Abstract
A growing translational literature suggests that adolescent exposure to anabolic-androgenic steroids (AASs) leads to increased aggression and impulsivity. However, little is known about the cognitive effects of AASs among AAS users or the differences between adolescent and adult onset users. This study provides a test of the effects of acute naturalistic AAS use and age of onset (adolescent vs. adult) on measures of inhibitory control, planning and attention, and decision making. Seventy one active adult male AAS uses completed self-report measures of impulsivity and aggress and a subsample (11 adolescent onset vs. 11 adult onset) matched on current age were administered four computerized test from the CANTAB battery and the Iowa Gambling Task. Multiple regression analyses and a series of 2 (Adolescent vs. Adult) X 2 (On-cycle vs. Off-cycle) analyses of variance (ANOVAs) were used to examine the differential effects of age of onset and acute drug use on cognition and behavior. Regression analyses revealed larger on-cycle effects for adolescent users than adult users. Subsample analyses indicated that on-cycle users performed less well on cognitive measures of inhibitory control and attention, but not on tests of planning or decision making. Adolescent onset was associated with a greater impulsivity and a greater acute sensitivity to AAS effects on attention. These preliminary findings suggest the possibility that acute AAS use is associated with some differences in inhibitory control and impulsivity and to a lesser degree aggression. These effects may be more potent for those initiating AAS use in adolescence.
Keywords: Anabolic-Androgenic Steroids, Early Onset, CANTAB, inhibitory control, executive function, impulsivity, aggression
Developmental Risks of Anabolic Steroid Use
Anabolic-androgenic steroids (AASs) are a family of synthetic androgenic hormones used by primarily exercising men to improve athletic performance and physical appearance (Bahrke & Yesalis, 2004). The developmental trajectory of those that use these and other appearance and performance enhancing drugs (APEDS) is largely unknown. While many of the acute psychiatric and physical effects of AAS use appears to be transitory (Evans, 2004), these negative outcomes may also partially motivate long-term use (Hildebrandt, Langenbucher, Carr, Sanjuan, & Park, 2006). However, the developmental risks of AASs remain largely undocumented and the existence of lasting psychiatric changes remains untested.
Large sample studies suggest that the majority of AAS use begins in adulthood (Hildebrandt, Langenbucher, Carr, & Sanjuan, 2007; Perry, Lund, Deninger, Kutscher, & Schneider, 2005), with population estimates suggesting a prevalence of about 3% of the adult US population (Centers for Disease Control and Prevention, 2004). Estimates among youth suggest 1–2% of male adolescents have used in the past 12 months (Johnston, O’Malley, Bachman, & Schulenberg, 2012). Rates of use are likely higher in non-US populations where AASs are less regulated (Galduroz, Noto, Nappo, & Carlini, 2005; Hakansson, Mickelsson, Wallin, & Berglund, 2012). The risk for developing AAS dependence is very high compared to other drugs of abuse, with drug dependence among current users estimated at about 30% (Kanayama, Hudson, & Pope, 2009). However, the construct validity of AAS dependence has been challenged (Hildebrandt, Lai, et al., 2011; Kanayama, Brower, Wood, Hudson, & Pope, 2009) and is complicated by the lack of information about important developmental transitions in the course of AAS use.
Anabolic-Androgenic Steroid Intoxication
AASs are notable among drugs of abuse because they lack reliable acute interoceptive changes and the euphoria characteristic of drug reinforcement (Fingerhood, Sullivan, Testa, & Jasinski, 1997; Su et al., 1993). Rather, intoxication is more accurately defined by the changes in threshold for a cluster of impulsive or rewarding behaviors including aggression, sexual behavior, exercise, and drug use (Hildebrandt, 2012). The increase in these behaviors have been documented in translational, observational, and experimental studies conducted in rodents (Oberlander & Henderson, 2012) and humans (Kanayama, Hudson, & Pope, 2010). Studies of AAS intoxication among experienced users remain absent in the literature.
State versus Trait Effects of Anabolic Steroid Use
Evidence from the rodent literature suggests that exposure to high doses of AASs at developmentally critical periods can have lasting effects on brain development and behavioral disinhibition (Cunningham, Lumia, & McGinnis, 2012). Animals administered high doses of AASs during adolescence experience persistent changes in impulsive aggression even after a single exposure (Farrell & McGinnis, 2004), whereas this behavior in adult animals are less robust (Salas-Ramirez, Montalto, & Sisk, 2008) and experiences a rapid decay (McGinnis, Lumia, & Possidente, 2002). Adolescent AAS exposure may promote greater impulsivity and aggression compared to adult onset. Specifically, it is plausible that adolescent exposure causes increases in trait levels of impulsivity and aggression. Adolescent AAS users report significantly higher levels of impulsive, aggressive, and risk taking behavior than controls (Bahrke, Yesalis, Kopstein, & Stephens, 2000; Beaver, Vaughn, Delisi, & Wright, 2008; vandenBerg, Neumark-Sztainer, Cafri, & Wall, 2007).
AAS use and Cognitive Changes
A recent study by Kanayama, Kean, Hudson, and Pope (2012) found a positive relationship between level of AAS exposure and impairments in visiospatial memory, but not other cognitive measures. The severity of impairment was correlated with lifetime exposure to AAS and consistent with a model where AASs induce neurodegenerative effects in the forbrain (Pieretti et al., 2013). One outstanding question raised by this finding is whether cognitive changes are affected by cycle status and age of onset. Hypogonadaism, a endocrine state often occurring during post-cycle recovery (Tan & Scally, 2009), is associated with decline in cognitive performance over time (Matousek & Sherwin, 2010). Conversely, testosterone supplementation to aging eugonadal men is associated with improvement in cognitive function (Holland, Bandelow, & Hogervorst, 2011). Thus, age and cycle status may differentially affect cognition related to trait and state aggression and impulsivity.
Hypotheses
Building upon the experimental animal findings and observational studies with adolescents, we hypothesized that age of onset would be associated with greater impairments cognitive functioning, particular measures associated with inhibitory control, compared to adult onset AAS use. Significant experimental and observational research implicates acute AASs use in the increased risk for aggressive and impulsive behavior. Consequently, we hypothesized that acute AAS use would be associated with reduced performance on cognitive measures of inhibitory control, planning, and attention.
Methods
Participants
Men from the original sample (N =71) described by Hildebrandt, Langenbucher, Lai, Loeb, and Hollander (2011) were included in this study and a subset of (n = 22) were used to test hypotheses about cognition. We recruited current (on-cycle or plans to go on-cycle in the next year) experienced (>1 AAS cycle) AAS users primarily from local gyms and newspaper adds. We recruited individuals based on cycle status (on-cycle vs. off cycle) and age of first AAS exposure (< 19 years old, > 22 years old, see Table 2) and matched them on baseline demographics using a mean matching algorithm for each group to protect sample from biases introduced by current age or cohort differences in demographics. We did not sample individuals who began during college because of some uncertainty about the threshold definition of adolescence (Spear, 2013). Cycle status was verified by random sampling of urine analysis (5 of 22 sampled) using gas chromatography and mass spectrometry (Anti-Doping Research, Inc.; Los Angeles, USA). All five samples confirmed self-reported cycle status. All procedures were approved by the Institutional Review Boards of the participating institutions.
Table 2.
Summary of Cognitive Assessment Battery Tasks
| Construct | Description | Outcomes | |
|---|---|---|---|
| Affective Go-No go (AGN; Murphy, Sahakian, Rubinsztein, Rogers, Robbins, & Paykel, 1999) | Behavioral Disinhibition in emotional contexts | The tasks uses six modes. He/shee is given a target category (i.e, positive, negative and neutral) and is asked to press the press pad when they see a word matching this category. | Outcome measures were response times (time taken to respond to each target), commission errors (responses to distractor stimuli). |
| Intra-Extra Dimensional Set Shift (IED; Downs, Roberts, Sahakian, Evenden, Morris, & Robbins, 1989) | Attention and set shifting | At each block, two sets of stimuli are presented in simple or compound variations. Participants progress through the test by satisfying a predetermined set of criteria, and learn the correct answer through feedback provided by the computer after each response. After six correct responses the rules are changed. | Outcome measures were the number of test trials completed (minimum moves) and the recorded latency to the first move providing estimates of cognitive processing speed (initial planning time). |
| Stockings of Cambridge (SOC) | Spatial Planning | The task presents participants with an upper and lower display of three colored balls. Participants are asked to replicate the patterns of colored balls presented in the upper display in the lower display. | Outcome variables include number of moves, number of correct trials, and response latency as measures of spatial planning and processing speed. |
| Stop Signal Task (SST) | Behavioral Inhibition | Participants are asked to click a button on the mouse pad in the direction of the arrows presented on the computer screen. Participants are then asked to continue to press the mouse pad when they see the arrows, however, when the stimuli is in conjunction with an auditory stimuli (a beep), participants must withhold (i.e., inhibit) their responses. | The outcome measures include distractor errors and reaction time. |
| Iowa Gambling Task (IGT; Bechara, Damasio, Damasio and Anderson, 1994) | Decision Making | Participants are provided four decks of cards and “gamble” to earn a profit by turning one of four cards. Decks have been predetermined to be either “disadvantageous” (costing the most in the long run) or “advantageous” (resulting in an overall gain). | A positive net score indicates advantageous performance on the IGT and a negative score indicates poor decision-making performance. |
Cognitive Testing
The Cambridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition, 2002; www.cantab.com) is a computerized neurocognitive testing battery and we used four tests to measure memory, learning, affective processing, motor speed, planning abilities and attention. Table 2 summaries each task and the associated construct.
Questionnaires
Participants completed the Barratt Impulsiveness Scale version-11 (BIS-11;(Patton et al., 1995) as a measure impulsivity, which has three subscales (attentional, motor, and non-planning impulsiveness) and demonstrated good internal consistency in this sample (α = 0.79 to 0.83). They also completed the Buss-Perry Aggression Questionnaire (BPAQ; Buss and Perry, 1992) as a measure of aggression (Physical Aggression, Verbal Aggression, Anger, and Hostility) and also demonstrated good internal consistency in this sample (α = 0.82 to 0.88).
Clinical Interviews
As reported in Hildebrandt, Langenbucher, et al., (2011), all clinical interviews were completed by trained research staff and co-rated by blind co-raters and reached high levels of inter-rater and test-retest reliability. The Structured Clinical Interview of Diagnosis (SCID-I; (First, Spitzer, Gibbon, & Williams, 2007) was used to assess for AAS dependence and comorbid SUDs. The Appearance and Performance Enhancing Drug Use Schedule (APEDUS), as described by Hildebrandt, Langenbucher, et al., (2011), is a semi-structured interview that includes 10 modules providing a comprehensive assessment of APED use and associated phenomena. For this sub-sample, inter-rater reliability was high for individual items and scales ranging from κ = .94 to 1.0. One-week test-retest reliability for these items range from r = .91 to .97 and for the age of onset item r = .97.
Statistical Tests
Primary statistical analyses were conducted using R version 2.15. We used multiple regression models for self-report measures of impulsivity and aggression with N = 71 men available from the original sample. For these models, we used age of onset as a continuous predictor. A 2 (On-cycle vs. Off-cycle) X 2 (Adolescent onset vs. Adult onset) factorial analysis of variance (ANOVA) was conducted including main effects and interaction (Cycle Status X Age of Onset). Data were screened for threats to ANOVA assumptions. After screening for the original model, AAS exposure (total number of cycles in weeks X average mg/week of AAS), SCID diagnosis of AAS dependence, SCID diagnosis of other substance use disorder, and current stimulant use were tested as covariates in the models. None of these variables had a significant effect and were subsequently dropped to conserve power.
Results
Cognitive Testing
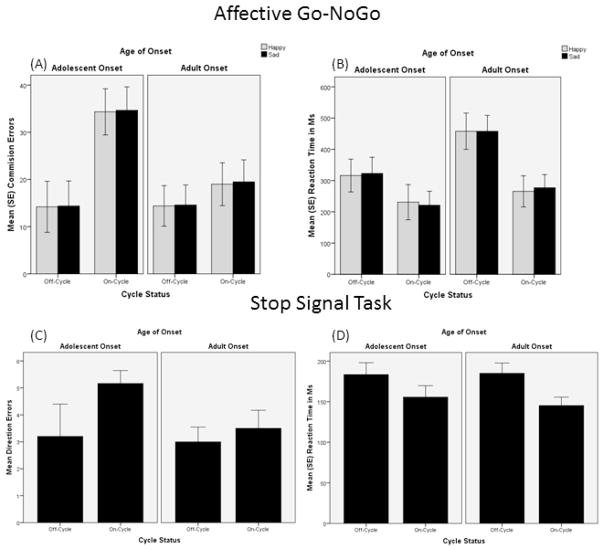
Figure 1 indicates that on-cycle AAS users had significantly more commission errors, but only for affective stimuli. On-cycle AAS users also responded more quickly to happy and sad nogo signals than off-cycle users. Age of onset was not significantly associated with differences in commission errors (η2 = .079–.120) or reaction time (η2 = .126 for happy; η2 = .187 for sad). None of the interaction effects were significant, but effect sizes were moderately sized for affective commission errors (η2 = .122 for happy; η2 = .127 for sad). All other interaction effect sizes were small (η2 = .012–.051).
Figure 1.
(A): Happy (F = 6.6, p < .05, η2 = .27) and Sad (F = 6.8. p < .05, η2 = .27) commission errors significantly differed by cycle status. (B): Happy (6.4, p < .05, η2 = .26) and Sad (F = 9.0, p < .01, η2 = .33) reaction times differed by cycle status. (C): No significant differences in number of direction errors. (D): Mean reaction times on stop signals significantly differed by cycle (F = 6.7, p < .05, η2 = .27). No other significant effects were observed across measures; however, moderate effect sizes were observed for age of onset (η2 = .08–.19) and age of onset X cycle status interactions (η2 = .12–.13) for commission errors.
Tables 3 and 4 summarize the group means and ANOVA results for the remaining cognitive performance measures. Results of the IED shift (stages complete), which measures attentional accuracy, indicated that on-cycle users successfully completed less stages, but this was not associated with more efficiency errors. The direction of cycle effects, however, was consistent with the general finding of less accurate planning. Adolescent onset users had poorer planning efficiency (SOC task), but not planning time. No other differences were observed.
Table 3.
Summary of Cognitive Performance by Age of Onset and Cycle Status (N = 22)
| Participant Characteristics | Adolescent Onset (<19) | Adult Onset (>22) | ||
|---|---|---|---|---|
|
| ||||
| On Cycle (n = 6) | Off Cycle (n = 5) | On-Cycle (n = 6) | Off-Cycle (n=5) | |
| Age of first AAS use | 16.5 (0.72) | 16.71 (1.12) | 25.54 (1.97) | 24.89 (2.10) |
| Lifetime AAS Exposure* | 224.67 (110.54) | 208.8 (75.28) | 201.50 (49.30) | 188.40 (75.14) |
| Stimulant Use | 50.00% | 40.00% | 66.67% | 60% |
| AAS Dependence | 16.67% | 20.00% | 16.67% | 0% |
| Co-Morbid SUD | 16.67% | 20.00% | 16.67% | 20.00% |
|
| ||||
| Cognitive Performance | ||||
|
| ||||
| IED (stages complete) | 4.83 (1.17) | 8.60 (1.95) | 6.67 (1.03) | 8.80 (2.17) |
| IED Shift (errors) | 36.00 (9.96) | 31.60 (10.11) | 30.17 (9.45) | 32.00 (8.09) |
| Stockings of Cambridge (min moves) | 4.00 (1.10) | 4.80 (1.48) | 6.67 (1.37) | 7.60 (1.14) |
| Initial Planning Time | 11,483 (5,312) | 12,670 (5,362) | 12,618 (5,228) | 12,929 (8,309) |
| Iowa Gambling Task (advantageous - disadvantageous) | −3.17 (20.61) | 0.6 (21.82) | 2.83 (19.79) | 2.6 (20.55) |
Note. IED = intra-extra dimensional set shift. Mean (standard deviation).
Table 4.
Summary of 2 (On-cycle vs. Off Cycle) X 2 (Adolescent vs. Adult Onset) Analyses of Variance Results (N = 22)
| Age of Onset | Cycle Status | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Participant Characteristics | F | p-value | η2 | F | p-value | η2 | F | p-value | η2 |
| Age of first AAS use | 159.46 | <.001 | 0.90 | 0.41 | 0.53 | 0.02 | 10 | 0.74 | 0.01 |
| Lifetime AAS Exposure | 0.38 | 0.54 | 0.02 | 0.17 | 0.69 | 0.01 | .00 | 0.97 | <0.01 |
| Stimulant Usea | 0.73 | 0.39 | - | 0.21 | 0.69 | - | 0.01 | 0.93 | - |
| AAS Dependencea | 0.00 | 1.0 | - | 0.01 | 0.95 | - | 0.00 | 0.99 | - |
| Co-Morbid SUDa | 0.00 | 1.0 | - | 0.00 | 1.0 | - | 0.00 | 1.0 | - |
|
| |||||||||
| Cognitive Performance | |||||||||
|
| |||||||||
| IED (stages complete) | 2.20 | 0.16 | 0.11 | 18.51 | <0.001 | 0.50 | 1.42 | 0.25 | 0.07 |
| IED Shift (errors) | 0.45 | 0.51 | 0.02 | 0.10 | 0.76 | 0.01 | 0.59 | 0.45 | 0.03 |
| Stockings of Cambridge (min moves) | 25.01 | <0.001 | 0.58 | 2.51 | 0.13 | 0.12 | 0.02 | 0.90 | 0.00 |
| Initial Planning Time | 0.07 | 0.79 | 0.00 | 0.08 | 0.78 | 0.01 | 0.03 | 0.87 | 0.00 |
| Iowa Gambling Task (advantageous - disadvantageous) | 0.21 | 0.66 | 0.01 | 0.04 | 0.84 | 0.00 | 0.05 | 0.82 | 0.00 |
Note. IED = intra-extra dimensional set shift. Mean (standard deviation).
Chi Square Statistic reported
Self-Report Impulsivity and Aggression
Table 5 summarizes regression models for self-reported impulsivity and aggression. For impulsivity, regression models explained between 40% and 67% of the variance. Interaction effects indicated that later onset AAS use was associated with more non-planning impulsivity on-cycle than off-cycle, but little effect was observed for early onset AAS use. The opposite pattern was true for self-reported attentional and motor impulsivity. Regression models explained 10% to 29% of the variance in aggression. Significant effects of cycle status indicated generally more aggression, although this was only significant for hostility and verbal aggression. Earlier onset of AAS use was associated with more hostility, but not other types of aggression. Significant interactions for the anger and verbal aggression scales indicated greater aggression scores among adolescent onset users when they were on-cycle. Supplementary data report same findings for the subsample of 22 AAS users matched on current age.
Table 5.
Regression Self-Reported Aggression and Impulsivity on Cycle Status, Age of Onset, and their Interaction
| BIS Total Score | BIS Attention | BIS Motor | BIS Non-Planning | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | B | SE | β | B | SE | β | B | SE | β | |
| Intercept | 59.050 | 1.361 | 17.772 | .489 | 19.475 | .463 | 21.803 | .721 | ||||
| Age of Onset | −1.817 | .309 | −.687*** | −.432 | .111 | −.403*** | −.266 | .105 | −.321* | −1.120 | .163 | −.927*** |
| On Cycle | 9.719 | 1.912 | .406*** | 4.419 | .687 | .455*** | 3.231 | .651 | .431*** | 2.070 | 1.013 | .189* |
| Interaction | .033 | .423 | .009 | −.531 | .152 | −.360** | −.355 | .144 | −.311* | .919 | .224 | .553*** |
| R2adj | .55 | .64 | .47 | .40 | ||||||||
| BPAQ Anger | BPAQ Hostility | BPAQ Physical | BPAQ Verbal | BPAQ Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | 15.251 | .616 | 15.630 | .683 | 13.855 | .629 | 12.657 | .526 | 57.393 | 1.949 | |||||
| Age of Onset | .040 | .140 | .045 | −.359 | .155 | −.373* | −.162 | .143 | −.189 | −.052 | .119 | −.064 | −.533 | .442 | −.184 |
| On Cycle | 1.692 | .865 | .208 | 3.226 | .960 | .370*** | 1.548 | .884 | .199 | 2.853 | .739 | .389*** | 9.318 | 2.739 | .356*** |
| Interaction | −.592 | .191 | −.480*** | .246 | .212 | .186 | −.188 | .195 | −.159 | −.422 | .163 | −.379* | −.957 | .605 | −.241 |
| R2adj | .21 | .15 | .10 | .29 | .23 | ||||||||||
Note. BIS=Barratt Impulsiveness Scale; BPAQ= Buss-Perry Aggression Questionnaire. All regression models had df 3,68 and included 71 male participants. Age of onset was centered (M = 26.35, SD = 9.51).
p < .05.
p < .01.
p < .001.
Discussion
The pattern of results generally suggested that early onset users were more impulsive, and demonstrated deficits in affective processing, behavioral disinhibition, and planning, but not decision making. Group differences in impulsivity and cognition are consistent with translational research AAS’s behavioral effects (Oberlander & Henderson, 2012). These data also implicate emotional triggers more explicitly in AAS intoxication and may be used to better understand individual differences in consequences of APED use. In particular, these data suggest that early onset users may have elevated risk for disinhibitory effects of AAS when triggered emotionally.
Cognitive effects
Inhibitory control
The cycle effects of AAS on cognition are consistent with predictions of general brain arousal (Pfaff, 2006), which operationalizes this arousal state by increased emotional reactivity, locomotion, and alertness and involves mechanisms mediated through androgen and estrogen receptors in the central nervous system (Garey et al., 2003). Increased reaction time to both positive and negative stimuli is suggestive of a cognitive state that is reactive broadly to emotional stimuli. Other studies using AGN methods have shown that shorter reaction times and commission errors are consistent with affect congruent states or mood bias (Elliott, Rubinsztein, Sahakian, Dolan, 2002). The shorter reaction times observed for SST go and stop trials are also consistent with the brain arousal hypothesis suggesting an increased alertness and ability to attend and react quickly to relevant stimuli. This finding is unusual compared to other impulsive substance abusing populations that have delayed reaction times ( e.g., Li, Luo, Yan, Bergquist, & Sinha, 2009).
The same disinhibitory effects found in the AGN were not evident in the SST where reaction times were faster but errors did not differ between groups. Differences in performance on these two tasks suggest that the disinhibitory effects AAS intoxication are primarily a result of emotional processing. When distractors were neutral, AAS users on-cycle responded more quickly and at a similar rate of accuracy as those individuals off-cycle. This finding could be interpreted as enhanced cognitive processing speed and may be a result of enhanced amygdala activation to all stimuli. A recent study by Ackermann et al. (2012) found testosterone levels in adult men were predictive of amygdala BOLD response and enhanced retrieval of both emotional and neutral stimuli, suggesting androgens assign significance broadly to stimuli.
Attentional processes
On-cycle AAS users’ ability to shift attention effectively was diminished and it indicates that on-cycle users tended to stick with a behavioral response pattern, even in the context of negative feedback. This perseverative responding has been shown to be mediated by testosterone in rodents (van Hest, van Haaren, & van de Poll, 1989) and linked to androgen mediated effects on dopaminergic neurons in medial-PFC (Kritzer, Brewer, Montalmant, Davenport, & Robinson, 2007). The number of set shifting errors was not significantly different between groups although the pattern of errors supports the general finding of set shifting difficulties. The reduced reaction times, but no difference in commission errors, observed during the SST suggests that active drug use may improve some attentional processes.
Planning and decision making
Measures of risky decision making and planning did not differ by study group. This finding is in contrast to observational data that suggest higher circulating testosterone is correlates with risky decision making in healthy males (Stanton, Liening, & Schultheiss, 2011). The observed effect may, however, be offset by differences in circulating estrogen. In an experimental study of letrozol (a potent aromatase inhibitor), risk taking under conditions of uncertainty increased among those whose estrogen levels were reduced and testosterone increased by blockade of aromatization (Goudriaan et al., 2010). Thus, differential levels of aromatization may contribute to variability in results.
Self-reported Aggression and Impulsivity
Impulsivity
Group differences in impulsivity indicated robust effects for both AAS cycle and age of onset, generally indicating that AASs increase attentional and behavioral impulsivity, but not planning. The latter finding is intuitive because experienced users engage in complex patterns of drug administration (Hildebrandt et al., 2007; Monaghan, 2002), which requires an intact ability to plan and control use. In contrast, adolescent onset users plan less frequently and avoid more complex decision making, independent of cycle status. The effects of AAS on motor impulsivity indicate that both AASs and age of onset have robust associations with the tendency to act quickly and in the moment. These findings are also consistent with the animal literature (Ambar & Chiavegatto, 2009; Kindlundh, Lindblom, Bergstrom, & Nyberg, 2003) and cross-sectional data on AAS users (Bahrke et al., 2000). Attentional differences between groups indicated that adolescent onset users experienced greater impairment in cognition on-cycle than the adults, despite AAS effects being present for both groups.
Aggression
The effects of AAS on aggression was consistent with published animal and human literatures (Trenton & Currier, 2005), although effect sizes were small to moderate. The differences in subscale effect sizes suggest that the effects of AASs are likely to be somewhat idiosyncratic, with a wide variety of non-AAS influences (Liu, Lewis, & Evans, 2013). The majority of participants in this study were middle aged men and we did not assess for lifetime aggressive behaviors. It is possible that AASs may have had a different impact on the expression of aggression at different developmental stages.
Study Limitations
The current study had a number of limitations, primarily associated with the small sample size, lack of control over individual exposure to AASs, and high degree of matching between groups in the subsample. Due to the small sample, even the robust effects should be interpreted with caution because of the reduced participant variability in population typically considered to be largely heterogeneous (Hildebrandt, Alfano, & Langenbucher, 2010; Hildebrandt et al., 2007). Furthermore, the between-subjects design does not allow us to quantify if the magnitude of individual cycle effects, which will be a necessary next step in this research. Finally, it is unclear how stable these effects are across AAS cycles, so it will be important for future studies to examine how cognition and behavior change over time in response to an AAS cycle and extend these effects to female AAS users.
Conclusions
Taken together, the results from this study support a primary role of AASs in altering emotional reactivity and overall responsiveness to relevant stimuli. These results have implications for defining AAS intoxication in terms of lower thresholds for emotional reactivity, disinhibited behavior, and attention. This observation stands in contrast to the simplistic “roid rage” stereotype of intoxication associated with AAS use. These findings are also consistent with clinical and theoretical models of AAS use (Hildebrandt, Lai, et al., 2011; Hildebrandt, Langenbucher, et al., 2011) that suggest disinhibition might be a better clinical marker of intoxication than any specific mood or behavior.
The finding that exposure to AASs in adolescence may lead to greater cognitive changes associated with acute AAS use is also of significant clinical value. Adolescence is characterized by significant changes in prefrontal regulation of limbic neurocircuitry, some of which is mediated by gonadal hormones and associated AR mechanisms (Bramen et al., 2012). Flooding these receptors with excess exogenous androgens (and estrogens via aromatization pathway) has potentially important developmental effects that will be important to study in future studies.
Table 1.
Summary of Demographic and Anthropometric Measures by Age of AAS Onset
| Adolescent Onset (n=11) | Adult Onset (n=11) | t | p | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 36.32 (4.51) | 38.03(5.95) | −.80 | .22 |
| BMI | 27.37 (4.22) | 28.59 (5.02) | −.62 | .27 |
| Body Fat % | 11.77 % (6.05) | 13.40 % (7.10) | −.58 | .28 |
| Income | 65,150 (38,234) | 70,505 (45,500) | −.30 | .38 |
| % with at least college degree | 72.72% | 100% | 1.93a | .06 |
| Marital Status | 2.66b | .62 | ||
| Single | 54.54% | 27.27% | ||
| Divorced | 9.09% | 18.18% | ||
| Separated | 0.0% | 9.09% | ||
| Living as married | 9.09% | 18.18% | ||
| Married | 27.27% | 27.27% | ||
| Employment | 2.9b | .58 | ||
| Full-time | 36.36% | 54.54% | ||
| Part-time | 45.45% | 27.27% | ||
| Student | 9.09% | 0.0% | ||
| Retired | 0.0% | 9.09% | ||
| Unemployed | 9.09% | 9.09% | ||
| Disabled | 0.0% | 0.0% | ||
| Race/ethnicity | 1.06b | .79 | ||
| White | 72.72% | 81.81% | ||
| African American or Black | 9.09% | 0.0% | ||
| American Indian or Alaskan Native | 0.0% | – | ||
| Asian | 9.09% | 9.09% | ||
| Hispanic or Latino | 9.09% | 9.09% | ||
| Sexual orientation | 1.05b | .59 | ||
| Primarily heterosexual | 90.90% | 81.81% | ||
| Primarily homosexual | 9.09% | 9.09% | ||
| Bisexual | 0.0% | 9.09% | ||
Note. Mean and standard deviation reported for continuous variables.
Z-score.
Chi-square test statistic.
Acknowledgments
Role of Funding Source
Tom Hildebrandt’s role on this project was supported by a grant from the National Institutes on Drug Abuse (DA022444 & DA024043-02A1) which had no further role in study design, data collection, analysis or interpretation of data, writing manuscript, or the decision to submit the paper for publication.
This research was supported in part by grants K23 DA024043 awarded to Dr. Hildebrandt and R03 DA022444 awarded to Drs. Hildebrandt and Langenbucher by that National Institute on Drug Abuse.
Footnotes
Contributors
All contents of the paper and the significant contribution of each author to the manuscript are consistent with guidelines on ethical publishing.
Drs. Hildebrandt, Berlin, and Langenbucher contributed to the study design, developed and wrote the protocol. Dr. Hildebrandt, Dr. Harty, and Ms. Flores contributed to the statistical analysis and the first draft of the manuscript. All remaining authors all contributed revision and approved the final manuscript.
Conflict of interest
None of the authors have any conflicts of interest for this manuscript.
Contributor Information
Tom Hildebrandt, Ichan School of Medicine at Mount Sinai.
James W. Langenbucher, Rutgers, The State University of New Jersey
Adrianne Flores, Ichan School of Medicine at Mount Sinai.
Seth Harty, University of Pittsburg Medical Center.
Heather A. Berlin, Ichan School of Medicine at Mount Sinai
References
- Anti-Doping Research, Inc.; Los Angeles, USA
- Ackermann S, Spalek K, Rasch B, Gschwind L, Coynel D, Fastenrath M, de Quervain DJ. Testosterone levels in healthy men are related to amygdala reactivity and memory performance. Psychoeuroendocrinology. 2012;37(9):1417–1424. doi: 10.1016/j.psyneuen.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Bahrke MS, Yesalis CE. Abuse of anabolic androgenic steroids and related substances in sport and exercise. Current Opinion in Pharmacology. 2004;4(6):614–620. doi: 10.1016/j.coph.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Bahrke MS, Yesalis CE, Kopstein AN, Stephens JA. Risk factors associated with anabolic-androgenic steroid use among adolescents. Sports Medicine. 2000;29(6):397–405. doi: 10.2165/00007256-200029060-00003. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Vaughn MG, Delisi M, Wright JP. Anabolic-androgenic steroid use and involvement in violent behavior in a nationally representative sample of young adult males in the United States. American Journal of Public Health. 2008;98(12):2185–2187. doi: 10.2105/AJPH.2008.137018. AJPH.2008.137018 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, Sowell ER. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS One. 2012;7(3):e33850. doi: 10.1371/journal.pone.0033850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss AH, Perry MP. The aggression questionnaire. Journal of Personality and Social Psychology. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Cambridge Cognition. Cambridge: Cambridge Cognition Limited; 2002. [Google Scholar]
- Cunningham RL, Lumia AR, McGinnis MY. Androgenic anabolic steroid exposure during adolescence: Ramifications for brain development and behavior. Hormones and Behavior. 2013;64(2):35–356. doi: 10.1016/j.yhbeh.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Archives of General Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Evans NA. Current concepts in anabolic-androgenic steroids. American Journal of Sports Medicine. 2004;32(2):534–542. doi: 10.1177/0363546503262202. [DOI] [PubMed] [Google Scholar]
- Fingerhood MI, Sullivan JT, Testa M, Jasinski DR. Abuse liability of testosterone. Journal of Psychopharmacology. 1997;11(1):59–63. doi: 10.1177/026988119701100115. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2007. (Vol. November, 2002) [Google Scholar]
- Galduroz JC, Noto AR, Nappo SA, Carlini EA. Household survey on drug abuse in Brazil: study involving the 107 major cities of the country--2001. Addictive Behaviors. 2005;30(3):545–556. doi: 10.1016/j.addbeh.2004.08.004. S0306-4603(04)00282-5 [pii] [DOI] [PubMed] [Google Scholar]
- Garey J, Goodwillie A, Frohlich J, Morgan M, Gustafsson JA, Smithies O, Pfaff DW. Genetic contributions to generalized arousal of brain and behavior. Proceedings of the National Academy of Sciences U S A. 2003;100(19):11019–11022. doi: 10.1073/pnas.1633773100. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson A, Mickelsson K, Wallin C, Berglund M. Anabolic androgenic steroids in the general population: user characteristics and associations with substance use. European Addiction Research. 2012;18(2):83–90. doi: 10.1159/000333037. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T. Anabolic-Androgenic Steroid Use and Misuse. In: Pfaff DW, editor. Neuroscience in the 21st Century. New York: Springer; 2012. pp. 2813–2832. [Google Scholar]
- Hildebrandt T, Alfano L, Langenbucher J. Body image disturbance among 1000 appearance and performance enhancing drug users. Journal of Psychiatric Research. 2010;44:841–846. doi: 10.1016/j.jpsychires.2010.01.001. http://dx.doi.org/10.1016/j.bbr.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt T, Lai JK, Langenbucher JW, Schneider M, Yehuda R, Pfaff DW. The diagnostic dilemma of pathological appearance and performance enhancing drug use. Drug and Alcohol Dependence. 2011;114(1):1–11. doi: 10.1016/j.drugalcdep.2010.09.018. S0376-8716(10)00333-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt T, Langenbucher J, Carr S, Sanjuan P, Park S. Predicting intentions for long-term anabolic-androgenic steroid use among men: a covariance structure model. Psychology of Addictive Behaviors. 2006;20(3):234–240. doi: 10.1037/0893-164X.20.3.234. 2006-10832-002 [pii] [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Langenbucher JW, Carr SJ, Sanjuan P. Modeling population heterogeneity in appearance- and performance-enhancing drug (APED) use: applications of mixture modeling in 400 regular APED users. Journal of Abnormal Psychology. 2007;116(4):717–733. doi: 10.1037/0021-843X.116.4.717. 2007-17062-005 [pii] [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Langenbucher JW, Lai JK, Loeb KL, Hollander E. Development and validation of the appearance and performance enhancing drug use schedule. Addictive Behaviors. 2011;36(10):949–958. doi: 10.1016/j.addbeh.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J, Bandelow S, Hogervorst E. Testosterone levels and cognition in elderly men: a review. Maturitas. 2011;69(4):322–337. doi: 10.1016/j.maturitas.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2011. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG., Jr Anabolic-androgenic steroid dependence: an emerging disorder. Addiction. 2009;104(12):1966–1978. doi: 10.1111/j.1360-0443.2009.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr Features of men with anabolic-androgenic steroid dependence: A comparison with nondependent AAS users and with AAS nonusers. [Article] Drug and Alcohol Dependence. 2009;102(1–3):130–137. doi: 10.1016/j.drugalcdep.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr Illicit anabolic-androgenic steroid use. Hormones and Behavior. 2010;58(1) doi: 10.1016/j.yhbeh.2009.09.006. S0018-506X(09)00195-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Kean J, Hudson JI, Pope HG., Jr Cognitive deficits in long-term anabolic-androgenic steroid users. Drug and Alcohol Dependence. 2012 doi: 10.1016/j.drugalcdep.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindlundh AM, Lindblom J, Bergstrom L, Nyberg F. The anabolic-androgenic steroid nandrolone induces alterations in the density of serotonergic 5HT1B and 5HT2 receptors in the male rat brain. Neuroscience. 2003;119(1):113–120. doi: 10.1016/s0306-4522(03)00120-9. S0306452203001209 [pii] [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Hormones and Behavior. 2007;51(2):183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Li CS, Luo X, Yan P, Bergquist K, Sinha R. Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcoholism: Clinical and Experimental Research. 2009;33(4):740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lewis G, Evans L. Understanding aggressive behaviour across the lifespan. Journal of Psychiatric and Mental Health Nursing. 2013;20(2):156–168. doi: 10.1111/j.1365-2850.2012.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matousek RH, Sherwin BB. Sex steroid hormones and cognitive functioning in healthy, older men. Hormones and Behavior. 2010;57(3):352–359. doi: 10.1016/j.yhbeh.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Possidente BP. Effects of withdrawal from anabolic androgenic steroids on aggression in adult male rats. Physiology and Behavior. 2002;75(4):541–549. doi: 10.1016/s0031-9384(02)00657-1. [DOI] [PubMed] [Google Scholar]
- Monaghan LF. Vocabularies of motive for illicit steroid use among bodybuilders. Social Science & Medicine. 2002;55(5):695–708. doi: 10.1016/s0277-9536(01)00195-2. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychological Medicine. 1999;29(6):1307–21. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Henderson LP. The Sturm und Drang of anabolic steroid use: angst, anxiety, and aggression. Trends in Cognitive Sciences. 2012;35(6):382–392. doi: 10.1016/j.tins.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Lund BC, Deninger MJ, Kutscher EC, Schneider J. Anabolic steroid use in weightlifters and bodybuilders: An internet survey of drug utilization. Clinical Journal of Sport Medicine. 2005;15(5):326–330. doi: 10.1097/01.jsm.0000180872.22426.bb. 00042752-200509000-00008 [pii] [DOI] [PubMed] [Google Scholar]
- Pfaff D. Brain Arousal and Information Theory: Neural and Genetic Mechanisms. Cambridge, MA: Harvard University Press; 2006. [Google Scholar]
- Pieretti S, Mastriota M, Tucci P, Battaglia G, Trabace L, Nicoletti F, Scaccianoce S. Brain nerve growth factor unbalance induced by anabolic androgenic steroids in rats. Medicine & Science in Sports & Exercise. 2013;45(1):29–35. doi: 10.1249/MSS.0b013e31826c60ea. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Youth risk behavior surveillance-United States, 2003. SS-2. Vol. 53. United States: CDC; 2004. [Google Scholar]
- Salas-Ramirez KY, Montalto PR, Sisk CL. Anabolic androgenic steroids differentially affect social behaviors in adolescent and adult male Syrian hamsters. Hormones and Behavior. 2008;53(2):378–385. doi: 10.1016/j.yhbeh.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurodevelopment. Journal of Adolescent Health. 2013;52(2):S7–S13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SJ, Liening SH, Schultheiss OC. Testosterone is positively associated with risk taking in the Iowa Gambling Task. Hormones and Behavior. 2011;59(2):252–256. doi: 10.1016/j.yhbeh.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Su TP, Pagliaro M, Schmidt PJ, Pickar D, Wolkowitz O, Rubinow DR. Neuropsychiatric effects of anabolic steroids in male normal volunteers. Journal of the American Medical Association. 1993;269(21):2760–2764. [PubMed] [Google Scholar]
- Tan RS, Scally MC. Anabolic steroid-induced hypogonadism--towards a unified hypothesis of anabolic steroid action. Medical Hypotheses. 2009;72(6):723–728. doi: 10.1016/j.mehy.2008.12.042. S0306-9877(09)00052-8 [pii] [DOI] [PubMed] [Google Scholar]
- Trenton AJ, Currier GW. Behavioural manifestations of anabolic steroid use. CNS Drugs. 2005;19(7):571–595. doi: 10.2165/00023210-200519070-00002. [DOI] [PubMed] [Google Scholar]
- van Hest A, van Haaren F, van de Poll NE. Perseverative responding in male and female Wistar rats: effects of gonadal hormones. Hormones and Behavior. 1989;23(1):57–67. doi: 10.1016/0018-506x(89)90074-3. [DOI] [PubMed] [Google Scholar]
- vandenBerg P, Neumark-Sztainer D, Cafri G, Wall M. Steroid use among adolescents: longitudinal findings from Project EAT. Pediatrics. 2007;119(3):476–486. doi: 10.1542/peds.2006-2529. 119/3/476 [pii] [DOI] [PubMed] [Google Scholar]