Abstract
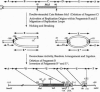
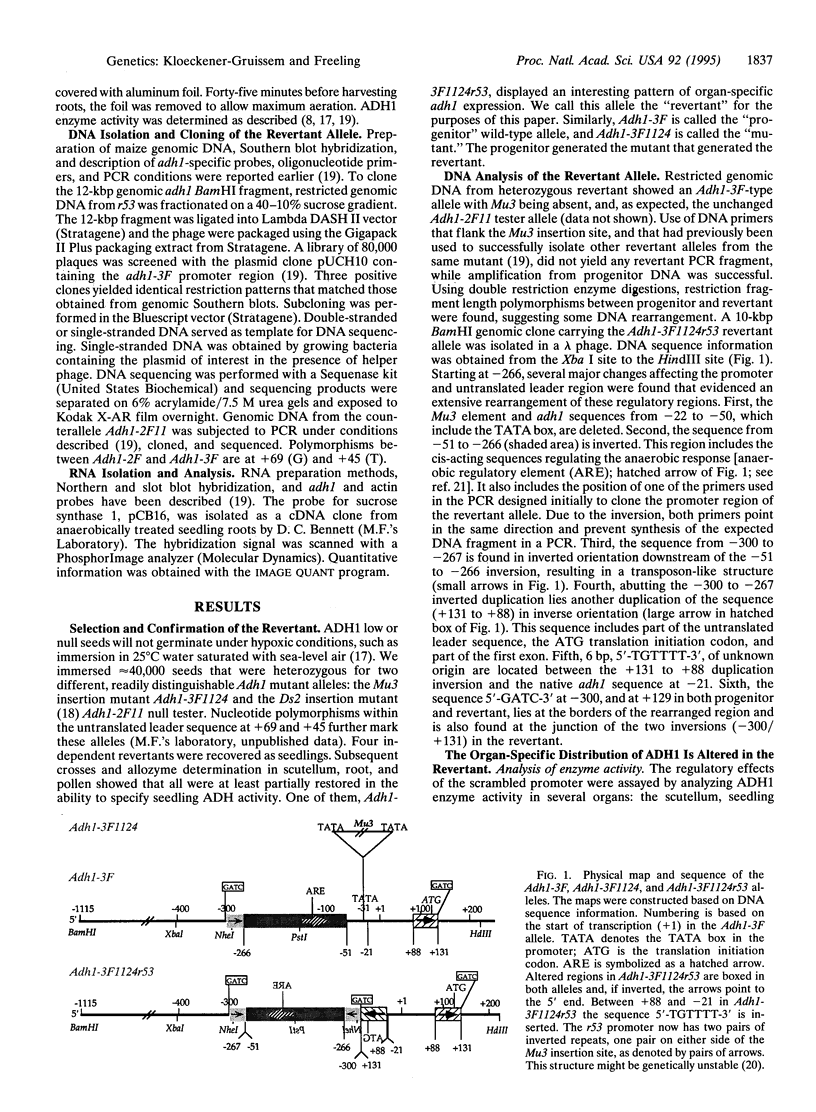
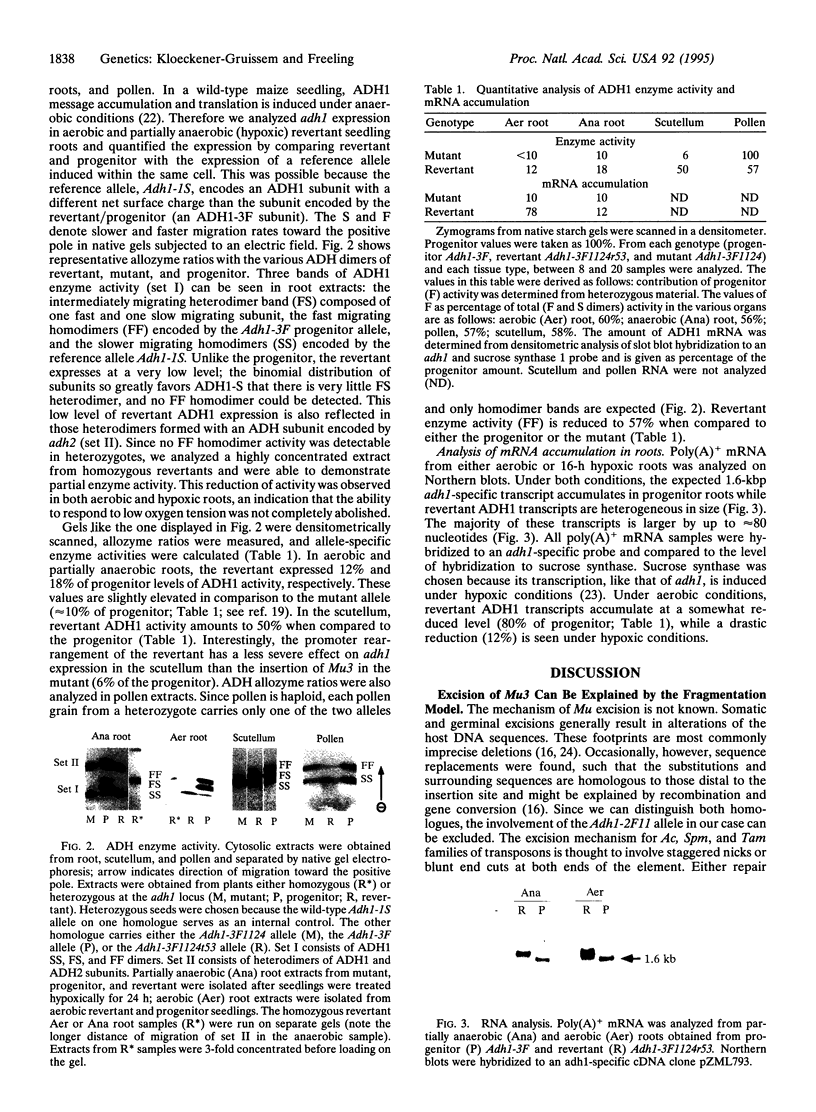
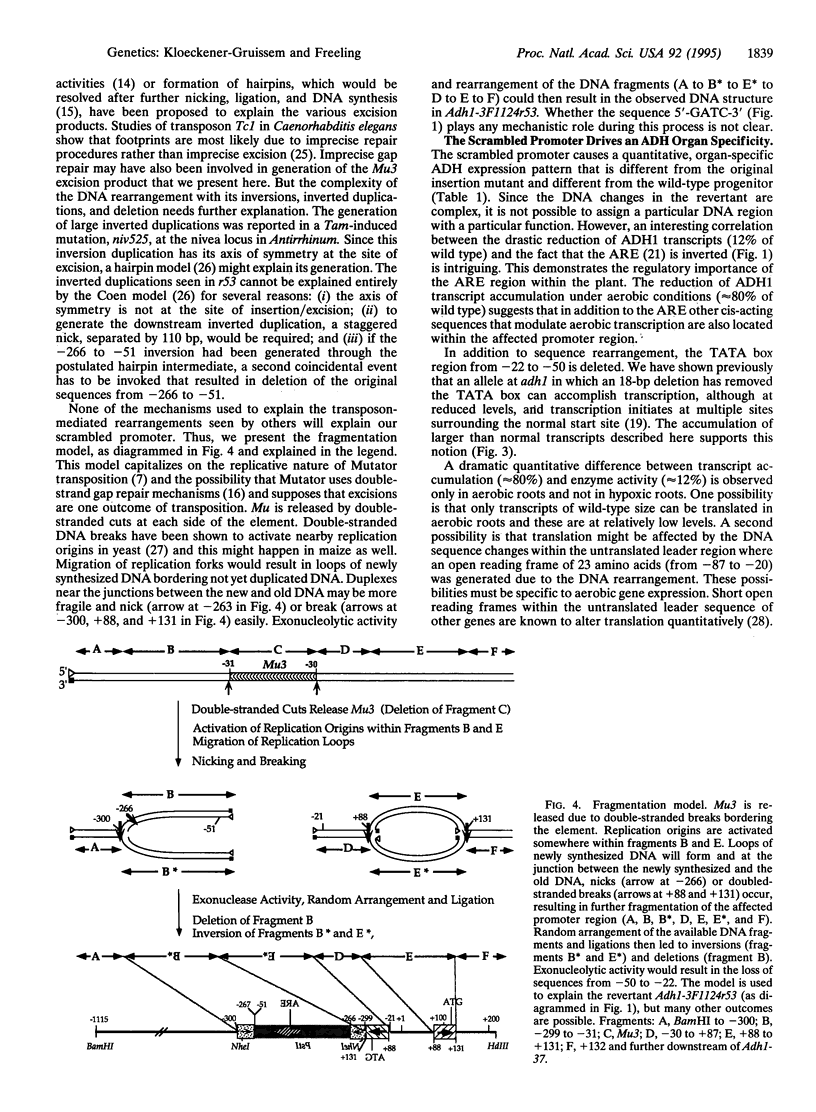
We have studied a germinal revertant of the Mutator (Mu3)-induced mutation (Adh1-3F1124) of the maize alcohol dehydrogenase 1 gene (adh1). Transposon Mu3 was inserted at the TATA box of the promoter. The excision of Mu3 caused a complex, multibreakpoint DNA rearrangement with deletion, inverted duplication, and inversions affecting 430 nucleotides in the promoter region. These changes led to an unusual pattern of adh1 gene expression: increased levels of enzyme activity in one organ, decreased levels in another, and almost unchanged levels in a third organ. The evolutionary impact of transposon-induced promoter scrambling on generation of allelic diversity is discussed. We present a fragmentation model to help explain how transposon excision could induce multiple breakpoint aberrations without involving a homologous chromosome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britt A. B., Walbot V. Germinal and somatic products of Mu1 excision from the Bronze-1 gene of Zea mays. Mol Gen Genet. 1991 Jun;227(2):267–276. doi: 10.1007/BF00259680. [DOI] [PubMed] [Google Scholar]
- Chandler V. L., Hardeman K. J. The Mu elements of Zea mays. Adv Genet. 1992;30:77–122. doi: 10.1016/s0065-2660(08)60319-3. [DOI] [PubMed] [Google Scholar]
- Chen C. H., Oishi K. K., Kloeckener-Gruissem B., Freeling M. Organ-specific expression of maize Adh1 is altered after a Mu transposon insertion. Genetics. 1987 Jul;116(3):469–477. doi: 10.1093/genetics/116.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen E. S., Carpenter R. A semi-dominant allele, niv-525, acts in trans to inhibit expression of its wild-type homologue in Antirrhinum majus. EMBO J. 1988 Apr;7(4):877–883. doi: 10.1002/j.1460-2075.1988.tb02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani R. D., Jr, Wessler S. R. An upstream open reading frame represses expression of Lc, a member of the R/B family of maize transcriptional activators. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8244–8248. doi: 10.1073/pnas.90.17.8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe R. K., Lachmansingh A. R., Freeling M. Transposon-mediated mutations in the untranslated leader of maize Adh1 that increase and decrease pollen-specific gene expression. Plant Cell. 1993 Mar;5(3):311–319. doi: 10.1105/tpc.5.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doseff A., Martienssen R., Sundaresan V. Somatic excision of the Mu1 transposable element of maize. Nucleic Acids Res. 1991 Feb 11;19(3):579–584. doi: 10.1093/nar/19.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan D. J. Eukaryotic transposable elements and genome evolution. Trends Genet. 1989 Apr;5(4):103–107. doi: 10.1016/0168-9525(89)90039-5. [DOI] [PubMed] [Google Scholar]
- Gierl A., Saedler H. The En/Spm transposable element of Zea mays. Plant Mol Biol. 1989 Sep;13(3):261–266. doi: 10.1007/BF00025313. [DOI] [PubMed] [Google Scholar]
- Gordenin D. A., Lobachev K. S., Degtyareva N. P., Malkova A. L., Perkins E., Resnick M. A. Inverted DNA repeats: a source of eukaryotic genomic instability. Mol Cell Biol. 1993 Sep;13(9):5315–5322. doi: 10.1128/mcb.13.9.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J. Ontogeny and phylogeny--revisited and reunited. Bioessays. 1992 Apr;14(4):275–279. doi: 10.1002/bies.950140413. [DOI] [PubMed] [Google Scholar]
- Kloeckener-Gruissem B., Vogel J. M., Freeling M. The TATA box promoter region of maize Adh1 affects its organ-specific expression. EMBO J. 1992 Jan;11(1):157–166. doi: 10.1002/j.1460-2075.1992.tb05038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister C., Jackson D., Martin C. Transposon-induced inversion in Antirrhinum modifies nivea gene expression to give a novel flower color pattern under the control of cycloidearadialis. Plant Cell. 1993 Nov;5(11):1541–1553. doi: 10.1105/tpc.5.11.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. The significance of responses of the genome to challenge. Science. 1984 Nov 16;226(4676):792–801. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H. The origin of footprints of the Tc1 transposon of Caenorhabditis elegans. EMBO J. 1991 Jul;10(7):1919–1925. doi: 10.1002/j.1460-2075.1991.tb07718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman M. K., Brewer B. J., Fangman W. L. Activation of a yeast replication origin near a double-stranded DNA break. Genes Dev. 1994 Mar 1;8(5):554–562. doi: 10.1101/gad.8.5.554. [DOI] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Saedler H., Nevers P. Transposition in plants: a molecular model. EMBO J. 1985 Mar;4(3):585–590. doi: 10.1002/j.1460-2075.1985.tb03670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeyer S. B., Hansen L. J., Chalker D. L. Integration specificity of retrotransposons and retroviruses. Annu Rev Genet. 1990;24:491–518. doi: 10.1146/annurev.ge.24.120190.002423. [DOI] [PubMed] [Google Scholar]
- Schneuwly S., Kuroiwa A., Gehring W. J. Molecular analysis of the dominant homeotic Antennapedia phenotype. EMBO J. 1987 Jan;6(1):201–206. doi: 10.1002/j.1460-2075.1987.tb04739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer B., Werr W., Starlinger P., Bennett D. C., Zokolica M., Freeling M. The Shrunken gene on chromosome 9 of Zea mays L is expressed in various plant tissues and encodes an anaerobic protein. Mol Gen Genet. 1986 Dec;205(3):461–468. doi: 10.1007/BF00338083. [DOI] [PubMed] [Google Scholar]
- Stavenhagen J. B., Robins D. M. An ancient provirus has imposed androgen regulation on the adjacent mouse sex-limited protein gene. Cell. 1988 Oct 21;55(2):247–254. doi: 10.1016/0092-8674(88)90047-5. [DOI] [PubMed] [Google Scholar]
- Walker J. C., Howard E. A., Dennis E. S., Peacock W. J. DNA sequences required for anaerobic expression of the maize alcohol dehydrogenase 1 gene. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6624–6628. doi: 10.1073/pnas.84.19.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler S. R. Phenotypic diversity mediated by the maize transposable elements Ac and Spm. Science. 1988 Oct 21;242(4877):399–405. doi: 10.1126/science.2845581. [DOI] [PubMed] [Google Scholar]