, IFN: interferon, CaMK: calcium calmodulin dependent protein kinase, HDAC: histone deacetylase inhibitor, PARP: poly-(ADP-ribose) polymerase.
CONCLUSION
High-risk and relapsed/refractory neuroblastoma remain challenging and children continue to have unacceptably low survival rates despite contemporary multimodal and intensive therapies2,3. While we have made clear advances in recent years with the addition of retinoic acid and anti-GD2 antibody therapy, pediatric oncologists and families continue to struggle with this disease and urgent novel therapies and/or combinations of therapies are necessary. A promising strategy for improving outcomes is to enhance the expression and function of NET by combining chemotherapy and other agents or treatments with 131I-mIBG, including ionizing radiation, phorbol esters, retinoids, interferon-gamma, cisplatin, and doxorubicin. The 30% response rate with mIBG therapy affords hope of a cure if this treatment modality can be fully understood and exploited as a targeted therapy. Limiting toxicities while improving outcomes remains a challenge in oncology but is one that is vitally important in neuroblastoma children who are already at significant risk of multiple toxicities after standard treatment.
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number T32HD043003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- Castleberry RP. Neuroblastoma. Eur J Cancer. 1997;33:1430–1437. doi: 10.1016/s0959-8049(97)00308-0. discussion 1437-1438. [DOI] [PubMed] [Google Scholar]
- Matthay KK. Intensification of therapy using hematopoietic stem-cell support for high-risk neuroblastoma. Pediatr Transplant. 1999;3:72–77. doi: 10.1034/j.1399-3046.1999.00070.x. [DOI] [PubMed] [Google Scholar]
- Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JS, Gains JE, Moroz V. A systematic review of (131)I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer. 2014;50:801–815. doi: 10.1016/j.ejca.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Treuner J, Feine U, Niethammer D. Scintigraphic imaging of neuroblastoma with [131-I]iodobenzylguanidine. Lancet. 1984;1:333–334. doi: 10.1016/s0140-6736(84)90375-1. [DOI] [PubMed] [Google Scholar]
- Dubois SG, Geier E, Batra V. Evaluation of Norepinephrine Transporter Expression and Metaiodobenzylguanidine Avidity in Neuroblastoma: A Report from the Children's Oncology Group. Int J Mol Imaging. 2012;2012:250834. doi: 10.1155/2012/250834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin S, Mairs RJ, McCluskey AG. Development of a real-time polymerase chain reaction assay for prediction of the uptake of meta-[(131)I]iodobenzylguanidine by neuroblastoma tumors. Clin Cancer Res. 2003;9:3338–3344. [PubMed] [Google Scholar]
- Bönisch H, Brüss M. The norepinephrine transporter in physiology and disease. Handb Exp Pharmacol. 2006:485–524. doi: 10.1007/3-540-29784-7_20. [DOI] [PubMed] [Google Scholar]
- Iversen ll. The uptake of noradrenaline by the isolated perfused rat heart. Br J Pharmacol Chemother. 1963;21:523–537. doi: 10.1111/j.1476-5381.1963.tb02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Ko BH, Paik JY. Characteristics and regulation of 123I-MIBG transport in cultured pulmonary endothelial cells. J Nucl Med. 2006;47:437–442. [PubMed] [Google Scholar]
- Buck J, Bruchelt G, Girgert R. Specific uptake of m-[125I]iodobenzylguanidine in the human neuroblastoma cell line SK-N-SH. Cancer Res. 1985;45:6366–6370. [PubMed] [Google Scholar]
- Armour A, Mairs RJ, Gaze MN. Modification of meta-iodobenzylguanidine uptake in neuroblastoma cells by elevated temperature. Br J Cancer. 1994;70:445–448. doi: 10.1038/bjc.1994.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets LA, Loesberg C, Janssen M. Active uptake and extravesicular storage of m-iodobenzylguanidine in human neuroblastoma SK-N-SH cells. Cancer Res. 1989;49:2941–2944. [PubMed] [Google Scholar]
- Mairs RJ, Gaze MN, Barrett A. The uptake and retention of metaiodobenzyl guanidine by the neuroblastoma cell line NB1-G. Br J Cancer. 1991;64:293–295. doi: 10.1038/bjc.1991.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönisch H, Brüss M. The noradrenaline transporter of the neuronal plasma membrane. Ann N Y Acad Sci. 1994;733:193–202. doi: 10.1111/j.1749-6632.1994.tb17269.x. [DOI] [PubMed] [Google Scholar]
- Kippenberger AG, Palmer DJ, Comer AM. Localization of the noradrenaline transporter in rat adrenal medulla and PC12 cells: evidence for its association with secretory granules in PC12 cells. J Neurochem. 1999;73:1024–1032. doi: 10.1046/j.1471-4159.1999.0731024.x. [DOI] [PubMed] [Google Scholar]
- Melikian HE, Ramamoorthy S, Tate CG. Inability to N-glycosylate the human norepinephrine transporter reduces protein stability, surface trafficking, and transport activity but not ligand recognition. Mol Pharmacol. 1996;50:266–276. [PubMed] [Google Scholar]
- Nguyen TT, Amara SG. N-linked oligosaccharides are required for cell surface expression of the norepinephrine transporter but do not influence substrate or inhibitor recognition. J Neurochem. 1996;67:645–655. doi: 10.1046/j.1471-4159.1996.67020645.x. [DOI] [PubMed] [Google Scholar]
- Wieland DM, Mangner TJ, Inbasekaran MN. Adrenal medulla imaging agents: a structure-distribution relationship study of radiolabeled aralkylguanidines. J Med Chem. 1984;27:149–155. doi: 10.1021/jm00368a008. [DOI] [PubMed] [Google Scholar]
- Mairs RJ, Cunningham SH, Russell J. No-carrier-added iodine-131-MIBG: Evaluation of a therapeutic preparation. J Nucl Med. 1995;36:1088–1095. [PubMed] [Google Scholar]
- Taal BG, Hoefnagel C, Boot H. Improved effect of 131I-MIBG treatment by predosing with non-radiolabeled MIBG in carcinoid patients, and studies in xenografted mice. Ann Oncol. 2000;11:1437–1443. doi: 10.1023/a:1026592025862. [DOI] [PubMed] [Google Scholar]
- Kölby L, Bernhardt P, Levin-Jakobsen AM. Uptake of meta-iodobenzylguanidine in neuroendocrine tumours is mediated by vesicular monoamine transporters. Br J Cancer. 2003;89:1383–1388. doi: 10.1038/sj.bjc.6601276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O, Jakobsen AM, Kölby L. Importance of vesicle proteins in the diagnosis and treatment of neuroendocrine tumors. Ann N Y Acad Sci. 2004;1014:280–283. doi: 10.1196/annals.1294.032. [DOI] [PubMed] [Google Scholar]
- Kölby L, Bernhardt P, Johanson V. Can quantification of VMAT and SSTR expression be helpful for planning radionuclide therapy of malignant pheochromocytomas. Ann N Y Acad Sci. 2006;1073:491–497. doi: 10.1196/annals.1353.051. [DOI] [PubMed] [Google Scholar]
- Smets LA, Janssen M, Metwally E. Extragranular storage of the neuron blocking agent meta-iodobenzylguanidine (MIBG) in human neuroblastoma cells. Biochem Pharmacol. 1990;39:1959–1964. doi: 10.1016/0006-2952(90)90615-r. [DOI] [PubMed] [Google Scholar]
- Montaldo PG, Lanciotti M, Casalaro A. Accumulation of m-iodobenzylguanidine by neuroblastoma cells results from independent uptake and storage mechanisms. Cancer Res. 1991;51:4342–4346. [PubMed] [Google Scholar]
- Gaze MN, Huxham IM, Mairs RJ. Intracellular localization of metaiodobenzyl guanidine in human neuroblastoma cells by electron spectroscopic imaging. Int J Cancer. 1991;47:875–880. doi: 10.1002/ijc.2910470615. [DOI] [PubMed] [Google Scholar]
- Lashford LS, Hancock JP, Kemshead JT. Meta-iodobenzylguanidine (mIBG) uptake and storage in the human neuroblastoma cell line SK-N-BE(2C) Int J Cancer. 1991;47:105–109. doi: 10.1002/ijc.2910470119. [DOI] [PubMed] [Google Scholar]
- Servidei T, Iavarone A, Lasorella A. Release mechanisms of [125I]meta-iodobenzylguanidine in neuroblastoma cells: Evidence of a carrier-mediated efflux. Eur J Cancer. 1995;31A:591–595. doi: 10.1016/0959-8049(95)00042-h. [DOI] [PubMed] [Google Scholar]
- Gaze MN, Wheldon TE, O'Donoghue JA. Multi-modality megatherapy with [131I]meta-iodobenzylguanidine, high dose melphalan and total body irradiation with bone marrow rescue: Feasibility study of a new strategy for advanced neuroblastoma. Eur J Cancer. 1995;31A:252–256. doi: 10.1016/0959-8049(94)e0036-4. [DOI] [PubMed] [Google Scholar]
- Shapiro B, Copp JE, Sisson JC. Iodine-131 metaiodobenzylguanidine for the locating of suspected pheochromocytoma: Experience in 400 cases. J Nucl Med. 1985;26:576–585. [PubMed] [Google Scholar]
- Shapiro B, Sisson JC, Eyre P. 131I-MIBG–a new agent in diagnosis and treatment of pheochromocytoma. Cardiology. 1985;72:137–142. doi: 10.1159/000173960. [DOI] [PubMed] [Google Scholar]
- Lewis IJ, Lashford LS, Fielding S. A phase I/II study of 131I mIBG in chemo-resistant neuroblastoma. The United Kingdom Children's Cancer Study Group (UKCCSG) Prog Clin Biol Res. 1991;366:463–469. [PubMed] [Google Scholar]
- Voûte PA, Hoefnagel CA, de Kraker J. Results of treatment with 131 I-metaiodobenzylguanidine (131 I-MIBG) in patients with neuroblastoma. Future prospects of zetotherapy. Prog Clin Biol Res. 1991;366:439–445. [PubMed] [Google Scholar]
- Matthay KK, DeSantes K, Hasegawa B. Phase I dose escalation of 131I-metaiodobenzylguanidine with autologous bone marrow support in refractory neuroblastoma. J Clin Oncol. 1998;16:229–236. doi: 10.1200/JCO.1998.16.1.229. [DOI] [PubMed] [Google Scholar]
- Garaventa A, Bellagamba O, Lo Piccolo MS. 131I-metaiodobenzylguanidine (131I-MIBG) therapy for residual neuroblastoma: A mono-institutional experience with 43 patients. Br J Cancer. 1999;81:1378–1384. doi: 10.1038/sj.bjc.6694223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani MR, Chiti A, Seregni E. Role of 131I-metaiodobenzylguanidine (MIBG) in the treatment of neuroendocrine tumours. Experience of the National Cancer Institute of Milan. Q J Nucl Med. 2000;44:77–87. [PubMed] [Google Scholar]
- Claudiani F, Stimamiglio P, Bertolazzi L. Radioiodinated meta-iodobenzylguanidine in the diagnosis of childhood neuroblastoma. Q J Nucl Med. 1995;39:21–24. [PubMed] [Google Scholar]
- Kang TI, Brophy P, Hickeson M. Targeted radiotherapy with submyeloablative doses of 131I-MIBG is effective for disease palliation in highly refractory neuroblastoma. J Pediatr Hematol Oncol. 2003;25:769–773. doi: 10.1097/00043426-200310000-00005. [DOI] [PubMed] [Google Scholar]
- Howard JP, Maris JM, Kersun LS. Tumor response and toxicity with multiple infusions of high dose 131I-MIBG for refractory neuroblastoma. Pediatr Blood Cancer. 2005;44:232–239. doi: 10.1002/pbc.20240. [DOI] [PubMed] [Google Scholar]
- Matthay KK, Quach A, Huberty J. Iodine-131–metaiodobenzylguanidine double infusion with autologous stem-cell rescue for neuroblastoma: A new approaches to neuroblastoma therapy phase I study. J Clin Oncol. 2009;27:1020–1025. doi: 10.1200/JCO.2007.15.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, McGlynn B, Saggio J. Safety and efficacy of tandem 131I-metaiodobenzylguanidine infusions in relapsed/refractory neuroblastoma. Pediatr Blood Cancer. 2011;57:1124–1129. doi: 10.1002/pbc.23062. [DOI] [PubMed] [Google Scholar]
- Corbett R, Pinkerton R, Tait D. [131I]metaiodobenzylguanidine and high-dose chemotherapy with bone marrow rescue in advanced neuroblastoma. J Nucl Biol Med. 1991;35:228–231. [PubMed] [Google Scholar]
- Castel V, Cañete A, Melero C. Results of the cooperative protocol (N-III-95) for metastatic relapses and refractory neuroblastoma. Med Pediatr Oncol. 2000;35:724–726. doi: 10.1002/1096-911x(20001201)35:6<724::aid-mpo53>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Matthay KK, Tan JC, Villablanca JG. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: A new approaches to Neuroblastoma Therapy Consortium Study. J Clin Oncol. 2006;24:500–506. doi: 10.1200/JCO.2005.03.6400. [DOI] [PubMed] [Google Scholar]
- Yanik GA, Levine JE, Matthay KK. Pilot study of iodine-131-metaiodobenzylguanidine in combination with myeloablative chemotherapy and autologous stem-cell support for the treatment of neuroblastoma. J Clin Oncol. 2002;20:2142–2149. doi: 10.1200/JCO.2002.08.124. [DOI] [PubMed] [Google Scholar]
- Mastrangelo R, Lasorella A, Troncone L. [131I]metaiodobenzylguanidine in neuroblastoma patients at diagnosis. J Nucl Biol Med. 1991;35:252–254. [PubMed] [Google Scholar]
- Mastrangelo S, Tornesello A, Diociaiuti L. Treatment of advanced neuroblastoma: Feasibility and therapeutic potential of a novel approach combining 131-I-MIBG and multiple drug chemotherapy. Br J Cancer. 2001;84:460–464. doi: 10.1054/bjoc.2000.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker G, Schoot RA, Caron HN. Toxicity of upfront (131)I-metaiodobenzylguanidine ((131)I-MIBG) therapy in newly diagnosed neuroblastoma patients: A retrospective analysis. Eur J Nucl Med Mol Imaging. 2013;40:1711–1717. doi: 10.1007/s00259-013-2510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kraker J, Hoefnagel CA, Caron H. First line targeted radiotherapy, a new concept in the treatment of advanced stage neuroblastoma. Eur J Cancer. 1995;31A:600–602. doi: 10.1016/0959-8049(95)00063-o. [DOI] [PubMed] [Google Scholar]
- Moyes JS, Babich JW, Carter R. Quantitative study of radioiodinated metaiodobenzylguanidine uptake in children with neuroblastoma: Correlation with tumor histopathology. J Nucl Med. 1989;30:474–480. [PubMed] [Google Scholar]
- Bruchelt G, Girgert R, Buck J. Cytotoxic effects of m-[131I]- and m-[125I]iodobenzylguanidine on the human neuroblastoma cell lines SK-N-SH and SK-N-LO. Cancer Res. 1988;48:2993–2997. [PubMed] [Google Scholar]
- Verberne HJ, de Bruin K, Habraken JB. No-carrier-added versus carrier-added123I-metaiodobenzylguanidine for the assessment of cardiac sympathetic nerve activity. Eur J Nucl Med Mol Imaging. 2006;33:483–490. doi: 10.1007/s00259-005-0022-1. [DOI] [PubMed] [Google Scholar]
- Mairs RJ, Russell J, Cunningham S. Enhanced tumour uptake and in vitro radiotoxicity of no-carrier-added [131I]meta-iodobenzylguanidine: implications for the targeted radiotherapy of neuroblastoma. Eur J Cancer. 1995;31A:576–581. doi: 10.1016/0959-8049(95)00052-k. [DOI] [PubMed] [Google Scholar]
- Barrett JA, Joyal JL, Hillier SM. Comparison of high-specific-activity ultratrace 123/131I-MIBG and carrier-added 123/131I-MIBG on efficacy, pharmacokinetics, and tissue distribution. Cancer Biother Radiopharm. 2010;25:299–308. doi: 10.1089/cbr.2009.0695. [DOI] [PubMed] [Google Scholar]
- Matthay KK, Weiss B, Villablanca JG. Dose escalation study of no-carrier-added 131I-metaiodobenzylguanidine for relapsed or refractory neuroblastoma: New approaches to neuroblastoma therapy consortium trial. J Nucl Med. 2012;53:1155–1163. doi: 10.2967/jnumed.111.098624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaventa A, Gambini C, Villavecchia G. Second malignancies in children with neuroblastoma after combined treatment with 131I-metaiodobenzylguanidine. Cancer. 2003;97:1332–1338. doi: 10.1002/cncr.11167. [DOI] [PubMed] [Google Scholar]
- Polishchuk AL, Dubois SG, Haas-Kogan D. Response, survival, and toxicity after iodine-131-metaiodobenzylguanidine therapy for neuroblastoma in preadolescents, adolescents, and adults. Cancer. 2011;117:4286–4293. doi: 10.1002/cncr.25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois SG, Messina J, Maris JM. Hematologic toxicity of high-dose iodine-131-metaiodobenzylguanidine therapy for advanced neuroblastoma. J Clin Oncol. 2004;22:2452–2460. doi: 10.1200/JCO.2004.08.058. [DOI] [PubMed] [Google Scholar]
- Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill C, Seymour C. Radiation-induced bystander and other non-targeted effects: Novel intervention points in cancer therapy. Curr Cancer Drug Targets. 2006;6:447–454. doi: 10.2174/156800906777723976. [DOI] [PubMed] [Google Scholar]
- Bishayee A, Rao DV, Bouchet LG. Protection by DMSO against cell death caused by intracellularly localized iodine-125, iodine-131 and polonium-210. Radiat Res. 2000;153:416–427. doi: 10.1667/0033-7587(2000)153[0416:pbdacd]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd M, Ross SC, Dorrens J. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with alpha-, beta-, and auger electron-emitting radionuclides. J Nucl Med. 2006;47:1007–1015. [PubMed] [Google Scholar]
- Smets LA, Bout B, Wisse J. Cytotoxic and antitumor effects of the norepinephrine analogue meta-iodo-benzylguanidine (MIBG) Cancer Chemother Pharmacol. 1988;21:9–13. doi: 10.1007/BF00262730. [DOI] [PubMed] [Google Scholar]
- Mairs RJ, Angerson WJ, Babich JW. Differential penetration of targeting agents into multicellular spheroids derived from human neuroblastoma. Prog Clin Biol Res. 1991;366:495–501. [PubMed] [Google Scholar]
- Wang SS, Hsiao R, Limpar MM. Destabilization of MYC/MYCN by the mitochondrial inhibitors, metaiodobenzylguanidine, metformin and phenformin. Int J Mol Med. 2014;33:35–42. doi: 10.3892/ijmm.2013.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaze MN, Mairs RJ, Boyack SM. 131I-meta-iodobenzylguanidine therapy in neuroblastoma spheroids of different sizes. Br J Cancer. 1992;66:1048–1052. doi: 10.1038/bjc.1992.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen J, Van Kuilenburg AB, Voûte PA. MIBG causes oxidative stress and up-regulation of anti-oxidant enzymes in the human neuroblastoma cell line SK-N-BE(2c) Int. J Cancer. 1997;72:486–490. doi: 10.1002/(sici)1097-0215(19970729)72:3<486::aid-ijc17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Cornelissen J, Wanders RJ, Van den Bogert C. Meta-iodobenzylguanidine (MIBG) inhibits malate and succinate driven mitochondrial ATP synthesis in the human neuroblastoma cell line SK-N-BE(2c) Eur. J Cancer. 1995;31A:582–586. doi: 10.1016/0959-8049(95)00045-k. [DOI] [PubMed] [Google Scholar]
- DuBois SG, Matthay KK. Radiolabeled metaiodobenzylguanidine for the treatment of neuroblastoma. Nucl Med Biol. 2008;35:S35–48. doi: 10.1016/j.nucmedbio.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldo PG, Carbone R, Cornaglia Ferraris P. Interferon-γ-induced differentiation of human neuroblastoma cells increases cellular uptake and halflife of metaiodobenzylguanidine. Cytotechnology. 1993;11:S140–143. doi: 10.1007/BF00746080. [DOI] [PubMed] [Google Scholar]
- Montaldo PG, Raffaghello L, Guarnaccia F. Increase of metaiodobenzylguanidine uptake and intracellular half-life during differentiation of human neuroblastoma cells. Int J Cancer. 1996;67:95–100. doi: 10.1002/(SICI)1097-0215(19960703)67:1<95::AID-IJC16>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Rutgers M, Buitenhuis CK, Hoefnagel CA. Targeting of meta-iodobenzylguanidine to SK-N-SH human neuroblastoma xenografts: Tissue distribution, metabolism and therapeutic efficacy. Int J Cancer. 2000;87:412–422. doi: 10.1002/1097-0215(20000801)87:3<412::aid-ijc16>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Matthay KK, Panina C, Huberty J. Correlation of tumor and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with (131)I-MIBG. J Nucl Med. 2001;42:1713–1721. [PubMed] [Google Scholar]
- Mairs RJ, Livingstone A, Gaze MN. Prediction of accumulation of 131I-labelled meta-iodobenzylguanidine in neuroblastoma cell lines by means of reverse transcription and polymerase chain reaction. Br J Cancer. 1994;70:97–101. doi: 10.1038/bjc.1994.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode HN, Bruchelt G, Seitz G. Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of monoamine transporters in neuroblastoma cell lines: Correlations to meta-iodobenzylguanidine (MIBG) uptake and tyrosine hydroxylase gene expression. Eur J Cancer. 1995;31A:586–590. doi: 10.1016/0959-8049(95)00039-l. [DOI] [PubMed] [Google Scholar]
- Smets LA, Janssen M, Rutgers M. Pharmacokinetics and intracellular distribution of the tumor-targeted radiopharmaceutical m-iodo-benzylguanidine in SK-N-SH neuroblastoma and PC-12 pheochromocytoma cells. Int J Cancer. 1991;48:609–615. doi: 10.1002/ijc.2910480421. [DOI] [PubMed] [Google Scholar]
- Höpfner M, Sutter AP, Huether A. A novel approach in the treatment of neuroendocrine gastrointestinal tumors: Additive antiproliferative effects of interferon-gamma and meta-iodobenzylguanidine. BMC Cancer. 2004;4:23. doi: 10.1186/1471-2407-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavarone A, Lasorella A, Servidei T. Uptake and storage of m-iodobenzylguanidine are frequent neuronal functions of human neuroblastoma cell lines. Cancer Res. 1993;53:304–309. [PubMed] [Google Scholar]
- Montaldo PG, Carbone R, Ponzoni M. gamma-Interferon increases metaiodobenzylguanidine incorporation and retention in human neuroblastoma cells. Cancer Res. 1992;52:4960–4964. [PubMed] [Google Scholar]
- Sun Z, Fan Y, Zha Q. Corticosterone up-regulates expression and function of norepinephrine transporter in SK-N-BE(2)C cells. J Neurochem. 2010;113:105–116. doi: 10.1111/j.1471-4159.2010.06587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour A, Cunningham SH, Gaze MN. The effect of cisplatin pretreatment on the accumulation of MIBG by neuroblastoma cells in vitro. Br J Cancer. 1997;75:470–476. doi: 10.1038/bjc.1997.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meco D, Lasorella A, Riccardi A. Influence of cisplatin and doxorubicin on 125I-meta-iodobenzylguanidine uptake in human neuroblastoma cell lines. Eur J Cancer. 1999;35:1227–1234. doi: 10.1016/s0959-8049(99)00078-7. [DOI] [PubMed] [Google Scholar]
- Mastrangelo R, Tornesello A, Riccardi R. A new approach in the treatment of stage IV neuroblastoma using a combination of [131I]meta-iodobenzylguanidine (MIBG) and cisplatin. Eur J Cancer. 1995;31A:606–611. doi: 10.1016/0959-8049(95)00048-n. [DOI] [PubMed] [Google Scholar]
- Mastrangelo R, Tornesello A, Lasorella A. Optimal use of the 131-I-metaiodobenzylguanidine and cisplatin combination in advanced neuroblastoma. J Neurooncol. 1997;31:153–158. doi: 10.1023/a:1005770405844. [DOI] [PubMed] [Google Scholar]
- Apparsundaram S, Galli A, DeFelice LJ. Acute regulation of norepinephrine transport: I. protein kinase C-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J Pharmacol Exp Ther. 1998;287:733–743. [PubMed] [Google Scholar]
- Chung HW, Park JW, Lee EJ. 131I-MIBG targeting of neuroblastoma cells is acutely enhanced by KCl stimulation through the calcium/calmodulin-dependent kinase pathway. Cancer Biother Radiopharm. 2013;28:488–493. doi: 10.1089/cbr.2012.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Yang X, Sottero TL. Cooperation of the HDAC inhibitor vorinostat and radiation in metastatic neuroblastoma: Efficacy and underlying mechanisms. Cancer Lett. 2011;306:223–229. doi: 10.1016/j.canlet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More SS, Itsara M, Yang X. Vorinostat increases expression of functional norepinephrine transporter in neuroblastoma in vitro and in vivo model systems. Clin Cancer Res. 2011;17:2339–2349. doi: 10.1158/1078-0432.CCR-10-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C, Tesson M, Babich JW. Radiosensitization of noradrenaline transporter-expressing tumour cells by proteasome inhibitors and the role of reactive oxygen species. EJNMMI Res. 2013;3:73. doi: 10.1186/2191-219X-3-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey AG, Boyd M, Ross SC. [131I]meta-iodobenzylguanidine and topotecan combination treatment of tumors expressing the noradrenaline transporter. Clin Cancer Res. 2005;11:7929–7937. doi: 10.1158/1078-0432.CCR-05-0982. [DOI] [PubMed] [Google Scholar]
- Nitschke R, Parkhurst J, Sullivan J. Topotecan in pediatric patients with recurrent and progressive solid tumors: A Pediatric Oncology Group phase II study. J Pediatr Hematol Oncol. 1998;20:315–318. doi: 10.1097/00043426-199807000-00006. [DOI] [PubMed] [Google Scholar]
- DuBois SG, Chesler L, Groshen S. Phase I study of vincristine, irinotecan, and 131I-metaiodobenzylguanidine for patients with relapsed or refractory neuroblastoma: A new approaches to neuroblastoma therapy trial. Clin Cancer Res. 2012;18:2679–2686. doi: 10.1158/1078-0432.CCR-11-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey AG, Mairs RJ, Tesson M. Inhibition of poly(ADP-Ribose) polymerase enhances the toxicity of 131I-metaiodobenzylguanidine/topotecan combination therapy to cells and xenografts that express the noradrenaline transporter. J Nucl Med. 2012;53:1146–1154. doi: 10.2967/jnumed.111.095943. [DOI] [PubMed] [Google Scholar]
- Mueller S, Bhargava S, Molinaro AM. Poly (ADP-Ribose) polymerase inhibitor MK-4827 together with radiation as a novel therapy for metastatic neuroblastoma. Anticancer Res. 2013;33:755–762. [PMC free article] [PubMed] [Google Scholar]
- Boyd M, Mairs RJ, Mairs SC. Expression in UVW glioma cells of the noradrenaline transporter gene, driven by the telomerase RNA promoter, induces active uptake of [131I]MIBG and clonogenic cell kill. Oncogene. 2001;20:7804–7808. doi: 10.1038/sj.onc.1204955. [DOI] [PubMed] [Google Scholar]
- Cunningham S, Boyd M, Brown MM. A gene therapy approach to enhance the targeted radiotherapy of neuroblastoma. Med Pediatr Oncol. 2000;35:708–711. doi: 10.1002/1096-911x(20001201)35:6<708::aid-mpo49>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Doubrovin MM, Doubrovina ES, Zanzonico P. In vivo imaging and quantitation of adoptively transferred human antigen-specific T cells transduced to express a human norepinephrine transporter gene. Cancer Res. 2007;67:11959–11969. doi: 10.1158/0008-5472.CAN-07-1250. [DOI] [PubMed] [Google Scholar]
- McCluskey AG, Mairs RJ, Sorensen A. Gamma irradiation and targeted radionuclides enhance the expression of the noradrenaline transporter transgene controlled by the radio-inducible p21(WAF1/CIP1) promoter. Radiat Res. 2013;179:282–292. doi: 10.1667/RR3030.1. [DOI] [PubMed] [Google Scholar]


