Abstract
5-HT2C receptors play a role in psychoaffective disorders and often contribute to the antidepressant and anxiolytic effects of psychotropic drugs. During stress, activation of these receptors exerts a negative feedback on serotonin (5-HT) release, probably by increasing the activity of GABAergic interneurons. However, to date, the GABA receptor types that mediate the 5-HT2C receptor-induced feedback inhibition are still unknown. To address this question, we assessed the inhibition of 5-HT turnover by a 5-HT2C receptor agonist (RO 60-0175) at the hippocampal level and under conditions of stress, after pharmacological or genetic inactivation of either GABA-A or GABA-B receptors in mice. Neither the GABA-B receptor antagonist phaclofen nor the specific genetic ablation of either GABA-B1a or GABA-B1b subunits altered the inhibitory effect of RO 60-0175, although 5-HT turnover was markedly decreased in GABA-B1a knock-out mice in both basal and stress conditions. In contrast, the 5-HT2C receptor-mediated inhibition of 5-HT turnover was reduced by the GABA-A receptor antagonist bicuculline. Because a significant effect of 5-HT2C receptor activation persisted in mutant mice deficient in the α3 subunit of GABA-A receptors, it can be inferred that non α3 subunit-containing GABA-A receptors, but not GABA-B receptors, mediate the 5-HT2C-induced inhibition of stress-induced increase of hippocampal 5-HT turnover in mice.
Keywords: bicuculline, phaclofen, GABA-A α3 subunit, GABA-B, knockout
Introduction
5-HT2C receptors are thought to play a key role in stress-related disorders (for a review see Martin et al., 2014), not only through their modulatory role on the corticotropin releasing hormone (CRH) neurons in the paraventricular nucleus of the hypothalamus (PVN) that regulates glucocorticoid secretion (Heisler et al., 2007), but also by modulating GABAergic neurotransmission. Indeed, several studies argue for a close interaction between excitatory 5-HT2C receptors, linked to Gαq proteins and phospholipase C, and the inhibitory GABAergic system. On the one hand, 5-HT2C receptors are expressed by GABA interneurons in various brain areas, including the raphe nuclei (Serrats et al., 2005; Boothman et al., 2006; Invernizzi et al., 2007; Liu et al., 2007; Quérée et al., 2009; Bubar et al., 2011). As expected, 5-HT2C receptor agonists increase the activity of GABA neurons in these nuclei (Boothman et al., 2006). Thus, under conditions of elevated 5-HT tone, such as after high doses of selective serotonin reuptake inhibitors (SSRIs; Cremers et al., 2007) or during restraint-stress (Mongeau et al., 2010), 5-HT2C receptor stimulation inhibits 5-HT release and turnover in the hippocampus, probably by activating GABA interneurons. Indeed, SSRIs increase extracellular GABA while 5-HT2C receptor stimulation decreases extracellular 5-HT in the dorsal raphe (Calcagno and Invernizzi, 2010). In keeping with this view, local infusion of either GABA-A or GABA-B receptor agonists into either the median or the dorsal raphe nuclei was found to decrease extracellular 5-HT concentration in the forebrain (e.g. the nucleus accumbens), but GABA-A receptor ligands appeared the most effective in this respect (Tao et al., 1996). Conversely, Cremers et al. (2007) showed that a GABA-B receptor antagonist, similarly to a 5-HT2C receptor antagonist, potentiated SSRI-induced 5-HT outflow in the hippocampus whereas a GABA-A receptor antagonist enhanced basal 5-HT outflow. Thus, it appears that both GABA-A and GABA-B receptors participate in the inhibitory control of 5-HT neurotransmission mediated by GABA. However, the data also suggest that the indirect negative feedback signal triggered by 5-HT2C receptor activation under elevated 5-HT tone (under SSRI treatment or stress conditions) is likely to be dependent on GABA-B rather than GABA-A receptors.
GABA-A receptors are ionotropic receptors composed of different subunits (α1–6, β1–3, γ1–3, δ, ε, θ, π and ρ1–3). Various alpha subunits are differentially expressed among brain regions, with the α3 subunit being the prominent in monoaminergic neurons (Fritschy et al., 1992). GABA-B receptors are metabotropic receptors composed of GABA-B1 and GABA-B2 subunits. The GABA-B1 subunit exists in two abundant isoforms, GABA-B1a and GABA-B1b that localize to pre- and post-synaptic elements, respectively (Gassmann and Bettler, 2012).
Inhibition of 5-HT release and 5-HT turnover by 5-HT2C receptor activation was previously observed to occur exclusively in a stress condition and in particular in the hippocampus, a brain area most relevant to stress disorders, and in which 5-HT2C receptor activation with the 5-HT2C/2B receptor agonist RO 60-0175 was previously shown to exert a clear-cut inhibition of 5-HT turnover during stress (Mongeau et al., 2010). The action of RO 60-0175 at 5-HT2C receptors was demonstrated in hippocampal and other areas with the selective 5-HT2C receptor antagonist SB 242,084, which also has an effect by itself during stress in the hippocampus, but not other areas (Mongeau et al., 2010).
Here we investigated whether specific subtypes of GABA-A and GABA-B receptors are involved in this modulation during stress. To this end, we used the following two strategies: (i) individual genetic ablation of either the GABA-B1a, GABA-B1b or the GABA-Aα3 subunit and (ii) pharmacological blockade, using either the GABA-B antagonist phaclofen or the GABA-A antagonist bicuculline.
Materials and methods
Animals
Animals were housed 3–6 per cage (33 × 15 × 13 cm) under standard laboratory conditions (12h light-dark cycle, lights on at 7:00 h, room temperature 21±1°C) with free access to food and water for at least one week before any treatment. All procedures implicating animals were conducted in strict agreement with the institutional guidelines for use of animals and their care, in compliance with national and international laws and policies (council directive no. 87-848, October 19, 1987, Ministère de l’Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection Animale, permission no. 75-977 to L.L.).
Neurochemical experiments with GABA-A receptor inactivation were performed on 2–4 month-old male mice of the C57BL/6J strain from a breeding center (CER Janvier, Le-Genest-St-Isle, France) and GABA-Aα3 subunit deficient mutants and wild-type littermates of the same C57BL/6J genetic background (Yee et al., 2005), raised in Paris. Neurochemical experiments with GABA-B receptor inactivation were performed on 2–4 month-old male mice of the BALB/c strain (CER Janvier) and mutant mice of the same genetic background but deficient in GABA-B1a or GABA-B1b subunits, and their wild-type littermates (Vigot et al., 2006).
Stress procedure
Mice were stressed by physical constraint for 45 minutes inside a 50-ml tube, perforated at the tip to allow breathing, as previously described (Mongeau et al., 2010). They were sacrificed immediately after the stress session and the hippocampi were rapidly dissected on ice for biochemical determinations. Previous studies showed that this restraint-stress enhances 5-HT release as well as 5-HT turnover. The increase in 5-HT turnover is exclusively due to increased 5-hydroxyindoleacetic acid (5-HIAA) tissue levels as 5-HT catabolism increases with 5-HT utilization (Mongeau et al., 2010).
Measurements of 5-HT and 5-HIAA
Tissue levels of 5-HT and its metabolite 5-HIAA were determined using high-pressure liquid chromatography (HPLC) with electrochemical detection (ED). Dissected hippocampi were weighed and immediately homogenized by sonication for 15 s in 250 µL of an extraction solution at 0–4°C (perchloric acid 0.1 M; EDTA 1.34 mM; sodium metabisulfite 0.05% w/v). Homogenates were centrifuged at 30,000 g for 20 min at 4°C and the supernatants were stored at −80°C. Samples were then thawed on ice, neutralized using 2 M KH2PO4/K2HPO4, pH 7.4, and endogenous ascorbic acid was removed using ascorbate oxidase (0.01 mg/ml; Boehringer-Mannheim, Germany). After centrifugation at 20,000 g for 10 min at 4°C, supernatants were collected and aliquots (10 µl) were automatically injected into the HPLC system. 5-HT and 5-HIAA were separated on a Beckman Coulter Ultrasphere 5 µm C18 (250 × 4.6 mm) column, protected by a Brownlee (3 cm, 5 µm) pre-column, using a mobile phase (K2HPO4 70 mM, triethylamine 3.1 mM, EDTA 0.1 mM, methanol 16 % v/v, octane sulphonate 1.05 mM, pH 3) at a flow rate of 1 ml/min. Compounds were oxidized by a coulometric electrochemical detector (ESA, Bedford, MA, USA) with an analytical cell (Model 5011) equipped with two electrodes set at +50 mV and +350 mV. The gain of the detector was set at 100 nA. The signal was sent to a computer and analyzed by an acquisition program (Empower 2, Waters, France). Results are expressed as ng 5-HT or 5-HIAA/g of fresh tissue and 5-HIAA/5-HT ratios (the turnover index which normally increases with utilization as extracellular 5-HT is metabolized into 5-HIAA).
Drugs
The 5-HT2C receptor agonist RO 60-0175 [(S-2-chloro-5-fluoro-indol-1-yl)-1-methylethylamine fumarate] (3 mg/kg; Tocris, Bristol, UK), the GABA-B receptor antagonist phaclofen (2 mg/kg; Sigma-Aldrich, France) and GABA-A receptor antagonist bicuculline (8 mg/kg; Sigma-Aldrich, France) were freshly prepared in saline (0.9% NaCl) solution with a brief sonication, and administered intraperitoneally (i.p.) 30 minutes before initiating the restraint-stress period. The doses chosen were previously shown to be effective at the respective drug targets (Zarrindast and Farahvash, 1994; Dalvi and Rodgers, 1996). We have assessed 5-HT2C receptor function after blockade or deletion of GABA receptors using a 3 mg/kg dose of RO 60-0175 previously shown to act on 5-HT2C receptors, using the selective 5-HT2C receptor antagonist SB 242,084 in neurochemical and behavioural assays (Mongeau et al. 2010, Kennett et al., 2000). Note that a dose of 6 mg/kg of RO 60-0175 inhibits totally the stress-induced increase of 5-HT turnover in C57BL/6J mice (Mongeau et al., 2010). However, in most brain areas, beside the dorsal raphe, RO 60-0175 is without effect by itself on extracellular 5-HT or 5-HT turnover in the basal conditions, that is - in absence of either stress or SSRI administration (Mongeau et al., 2010; Millan et al., 1998; Gobert et al., 2000; Calcagno and Invernizzi, 2010).
Statistical calculations
Most data were compared in pairs using the two-tailed Student’s t-test. Once the inhibitory effect of RO 60-0175 on 5-HT turnover had been verified in each mouse strain (the C57BL/6J and the BALB/c strains being necessary to study the effects of GABA-A and GABA-B receptors inactivation, respectively), data were represented as percentages of respective controls and compared using the one-tailed Student’s t-test.
Results
Effects of stress and 5-HT2C receptor activation on 5-HT turnover in BALB/c vs C57BL/6J mice
The 5-HT2C receptor-mediated inhibition of stress-induced increase in 5-HT turnover was evaluated in the hippocampus of both BALB/c and C57BL/6J mice, because both strains were used to investigate the effects of genetic ablation of either GABA-A or GABA-B receptors on the 5-HT2C-evoked response. Studies with selective GABA-A and GABA-B receptor antagonists were also performed in these mouse strains.
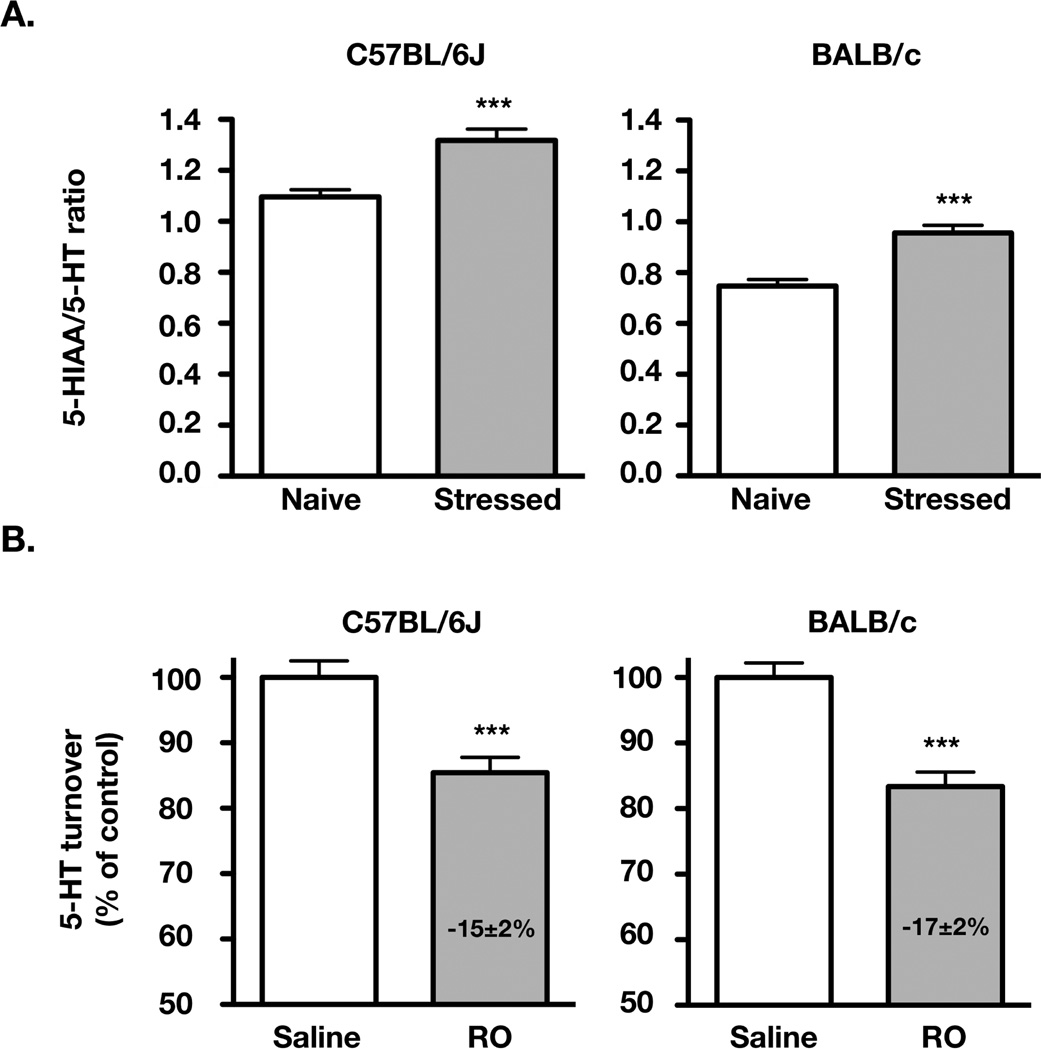
As shown in Fig. 1A, restraint-stress induced a similar increase in 5-HT turnover in C57BL/6J mice (+20±4 % in 5-HIAA/5-HT ratio, compared to naive mice) and BALB/c mice (+28±4 %; means ± S.E.M., n= 14–17 and 20–29, respectively). As expected from our previous study (Mongeau et al., 2010), RO 60-0175 at 3 mg/kg induced a significant decrease (−15±2%; p<0.001) of the overall 5-HIAA/5-HT ratio in C57BL6/J mice. A similar reduction (−17±2%; p<0.001) was observed in BALB/c mice (Fig. 1B). These reductions may appear small, but it is important to consider that only the enhancement of 5-HT turnover by stress can be modulated by RO 60-0175 (Mongeau et al., 2010). We have estimated this stress-induced increase in 5-HT turnover (i.e. the absolute increase in 5-HIAA/5-HT ratio caused by stress over baseline value from naïve mice). We found it to be similarly inhibited (by nearly 70%) in both strains following RO 60-0175 administration (C57BL6/J: saline = 0.200±0.035; RO = 0.065±0.033 n= 14–16; p<0.01; BALB/c: saline = 0.217±0.022; RO = 0.052±0.021; n= 22–28; p<0.0001). The 3 mg/kg dose of RO 60-0175 was thus effective in reducing 5-HT turnover without inducing maximal inhibitory effects in either strains of mice.
Figure 1. 5-HT2C receptor-mediated inhibition by RO 60-0175 of the stress-induced increase in 5-HT turnover in BALB/c and C57BL/6J mice.
A) Effect of restraint stress on the 5-HT turnover [5-HIAA/5-HT ratio; means ± S.E.M.] in the hippocampus of C57BL/6J (n= 14–17) and BALB/c (n= 20–29) WT mice. B) The effects of RO 60-0175 (RO; 3 mg/kg i.p.) are presented as a percentage (means ± S.E.M.) of the 5-HIAA/5-HT ratio of saline-treated mice of the C57BL/6J (n=14–16) and BALB/c (n=22–31) strain. ***p<0.001, two-tail Student’s t-test.
Effects of GABA-B and/or GABA-A receptors inactivation on the 5-HT2C receptor-mediated inhibition of 5-HT turnover in stressed mice
In naive animals, the basal value of 5-HIAA/5-HT ratio did not significantly differ in mutant mice lacking the GABA-Aα3 subunit and littermate WT mice of the C57BL/6J strain (GABA-Aα3−/−= 1.41±0.05, n= 4; WT = 1.35±0.05, n= 4, n.s.). Similarly, genetic ablation of the GABA-B1b subunit had no effect on basal 5-HT turnover in the hippocampus of BALB/c mice (GABA-B1b−/−= 0.78±0.05, WT= 0.72±0.05, means ± S.E.M., n=6–7; n.s.). In contrast, mutants with genetic ablation of the GABA-B1a subunit displayed a significant decrease of 5-HT turnover compared to BALB/c WT mice (GABA-B1a−/−= 0.60±0.04, WT= 0.80±0.04, means ± S.E.M., n= 6–9; p<0.01, Student’s t-test)
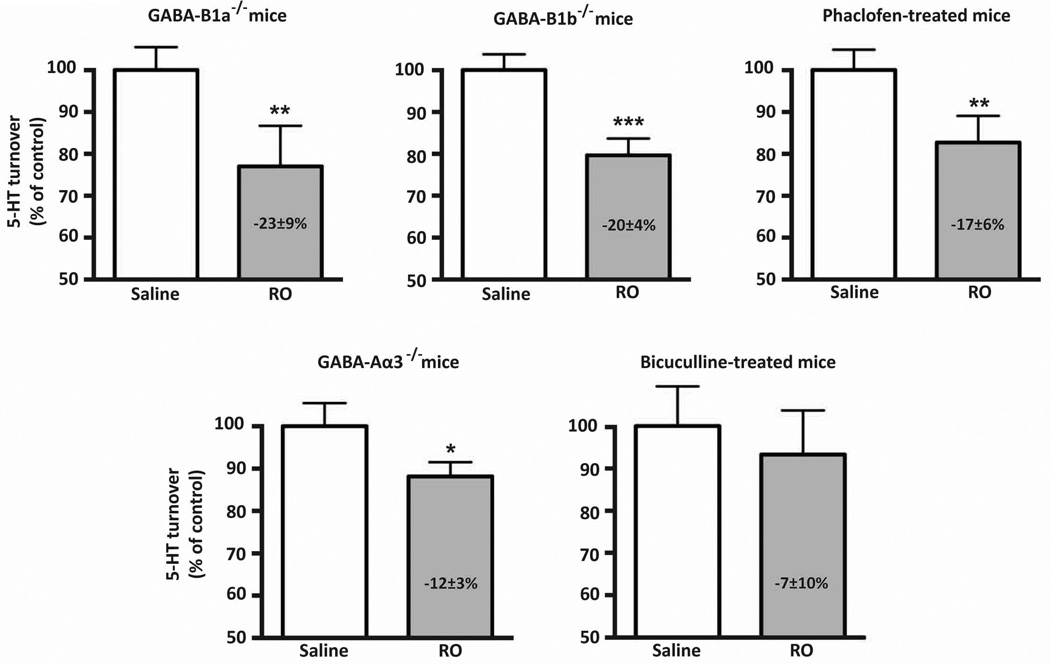
As shown in Fig. 2, in the stress conditions, administration of the 5-HT2C receptor agonist RO 60-0175 reduced 5-HT turnover by about 20% in both GABA-B1a−/− and GABA-B1b−/− mice, similarly to the effect normally observed in BALB/c mice (Fig. 1B). Furthermore, a similar significant reduction by RO 60-0175 of 5-HT turnover was observed after pharmacological blockade of GABA-B receptors with phaclofen in stressed BALB/c WT mice (Fig. 2). In contrast, although RO 60-0715 no longer significantly decreased 5-HT turnover when GABA-A receptors were blocked by bicuculline, an inhibitory effect of the 5-HT2C receptor agonist occurred in stressed GABA-A deficient mice of the GABA-Aα3−/− genotype (−12%; p=0.05; Fig. 2) as in C57BL6/J wild-type mice (Fig. 1B).
Figure 2. Consequences of pharmacological or genetic inactivation of specific GABA receptors on 5-HT2C receptor-mediated inhibition of 5-HT turnover in stressed mice.
The effect of RO 60-0175 (RO; 3 mg/kg, i.p.) on 5-HT turnover under conditions of stress was determined in mice with genetic ablation of either GABA-B1a, GABA-B1b or GABA-Aα3 receptor subunit and in WT mice after pharmacological blockade of GABA-A or GABA-B receptors with bicuculline (8 mg/kg, i.p.) or phaclofen (2 mg/kg, i.p.), respectively. Results are presented as a percentage of the 5-HIAA/5-HT ratio in saline-treated mice of the same genotype (means ± S.E.M.; n= 4–8). * p≤0.05, ** p<0.01, one-tail Student’s t-test.
Discussion
As previously stated, the inhibition of stress-induced increase in 5-HT turnover by 5-HT2C receptor activation is most likely mediated by the GABAergic rather than the CRH system (Mongeau et al., 2010). Indeed, one would expect an increase rather than a reduction of the stress-induced enhancement of 5-HT release in response to 5-HT2C receptor activation by RO 60-0175 if the 5-HT2C receptor effect was to occur through CRH, a neurohormone that increase 5-HT neurons activity (Mo et al., 2008; Martin et al., 2014). Moreover, an indirect negative feedback control of monoaminergic neurotransmission has been shown to occur via GABAergic interneurons. More specifically, an inhibitory effect of 5-HT2A/2C receptor agonists on 5-HT neurotransmission through GABAergic neurotransmission has been demonstrated using electrophysiological approaches (Boothman et al., 2006; Invernizzi et al., 2007; Quérée et al., 2009). Because Cremers and colleagues (2007) found that the GABA-B receptor antagonist phaclofen, like the 5-HT2C receptor antagonists, potentiates the effect of the SSRI citalopram on extracellular 5-HT levels in the ventral hippocampus, it was hypothesized that GABA-B receptors were involved in the 5-HT2C receptor-mediated inhibition of stress-induced increase of 5-HT turnover (Mongeau et al., 2010). However, our present results clearly show that neither the administration of phaclofen nor the specific genetic ablation of GABA-B1a or GABA-B1b subunits prevented this 5-HT2C receptor-mediated inhibition. This suggests that the 5-HT2C receptors that control 5-HT turnover during stress are different from those that regulate 5-HT outflow during acute SSRI treatment.
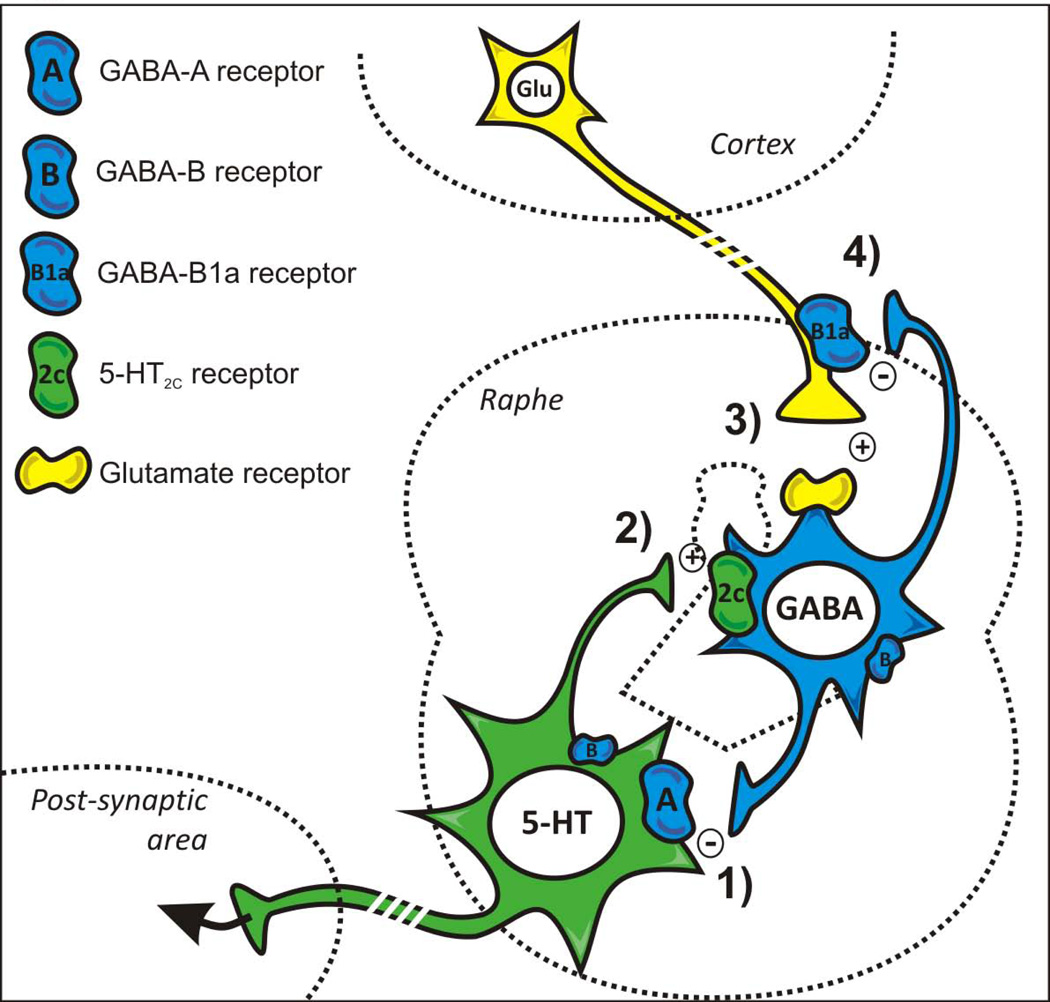
Interestingly, however, GABA-B receptors do appear to regulate 5-HT turnover under basal conditions. Mice lacking the GABA-B1a subunit (but not those lacking GABA-B1b) have a significant decrease in 5-HT turnover compared to WT littermates. Glutamatergic terminals mainly express the GABA-B1a subunit (Vigot et al., 2006) and cortical glutamatergic neurons project to inhibitory GABAergic interneurons in the raphe area (Jankowski and Sesack, 2004). The reduction in 5-HT turnover observed in GABA-B1a−/− mice might thus be explained by a stronger excitatory glutamatergic input onto GABAergic interneurons inhibiting 5-HT neurons in raphe nuclei, resulting from a lack of negative feedback on glutamatergic terminals via GABA-B heteroreceptors (Fig. 3). In keeping with this view, previous studies have shown increased 5-HT neurotransmission following glutamatergic receptor blockade (Lopez-Gil et al., 2007).
Figure 3. Schematic representation of GABAergic interneuron-mediated inhibition of serotonergic neuron activity.
GABAergic interneurons are known to inhibit 5-HT neuronal activity via GABA-A receptors (1). Because of 5-HT release during stress, 5-HT2C receptors expressed by GABAergic interneurons are activated (2), which leads to a GABA-mediated negative feedback control of 5-HT neuron during stress (Mongeau et al., 2010). Glutamatergic (Glu) projections from forebrain (cortex) areas also excite GABAergic interneurons (3), but inhibitory terminal GABA-B1a subunit containing heteroreceptors exert a presynaptic modulation on these projections (4). Accordingly, the excitatory influence of cortical glutamatergic projections onto GABA interneurons is larger in GABA-B1a−/− mice, which would account for a reduced 5-HT turnover in these mutants.
In contrast to phaclofen, the GABA-A receptor antagonist bicuculline prevented the inhibitory effect of RO 60-0175 in the stress condition, indicating that GABA-A receptors were involved in this 5-HT2C receptor-mediated response. Previous pharmacological studies in behaving mice also suggested that GABA-A receptor involvement in 5-HT2C responses (Donatti and Leite-Panissi, 2009; de Oliveira Sergio et al., 2011). The GABA-Aα3 subunit is densely expressed in serotonergic neurons (Fritschy et al., 1992) and is potentially involved in the regulation of stress and anxiety (Dias et al., 2005; Judge et al., 2006; Marowsky et al., 2012). We therefore hypothesized that genetic ablation of the GABA-Aα3 subunit should mimic the effect of bicuculline. However, a significant effect of RO 60-0175 on the stress-induced increase of 5-HT turnover was still observed in mice lacking the GABA-Aα3 subunit. 5-HT neurons in the dorsal raphe nucleus express other alpha subunit isoforms (Pirker et al., 2000). Therefore our results do not exclude that GABA-A receptors on 5-HT neurons mediate the inhibitory response to 5-HT2C receptor activation (Fig. 3). Alternatively, it is possible that GABA-A receptors on GABAergic interneurons expressing the GABA-Aα1 subunit (Gao et al., 1993) contribute to the inhibitory response. Considering that bicuculline is not specific for any of the GABA-A receptor subtypes, our data show that the α3 subunit is, at the very least, not indispensable to the 5-HT2C receptor-mediated response. Because of the multiple subunits constituting the GABA-A receptors throughout the brain (Rudolph and Möhler, 2006), it will remain difficult to determine precisely which GABA-A receptor subtype(s) actually mediate(s) the 5-HT2C receptor-induced negative feedback control of 5-HT neurotransmission in stressed mice.
To conclude, during stress, in addition to the 5-HT2C receptor activation that increases CRH release and produces CRH2 receptor-mediated excitation of 5-HT neurons (Day et al., 2004; Martin et al., 2014), activation of 5-HT2C receptors on GABAergic neurons can trigger a negative-feedback control of serotonergic transmission mainly through GABA-A receptors on 5-HT neurons.
Acknowledgements
This research was supported by European Community DevAnx (Health: F2-2007-201714) Program, by INSERM, Université Pierre et Marie Curie and ANR contract “Sercedit” (ANR-06-Neuro-045-01). CBP Martin was recipient of a fellowship from the French Ministère de la Recherche during performance of this work. U Rudolph was supported by Award Number R01 MH095905 of the National Institute of Mental Health.
Footnotes
ARRIVE guidelines have been followed:
Yes
=> if No, skip complete sentence
=> if Yes, insert "All experiments were conducted in compliance with the ARRIVE guidelines."
Conflicts of interest: U Rudolph has performed professional services for Sunovion and for Concert Pharmaceuticals. The other authors declare no conflict of interest. The funding agencies did not have any role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
=> if 'none', insert "The authors have no conflict of interest to declare."
=> otherwise insert info unless it is already included
References
- Boothman L, Raley J, Denk F, Hirani E, Sharp T. In vivo evidence that 5-HT(2C) receptors inhibit 5-HT neuronal activity via a GABAergic mechanism. Br. J. Pharmacol. 2006;149:861–869. doi: 10.1038/sj.bjp.0706935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Stutz SJ, Cunningham KA. 5-HT(2C) receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PLoS ONE. 2011;6:e20508. doi: 10.1371/journal.pone.0020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno E, Invernizzi RW. Strain-dependent serotonin neuron feedback control: role of serotonin2C receptors. J. Neurochem. 2010;114:1701–1710. doi: 10.1111/j.1471-4159.2010.06880.x. [DOI] [PubMed] [Google Scholar]
- Cremers TIFH, Rea K, Bosker FJ, Wikstrom HV, Hogg S, Mørk A, Westerink BHC. Augmentation of SSRI effects on serotonin by 5-HT2C antagonists: mechanistic studies. Neuropsychopharmacology. 2007;32:1550–1557. doi: 10.1038/sj.npp.1301287. [DOI] [PubMed] [Google Scholar]
- Dalvi A, Rodgers RJ. GABAergic influences on plus-maze behaviour in mice. Psychopharmacology. 1996;128:380–397. doi: 10.1007/s002130050148. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5-HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J. Comp. Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Sheppard WFA, Fradley RL, Garrett EM, Stanley JL, Tye SJ, Goodacre S, Lincoln RJ, Cook SM, Conley R, Hallett D, Humphries AC, Thompson SA, Wafford KA, Street LJ, Castro JL, Whiting PJ, Rosahl TW, Atack JR, McKernan RM, Dawson GR, Reynolds DS. Evidence for a significant role of alpha 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J. Neurosci. 2005;25:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatti AF, Leite-Panissi CRA. GABAergic antagonist blocks the reduction of tonic immobility behavior induced by activation of 5-HT2 receptors in the basolateral nucleus of the amygdala in guinea pigs. Brain Res. Bull. 2009;79:358–364. doi: 10.1016/j.brainresbull.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Fritschy J-M, Benke D, Mertens S, Oertel WH, Bachi T, Möhler H. Five subtypes of type A gamma-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc. Natl. Acad. Sci. USA. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Fritschy J-M, Benke D, Möhler H. Neuron-specific expression of GABAA-receptor subtypes: differential association of the alpha 1- and alpha 3-subunits with serotonergic and GABAergic neurons. Neuroscience. 1993;54:881–892. doi: 10.1016/0306-4522(93)90582-z. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Bettler B. Regulation of neuronal GABA(B) receptor functions by subunit composition. Nat. Rev. Neurosci. 2012;13:380–394. doi: 10.1038/nrn3249. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, Yeo GSH, O'Rahilly S, Colmers WF, Elmquist JK, Tecott LH. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J. Neurosci. 2007;27:6956–6964. doi: 10.1523/JNEUROSCI.2584-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernizzi RW, Pierucci M, Calcagno E, Di Giovanni G, Di Matteo V, Benigno A, Esposito E. Selective activation of 5-HT(2C) receptors stimulates GABA-ergic function in the rat substantia nigra pars reticulata: a combined in vivo electrophysiological and neurochemical study. Neuroscience. 2007;144:1523–1535. doi: 10.1016/j.neuroscience.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J. Comp. Neurol. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Young RL, Gartside SE. GABA(A) receptor modulation of 5-HT neuronal firing in the median raphe nucleus: implications for the action of anxiolytics. Eur. Neuropsychopharmacol. 2006;16:612–619. doi: 10.1016/j.euroneuro.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Kennett G, Lightowler S, Trail B, Bright F, Bromidge S. Effects of RO 60 0175, a 5-HT(2C) receptor agonist, in three animal models of anxiety. Eur. J. Pharmacol. 2000;387:197–204. doi: 10.1016/s0014-2999(99)00706-2. [DOI] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1677–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Rudolph U, Fritschy J-M, Arand M. Tonic inhibition in principal cells of the amygdala: a central role for α3 subunit-containing GABAA receptors. J. Neurosci. 2012;32:8611–8619. doi: 10.1523/JNEUROSCI.4404-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CBP, Hamon M, Lanfumey L, Mongeau R. Controversies on the role of 5-HT2C receptors in the mechanisms of action of antidepressant drugs. Neurosci. Biobehav. Rev. 2014;42:208–223. doi: 10.1016/j.neubiorev.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Mo B, Feng N, Renner K, Forster G. Restraint stress increases serotonin release in the central nucleus of the amygdala via activation of corticotropin-releasing factor receptors. Brain. Res. Bull. 2008;76:493–498. doi: 10.1016/j.brainresbull.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeau R, Martin CBP, Chevarin C, Maldonado R, Hamon M, Robledo P, Lanfumey L. 5-HT2C receptor activation prevents stress-induced enhancement of brain 5-HT turnover and extracellular levels in the mouse brain: modulation by chronic paroxetine treatment. J. Neurochem. 2010;115:438–449. doi: 10.1111/j.1471-4159.2010.06932.x. [DOI] [PubMed] [Google Scholar]
- de Oliveira Sergio T, de Bortoli VC, Zangrossi H. Serotonin-2A receptor regulation of panic-like behavior in the rat dorsal periaqueductal gray matter: the role of GABA. Psychopharmacology. 2011;218:725–732. doi: 10.1007/s00213-011-2369-2. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Quérée P, Peters S, Sharp T. Further pharmacological characterization of 5-HT(2C) receptor agonist-induced inhibition of 5-HT neuronal activity in the dorsal raphe nucleus in vivo. BrJPharmacol. 2009;158:1477–1485. doi: 10.1111/j.1476-5381.2009.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr. Opin. Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Serrats J, Mengod G, Cortés R. Expression of serotonin 5-HT2C receptors in GABAergic cells of the anterior raphe nuclei. J. Chem. Neuroanat. 2005;29:83–91. doi: 10.1016/j.jchemneu.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Differential regulation of 5-hydroxytryptamine release by GABAA and GABAB receptors in midbrain raphe nuclei and forebrain of rats. Br. J. Pharmacol. 1996;119:1375–1384. doi: 10.1111/j.1476-5381.1996.tb16049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Bräuner-Osborne H, Turecek R, Shigemoto R, Zhang Y-P, Luján R, Jacobson LH, Biermann B, Fritschy J-M, Vacher C-M, Müller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Keist R, Boehmer von L, Studer R, Benke D, Hagenbuch N, Dong Y, Malenka RC, Fritschy J-M, Bluethmann H, Feldon J, Möhler H, Rudolph U. A schizophrenia-related sensorimotor deficit links alpha 3-containing GABAA receptors to a dopamine hyperfunction. Proc. Natl. Acad. Sci. USA. 2005;102:17154–17159. doi: 10.1073/pnas.0508752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Farahvash H. Effects of GABA-ergic drugs on penile erection induced by apomorphine in rats. Psychopharmacology. 1994;115:249–253. doi: 10.1007/BF02244779. [DOI] [PubMed] [Google Scholar]