Abstract
Microglia and astroglia play critical roles in the development, function and survival of neurons in the CNS. However, under inflammatory conditions the role of astrogliosis in the inflammatory process and its effects on neurons remains unclear. Here, we used several types of cell cultures treated with the bacterial inflammogen LPS to address these questions. We found that the presence of astroglia reduced inflammation-driven neurotoxicity, suggesting that astrogliosis is principally neuroprotective. Neutralization of supernatant glial cell line-derived neurotrophic factor (GDNF) released from astroglia significantly reduced this neuroprotective effect during inflammation. To determine the immunological role of astroglia, we optimized a highly-enriched astroglial culture protocol and demonstrated that LPS failed to induce the synthesis and release of TNF-α and iNOS/NO. Instead we found significant enhancement of TNF-α and iNOS expression in highly-enriched astroglial cultures required the presence of 0.5 to 1% microglia, respectively. Thus suggesting that microglial-astroglial interactions are required for LPS to induce the expression of pro-inflammatory factors and GDNF from astroglia. Specifically, we found that microglia-derived TNF-α plays a pivotal role as a paracrine signal to regulate the neuroprotective functions of astrogliosis. Taken together, these findings suggest that astroglia may not possess the ability to directly recognize the innate immune stimuli LPS, but rather depend on cross-talk with microglia to elicit release of neurotrophic factors as a counterbalance to support neuronal survival from the collateral damage generated by activated microglia during neuroinflammation.
Keywords: Astroglia, Microglia, GDNF, glial interaction, neuroinflammation, neuroprotection
Introduction
In the central nervous system (CNS) the immune response to brain insults are initiated by microglia and astroglia. Due to their different ontogenic origins, microglia and astroglia play different roles during neuroinflammation. Microglia are considered ‘professional’ immune cells due to their myeloid lineage, whereby they can detect and clear pathogens and damaged cells through pattern recognition receptors and phagocytosis. Astroglia, on the other hand, are derived from a neuroectoderm lineage and assist with the maintenance of CNS homeostasis including immune regulation. Although astrogliosis is routinely used as a hallmark of neuropathological conditions and trauma, the mechanisms of how astroglia become activated and their roles during neuroinflammation remain uncertain.
To date, most of the knowledge regarding the immune function of astroglia was obtained from in vitro studies. Although pro-inflammatory factors such as TNF-α, IL-1β and NO were detected in astroglial cultures treated with the bacterial inflammogen lipopolysaccharide (LPS) (Carpentier et al. 2005; Chung and Benveniste 1990; Esen et al. 2004; Galea et al. 1994; Krasowska-Zoladek et al. 2007; Lieberman et al. 1989), a recent finding takes into question whether astroglia are capable of independently detecting LPS to stimulate their activation (Holm et al. 2012). Many believe that the activation of ~0.5–10% microglial contamination in these cultures may have mistakenly been attributed to astroglia (Giulian et al. 1994; Saura 2007), thus challenging whether reactive astrogliosis generates high levels of cytotoxic factors resulting in a deleterious role towards neurons (Tacconi 1998). Studies have shown that astrogliosis can serve a neuroprotective role by preserving bioenergetic (Kajihara et al. 2001) and trophic support (Goss et al. 1998), preventing excitatory neurotoxicity (Chorna et al. 2004; Hansson et al. 2000), modulating free radical oxidation (Dringen 2000; Gegg et al. 2003) and apoptosis (Nakase et al. 2004) in neurons.
The purpose of this study was to accurately discriminate the ability of astroglia to detect and respond to LPS and to determine the role of astrogliosis in neuroinflammation. To reduce the confounding factors associated with microglial contamination, we used a modified highly-enriched astroglial culture protocol containing less than 0.005% microglial contamination. Here we show that astroglia are beneficial to DAnergic neuron survival in neuroinflammatory conditions by secreting glial cell line-derived neurotrophic factor (GDNF) in response to paracrine signaling of TNF-α released by activated microglia. Interestingly, we found that LPS failed to induce TNF-α and NO release in highly-enriched astroglial cultures, but low levels of astroglial iNOS could be induced in the presence of activated microglia. These results suggest that although, in the presence of activated microglia, astroglia may generate small amount of potentially cytotoxic factors, the GDNF they produce is significantly more protective. Most importantly, our results further confirm the immunomodulatory role of glial cross-talk during LPS-induced neuroinflammation.
Materials and Methods
Animals
Timed-pregnant Fisher 344 rats at day 14 of gestation were purchased from Charles River Laboratories (Raleigh, NC, USA). Timed-pregnant C57BL/6J and B6.129S-Tnftm1Gkl/J (TNF-α deficient) mice were generated by our institute’s animal husbandry staff using breeders obtained from Jackson Laboratories (Bar Harbor, ME, USA). TNF R1/R2 knockout mice were kindly gifted from Dr. Perry Blackshear at National Institute of Environmental Health Sciences. Dams were housed in polycarbonate cages in a facility with 12 h artificial light-dark cycle and provided fresh deionized water and NIH 31 chow ad libitum. Animal procedures were conducted in strict accordance with the National Institutes of Health animal care and use guidelines.
Reagents
Poly-D-lysine, cytosine β-D-arabinofuranoside (Ara-c), L-leucine methyl ester (LME) and 3,3′-diaminobenzidine, mazindo and urea-hydrogen peroxide tablets were purchased from Sigma-Aldrich (St. Louis, MO, USA). Lipopolysaccharide (LPS; E. coli strain O111:B4) was purchased from Calbiochem (San Diego, CA, USA). Cell culture ingredients were obtained from Life Technologies (Grand Island, NY, USA). Suberoylanilide hydroxamic acid was purchased from Cayman Chemical (Ann Arbor, MI, USA). Anti-GFAP and antibody diluent were purchased from DAKO (Carpinteria, CA, USA). Anti-Iba1 antibody was purchased from Wako Pure Chemicals (Richmond, VA, USA). Anti-iNOS and anti-S100b antibodies were purchased from Abcam (Cambridge, MA, USA). TNF-α ELISA kit and Anti-GDNF and isotype control antibodies were purchased from R&D Systems (Minneapolis, MN, USA). Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 594 goat anti-rabbit IgG were purchased from Invitrogen (Carlsbad, CA, USA). Goat anti-rabbit biotinylated secondary antibody was purchased from Vector Laboratory (Burlingame, CA, USA).
Primary mixed glia and highly-enriched astroglial cultures
Primary mixed glia cultures were prepared from rat pups at postnatal day 1–3 as previously described (Chen et al. 2013). Briefly, cortices and midbrain were isolated and triturated into a single cell suspension. Cells were centrifuged and resuspended in warm mixed glia culture maintenance medium [DMEM-F12 (1:1) media supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 μM non-essential amino acids, 50 U/ml penicillin, and 50 μg/ml streptomycin] and plated on poly-D-lysine-coated 6- or 24-well plates at 1×106 or 1×105 cells/well, respectively. Mixed glia culture media was refreshed every three days until treatment seven days after seeding. Highly-enriched astroglial cultures were derived from mixed glial cultures supplemented with 1 mM of L-leucine methyl ester (LME) 72 hours after seeding for 5–7 days. Immunocytochemistry revealed mixed glial cultures contained ~15% microglia (Iba-1-immunoreactive cells), ~3% oligodendrocytes (MBP-immunoreactive cells) and ~80% astroglia (GFAP-immunereactive cells), whereas highly-enriched astroglial cultures contained less than 0.005% microglia, and more than 99.9% astroglia.
Microglia-enriched cultures
Primary microglia-enriched cultures were prepared from rat pups at postnatal day 1–3 as previously described (Chen et al. 2013). Briefly, mixed glia cultures were plated on poly-D-lysine-coated 150 cm3 flasks at 5 × 107 cells/flask and maintained in fresh mixed glia culture medium changed every three days for two weeks. Microglia were mechanically isolated by shaking cultures in incubator orbital shaker (180 rpm for 40 minutes) and re-plated on poly-D-lysine-coated 24-well plates at 5×105 cells/well for the collection of microglia conditioned media or added to highly-enriched astroglia cultures for reconstitution studies. Immunocytochemistry revealed microglia-enriched cultures contained less than 1% contamination of astroglia (GFAP-immunoreactive cells) (McCarthy and de Vellis 1980).
Neuron-glia and reconstituted neuron-microglia cultures
Mesencephalic neuron-glia cultures were prepared from rat embryos at gestation day 14 as described previously (Chen et al. 2013). Briefly, midbrain tissues were triturated into single cell suspension and seeded on poly-D-lysine-coated 24-well plates at 5 × 105 cells/well. For neuron-microglia cultures, dividing glia were depleted from neuron-glia cultures 48 hours after seeding with 8–10 μM of cytosine β-D-arabinofuranoside (Ara-C) for three days. Neuron-enriched cultures were reconstituted with enriched-microglia cultures to a ratio of 90% neurons and 10% microglia and treated 24 hours later.
Dopamine uptake assay
[3H] Dopamine (DA) uptake assay was performed as described previously (Liu et al. 2002). Briefly, the rate of uptake of radiolabeled DA by DAnergic neuron cultures was measured for 21 minutes at 37°C. Cells were washed and lysed to release internalized radiolabeled DA and quantified with a liquid scintillation counter (Tri-Carb 4000; Packard, Meriden, CT, USA). Nonspecific [3H] DA uptake was accounted for by competitively inhibiting DA uptake with 10 μM of mazindol.
Astrocytoma cultures
Mycoplasma-free rat C6 astrocytoma cell line was purchased from ATCC (Manassas, VA, USA) and maintained in cell line media (DMEM supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin). Cells were split on T75 flasks and utilized at passages 3–5 for experiments on 24-well plates seeded at 1×105 cells/well and treated 24 hours later.
Immunofluorescence and immunocytochemical staining
For immunofluorescence, mixed glia and highly-enriched astroglia cultures were fixed with 3.7% formaldehyde in PBS for 20 minutes and incubated for 20 min in blocking solution (BSA 1%/Triton X-100 0.4%/Normal Goat Serum 4% in PBS) to block non-specific binding. Cells were immunostained overnight at 4°C with rabbit polyclonal antibodies against either Iba-1 (1:750; microglial marker), GFAP (1:1000; astroglial marker) or S100b (1:200; astroglial marker) diluted in Antibody Diluent (DAKO) and co-labeled with a mouse monoclonal antibody against iNOS (1: 250). Antibodies were detected and visualized using Alexa Fluor 488 goat anti-rabbit IgG (1:750), Alexa Fluor 594 goat anti-rabbit IgG (1:750) or Alexa Fluor 594 goat anti-mouse IgG (1:750) secondary antibodies.
For immunohistochemistry, fixed cultures were treated for 10 minutes with 1% hydrogen peroxide and immunostained overnight at 4°C with rabbit polyclonal antibodies against either TH (1:3000; DAnergic neuron marker) or Iba-1 (1:750; microglia marker). Antibodies were detected with biotinylated goat anti-rabbit secondary antibody (1:227; Vector Laboratory), amplified with Vectastain ABC reagents (Vector Laboratory) and visualized with 3,3′-diaminobenzidine (DAB).
Cell counting
Immunoreactive cells against TH or Iba-1 were manually counted by two investigators in a blind fashion under microscopy and averaged. The percentage of microglial contamination was calculated by dividing the number of microglia by total number of cells per well.
RNA analysis
Total RNA was extracted from cultures with Qiagen RNeasy Minikit and reverse transcribed with an oligo dT primer. Real-time PCR amplification was performed using SYBR Green PCR Master Mix and an ABI 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) according to manufacturer’s protocols. The primers were designed using Vector NTI software (v.11, Invitrogen, Carlsbad, CA, USA) and validated for efficacy through melting curve analyses. Rat GDNF F2 (5′-CAGAGGGAAAGGTCGCAGAGG-3′; 300 nM), Rat GDNF R2 (5′-TAGCCCAAACCCAAGTCAGTG-3′; 300 nM), Rat BDNF F1 (5′-CGATGCCAGTTGCTTTGTCTTC-3′; 300 nM), Rat BDNF R1 (5′-AAGTTCGGCTTTGCTCAGTGG-3′; 300 nM), Mouse/Rat GAPDH F2 (5′-TTCAACGGCACAGTCAAGGC-3′; 300 nM), Mouse/Rat GAPDH R2 (5′-GACTCCACGACATACTCAGCACC-3′; 300 nM), Rat TNF-α F4 (5′ CCAGACCCTCACACTCAGATCATC 3′; 300 nM), Rat TNF-α R4 (5′ CCTCCGCTTGGTGGTTTGCT 3′; 300 nM), Rat iNOS F1(5′ GAGTGAGGAGCAGGTTGAGGATTAC 3′; 300 nM), Rat iNOS R1 (5′ AGGAAAAGACCGCACCGAAG 3′; 300 nM), Mouse GDNF F2 (5′ GGCTGACCTTGAACTTACTGCTTG 3′; 300 nM), and Mouse GDNF R2 (5′ CCTGTGGATACGGTGTGATTGAT 3′; 300 nM) primers were used to amplify rat GDNF [GenBank: NM_019139], rat BDNF [GenBank: NM_012513], Mouse/Rat GAPDH [GenBank: NM_017008 (rat); NM_008084 (mouse)], rat TNF-α [GenBank: NM_012675], rat iNOS [GenBank: NM_012611], and mouse GDNF [GenBank: NM_010275] genes. Amplifications were done at 95°C for 10 seconds, 55°C for 30 seconds, and 72°C for 30 seconds for 40 cycles. All samples were tested in triplicate from at least three independent experiments and normalized with GAPDH using the 2−ΔΔCt method. Fold changes for each treatment were normalized in percentage to the maximum expression.
Measurement of nitrite and TNF-α in culture supernatant
Cell culture supernatant was collected at 3 and 24 hours after treatment for TNF-α and NO assays, respectively. NO was assessed by measuring nitrite in 50 μl of supernatants using Griess reagent as described previously (Liu et al. 2000). TNFα was measured in accordance to the manufacturer’s instructions using an enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems. Both colorimetric assays were quantified using a spectrophotometer.
Statistics
Data were presented as the mean ± SEM. Comparison of more than two groups were performed using one-way ANOVA followed by Bonferroni post hoc multiple comparison test. Comparisons of more than two parameters were performed by two-way ANOVA analysis followed by Bonferroni post hoc multiple comparison test. Data were analyzed using Prism (v6.00, GraphPad, San Diego, CA). P-values less than or equal to 0.05 were considered statistically significant.
Results
Astroglia assist in the survival of DAnergic neurons upon LPS stimulation
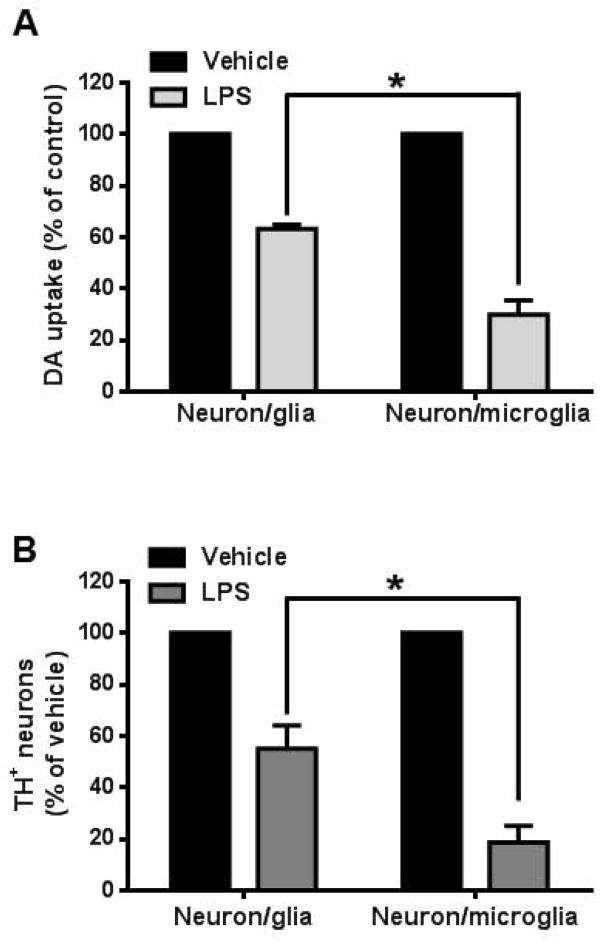
Reactive astrogliosis is observed in neuroinflammation and serves as a marker for several neuropathologies (O’Callaghan and Sriram 2005; Zhang et al. 2010), yet its role is not fully understood. To address this issue, we treated mesencephalic neuron-glia (composed of neurons, astroglia and microglia) and neuron-microglia cultures (absent of astroglia) containing an identical number of microglia with LPS and measured [3H] DA uptake capacity to assess functional changes of DAnergic neurons. LPS treatment produced significantly greater reduction in [3H] DA uptake in neuron-microglia cultures than that in neuron-glia cultures (Fig. 1A). Correspondingly, a significantly greater loss in the number TH-immunoreactive DAnergic neurons was also observed in neuron-microglia cultures (Fig. 1B). The TH-immunoreactive DAnergic neuron number decreased about 45% in LPS-treated neuron-glia cultures compared to that of in vehicle-treated cultures (Vehicle group: 216±16 cells/well vs. LPS group: 119±8 cells/well). In neuron-microglia cultures, the DAnergic neuron number decreased about 80% in LPS-treated cultures (Vehicle group: 296±12 cells/well vs. LPS group: 58±9 cells/well).
Figure 1.
Astroglia improve the outcome of LPS-mediated DAnergic neurodegeneration in neuron-glia cultures. (A) Neuron-glia and neuron-microglia cultures were treated with vehicle or of LPS (15 ng/ml). After 7 days, [3H] DA uptake assay was performed to assess DA neuron function. DA uptake function of DAnergic neurons had significantly less reduction in DA uptake in LPS-treated (15 ng/ml for seven days) neuron-glia (containing neuron, microglia and astroglia) cultures compared to neuron-microglia (absence of astroglia) cultures (F(1,12)= 188.4, P< 0.0001; t=19.41, *P < 0.05, post hoc analysis by Bonferroni t-test). (B) After 7 days, cultures were also subjected to immunocytochemical staining with anti-tyrosine hydroxylase (TH) antibody. The number of TH immunoreactive cells were significantly less depleted in LPS-treated neuron-glia cultures than neuron-microglia (F(1,12)= 60.71, P< 0.0001; t=11.02, *P < 0.05 post hoc analysis by Bonferroni t-test). Data show mean ± SEM from four experiments, each done in triplicate.
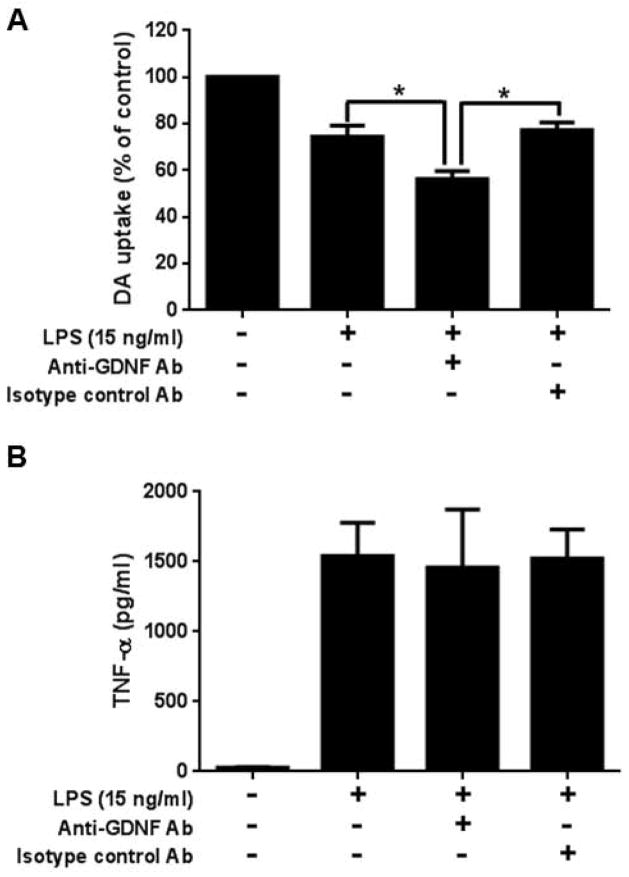
Astroglia are capable of producing neurotrophic factors, such as glial-derived neurotrophic factor (GDNF), known to promote DAnergic neuronal survival (Lin et al. 1993). To evaluate if GDNF participates in the astroglial-mediated neuroprotection during neuroinflammation, GDNF neutralization antibody was added into neuron-glia cultures prior to LPS stimulation (Fig. 2A). ANOVA analysis showed that there was a significant effect between groups (F(3, 12) = 111.5, P< 0.0001), and post hoc analyses revealed that GDNF-neutralizing antibody significantly reduced [3H] DA uptake capacity compared to the antibody isotype control (t= 10.40, P<0.05). To rule out the possibility that the reduction of [3H] DA uptake capacity in GDNF-neutralizing antibody–treated cultures could be related to the augmentation of the enhanced release of pro-inflammatory factors, such as TNF-α when incubation with GDNF-neutralizing antibody. The result showed that treatment of GDNF neutralization antibody failed to augment TNF-α level in the culture supernatant (Figure 2B). Collectively, these results demonstrated that astroglia play a neuroprotective role under a neuroinflammatory condition by releasing the neurotrophic factor GDNF.
Figure 2.
Neuron-glia cultures incubated an hour prior to LPS stimulation (15 ng/ml for seven days) with 20 μg/ml of anti-GDNF antibody displayed a significant reduction in DA uptake function (A) (LPS vs. GDNF Ab, t=6.334, *P < 0.05; GDNF Ab vs. isotype control IgG, t=10.40, *P < 0.05, post hoc analysis by Bonferroni t-test), but not altered the culture supernatant TNF-α level (B) compared to stimulated cultures with and without isotype control antibodies. Data show mean ± SEM from four independent experiments done in duplicate.
Microglia are required for LPS-induced increase in GDNF production by astroglia
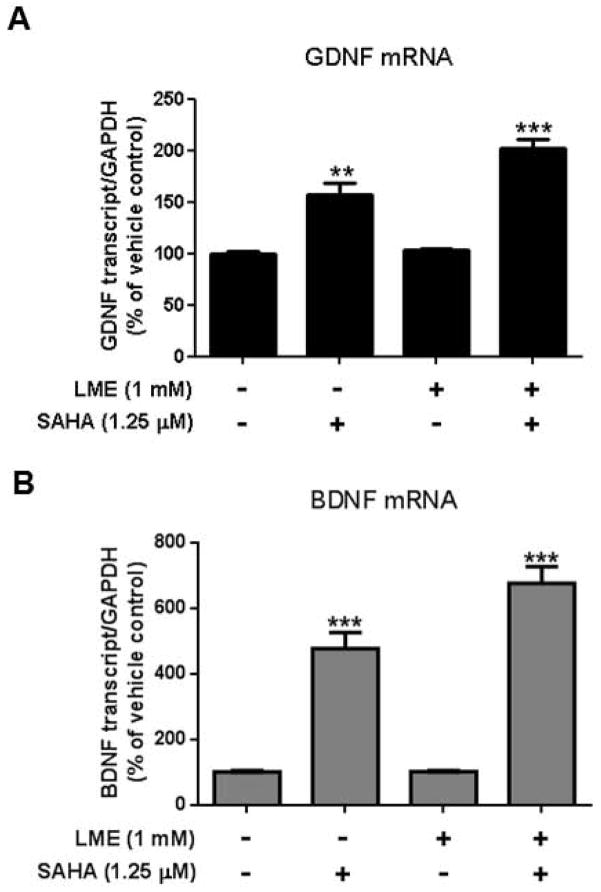
Enriched astroglial cultures with as little as 1% microglia contamination are sufficient to confound the results of a study (Okun et al. 2009). In order to prepare near pure astroglial cultures, the use of chemical, mechanical or genetic methods have been employed in attempts to ablate microglia (Crocker et al. 2008; Falsig et al. 2006; Foo et al. 2011; Hamby et al. 2006; Holm et al. 2012; Kanai et al. 2006; Losciuto et al. 2012; Pascual et al. 2012; Tabernero et al. 1996). However, many of these methods are costly, require specialized equipment or transgenic mice, take as long as three to four weeks to enrich, and fail to destroy microglia protected beneath the astroglial monolayer (Crocker et al. 2008). By modifying an established protocol (Giulian and Baker 1986) that selectively destroys microglia from mixed glial cultures with 1mM of leu-leu-methyl ester (LME) application three days after seeding, we generated a quick and thrifty method to produce highly-enriched astroglial cultures with minimal microglial contamination. The purity of cultures was verified seven days after seeding by immunofluorescent staining using antibody against the microglial marker Iba-1 (Fig. 3A). Direct cell counts of Iba-1-immunoreactive cells revealed that after four days of treatment with 1 mM of LME, the percentage of microglia in mixed glial cultures were reduced from 13.46 ± 0.04% to 0.004 ± 0.001% and showed no significant recovery until 7 days after LME was removed (Fig. 3B). The selectivity of LME treatment in our highly-enriched astroglial cultures was verified by a lack of change in astroglial morphology and a preserved ability of astroglia to express GDNF mRNA in response to the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) (Fig. 4).
Figure 3.
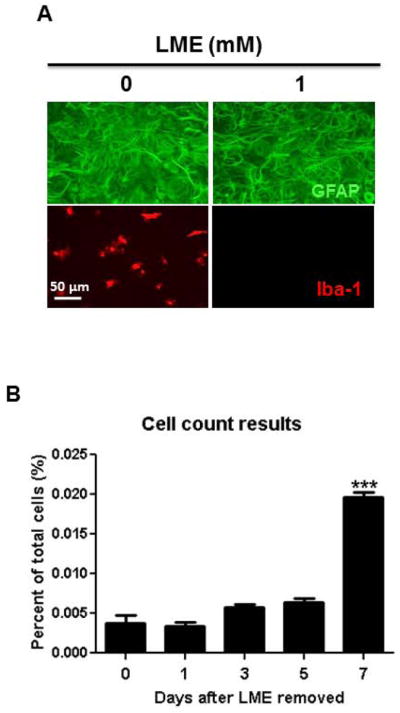
Assessment of microglia presence in highly-enriched astroglial cultures. (A) Three days after seeding, primary rat mixed glia cultures were treated with various concentrations of LME (1 mM) for 4 days. Cultures were immunostained using antibodies against GFAP (green, for astroglia) and Iba-1 (red, for microglia). Scale bar: 50 μm. (B) LME was removed from highly-enriched astroglial cultures and Iba1-positive cells were counted at different times thereafter to assess the repopulation of contaminating microglia. t=17.53, *** P < 0.0001, post hoc analysis by Bonferroni t-test compared to Day 0.
Figure 4.
Astroglial function in the presence of LME was assessed by their ability to stimulate greater GDNF mRNA levels by the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) (Chen et al. 2012). Mixed glial cultures (containing both microglia and astroglia) and highly-enriched astroglial cultures were treated with 1.25 μM of SAHA for 12 hours and changes in GDNF (A) and BDNF (B) mRNA expression levels were assessed. The basal level of GDNF or BDNF mRNA in mixed glia and highly-enriched astroglial cultures is comparable. To emphasize the effect of SAHA treatment, we set vehicle-treated group as 100%. Values were present as mean ± SEM from three independent experiments, with duplicates. ** P <0.001, *** P < 0.0001, Bonferroni t-test compared to basal expression levels of vehicle-treated mixed glia culture.
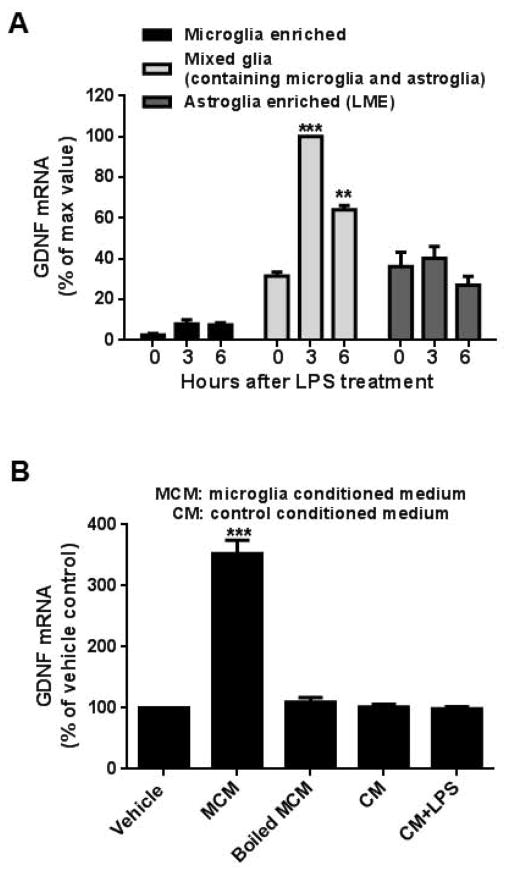
To investigate the mechanism of how GDNF expression is increased after LPS stimulation, microglia enriched, mixed glia (containing microglia and astroglia) and highly-enriched astroglial cultures were treated with LPS and their GDNF mRNA expression profiles were assessed (Fig. 5A). Interestingly, although both highly-enriched astroglial and, to a lesser extent, microglial-enriched cultures constitutively express GDNF mRNA, LPS stimulation failed to further increase their expression. In contrast, mixed glia cultures showed a significant increase in GDNF mRNA expression after LPS stimulation, suggesting that the interaction between microglia and astroglia is necessary for the enhancement of GDNF expression.
Figure 5.
Microglia-derived soluble factors from LPS stimulated cultures are required in the upregulation of GDNF mRNA during astrogliosis. (A) GDNF mRNA was significantly increased 3 and 6 hours after LPS stimulation (15 ng/ml) in mixed glial cultures (containing both microglia and astroglia) [3 hours, t=13.46, ***P<0.0001; 6 hours, t=6.402, **P<0.01, post hoc analysis by Bonferroni t-test compared to 0 hour value] but not in enriched microglia or highly-enriched astroglial cultures. Due to the great difference of GDNF mRNA level among these cultures, we set maximum value as 100%. Data were presented as percent of GDNF mRNA expression with respect to the expression of LPS-treated mixed glia cultures at 3 hours. (B) Highly-enriched astroglial cultures treated for 3 hours with microglia conditioned medium (MCM) from LPS-stimulated enriched microglia collected after 24 hours showed a significant increase in GDNF mRNA expression (t=28.47, ***P<0.0001, post hoc analysis by Bonferroni t-test compared to vehicle treated cultures). No changes were observed when treated with boiled MCM, unstimulated microglial conditioned medium or unstimulated microglial conditioned media plus LPS (15 ng/ml) compared to the vehicle control. To emphasize the effect of MCM treatment, we set vehicle-treated group as 100%. Data were presented as percent of GDNF mRNA expression with respect to the expression of the vehicle control. Data show mean ± SEM from three independent experiments, each done in duplicate.
To test the possibility that paracrine factors released from activated microglia during LPS stimulation mediate this interaction with astroglia, we added either LPS-treated microglial conditioned medium (MCM) or boiled MCM to highly-enriched astroglial cultures and assessed changes in GDNF mRNA. Interestingly, only highly-enriched astroglial cultures treated with MCM expressed significantly higher GDNF mRNA levels (t= 28.47, P < 0.0001), suggesting that the putative paracrine signal was most likely soluble proteins capable of being denatured (Fig. 5B).
TNF-α is critical for LPS-induced increase in astroglial GDNF expression
Among the multiple soluble factors released by microglia after LPS stimulation, the cytokine TNF-α is one of the earliest released and most abundant proinflammatory factors. To assess if TNF-α modulates the increased production of astroglial GDNF, we compared GDNF mRNA expression in LPS-treated mixed glia cultures derived from both TNF-α- and TNF R1/R2-deficient mice (Fig. 6A and 6B). Expression of GDNF mRNA three hours after LPS stimulation was significantly reduced in both TNF-α−/− (t = 5.031, P < 0.001) and TNF R1/R2−/− (t = 31.96, P < 0.0001) mixed glia cultures compared to wild type controls, indicating that TNF-α partly modulates changes in astroglial GDNF expression during LPS stimulation.
Figure 6.
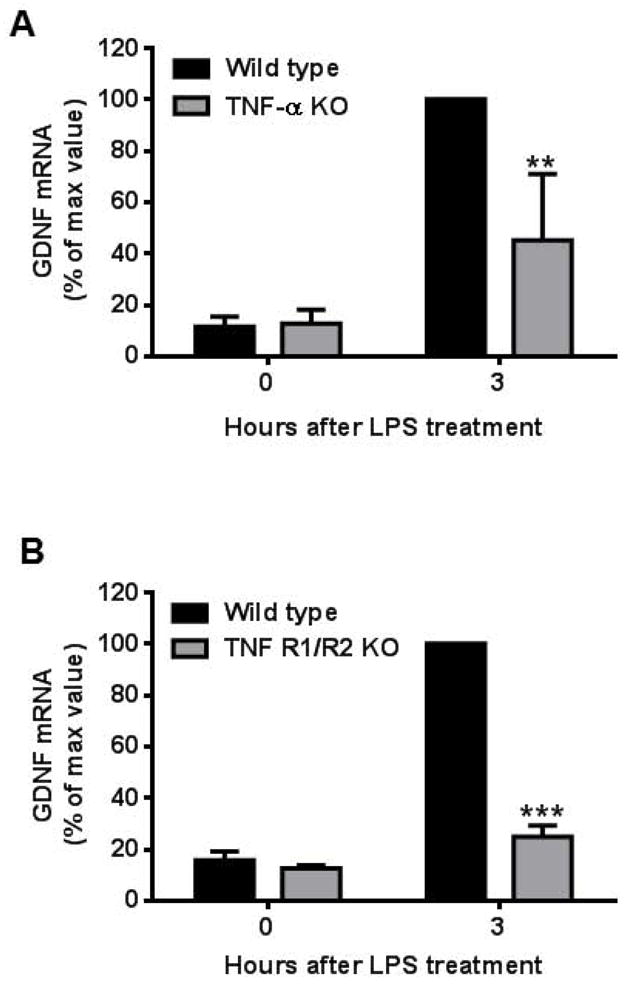
TNF-α is pivotal in LPS-induced increase in astroglial GDNF expression. Mixed glia cultures prepared from (A) TNF-α KO or (B) TNF R1/R2 KO mice showed a significant decrease in GDNF mRNA expression 3 hours after LPS (15 ng/ml) stimulation compared to cultures from their respective wild type controls [TNF-α KO, t=5.031, **P<0.001; TNF R1/R2 KO, t=31.96, ***P<0.0001, post hoc analysis by Bonferroni t-test compared to wild type treated with LPS for 3 hour cultures]. Due to the great difference of GDNF mRNA level between 0 and 3 hours after LPS stimulation, we set maximum value as 100%. Data were presented as percent of GDNF mRNA expression with respect to the expression of the wild type cultures treated with LPS for 3 hours. Data shows mean ± SEM from three independent experiments, each done in duplicate.
LPS fails to stimulate NO and TNF-α production in highly-enriched astroglia
Astroglial enriched primary cultures have been widely used to study the immune function of astroglia; however, the effects from microglial contamination are usually discounted. Therefore, in order to properly assess the role of astrogliosis in LPS-induced neuroinflammation we measured the temporal release of TNF-α and NO by mixed glia, enriched astroglia, highly-enriched astroglial cultures, and a C6 rat astrocytoma cell line stimulated with 100 ng/ml of LPS. We detected negligible levels of TNF-α (Fig. 7A) and NO (Fig. 7B) in highly-enriched astroglial cultures and C6 cell lines after LPS stimulation, yet their release was significantly increased in LPS-treated mixed glial cultures and in enriched astroglial cultures (~4% microglia) derived by the traditional ‘shake-off’ method (McCarthy and de Vellis 1980).
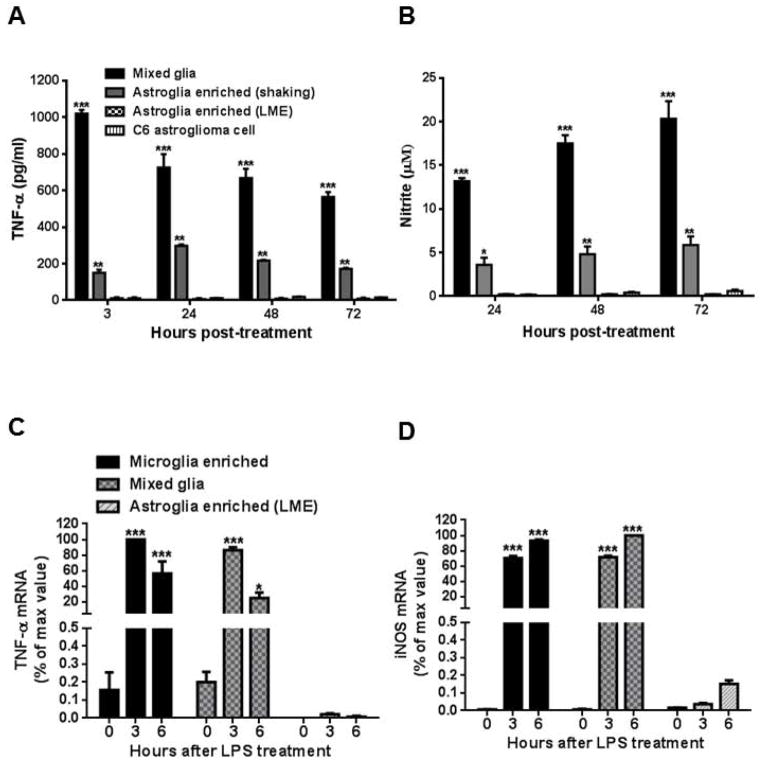
Figure 7.
LPS was unable to elicit the release of TNF-α or NO from either highly-enriched astroglial or astrocytoma cultures. Highly-enriched astroglia (LME-treated method) and C6 astrocytoma cultures treated with 100 ng/ml of LPS secreted significantly negligible levels of NO (A) and TNF-α (B) compared to mixed glia or enriched astroglia (shake-off method) cultures (** P < 0.001, *** P < 0.0001, post hoc analysis by Bonferroni t-test). A similar trend for TNF-α (C) and iNOS (D) mRNA expression was observed in enriched microglia, mixed glia and highly-enriched astroglial cultures (* P < 0.05, *** P < 0.0001, post hoc analysis by Bonferroni t-test compared to highly-enriched astroglial cultures). Due to the great difference of TNF and iNOS mRNA levels among these cultures, we set maximum value as 100%. Data were normalized with respect to the maximum value of microglia enriched culture. Data show mean ± SEM from three independent experiments, each with triplicates.
Since the TNF-α and NO levels detected in LPS-stimulated highly-enriched astroglial cultures were below the detection limitation threshold, mRNA expression levels for TNF-α and iNOS were also assessed for mixed glia, enriched microglia and highly-enriched astroglial cultures at 0, 3 and 6 hours after LPS stimulation. As expected, expression levels of mRNA were significantly increased for both TNF-α (Fig. 7C) and iNOS (Fig. 7D) in microglia-containing cultures after LPS treatment and showed comparably negligible expression levels when compared to highly-enriched astroglial cultures at 3 and 6 hours after LPS treatment. These results indicated that LPS is insufficient to induce an adequate astroglial immune response-related release of NO and TNF-α.
Microglia are the major source of NO and TNF-α in ‘enriched astroglial’ cultures
Although most studies acknowledge the potential confounding role of residual microglia in their cultures, the degree of microglial contamination sufficient to affect the results of astroglial immune studies have not been discerned. To address this issue, we reconstituted highly enriched astroglial cultures with 0.5 to 20% enriched microglia with respect to the total number of cells and detected the ability of LPS to produce detectable levels of TNF-α and NO (by measuring level of nitrite in supernatant). We found that as little as 0.5% and 1% microglial contamination produced significantly detectable levels of TNF-α (Fig. 8A; t=4.81, P < 0.0001) and NO (Fig. 8B; t=5.63, P < 0.0001), respectively, compared to highly enriched astroglial cultures after LPS stimulation. TNF-α and NO levels continued to increase in the presence of greater microglia numbers (TNF-α: F(6, 35) = 3003, P < 0.0001; NO: F(6, 35) = 1047, P < 0.0001; Fig. 8). These results demonstrate that miniscule numbers of microglia in astroglial cultures could significantly confound the outcome of a study.
Figure 8.
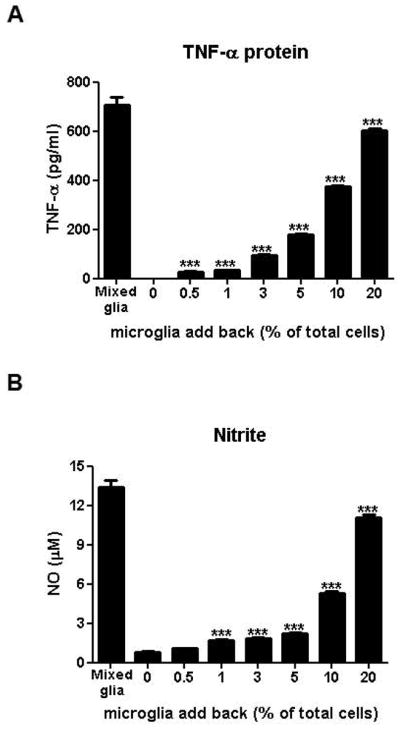
Microglia are the major source of LPS-elicited TNF-α and NO in enriched ‘astroglial’ cultures. Reconstituted highly-enriched astroglial cultures with 0.5 to 20% microglia were stimulated with LPS (100 ng/ml) and secreted TNF-α (A) and NO (B) levels were determined 3 and 24 hours after LPS treatment, respectively. Data were presented as mean ± SEM from three independent experiments, each done in triplicates. *** P < 0.0001, one-way ANOVA followed by Bonferroni post hoc multiple comparison test.
Microglia are required for astroglial NO and TNF-α production upon LPS stimulation
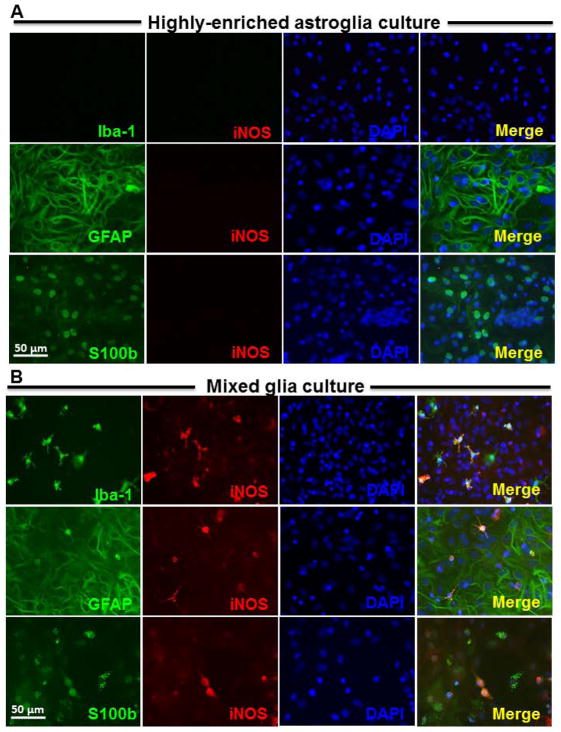
To further demonstrate how microglia may modulate astroglia during inflammation, we used double immunofluorescence staining to examine the cell type specific expression of iNOS in mixed glia and highly-enriched astroglia cultures after LPS stimulation. We found that iNOS-immunoreactivity was not detected in stimulated highly enriched astroglial cultures (Fig. 9A) and was localized exclusively to Iba1-immunoreactive microglia in mixed glia cultures 24 hours after LPS stimulation (data not shown). Interestingly, double staining of GFAP/iNOS or S100b/iNOS showed that these antigens were co-localized in 1% astroglia at 72 hours after LPS stimulation in mixed glia cultures (Fig. 9B). This finding indicates that a subset of astroglia are capable of expressing iNOS, albeit delayed, in the presence of microglia.
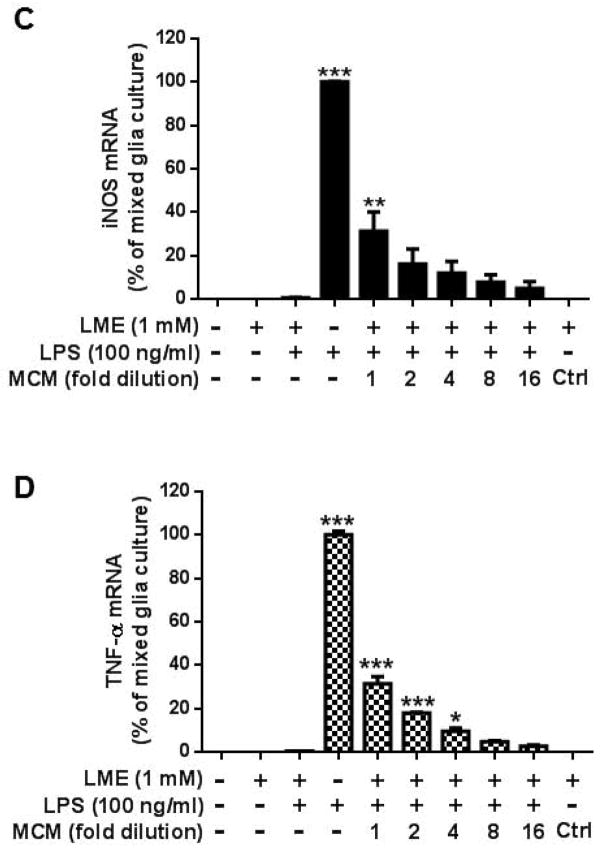
Figure 9.
Activated microglia are required for the expression of proinflammatory factors during LPS-induced astrogliosis. Highly-enriched astroglial (A) and mixed glial (B) cultures were treated with 100 ng/ml of LPS for 72 hours. Cultures were immunostained with antibodies against Iba-1 (green, for microglia), iNOS (red) and DAPI (blue) in upper panel; or GFAP (green, for astroglia), iNOS (red) and DAPI (blue) in middle panel; or S100b (green, for astroglia), iNOS (red) and DAPI (blue) in lower panel. Scale bar: 50 μm. Mixed glia and highly-enriched astroglial cultures were treated with vehicle, LPS, microglia conditioned medium which was collected from enriched microglia cultures at 24 hours after LPS treatment, and control conditioned medium (ctrl). After 3 and 6 hours, cultures were subjected to real-time PCR assay for (C) TNF-α and (D) iNOS mRNA expressions. Value of mixed glia cultures-treated with LPS was set as 100%. Data show mean ± SEM from three independent experiments, each done in duplicate. ***P<0.001, **P<0.01, post hoc analysis by Bonferroni t-test compared to vehicle treated highly-enriched astroglial cultures.
To determine whether microglial paracrine signals released in response to LPS stimulation modulate astroglial iNOS expression, we treated mixed glia (i.e., absent of LME) and highly-enriched astroglial cultures with different amount of microglia conditioned medium (MCM) collected from LPS-treated microglia-enriched cultures. As expected, LPS increased the levels of mRNA for both TNF-α and iNOS in mixed glial cultures, but not in highly-enriched astroglial cultures. It is interesting to observe that highly-enriched astroglial cultures could induce TNF-α (Fig. 9C; F(9, 15) = 459.9, P < 0.0001) and iNOS (Fig. 9D; NO: F(9, 15) = 62.84, P < 0.0001) mRNA expression in the presence of MCM in a concentration dependent manner. These findings further support that astroglia are incapable of detecting LPS and require microglia-mediated paracrine signaling to stimulate their activation.
Discussion
Results from this study provide interesting insights into the mechanism of regulation and functional role of reactive astrogliosis in pathological conditions. For the functional role, we demonstrated that astrogliosis could enhance the survival of neurons in inflammatory conditions in LPS-treated primary neuron-glia cultures. Whereby the neuroprotective effects from released neurotrophic factors outweigh the neurotoxic effects from released cytotoxic factors. For the mechanism of regulation, we discovered that presence of microglia is required for the production of proinflammatory and neurotrophic factors during astrogliosis. Highly enriched astroglial cultures failed to respond to LPS stimulation, yet we identified TNF-α released from activated microglia a critical secondary messenger for the activation of astrogliosis.
In pathological conditions, the role of reactive astrogliosis as supportive or detrimental to neuronal survival remains undefined (O’Callaghan and Sriram 2005; Zhang et al. 2010). Here, we demonstrate that astrogliosis protected DAnergic neurons against LPS-induced damage; whereby the reduction of DA uptake and loss of TH-immunoreactive cells was greater in reconstituted neuron-microglia cultures (lacking astroglia) compared to neuron-glia cultures (containing astroglia). Further studies suggested that GDNF released during astrogliosis could account for the neuroprotection. This conclusion was supported by the finding that LPS-treated neuron-glia cultures treated with GDNF neutralization antibody exacerbated the loss of DAnergic neurons. Besides direct action on neurons, anti-inflammatory effect of GDNF can also contribute to its neuroprotective effect. It was reported that exogenous GDNF suppresses the activation of microglia and reduce NO generation in LPS-treated midbrain organotypic cultures, resulting in reduced inflammation-mediated DA neurodegeneration (Xing et al. 2010). In addition, GDNF can reduce extracellular ROS production and microglial phagocytic activity in Zymosan A stimulated cultures (Rocha et al. 2012). Although we found that astrogliosis may secrete small amounts of cytotoxic factors (Fig. 9), our findings favor the possibility that the expression of neurotrophic factors by astrogliosis support DAnergic neuron survival during neuroinflammatory conditions.
LPS was previously thought to directly up-regulate the expression of GDNF in primary astroglial cultures (Kuno et al. 2006; Remy et al. 2003). Due to the inability of LPS to directly activate highly-enriched astroglial cultures in our study (Fig. 7), we suspected that the way LPS upregulates GDNF may not represent a direct action on astroglia, but rather through microglia. Three potential scenarios were considered. First, contaminating microglia in astroglial cultures generate the increased GDNF expression. Second, LPS can directly stimulate astroglia to produce GDNF. Third, activated microglia can stimulate astroglia to produce GDNF. We found that microglia, both non-stimulated and stimulated with LPS, expressed insignificant levels of GDNF mRNA; whereas highly-enriched astroglial cultures constitutively express GDNF mRNA yet showed no difference in expression after LPS stimulation. Instead, astroglia incubated with LPS-treated microglial conditioned medium significantly increased GDNF mRNA expression. This effect was reversed by boiling the conditioned medium (i.e., denaturing the proteins), thus we suspected that cytokines released by LPS-activated microglia might trigger astroglial activation and induce the expression of GDNF. Among these factors, TNF-α has previously been shown to partake in the autocrine regulation of GDNF production in activated astroglia (Figiel 2008; Kuno et al. 2006). By treating mixed glial cultures derived from both TNF-α deficient and TNF-α receptors 1 and 2 deficient mice with LPS, we showed that microglial-derived TNF-α is a key modulator of GDNF regulation in astroglia.
Several studies have concluded that astroglia can release TNF-α and NO upon stimulation with LPS (Carpentier et al. 2005; Chung and Benveniste 1990; Esen et al. 2004; Galea et al. 1994; Krasowska-Zoladek et al. 2007; Lieberman et al. 1989). Only a few studies used double-immunofluorescent staining or in situ hybridization to validate that TNF-α and iNOS were produced by astroglia rather than contaminating microglia (Lafortune et al. 1996). Interestingly, the expression of TNF-α (Giulian et al. 1994; Hetier et al. 1990; Welser-Alves and Milner 2013) and iNOS (Saura 2007; Vincent et al. 1997; von Bernhardi and Eugenin 2004) was co-localized to microglia marker and only a few studies noted a delayed, faint and heterogenous expression of iNOS in astroglia (Cassina et al. 2002; Galea et al. 1994). Consistent with these findings, we found that highly-enriched astroglial cultures generated negligible levels of TNF-α or NO upon stimulation with LPS, suggesting that these cytotoxic factors are almost exclusively attributed to microglia contamination. We verified this by reconstituting highly-enriched astroglial cultures with various concentrations of microglia and found that as little as 0.5 and 1% microglia into these cultures was capable of confounding the results of TNF-α and NO expression, respectively, which is similar to a previous study (Okun et al. 2009). Interestingly, the presence of microglia during LPS stimulation could induce weak levels of iNOS and TNF-α mRNA expression in astroglia, most likely as a result of secondary signaling through paracrine messengers derived from activated microglia. Furthermore, these findings confirm that the TNF-α, which modulates the upregulation of GDNF, is released through paracrine signaling by microglia. We suspect that previous reports indicating that the autocrine release of TNF-α from astroglia to upregulate GDNF maybe a result of contaminating microglia in their primary astroglia cultures (Figiel 2008; Kuno et al. 2006).
It is important to point out that although this study describes that microglia are essential for LPS-induced GDNF production from astrogia, other toxins may increase the production of pro-inflammatory factors in a direct fashion. It has been reported that astroglia can release cytotoxic pro-inflammatory factors such as cytokines and chemokines in response to different brain insults, such as infections, stroke and traumatic brain injuries (Dong and Benveniste 2001; Farina et al. 2007). Pattern recognition receptors (PRRs) expressed on astroglia, like Toll-like receptors (TLRs), NOD-like receptors, scavenger receptors and integrins can recognize pathogenic antigens (HIV Tat, dsRNA and etc.) or endogenous antigens released from damaged cells (nuclear or cytosolic proteins) to trigger the expression of pro-inflammatory factors from astroglia (Bowman et al. 2003; Farina et al. 2007; Kutsch et al. 2000; Park et al. 2006).
Our finding that astroglia failed to respond to LPS stimulation merits further discussion. LPS is recognized by TLR-4/MD2, membrane bound CD14, the β2-integrin receptor CD11b/CD18 and class A scavenger receptors (SR-As) that are all localized almost exclusively on the surface of innate immune cells. Microglia constitutively express high levels of these receptors (Chakravarty and Herkenham 2005; Laflamme and Rivest 2001; Lehnardt et al. 2002; Lehnardt et al. 2003), whereas astroglia lack membrane bound CD14 (Holm et al. 2012), CD11b/CD18 (Gasque et al. 1996) and SR-As receptors (Bell et al. 1994). Yet, the expression of TLR-4 on astroglia remains controversial. Although several groups have published that TLR-4 is undetectable on astroglia (Falsig et al. 2004; Farina et al. 2005; Lehnardt et al. 2002; Sola et al. 2002), one study was able to detect low-levels of TLR-4 mRNA expression in astroglia using in situ hybridization (Chakravarty and Herkenham 2005). These findings most likely explain that astroglia either lack or express insufficiently levels of membrane TLR-4 to generate a signal transduction to LPS. The most compelling evidence supporting this theory is that biotinylated (Holm et al. 2012) and FITC-labeled LPS (Lehnardt et al. 2002; Pascual et al. 2012) are neither bound nor internalized by astroglia.
Finally, a couple of issues not directly linking to the present study are worth noting. First, in addition to micorglia and astroglia, NG2+ glial cells also participated in regulating CNS functions under normal and diseased conditions (Levine et al. 2001). NG2+ cells have been thought as oligodendrocyte precursor cells (OPCs) and even considered as a fourth class of glial cells in the CNS (Karram et al. 2008). Several studies indicated the heterogeneity of this cell population (Chittajallu et al. 2004; Kitada and Rowitch 2006; Mallon et al. 2002). In respond to a variety of neuropathological insults such as traumatic brain injuries, stroke and viral infections, NG2+ cells can rapidly divide to myelin-forming oligodendrocytes, undergo morphological changes (e.g. enlarge cell body size and hypertrophied processes) and up-regulate of NG2 expression in OPCs and astroglia, which is an important component of glial scar formation (Levine et al. 1998; Levine et al. 2001). Recent studies also indicated that activated NG2+ cells expressed pro-inflammatory mediators such as TNF-α, IL-1β and iNOS. These properties of these NG2+ cells are similar to microglia/macrophage or pericytes in the blood-brain barrier (Alcendor et al. 2012; Fiedorowicz et al. 2008; Gao et al. 2010; Zhu et al. 2012). But how these cells are regulated and whether they interact with other glial cells during inflammation require further investigation. Second, although primary microglia culture has been widely used for studying the biological properties and function of microglia in vitro, several lines of evidence have indicated that the properties of primary cultured microglia are more closely related to activated macrophage than microglia in vivo (Biber et al. 2014; Melief et al. 2012; Schmid et al. 2009). By using microarray and cell surface molecular identification to compare molecular and functional signatures between fresh-sorted adult microglia and primary cultured microglia, recent reports indicated that microglial cells cultured in serum-containing medium lost their typical molecular signature determined in fresh-sorted adult microglia (Beutner et al. 2013; Butovsky et al. 2014; Salimi et al. 2003). Thus, whether the cross talk between microglia and astroglia during neuroinflammation from this report can also be observed in vivo requires further study.
In summary, this study demonstrates that highly-enriched astroglial cultures are incapable of responding to LPS, but instead become activated through their interactions with microglia to express neurotrophic factors to protect DAnergic neurons and modulate neuroinflammation—supporting our glial cross-talk activation hypothesis (Zhang et al. 2010). Although LPS can enter the brain via Gram-negative bacterial encephalopathies, LPS derived from peripheral infections rarely penetrates the blood-brain barrier (Banks and Robinson 2010), but rather activate microglia through the vesicular transcytosis of proinflammatory factors generated by peripheral immune cells. Other endogenous innate immune stimuli released during stroke, traumatic brain injury and exposures to xenobiotic could function in a very similar manner by activating microglia to release TNF-α, resulting in the subsequent activation of astroglia to express neurotrophic factors. In part, this study permits us to re-think the role of astrogliosis from an event that partakes in collateral neuronal damage to an event that helps regulate inflammation to minimize this damage. Moreover, this study serves as an additional caution that even a miniscule number of microglia may confound the results in studies that investigate the function of astroglia.
Main points.
Microglia modulate astroglial in response to neuroinflammation through paracrine signaling
Microglia driven astrogliosis during neuroinflammation is neuroprotective rather than neurotoxic
Acknowledgments
We thank Drs. Jean Harry and Christopher McPherson for critical reading of the manuscript. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Conflicts of interests
The authors have declared that no conflict of interest exists.
References
- Alcendor DJ, Charest AM, Zhu WQ, Vigil HE, Knobel SM. Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus. J Neuroinflammation. 2012;9:95. doi: 10.1186/1742-2094-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Robinson SM. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav Immun. 2010;24:102–9. doi: 10.1016/j.bbi.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MD, Lopez-Gonzalez R, Lawson L, Hughes D, Fraser I, Gordon S, Perry VH. Upregulation of the macrophage scavenger receptor in response to different forms of injury in the CNS. Journal of neurocytology. 1994;23:605–13. doi: 10.1007/BF01191555. [DOI] [PubMed] [Google Scholar]
- Beutner C, Linnartz-Gerlach B, Schmidt SV, Beyer M, Mallmann MR, Staratschek-Jox A, Schultze JL, Neumann H. Unique transcriptome signature of mouse microglia. Glia. 2013;61:1429–42. doi: 10.1002/glia.22524. [DOI] [PubMed] [Google Scholar]
- Biber K, Owens T, Boddeke E. What is microglia neurotoxicity (Not)? Glia. 2014;62:841–54. doi: 10.1002/glia.22654. [DOI] [PubMed] [Google Scholar]
- Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–91. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nature neuroscience. 2014;17:131–43. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–74. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- Cassina P, Peluffo H, Pehar M, Martinez-Palma L, Ressia A, Beckman JS, Estevez AG, Barbeito L. Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J Neurosci Res. 2002;67:21–9. doi: 10.1002/jnr.10107. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25:1788–96. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Oyarzabal EA, Hong JS. Preparation of rodent primary cultures for neuron-glia, mixed glia, enriched microglia, and reconstituted cultures with microglia. Methods in molecular biology. 2013;1041:231–40. doi: 10.1007/978-1-62703-520-0_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Wu HM, Ossola B, Schendzielorz N, Wilson BC, Chu CH, Chen SL, Wang Q, Zhang D, Qian L, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, protects dopaminergic neurons from neurotoxin-induced damage. British journal of pharmacology. 2012;165:494–505. doi: 10.1111/j.1476-5381.2011.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. The Journal of physiology. 2004;561:109–22. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorna NE, Santiago-Perez LI, Erb L, Seye CI, Neary JT, Sun GY, Weisman GA, Gonzalez FA. P2Y receptors activate neuroprotective mechanisms in astrocytic cells. J Neurochem. 2004;91:119–32. doi: 10.1111/j.1471-4159.2004.02699.x. [DOI] [PubMed] [Google Scholar]
- Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. Journal of immunology. 1990;144:2999–3007. [PubMed] [Google Scholar]
- Crocker SJ, Frausto RF, Whitton JL, Milner R. A novel method to establish microglia-free astrocyte cultures: comparison of matrix metalloproteinase expression profiles in pure cultures of astrocytes and microglia. Glia. 2008;56:1187–98. doi: 10.1002/glia.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–71. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J Neurochem. 2004;88:746–58. doi: 10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed] [Google Scholar]
- Falsig J, Porzgen P, Lotharius J, Leist M. Specific modulation of astrocyte inflammation by inhibition of mixed lineage kinases with CEP-1347. J Immunol. 2004;173:2762–70. doi: 10.4049/jimmunol.173.4.2762. [DOI] [PubMed] [Google Scholar]
- Falsig J, Porzgen P, Lund S, Schrattenholz A, Leist M. The inflammatory transcriptome of reactive murine astrocytes and implications for their innate immune function. J Neurochem. 2006;96:893–907. doi: 10.1111/j.1471-4159.2005.03622.x. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in immunology. 2007;28:138–45. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159:12–9. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz A, Figiel I, Zaremba M, Dzwonek K, Schliebs R, Oderfeld-Nowak B. Trimethyltin-evoked apoptosis of murine hippocampal granule neurons is accompanied by the expression of interleukin-1beta and interleukin-1 receptor antagonist in cells of ameboid phenotype, the majority of which are NG2-positive. Brain research bulletin. 2008;77:19–26. doi: 10.1016/j.brainresbull.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Figiel I. Pro-inflammatory cytokine TNF-alpha as a neuroprotective agent in the brain. Acta neurobiologiae experimentalis. 2008;68:526–34. doi: 10.55782/ane-2008-1720. [DOI] [PubMed] [Google Scholar]
- Foo LC, Allen NJ, Bushong EA, Ventura PB, Chung WS, Zhou L, Cahoy JD, Daneman R, Zong H, Ellisman MH, et al. Development of a method for the purification and culture of rodent astrocytes. Neuron. 2011;71:799–811. doi: 10.1016/j.neuron.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea E, Reis DJ, Feinstein DL. Cloning and expression of inducible nitric oxide synthase from rat astrocytes. J Neurosci Res. 1994;37:406–14. doi: 10.1002/jnr.490370313. [DOI] [PubMed] [Google Scholar]
- Gao Q, Lu J, Huo Y, Baby N, Ling EA, Dheen ST. NG2, a member of chondroitin sulfate proteoglycans family mediates the inflammatory response of activated microglia. Neuroscience. 2010;165:386–94. doi: 10.1016/j.neuroscience.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Gasque P, Chan P, Mauger C, Schouft MT, Singhrao S, Dierich MP, Morgan BP, Fontaine M. Identification and characterization of complement C3 receptors on human astrocytes. Journal of immunology. 1996;156:2247–55. [PubMed] [Google Scholar]
- Gegg ME, Beltran B, Salas-Pino S, Bolanos JP, Clark JB, Moncada S, Heales SJ. Differential effect of nitric oxide on glutathione metabolism and mitochondrial function in astrocytes and neurones: implications for neuroprotection/neurodegeneration? J Neurochem. 2003;86:228–37. doi: 10.1046/j.1471-4159.2003.01821.x. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1986;6:2163–78. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Li J, Li X, George J, Rutecki PA. The impact of microglia-derived cytokines upon gliosis in the CNS. Developmental neuroscience. 1994;16:128–36. doi: 10.1159/000112099. [DOI] [PubMed] [Google Scholar]
- Goss JR, O’Malley ME, Zou L, Styren SD, Kochanek PM, DeKosky ST. Astrocytes are the major source of nerve growth factor upregulation following traumatic brain injury in the rat. Experimental neurology. 1998;149:301–9. doi: 10.1006/exnr.1997.6712. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Uliasz TF, Hewett SJ, Hewett JA. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. Journal of neuroscience methods. 2006;150:128–37. doi: 10.1016/j.jneumeth.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hansson E, Muyderman H, Leonova J, Allansson L, Sinclair J, Blomstrand F, Thorlin T, Nilsson M, Ronnback L. Astroglia and glutamate in physiology and pathology: aspects on glutamate transport, glutamate-induced cell swelling and gap-junction communication. Neurochem Int. 2000;37:317–29. doi: 10.1016/s0197-0186(00)00033-4. [DOI] [PubMed] [Google Scholar]
- Hetier E, Ayala J, Bousseau A, Denefle P, Prochiantz A. Amoeboid Microglial Cells and not Astrocytes Synthesize TNF-alpha in Swiss Mouse Brain Cell Cultures. Eur J Neurosci. 1990;2:762–768. doi: 10.1111/j.1460-9568.1990.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Holm TH, Draeby D, Owens T. Microglia are required for astroglial Toll-like receptor 4 response and for optimal TLR2 and TLR3 response. Glia. 2012;60:630–8. doi: 10.1002/glia.22296. [DOI] [PubMed] [Google Scholar]
- Kajihara H, Tsutsumi E, Kinoshita A, Nakano J, Takagi K, Takeo S. Activated astrocytes with glycogen accumulation in ischemic penumbra during the early stage of brain infarction: immunohistochemical and electron microscopic studies. Brain Res. 2001;909:92–101. doi: 10.1016/s0006-8993(01)02640-3. [DOI] [PubMed] [Google Scholar]
- Kanai T, Uraushihara K, Totsuka T, Nemoto Y, Fujii R, Kawamura T, Makita S, Sawada D, Yagita H, Okumura K, et al. Ameliorating effect of saporin-conjugated anti-CD11b monoclonal antibody in a murine T-cell-mediated chronic colitis. J Gastroenterol Hepatol. 2006;21:1136–42. doi: 10.1111/j.1440-1746.2006.04391.x. [DOI] [PubMed] [Google Scholar]
- Karram K, Goebbels S, Schwab M, Jennissen K, Seifert G, Steinhauser C, Nave KA, Trotter J. NG2-expressing cells in the nervous system revealed by the NG2-EYFP-knockin mouse. Genesis. 2008;46:743–57. doi: 10.1002/dvg.20440. [DOI] [PubMed] [Google Scholar]
- Kitada M, Rowitch DH. Transcription factor co-expression patterns indicate heterogeneity of oligodendroglial subpopulations in adult spinal cord. Glia. 2006;54:35–46. doi: 10.1002/glia.20354. [DOI] [PubMed] [Google Scholar]
- Krasowska-Zoladek A, Banaszewska M, Kraszpulski M, Konat GW. Kinetics of inflammatory response of astrocytes induced by TLR 3 and TLR4 ligation. J Neurosci Res. 2007;85:205–12. doi: 10.1002/jnr.21088. [DOI] [PubMed] [Google Scholar]
- Kuno R, Yoshida Y, Nitta A, Nabeshima T, Wang J, Sonobe Y, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. The role of TNF-alpha and its receptors in the production of NGF and GDNF by astrocytes. Brain Res. 2006;1116:12–8. doi: 10.1016/j.brainres.2006.07.120. [DOI] [PubMed] [Google Scholar]
- Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000;74:9214–21. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Rivest S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. Faseb J. 2001;15:155–163. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- Lafortune L, Nalbantoglu J, Antel JP. Expression of tumor necrosis factor alpha (TNF alpha) and interleukin 6 (IL-6) mRNA in adult human astrocytes: comparison with adult microglia and fetal astrocytes. J Neuropathol Exp Neurol. 1996;55:515–21. doi: 10.1097/00005072-199605000-00003. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–86. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–9. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Enquist LW, Card JP. Reactions of oligodendrocyte precursor cells to alpha herpesvirus infection of the central nervous system. Glia. 1998;23:316–28. [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends in neurosciences. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci U S A. 1989;86:6348–52. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Liu B, Du L, Hong J-S. Naloxone Protects Rat Dopaminergic Neurons against Inflammatory Damage through Inhibition of Microglia Activation and Superoxide Generation. Journal of Pharmacology and Experimental Therapeutics. 2000;293:607–617. [PubMed] [Google Scholar]
- Liu Y, Qin L, Wilson BC, An L, Hong JS, Liu B. Inhibition by naloxone stereoisomers of beta-amyloid peptide (1–42)-induced superoxide production in microglia and degeneration of cortical and mesencephalic neurons. J Pharmacol Exp Ther. 2002;302:1212–9. doi: 10.1124/jpet.102.035956. [DOI] [PubMed] [Google Scholar]
- Losciuto S, Dorban G, Gabel S, Gustin A, Hoenen C, Grandbarbe L, Heuschling P, Heurtaux T. An efficient method to limit microglia-dependent effects in astroglial cultures. J Neurosci Methods. 2012;207:59–71. doi: 10.1016/j.jneumeth.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:876–85. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. The Journal of cell biology. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief J, Koning N, Schuurman KG, Van De Garde MD, Smolders J, Hoek RM, Van Eijk M, Hamann J, Huitinga I. Phenotyping primary human microglia: tight regulation of LPS responsiveness. Glia. 2012;60:1506–17. doi: 10.1002/glia.22370. [DOI] [PubMed] [Google Scholar]
- Nakase T, Sohl G, Theis M, Willecke K, Naus CC. Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. The American journal of pathology. 2004;164:2067–75. doi: 10.1016/S0002-9440(10)63765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan JP, Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert opinion on drug safety. 2005;4:433–42. doi: 10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain research reviews. 2009;59:278–92. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248–56. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 2012;109:E197–205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy S, Naveilhan P, Paille V, Brachet P, Neveu I. Lipopolysaccharide and TNFalpha regulate the expression of GDNF, neurturin and their receptors. Neuroreport. 2003;14:1529–34. doi: 10.1097/00001756-200308060-00026. [DOI] [PubMed] [Google Scholar]
- Rocha SM, Cristovao AC, Campos FL, Fonseca CP, Baltazar G. Astrocyte-derived GDNF is a potent inhibitor of microglial activation. Neurobiology of disease. 2012;47:407–15. doi: 10.1016/j.nbd.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Salimi K, Moser KV, Marksteiner J, Reindl M, Humpel C. GDNF and TGF-beta1 promote cell survival in serum-free cultures of primary rat microglia. Cell and tissue research. 2003;312:135–9. doi: 10.1007/s00441-003-0711-7. [DOI] [PubMed] [Google Scholar]
- Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CD, Melchior B, Masek K, Puntambekar SS, Danielson PE, Lo DD, Sutcliffe JG, Carson MJ. Differential gene expression in LPS/IFNgamma activated microglia and macrophages: in vitro versus in vivo. J Neurochem. 2009;109(Suppl 1):117–25. doi: 10.1111/j.1471-4159.2009.05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola C, Casal C, Tusell JM, Serratosa J. Astrocytes enhance lipopolysaccharide-induced nitric oxide production by microglial cells. Eur J Neurosci. 2002;16:1275–83. doi: 10.1046/j.1460-9568.2002.02199.x. [DOI] [PubMed] [Google Scholar]
- Tabernero A, Orfao A, Medina JM. Astrocyte differentiation in primary culture followed by flow cytometry. Neurosci Res. 1996;24:131–8. doi: 10.1016/0168-0102(95)00981-7. [DOI] [PubMed] [Google Scholar]
- Tacconi MT. Neuronal death: is there a role for astrocytes? Neurochem Res. 1998;23:759–65. doi: 10.1023/a:1022463527474. [DOI] [PubMed] [Google Scholar]
- Vincent VA, Tilders FJ, Van Dam AM. Inhibition of endotoxin-induced nitric oxide synthase production in microglial cells by the presence of astroglial cells: a role for transforming growth factor beta. Glia. 1997;19:190–8. doi: 10.1002/(sici)1098-1136(199703)19:3<190::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- von Bernhardi R, Eugenin J. Microglial reactivity to beta-amyloid is modulated by astrocytes and proinflammatory factors. Brain Res. 2004;1025:186–93. doi: 10.1016/j.brainres.2004.07.084. [DOI] [PubMed] [Google Scholar]
- Welser-Alves JV, Milner R. Microglia are the major source of TNF-alpha and TGF-beta1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem Int. 2013;63:47–53. doi: 10.1016/j.neuint.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, Xin T, Zhao L, Hunter RL, Chen Y, Bing G. Glial cell line-derived neurotrophic factor protects midbrain dopaminergic neurons against lipopolysaccharide neurotoxicity. Journal of neuroimmunology. 2010;225:43–51. doi: 10.1016/j.jneuroim.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Hu X, Qian L, O’Callaghan JP, Hong JS. Astrogliosis in CNS pathologies: is there a role for microglia? Molecular neurobiology. 2010;41:232–41. doi: 10.1007/s12035-010-8098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Xiang P, Guo K, Wang A, Lu J, Tay SS, Jiang H, He BP. Microglia/monocytes with NG2 expression have no phagocytic function in the cortex after LPS focal injection into the rat brain. Glia. 2012;60:1417–26. doi: 10.1002/glia.22362. [DOI] [PubMed] [Google Scholar]