Abstract
Objective
To evaluate patterns in actigraphy-defined sleep measures across the menstrual cycle, testing the hypothesis that sleep would be more disrupted in the premenstrual period, i.e. in the 14 days prior to menses.
Methods
A community-based, longitudinal study of wrist actigraphy-derived sleep measures was conducted with 163 women (58 African-American, 78 White, and 27 Chinese) of late reproductive age (mean=51.5, SD=2.0 years) from the Study of Women's Health Across the Nation (SWAN) Sleep Study. Daily measures of sleep [sleep efficiency (%) and total sleep time (minutes)] and movement during sleep [mean activity score (counts)] were characterized using wrist actigraphy across a menstrual cycle or 35 days, whichever was shorter. Data were standardized to 28 days to account for the variation of unequal cycle lengths and divided into four weekly segments for analyses.
Results
Sleep efficiency percentage declined gradually across the menstrual cycle, but the decline became pronounced in fourth week, the premenstrual period. Compared with third week, sleep efficiency declined by 5% (p<0.0001) and mean total sleep time was 25 minutes less (p=0.0002) in fourth week. No significant mean differences were found when comparing the means of second week versus third week. The association of weekly segments with sleep efficiency or minutes of total sleep time was modified by sociodemographic and lifestyle factors, including body mass index (BMI), race, study site, financial strain, marital status, and smoking.
Conclusions
Sleep varied systematically across the menstrual cycle in women of late reproductive age, including a gradual decline in sleep efficiency across all weeks, with a more marked change premenstrually during the last week of the menstrual cycle. These sleep changes may be modifiable by altering lifestyle factors.
Keywords: SWAN, menopause, sleep study, actigraphy, menstrual cycle, semiparametric stochastic mixed models
INTRODUCTION
Shifts in hormone levels and changes in hormone patterns across the menstrual cycle have been hypothesized to be associated with transient alterations in sleep patterns. In menstruating young adult women, the menstrual cycle is characterized by major shifts in hormone levels occurring around the mid-cycle, with marked increases followed by declines in estradiol and, subsequently, marked increases in gonadotropins, especially luteinizing hormone (LH). Post-ovulation, the pre-ovulatory increase in estradiol is accompanied by a three to ten-fold increase in progesterone levels. In the week preceeding menstruation, progesterone and estradiol levels fall. However, in midlife women, anovulatory events are increasingly frequent and the proportion of days before and after the LH surge begins to shift1–3, which has lead to questions concerning the role of reproductive aging in the reporting of sleep disturbances in women4–5.
Prior studies have reported sleep disturbances during the premenstrual week and during the first few days of menstruation compared with other times in the cycle6–7. The National Sleep Foundation Sleep in America survey (2007) identified that 25%–33% of menstruating women complained of sleep disturbances during the premenstrual week or during menses; women who reported severe premenstrual symptoms (PMS) also experienced symptoms of insomnia and daytime sleepiness6. Smaller studies of women with premenstrual mood disorders have identified substantial sleep disturbances, including either hypersomnia or insomnia during the premenstrual period8–9. The extent to which findings from these studies can be extrapolated to midlife women as they approach menopause remains in question, thereby limiting our understanding of the extent to which increased sleep disturbances in midlife women are associated with alterations in menstrual cycles and hormone dynamics4, 10.
Methodological issues (e.g., statistical: non-parametric functional form, and between- and within- subject variation) have made the development of an informative body of literature challenging11. Many studies have used polysomnography (PSG) data to study sleep patterns objectively, but few studies have characterized sleep alterations across the entire menstrual cycle12. Appropriately-designed studies with multiple nights of PSG throughout a menstrual cycle are costly, difficult to implement, and typically result in studies with small samples sizes of highly selected groups.
Actigraphy provides unique real-time information on sleep and activity, and has been widely used to document sleep-wake patterns objectively13–14. Therefore, wrist actigraphy data acquired during the Study of Women’s Health Across the Nation (SWAN) Sleep Study were used to generate daily sleep measures (total sleep time and sleep efficiency percentage) and a measure of movement (mean activity score) across a menstrual cycle in midlife women. We hypothesized that mean profiles of these sleep measures would change, consistent with different phases of the menstrual cycle and in particular with the premenstrual period, which is a time of notable sleep complaints in midlife women.
METHODS
Study Population
Data for this report came from the SWAN Sleep Study, a comprehensive study of sleep nested within the ongoing longitudinal SWAN cohort study and conducted at four of the seven SWAN clinical sites during 2003–2005, across a time frame that overlapped with the 5th, 6th, and 7th annual core SWAN assessments that have been described in detail15.
SWAN Study Design and Participants
SWAN is a multi-ethnic, community-based, multi-site cohort study of the menopausal transition. A total of 3,302 women were enrolled for longitudinal evaluation from a community based survey conducted at seven sites: Boston, MA, Chicago, IL, Detroit area, MI, Los Angeles and Oakland, CA, Newark, NJ, and Pittsburgh, PA. Each site recruited a sample of White and minority women. Eligibility criteria were: aged 42–52 years, not pregnant, not using exogenous hormones in the three months prior to interview, an intact uterus and at least one ovary, at least one menstrual period in the three months prior to the baseline interview and self-identifying as White or the site’s designated racial/ethnic group. Each site’s institutional review board approved the study protocol, and all women gave written informed consent to participate.
SWAN baseline and follow-up assessments consisted of detailed questions about medical, reproductive and menstrual history, lifestyle and psychosocial factors, physical and psychological symptoms, vasomotor symptoms and additional factors that might be related to menopause and sleep. Measurements of height and weight were obtained using a common protocol.
SWAN Sleep Study Design and Participants
A total of 370 women aged 48–59 years were enrolled from the Chicago, Detroit area, Oakland, and Pittsburgh SWAN sites, including 328 pre- and peri-menopausal and 42 postmenopausal African-American, White and Chinese women. Two women did not complete the Sleep Study. Eligibility for the Sleep Study was based on information from annual core SWAN assessments. Initially, women were recruited who were pre- or peri-menopausal as defined by the SWAN core study, although later, postmenopausal women were included. Women who had a hysterectomy (<1%) or were using hormone therapy (approximately 23% of the cohort) were excluded. Also excluded were women being treated for cancer or those who worked night or rotating shifts (exclusion rates for these factors were between 1–3%). Overall, approximately 30% of eligible participants declined participation in the Sleep Study. Sleep Study participants did not differ markedly from other SWAN participants at the four Sleep Study sites with regard to self-assessed sleep quality, race, self-reported health status, or proportion with depressive symptoms4, 16–17.
Sleep Study Protocol
The sleep measures included wrist actigraphy, PSG and sleep diaries. Among those women who were still menstruating, the sleep protocol was initiated within seven days of the onset of menses. In-home PSG studies were conducted on the first three nights of the protocol, while wrist actigraphy and sleep diary data were collected daily for an entire menstrual cycle or 35 days, whichever was shorter.
Sleep and movement outcome measures
Wrist movements of the non-dominant arm were recorded using an Actiwatch-64 (AW-64™ MiniMitter by Philips Respironics, Bend, OR), and data were analyzed with the Actiware software (version 3.4 and 5.0, Mini-Mitter Co. Inc.). Batteries were replaced around the 14th day of recording, and data were collected for the period up to the next menstrual bleed or up to 35 days, whichever came first. Participants were asked to wear the actigraph 24 hours/day, removing it only for bathing or swimming. When the actigraph was removed, the times were noted on the daily diaries (two questions in bedtime diary: 1) Today, I took off my actigraph (yes or no), 2) If yes, when were time off and time on?). Electronic data were sent to the University of Pittsburgh study site for processing, evaluation of data quality and scoring. Recording procedures and staff training were designed to reduce the incidence of missing data. Self-report data were checked and verified in the presence of participants, and procedures were implemented to prevent the loss of actigraphy data. A study to assess reproducibility as well as inter- and intra-rater reliability among two scorers was performed. For inconsistent data, actigraphy variables were further checked graphically by examining logic and ranges. If extreme data values were found, the corresponding diary data were further examined to identify root causes. Those extreme data values were excluded in the analysis. In the data preparation stage, the fragmentation index is used to screen the data, which is an index of restlessness during sleep, with higher values indicating more restlessness. Fragmentation index is calculated by summing the number of minutes with movement and expressing them as a percentage of the immobile phase18.
Actigraphy data were recorded continuously and the total activity count was determined using one-minute sampling epochs. The actigraphy-based sleep-wake variables were determined using the Actiware software program with total activity counts above the threshold sensitivity value of 40 counts/per epoch. In the Actiware, “sleep start” represents the time of sleep onset and is determined by the first 10-minute period in which no more than one epoch is scored as mobile. “Sleep end” represents the time of sleep termination and is determined by the last 10-minute period in which no more than one epoch is scored as mobile.
The actigraphy variables being reported herein include: 1) Total Sleep Time (minutes), which reflects the sum of those epochs between Sleep Start and Sleep End from the diaries that were designated as "sleeping." 2) Sleep Efficiency (percent %), which is an index of the amount of time in bed actually spent sleeping, is determined by dividing total-sleep-time by the time-in-bed, multiplied by 100. 3) Mean Activity Score, which reflects the magnitude of activity on a per-epoch-basis during sleep as determined by dividing the total-activity-score by the number of epochs during the assumed sleep period, the interval between actigraphically defined sleep start and sleep end.
Sociodemographic and health-related variables from the SWAN Core Study were collected at the baseline and annual core SWAN assessments. Information about age, marital status (single/never married, married or living as married, separated/widowed/divorced), educational attainment (high school graduate or less, some college, college graduate, graduate studies) and self-reported health status (poor, fair, good, very good, excellent) was obtained from questionnaires. A three-level response to a question about difficulty in paying for basics including food, shelter, and health care (very, somewhat, or not very difficult) was used as an indicator of financial strain.
The Perceived Stress Scale (PSS), reflecting the degree to which situations in one’s life were appraised as stressful, was summarized from 4 interview questions with scores between 1 to 5 for each question19 and was treated as dichotomous variable with the cut-points at <15, >=16. Body mass index (BMI) was computed as weight in kilograms divided by height in meters squared and was treated as dichotomous variable: non-obese versus obese (cut-off at 30)20. Study site was included in statistical models as an indicator variable in combination with the race/ethnicity variable responses.
Menopause related variables were obtained from the SWAN Core annual visit that preceded the sleep study. Using menstrual bleeding criteria, participants were classified into one of the following four categories: premenopausal (no change in menstrual bleeding regularity), early perimenopausal (menses in the preceding 3 months with an increase in bleeding irregularity in the previous 12 months), or late perimenopausal (menses in the previous 12 months, but not the previous 3 months, and postmenopausal (12 months ammenorrheic)21–23.
Variables from Sleep Study, questionnaires or daily Sleep Study diaries
Apnea/Hypopnea Index (AHI). The first night of the sleep protocol also included instrumentation to screen for the presence of sleep disordered breathing. The AHI is based on the number of apnea events plus the number of hypopnea events per hour of sleep24.
Depressive symptoms were evaluated with the 16-item Inventory of Depressive Symptoms (IDS) administered on the last day of the Sleep Study protocol25. Data analysis with the IDS was based on scores in which the contribution from the four sleep-related items had been removed.
The Sleep Study diaries were completed twice a day: at Awaketime and at Bedtime. Women were classified as having vasomotor symptoms on a given day if they reported an affirmative response to the question about cold sweats, hot flashes/flushes or night sweats on their daily diaries26. In the morning (waketime) diaries, women recorded mood and behavioral symptoms, including how rested, blue/down, and anxious/tense they felt, and whether they had experienced worries (coded present/absent) during the night. In the bedtime diaries, daytime fatigue/feeling tired and pain/discomfort were recorded and coded as “present” or “absent”.
Daily medication use affecting sleep (both prescription and over-the-counter) were recorded at Sleep Study protocol inception and on daily diaries. These were coded according to the World Health Organization Anatomical Therapeutic Chemical (ATC) classification27. In this report, medications that affect sleep were considered to be those products associated with the following ATC classification codes: N02A (opioids), N03A (antiepileptics), N05B (anxiolytics), N05C (hypnotics and sedatives), N06A (antidepressants) and R06A (antihistamines). Daily use of medications that affect sleep was dichotomized as “present” or “absent”. Daily exercise was defined according to a 4-level response (0=none; 1=light; 2=medium; 3=heavy). Daily responses about smoking frequency, alcohol consumption, and caffeine consumption were determined from the sleep diaries. Current smokers were those who reported occasional smoking at least seven cigarettes in the two-week period initiated by the sleep protocol. Sleep diary variables were treated as the time-varying covariate in analysis.
Data analyses
To be eligible for analyses, women had to satisfy the criteria that they: 1) had actigraphy and diary data after day-14 of the menstrual cycle and extending to the next menses or 35 days, whichever came first, with a minimum of ten observations; 2) were still menstruating (Pre-, early Peri- and late Perimenopause), 3) had completed sleep diaries allowing us to determine onset of subsequent menstrual bleeding episode and daily sleep start and sleep in parallel with their actigraphy data. Of the 370 women enrolled in SWAN Sleep Study, 347 provided wrist actigraphy information as well as the appropriate accompanying daily diaries, necessary for deriving the sleep variables and documenting menstruation. Of these, 285 women were still menstruating. Thirteen women were excluded because more than 50% of their observed values for fragmentation index, mean activity score or actual wake time were zeros, which was not a valid value for a participant wearing a functioning actigraph. For comparisons of follicular and luteal phases, an additional 109 women were excluded because they had no clearly identified first day of menstrual cycle or no data after day-14 of the menstrual cycle. Overall, 163 women met the criteria and contributed 3047 observations (mean = 18.7 days per woman) to the analysis. The average cycle length was 25.9 days with standard deviation 6.8 days.
To address the variability in menstrual cycle lengths, the menstrual cycle data were standardized uniformly to a reference 28-day period by weighting the data through a scale factor 28/cyclei where cyclei was the actual cycle length for ith woman28–29. The standardized data were then segmented into four 7-day weekly units. Information from the first week (days ≤7) were not included in analyses as other components of the protocol, such as habituating to overnight sleep assessments, may have disturbed actigraphy-assessed sleep30.
Statistical methods
Univariate statistics, including means, standard deviations, medians and interquartile ranges (IQR) were computed for continuous variables and frequencies were determined for categorical variables. Variables with highly skewed distributions were transformed or categorized. For modeling, the actigraphy-based variable mean activity score, as well as BMI and AHI, were transformed using a natural logarithm. Statistical significance was based on p-values from two-sided tests and defined as a value of p < 0.05.
First, sleep measures were plotted for 163 women to explore the day-to-day patterns of change across a complete menstrual cycle. The analysis showed that the relationships between actigraphy outcomes and time (day of menstrual cycle) were nonlinear over time and could not be appropriately modeled by quadratic or cubic terms. To allow for the flexibility in the functional form of sleep measures, semi-parametric stochastic mixed effect models with smoothed splines were used to fit the mean profiles across time29. As an extension of linear mixed models for longitudinal data, this method uses parametric fixed effects to represent covariate effects, a smooth function to model the time effect, and random effects and stochastic processes to model the within-woman correlations. Thus, this method is particularly powerful to model longitudinal data and biological signals with flexible functional forms without the assumption of parametric trend (for example, linear or quadratic form). Bootstrapping was used to fit upper and lower 95% confidence intervals (CI) around the mean profile 53–54.
To compare segment-specific differences in sleep measures, a piece-wise linear mixed effect model was used by placing nodes at the points corresponding to day 14 and day 21 to generate weekly segments. The day 14 node was approximately mid-cycle, and it was assumed that peri-ovulatory events could be demonstrated by comparing means and slopes from the second week (8~14 days) with means and slopes of the third week (14~21 days). Similarly, a node was placed at day 21, and it was assumed that this represented the initiation of the premenstrual period (fourth week, 21~28 days). The slopes and the least squares means (LSmeans) were calculated for each of the three weeks. F-tests from repeated measures ANOVA with orthogonal contrast were used to assess the importance of changes in the LSmeans and slopes of these weekly segments (See Appendix I in the Supplemental Digital Content).
Covariates eligible for inclusion in all models included as fixed variables chronological age, body mass index (BMI), menopausal status, health status, perceived stress, race, financial strain, marital status, education and site. Time-varying variables from the Sleep Study included AHI, symptoms of depression, smoking, VMS, exercise, alcohol and caffeine consumption, and use of medications that affect sleep. Covariates were retained in final models only if they were statistically significant at the p<0.05 level. Interaction terms with the segment variable and each covariate were evaluated in the models.
SAS 9.3 and Macro facilities (SAS Institute, Cary, North Carolina) were used to perform the statistical analyses and plot the findings.
RESULTS
The mean age of this perimenopausal sample of still-menstruating women (n=163) was 51.5 years (standard deviation (SD) = 2 years) (Table 1). The median BMI was 27.6 kg/m2 [interquartile range (IQR=8.9)]. Women self-identified as African-American (35.6%, n=58), White (47.9%, n=78), and Chinese (16.7%, n=27). About sixteen percent of women reported smoking while 62.1% reported having any vasomotor symptoms during their observed menstrual cycle.
Table 1.
Selected descriptive characteristics of SWAN Sleep Study, N = 163
| Variable | Description | Median (IQRa) |
|---|---|---|
| Age at sleep | Years | 51.5 (2.0)b |
| Length of menstrual cycle | days | 25.9 (6.8)b |
| BMI:Body mass index | Kg/m2 | 27.6 (8.9) |
| AHI: Apnea-hypopnea Index | Events/hour | 4.7 (9.8) |
| N (%) | ||
| Site | Detroit area, MI | 34 (20.9%) |
| Chicago, IL | 33 (20.3%) | |
| Oakland, CA | 50 (30.7%) | |
| Pittsburgh, PA | 46 (28.2%) | |
| Race/Ethnicity | African American | 58 (35.6%) |
| Chinese | 27 (16.6%) | |
| White | 78 (47.9%) | |
| Financial Strain (How hard paying for basics) | Very hard | 7 (4.3%) |
| Somewhat hard | 40 (24.5%) | |
| Not hard | 116 (71.2%) | |
| Obesity Status | BMI<30 | 101 (62.0%) |
| BMI>=30 | 62 (38.0%) | |
| Marital Status | Single | 24 (14.7%) |
| Married | 101 (62.0%) | |
| Not married | 38 (23.3%) | |
| Alcohol consumption(cups)c | None | 137 (84.1%) |
| Any | 26 (15.9%) | |
| Caffeine consumption(cups)c | None | 33 (20.3%) |
| Any | 130 (79.7%) | |
| Smoking frequencyc | None | 147 (90.2%) |
| Any | 16 (9.8%) |
IQR: interquartile range
The statistics is mean (standard deviation)
Variables from sleep daily diaries
Across the standardized menstrual cycles (days 8 –28), the median actigraphy-assessed sleep efficiency was 82% and was highly variable with an IQR of 15.5%, which is slightly lower than the normally expected value 85%. The median total sleep time was just over 6 hours per night (median 363 minutes, IQR of 107). The median time in bed was 455 minutes with IQR of 108; the median mean activity score was 14 per epoch during sleep that was also highly variable with an IQR of 14. Week-to-week differences in time in bed were not significant (F3,494 = 0.87; p-value = 0.45).
Sleep and menstrual cycle segment analysis
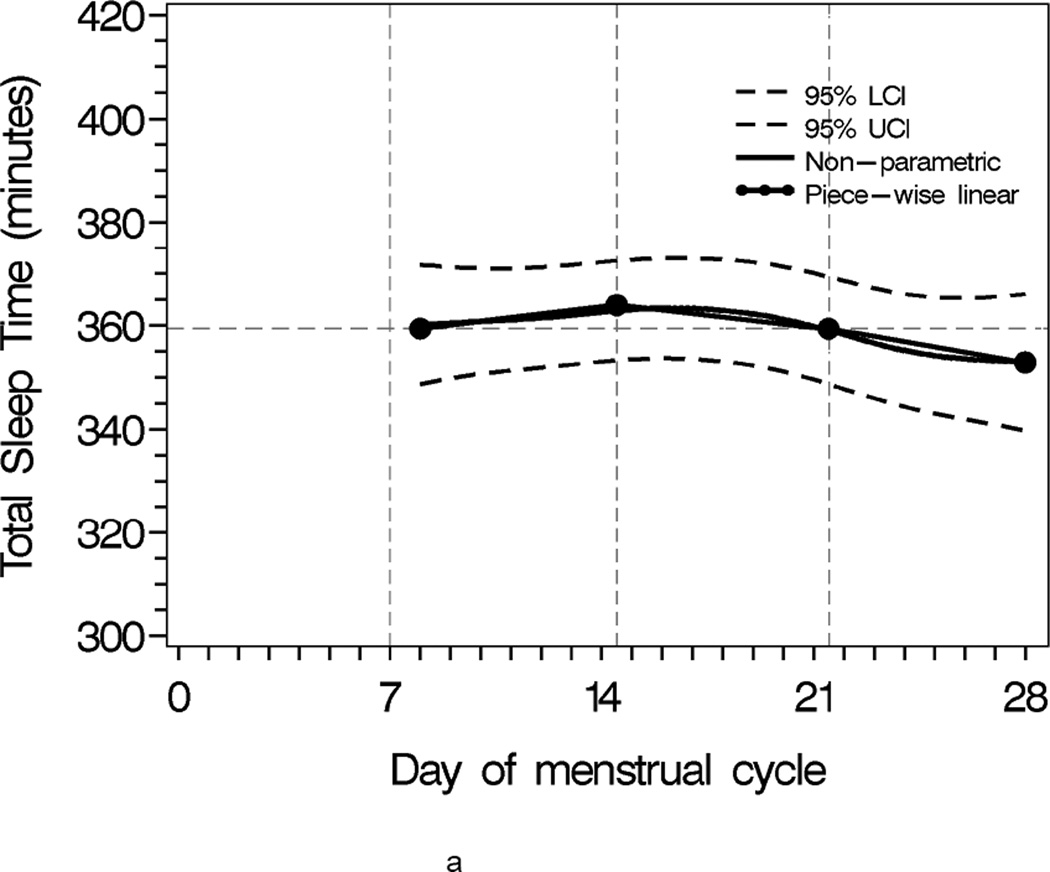
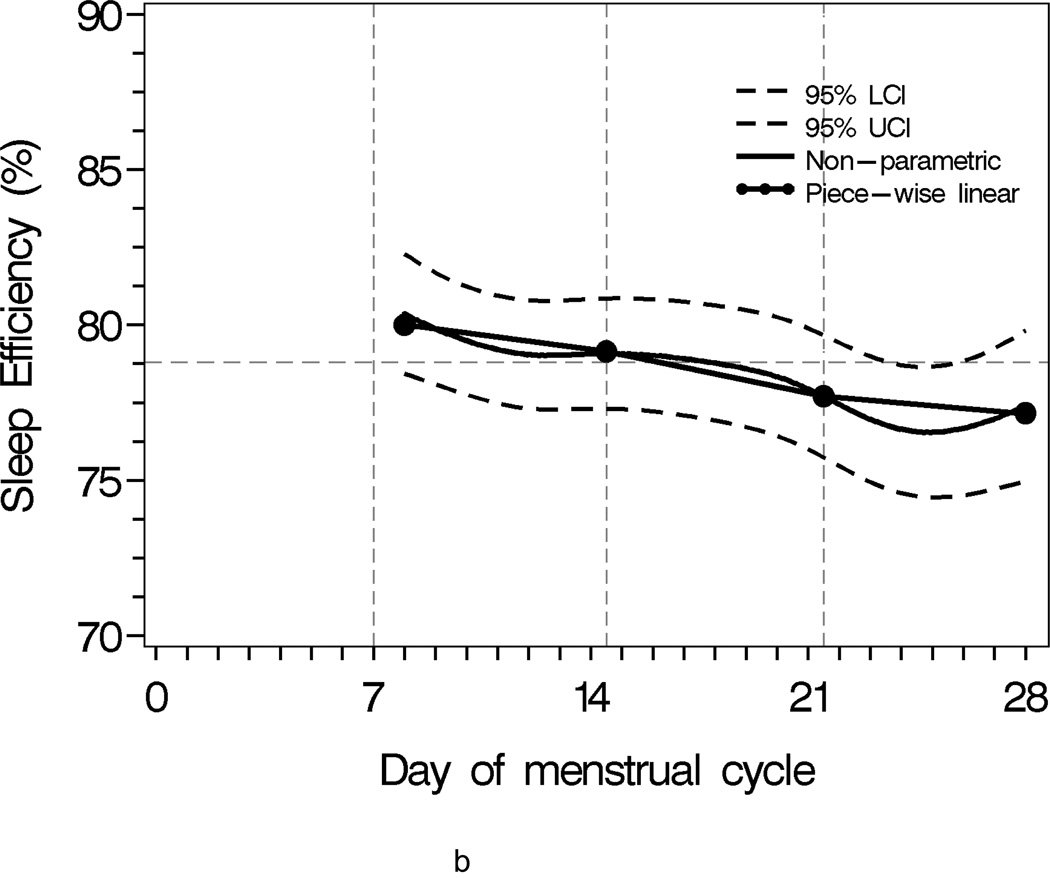
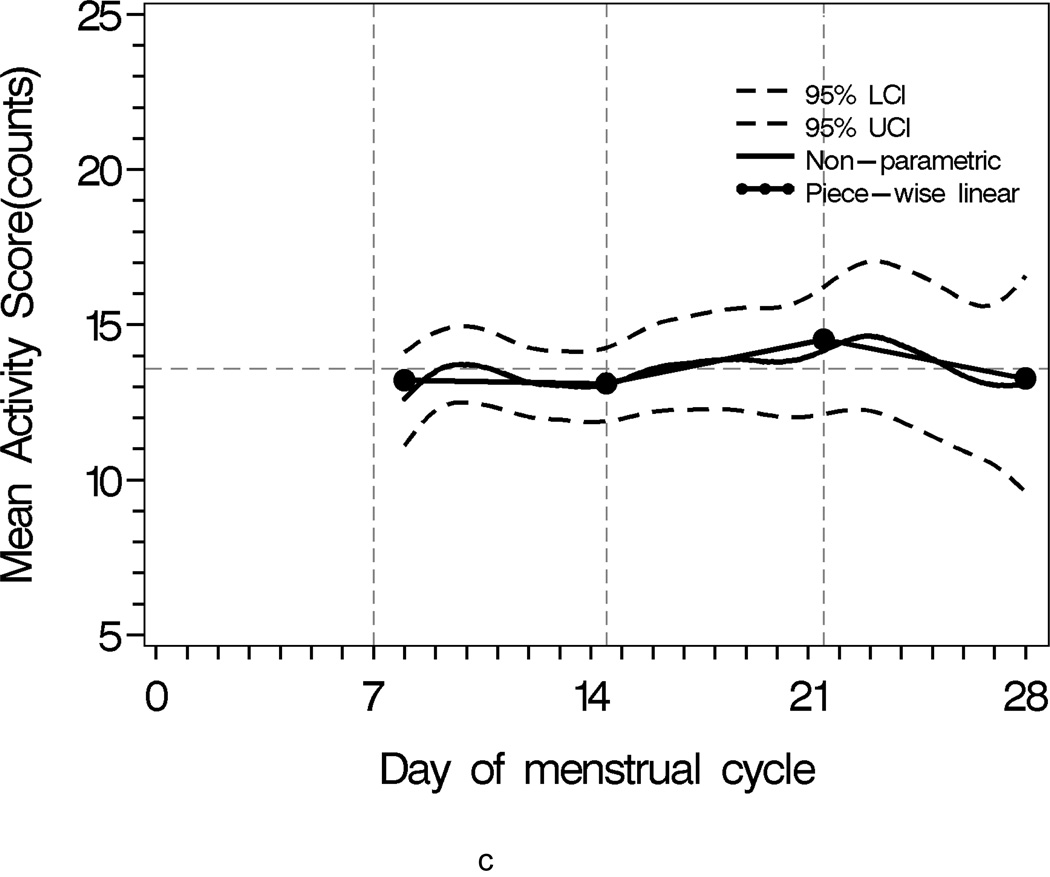
Sleep progressively worsened over the menstrual cycle, as characterized by lower sleep efficiency, shorter sleep time, and increased activity during sleep(Figure 1). The comparison of second week and third week was of interest as the interaction of two segments approximated the mid-cycle node when the ovulatory event likely occurred (Table 2).
Figure 1.
Population sleep profiles across menstrual cycle with 95% upper and lower confidence bands overlaid with segment-specific slopes of the piecewise linear mixed effects regression models for individual weekly segments. N = 163
a Total Sleep Time
b Sleep Efficiency
c Mean Activity Score
Table 2.
Adjusteda LSmeans (SE)b for 7-day segments and comparisons of LSmean difference (SE) between segments, N = 163
| Measures | LSmean (SE) | Difference between segments LSmeans(SE), p-value |
F-value in mean comparisons |
|||
|---|---|---|---|---|---|---|
| 2nd week segment |
3rd week segment |
4th week segment |
Segments week 3 vs. week 2 |
Segments week 4 vs. week 3 |
||
| Total Sleep Time (min) |
351.6 (8.7) |
363.0 (9.2) |
337.9 (9.1) |
11.5(6.4) p= 0.07 |
−25.2(6.7) p=0.0002 |
F2,2879=7.08 p=0.0009 |
| Sleep Efficiency (%) |
73.4 (1.7) |
74.7 (1.8) |
69.1 (1.8) |
1.3 (1.2) p= 0.3 |
−5.4(1.3) p<0.0001 |
F2,2879=11.09 P<0.0001 |
|
logMean activity score as counts |
2.7 (0.1) |
2.7 (0.1) |
2.6 (0.1) |
0.1(0.07) p= 0.2 |
0.05(0.08) p= 0.5 |
F2,2623=1.99 P=0.2 |
The covariates include chronological age, body mass index, site, race, difficulty in paying for basics, menopausal status, marital status, education, health history, perceived stress, AHI, the IDS without sleep items, daily smoking, VMS, exercise, alcohol and caffeine consumption, sleep medication.
LSmeans: Least Square Means; SE: Standard error
The total activity score progressively increased over the menstrual cycle and culminated in much greater activity score variability in the fourth week (Figure 1c). The interquartile range (IQR) for the fourth week was 14.73, which was 16.72% higher than the second week (IQR = 12.62) and 18.98% higher than the third week (IQR = 12.38). The mean width of 95% confidence bands for the second, third and fourth week were 2.44, 3.16, 5.16, respectively. All pair-wise comparisons of these bands were significant at p = 0.05.
Mean minutes of total sleep time, sleep efficiency and mean activity score did not differ significantly between second and third weeks. However, total sleep time tended to be 11.5 minutes longer in the third week compared to the second week (p <0.07). In contrast, mean total sleep time and sleep efficiency were significantly different when comparing the third to the fourth week. On average, total sleep time was 25 minutes shorter in the fourth week compared to the third week (p=0.0002), resulting in a 5% lower sleep efficiency in the fourth week compared to the third week (p<0.0001). The F-statistics for the week-to-week mean comparisons were F2,2879=7.08 with p=0.0009 for total sleep time, F2,2623=11.09 with p<0.0001 for sleep efficiency, F2,2497=1.99 with p=0.15 for mean activity score, respectively (Table 2).
In statistical models that included health, social, and daily health behavior measures, menstrual cycle weeks were significantly associated with minutes of total sleep time and sleep efficiency with p-values < 0.0009 and <0.0001, respectively. Hot flashes and night sweats were not significant in any of the models. Notably, interactions were present for menstrual cycle week with smoking for all three sleep measures, and with several covariates from all three domains of health status, social dimensions, and health behaviors for sleep efficiency (See Table A of Appendix II in the Supplemental Digital Content).
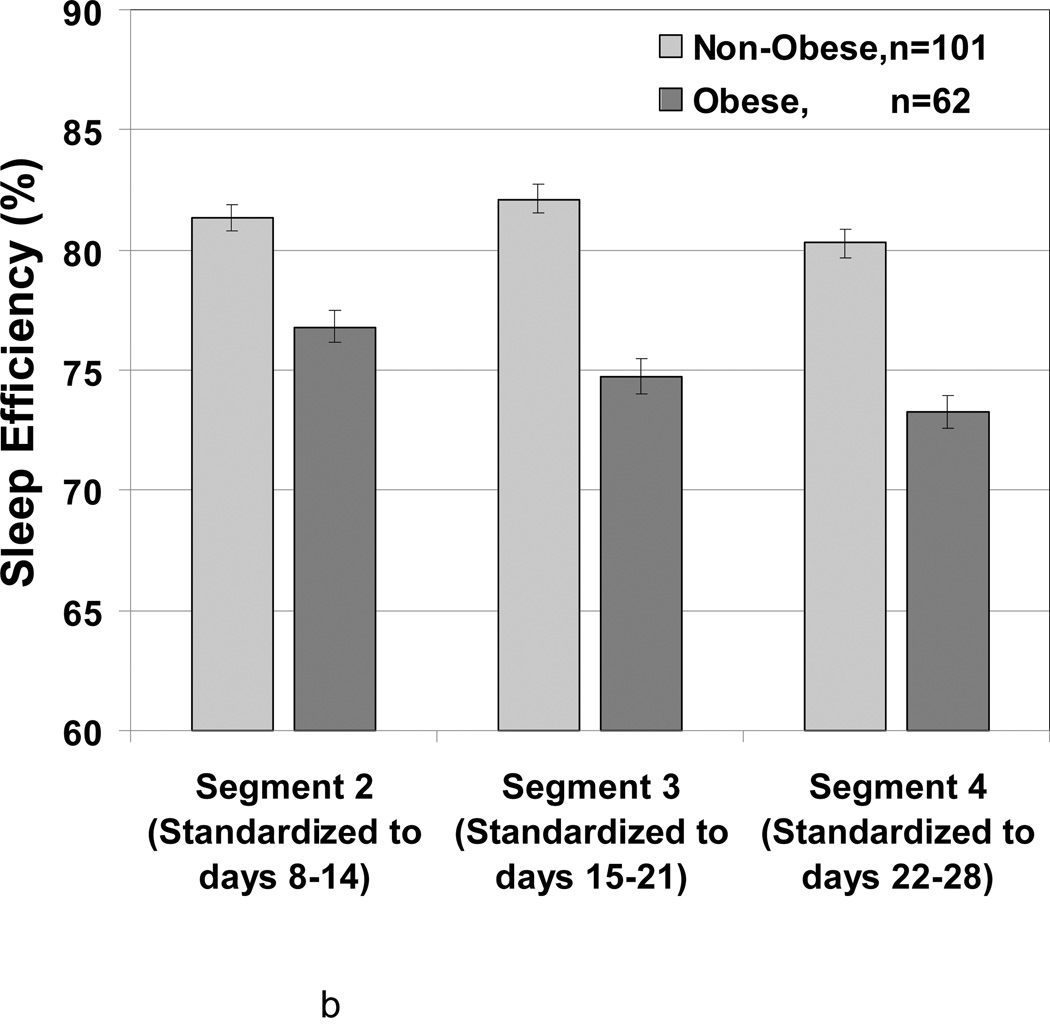
A significant interaction (p = 0.0031) was observed between timing segment and smoking for sleep efficiency (Figure 2a). Smokers and non-smokers had similar sleep efficiencies in the third week (74.0% versus 79.6%), while smokers had markedly lower sleep efficiency than non-smokers in the second week (69.2% versus 80.6%) and fourth week (65.0% versus 78.5%). Within smoker group (and non-smoker group), sleep efficiency was significantly lower in fourth week than third week with p=0.02 and 0.04, respectively. However, no significant differences were observed between second and third week (p>0.7). A significant interaction was observed between week and BMI (p=0.01) for sleep efficiency (Figure 2b). Sleep efficiencies for non-obese women (BMI<30) in second, third and fourth weeks were 81.3%, 82.14%, 80.31%, respectively. For this group, sleep efficiency was significantly lower at premenstruation (i.e., fourth week) in contrast to the prior week. For obese women (BMI ≥30), sleep efficiency at second, third and fourth weeks were 76.8%, 74.7%, 73.3%, respectively and premenstruation week four had the lowest sleep efficiency.
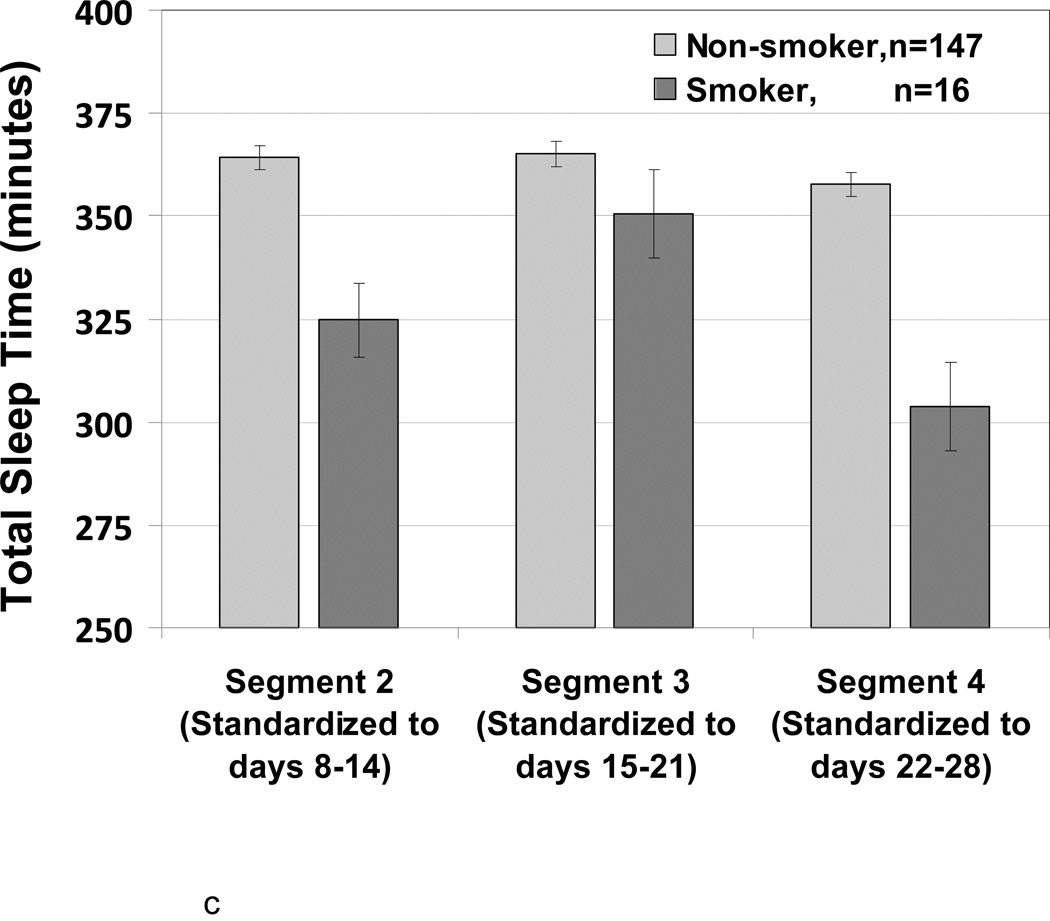
Figure 2.
Adjusted LSMeans (SE) showing the association of menstrual cycle segment according to smoking status and body size. N = 163
a Sleep Efficiency according to Smoking
b Sleep Efficiency according to Obesity
c Total Sleep Time (minutes) according to Smoking
For total sleep time (Figure 2c), the interaction between week and smoking was also significant (p=0.03). For non-smokers, total sleep times at second, third and fourth weeks were 364.2, 365.0, and 357.6 minutes, respectively. The premenstruation week four had slightly shorter sleep time in contrast to the prior 2 weeks (p=0.08). For smokers, total sleep times at second, third and fourth weeks were 324.6, 350.5, and 303.8 minutes, respectively. The premenstrual week four had markedly shorter sleep time (by about 50 minutes) than the previous week (p = 0.002).
The interaction between week and smoking in mean activity score model was also significant (p=0.003). Premenstruation week four had a slightly lower log-mean activity score 2.6 in contrast to second and third weeks with scores 2.8 and 2.7, respectively. However, the CV plot and the Signal-to-Noise Ratio (SNR) showed the largest variation (or lowest SNR) around day 14 and last week (See Figure A and Figure B of Appendix III in the Supplemental Digital Content).
For all models of sleep efficiency, total sleep time and mean activity score, the interaction effects among smoking, BMI, ethnicity and alcohol consumption were not significant (data not shown).
DISCUSSION
The time-series actigraphy data analysis31 allowed us to evaluate variability in sleep profiles across the menstrual cycle. This analysis was performed using data from one menstrual cycle in 163 healthy menstruating women with mean cycle length 25.9 days (standard deviation 6.8 days) in the SWAN Sleep Study. Actigraphy-derived sleep measures demonstrated that sleep efficiency declined over the course of the cycle (Figure 1b) with the most prominent decrease in fourth week, the premenstrual period. We observed less efficient sleep and shorter sleep durations in the final 7-day week of the menstrual cycle, a time period in which progesterone and estradiol levels decline32–34. In contrast, no parallel evidence of less favorable sleep around the approximate mid-cycle node was observed. Importantly, the study identified potentially important modifiable risk factors that influenced the menstrual segment's association with the sleep measures. Smoking, in particular, enhanced the relation of the menstrual cycle to sleep efficiency and total sleep time.
The previous studies of sleep disruption using sleep electroencephalogram (EEG) have reported changes in EEG power density in the 14.25–15.0 hertz band during non-rapid eye movement sleep in association with changes in progesterone levels during the luteal phase of the menstrual cycle, but no significant variation in objective measures of visually-scored sleep duration or continuity across the menstrual cycle35–36.
We tested the mean and slope differences in sleep measures between weekly segments of the menstrual cycle. The knots of each segment were determined based on assumptions about the timing of major shifts in reproductive hormone concentrations in regularly menstruating adult women. Significant differences were observed in sleep efficiency and total sleep time, but not in time in bed. Therefore, the change in sleep efficiency reflects decreased sleep time, rather than increased time in bed.
Fluctuations in reproductive hormones appear to affect sleep through women’s lifespan from adulthood to menopause 37–38. Ideally, we would have concurrent hormone measures to relate directly to the sleep measures and corroborate the use of the time-based segments. Unfortunately, specimens of concurrent hormone were not collected because of the costs for collection and assay, as well as the ongoing daily burden on the study participants. However, an earlier report from the SWAN Core study identified that the menstrual cycle pattern of increased urinary progesterone metabolite was associated with self-identified increase in trouble sleeping in perimenopausal women, and increased follicle stimulating hormone (FSH) levels was associated with increased trouble sleeping in premenopausal women10. This association between increased progesterone [pregnanediol glucuronide (PdG)] excretion and trouble sleeping contrasts with other studies which suggest that high progesterone levels facilitate sleep11.
In the absence of measures of hormones to describe the LH surge and rapid rise in progesterone, we assumed that ovulation occured at the mid-cyle and that comparisons of time segments were sufficiently sensitive to identify important changes in the sleep characteristics of the mid-cycle37. Our failure to observe changes in sleep measures between second and third week may mean that ovulation itself did not influence sleep, that misclassification of day of ovulation limited our ability to detect such an association, or that for some women, changes were associated with marked decreases in progesterone and estradiol that occurred in the last week of the cycle. Had statistically significant mid-cycle differences been observed, other factors apart from changes in LH and progesterone levels might be candidates for their contribution to sleep changes. For example, readily discernible changes in body temperature at the mid-cycle might be associated with alterations in sleep behaviors and need to be further explored. Notably, significant effects of hot flashes and night sweats on sleep were not observed in this study.
The importance of covariates in relation to menstrual cycle effects on sleep
Many sleep studies have adjusted for the presence of covariates such as smoking or body size. Our data analyses suggest that these covariates as well as race/ethnicity and financial strain are important effect modifiers of our proxy measures (time segments) for pronounced hormone changes and explained contrasting findings. The potential for smoking to be an effect modifier of the hormonal effect on sleep is not surprising given previous research. Smoking has effects on various metabolic and biological processes in the body and associated with early menopause39–41, higher FSH levels42 and lower ovarian follicle density43. Potential mechanisms of smoking’s toxic effects on the ovary include effect of cigarette smoke on oocyte quantity44, oocyte quality45, disruption of endocrine function46, or nicotine on sleep by acting on various neurotransmitter systems47, and reduction in oxygen-carrying capacity48–49.
In addition to smoking, this study also showed that body mass affected sleep quality. Both obese and non-obese women had significantly lower sleep efficiency in the premenstrual week in contrast to prior weeks. However, obese women had markedly lower sleep efficiency than non-obese women in the fourth week, which might have been due to the influence of body size on hormone patterns50, especially on FSH changes10, 51–52. For example, non-obese women have a significantly faster rate of change in FSH and higher FSH level during the menopausal transition. Sleep efficiency was significantly lower in those who had a slower FSH change4 and FSH level was significantly positively related to trouble sleeping10. Change in E2 was not related to sleep pathology4. Levels of estrone conjugates (E1c) and LH had no significant associations with trouble sleeping10.
For this healthy group of women, we found that larger body size, higher AHI, greater financial strain and smoking were associated with poorer sleep quality in the premenstrual phase. In contrast, hot flashes/cold sweats, stress and depression did not appear to affect sleep in this phase specifically, as determined by the absence of segment-by-variable interactions. However, other studies have shown that women with severe premenstrual syndrome (PMS) had poorer sleep quality in the premenstrual phase12, 36.
Impact of data loss
As noted above, the analysis excluded 7 days’ data from the first week. To evaluate the potential impact of this type of data loss we conducted a simulation study. The simulation showed that the intraclass correlation coefficient (ICC) was 0.90 using three weeks of data (21 days) and 0.80 using second week and third week data only (14 days). Another simulation using PSG data showed that about 125 women gave 95% coverage probability (i.e., the proportion of the simulation runs in which the estimated interval contained the true value of parameter over all the simulation runs) to detect “night” effects30. This suggests that the analytical dataset (163 women with three weeks data) was of sufficient sample size to minimize the estimation bias due to data loss by excluding some ineligible women and first week data.
Strengths and limitations
This study had unique strengths. It was an in-home-based sleep study with a substantial sample size that also included objective daily assessment of sleep duration across a menstrual cycle. This study was conducted in a sample of healthy middle-aged women to characterize the normal physiological and psychological events of the menopause in relation to sleep, but care must be exercised in extrapolating these findings to studies directed toward samples or study designs that are highly enriched for sleep pathology (e.g., a case-control study of insomnia). The Sleep Study was able to capitalize on the availability of a wide range of information about covariates from the core SWAN study. The daily sleep diaries were developed to not only record when sleep began and ended but also to consider time varying health behaviors as covariates with day-to-day variation that could influence associations between menstrual cycle phase and sleep. Additional strengths of this study included; standardization of cycle length to account for the unequal cycle28, 12 and use of semi-parametric stochastic mixed effect models29 that took into account the between- and within-woman effects with flexible functional form where linear or quadratic term might be inappropriate.
Nonetheless, the study also had limitations. The availability of data from only one menstrual cycle precludes exploring potential cycle-to-cycle variation or periodic patterns in this specific age group. Because African American women were recruited by three Sleep Study sites but only one site recruited Chinese women, a condition imposed by the design of the parent study, we could not disaggregate sources of variation associated specifically with study site and race/ethnicity. Furthermore, the study had only 16 smokers, thus the smoking effect should be interpreted cautiously and needs to be confirmed in future studies.
The size of the differences between third week and fourth week were small to moderate for some outcomes, including mean activity counts and a total sleep time. These findings are, nevertheless, important for understanding and furthering research on the etiology of sleep disturbances during midlife, a significant problem for many women, with potential effects on health. Furthermore, we have previously reported that self-reported trouble sleeping by these women was more prevalent in this premenstrual week, suggesting that the difference in total sleep time on average between third and fourth weeks of the menstrual cycle has clinical significance10.
CONCLUSIONS
Profiles of two daily measures of sleep (sleep efficiency in percentage, and total sleep time in minutes) and one measure of movement during sleep (mean activity score in counts) differed across a 28-day menstrual cycle. No significant mean and slope differences were found between second week and third week, which corresponded to mid-cycle. In the premenstrual period (fourth week), women slept worse as quantified by shorter total sleep time and lower sleep efficiency. The presence of significant effect modifications indicated that complex patterns exist between proxy measures of hormone status with lifestyle factors (such as smoking) and body size in relation to sleep.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all participants in the Study of Women’s Health Across the Nation (SWAN) and SWAN Sleep Study.
In memoriam: The authors thank Dr. MaryFran Sowers for her significant contributions and leading work in SWAN and SWAN Sleep Study.
Funding support: This work from the SWAN Sleep Study was supported by the National Institute on Aging (R01AG019360, R01AG019361, R01AG019362, R01AG019363). The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH); DHHS, through the National Institute on Aging (NIA); the National Institute of Nursing Research (NINR); and the NIH Office of Research on Women’s Health (ORWH) (U01NR004061, U01AG17104, U01AG017719, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). Sleep data were processed with the support of RR024153. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Footnotes
Conflict of Interest and Source of Funding: None of the work was supported from commercial sources. Dr.Daniel J Buysse has served as a paid consultant for Merck, Pfizer, Purdue Pharma, Philips Respironics, and General Sleep Corporation. Consulting fees for each company are less than $10,000 annually. Dr. Buysse has been paid for continuing education lectures supported by Astellas and Servier, and for on-line educational presentations supported by CME Outfittters and Medscape. For the remaining authors none were declared.
REFERENCES
- 1.Hale GE, Manconi F, Luscombe G, Fraser IS. Quantitative measurements of menstrual blood loss in ovulatory and anovulatory cycles in middle- and late-reproductive age and the menopausal transition. Obstet Gynecol. 2010;115(2 Pt 1):249–256. doi: 10.1097/AOG.0b013e3181ca4b3a. [DOI] [PubMed] [Google Scholar]
- 2.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92(8):3060–3067. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 3.Kapen S, Boyar R, Hellman L, Weitzman ED. The relationship of luteinizing hormone secretion to sleep in women during the early follicular phase: effects of sleep reversal and a prolonged three-hour sleep-wake schedule. J Clin Endocrinol Metab. 1976;42(6):1031–1040. doi: 10.1210/jcem-42-6-1031. [DOI] [PubMed] [Google Scholar]
- 4.Sowers MF, Zheng H, Kravitz HM, Matthews K, Bromberger JT, Gold EB, Owens J, Consens F, Hall MH. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31(10):1339–1349. [PMC free article] [PubMed] [Google Scholar]
- 5.Hollander LE, Freeman EW, Sammel MD, Berlin JA, Grisso JA, Battistini M. Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol. 2001;98(3):391–397. doi: 10.1016/s0029-7844(01)01485-5. [DOI] [PubMed] [Google Scholar]
- 6.National Sleep Foundation. Sleep in America poll 2007. Washington, DC: [Accessed March 5–6, 2007]. Available at http://www.sleepfoundation.org/.. [Google Scholar]
- 7.Baker FC, Driver HS. Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosom Res. 2004;56(2):239–243. doi: 10.1016/S0022-3999(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 8.Baker FC, Lamarche LJ, Iacovides S, Colrain IM. Sleep and menstrual-related disorders. Sleep Medicine Clinics. 2008;3(1):25–35. [Google Scholar]
- 9.Lamache LJ, Driver HS, Wiebe S, Crawford L, DE Koninck JM. Nocturnal sleep, daytime sleepiness, and napping among women with significant emotional / behavioral premenstrual symptoms. J Sleep Res. 2007;16(3):262–268. doi: 10.1111/j.1365-2869.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- 10.Kravitz HM, Janssen I, Santoro N, Bromberger JT, Schocken M, Everson-Rose SA, Karavolos K, Powell LH. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005;165(2):2370–2376. doi: 10.1001/archinte.165.20.2370. [DOI] [PubMed] [Google Scholar]
- 11.Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep. 1999;22(5):540–555. [PubMed] [Google Scholar]
- 12.Shechter A, Boivin DB. Sleep, Hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int J Endocrinol. 2010 doi: 10.1155/2010/259345. Article ID 259345, 17 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paquet J, Kawinska A, Carrier J. Wake detection capacity of actigraphy during sleep. Sleep. 2007;30(10):1362–1369. doi: 10.1093/sleep/30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCall C, McCall WV. Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. J Sleep Res. 2012;21(1):122–127. doi: 10.1111/j.1365-2869.2011.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sowers MF, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. Design, survey sampling and recruitment methods of SWAN: A multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition chapter 11. In: Wren J, Lobo RA, Kelsey J, editors. Menopause: Biology and Pathobiology. Academic Press; 2000. pp. 175–188. [Google Scholar]
- 16.Kravitz HM, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA, Sowers MF. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31(7):979–990. [PMC free article] [PubMed] [Google Scholar]
- 17.Hall MH, Matthews KA, Kravitz HM, Gold EB, Buysse DJ, Bromberger JT, Owens JF, Sowers MF. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 18.Mezick EJ, Matthews KA, Hall M, Kamarck TW, Buysse DJ, Owens JF, Reis SE. Intra-Individual Variability in Sleep Duration and Fragmentation: Associations with Stress. Psychoneuroendocrinology. 2009;34(9):1346–1354. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 20.WHO: World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. Geneva: 2007. [PubMed] [Google Scholar]
- 21.Brambilla DJ, McKinlay SM, Johannes CB. Defining the perimenopause for application in epidemiologic investigations. Am J Epidemiol. 1994;140(12):1091–1095. doi: 10.1093/oxfordjournals.aje.a117209. [DOI] [PubMed] [Google Scholar]
- 22.Dudley EC, Hopper JL, Taffe J, Guthrie JR, Burger HG, Dennerstein L. Using longitudinal data to define the perimenopause by menstrual cycle characteristics. Climacteric. 1998;1(1):18–25. doi: 10.3109/13697139809080677. [DOI] [PubMed] [Google Scholar]
- 23.WHO: World Health Organization Scientific Group. Research on the menopause in the 1990s. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1996;866:1–107. [PubMed] [Google Scholar]
- 24.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 25.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation of patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 26.Monk TH, Reynolds CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, Machen MA, Petrie SR, Ritenour AM. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3(1):111–120. [PubMed] [Google Scholar]
- 27.WHO: World Health Organization. [Accessed December 10, 2007];Guidelines for ATC Classification. 2007 Available at: http://www.whocc.no/atcddd.
- 28.Sowers MF, Crutchfield M, Shapiro B, Zhang B, Pietra ML, Randolph JF, Schork MA. Urinary ovarian and gonadotrophin hormone levels in premenopausal women with low bone mass. J Bone Miner Res. 1998;13:1191–1202. doi: 10.1359/jbmr.1998.13.7.1191. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Lin X, Raz J, Sowers MF. Semiparametric stochastic mixed models for longitudinal data. J. Amer. Statist. Assoc. 1998;93(442):710–719. [Google Scholar]
- 30.Zheng H, Sowers MF, Buysse DJ, Consens F, Kravitz HM, Matthews KA, Owens JF, Gold EB, Hall MH. Sources of variability in epidemiological studies of sleep using repeated nights of in-home polysomnography: SWAN Sleep Study. J Clin Sleep Med. 2012;8(1):87–96. doi: 10.5664/jcsm.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak C. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 32.Stahl ML, Orr WC, Males JL. Progesterone levels and sleep-related breathing during menstrual cycles of normal women. Sleep. 1985;8(3):227–230. doi: 10.1093/sleep/8.3.227. [DOI] [PubMed] [Google Scholar]
- 33.Chuong CJ, Kim SR, Taskin O, Karacan I. Sleep pattern changes in menstrual cycles of women with premenstrual syndrome: a preliminary study. Am J Obstet Gynecol. 1997;177(3):554–558. doi: 10.1016/s0002-9378(97)70145-5. [DOI] [PubMed] [Google Scholar]
- 34.Teofilo L, Lee-Chiong . Sleep Medicine Essentials. Wiley-Blackwell; 2010. [Google Scholar]
- 35.Driver HS, Dijk DJ, Werth E, Biedermann K, Borbély AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81(2):728–735. doi: 10.1210/jcem.81.2.8636295. [DOI] [PubMed] [Google Scholar]
- 36.Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30:1283–1291. doi: 10.1093/sleep/30.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moline ML, Broch L, Zak R. Sleep in women across the life cycle from adulthood through menopause. Med Clin North Am. 2004;88(3):705–736. doi: 10.1016/j.mcna.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Dzaja A, Arber S, Hislop J, Kerkhofs M, Kopp C, Pollmächer T, Polo-Kantola P, Skene DJ, Stenuit P, Tobler I, Porkka-Heiskanen T. Women’s sleep in health and disease. J Psychiatr Res. 2005;39:55–76. doi: 10.1016/j.jpsychires.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Midgette AS, Baron JA. Cigarette smoking and the risk of natural menopause. Epidemiology. 1990;1(6):474–480. doi: 10.1097/00001648-199011000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997;145(2):124–133. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- 41.Harlow SD, Mitchell ES, Crawford S, Nan B, Little R, Taffe J. The ReSTAGE collaboration: defining optimal bleeding criteria for onset of early menopausal transition. Fertil Steril. 2008;89(1):129–140. doi: 10.1016/j.fertnstert.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinney A, Kline J, Kelly A, Reuss ML, Levin B. Smoking, alcohol and caffeine in relation to ovarian age during the reproductive years. Hum Reprod. 2007;22(4):1175–1185. doi: 10.1093/humrep/del496. [DOI] [PubMed] [Google Scholar]
- 43.Westhoff C, Murphy P, Heller D. Predictors of ovarian follicle number. Fertil Steril. 2000;74(4):624–628. doi: 10.1016/s0015-0282(00)01527-2. [DOI] [PubMed] [Google Scholar]
- 44.Mattison DR, Thorgeirsson SS. Smoking and industrial pollution, and their effects on menopause and ovarian cancer. Lancet. 1978;1(8057):187–188. doi: 10.1016/s0140-6736(78)90617-7. [DOI] [PubMed] [Google Scholar]
- 45.Paszkowski T, Clarke RN, Hornstein MD. Smoking induces oxidative stress inside the Graafian follicle. Hum Reprod. 2002;17(4):921–925. doi: 10.1093/humrep/17.4.921. [DOI] [PubMed] [Google Scholar]
- 46.Valdez KE, Petroff BK. Potential roles of the aryl hydrocarbon receptor in female reproductive senescence. Reprod Biol. 2004;4(3):243–258. [PubMed] [Google Scholar]
- 47.Jaehne A, Loessl B, Barkai Z, Riemann D, Hornyak M. Effects of nicotine on sleep during consumption, withdrawal and replacement therapy. Sleep Med Rev. 2009;13(5):363–377. doi: 10.1016/j.smrv.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Morgado PB, Chen HC, Patel V, Herbert L, Kohner EM. The acute effect of smoking on retinal blood flow in subjects with and without diabetes. Ophthalmology. 1994;101(7):1220–1226. doi: 10.1016/s0161-6420(94)31185-7. [DOI] [PubMed] [Google Scholar]
- 49.Kapoor D, Jones TH. Smoking and hormones in health and endocrine disorders. Eur J Endocrinol. 2005;152:491–499. doi: 10.1530/eje.1.01867. [DOI] [PubMed] [Google Scholar]
- 50.Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, Finkelstein JS, Greendale GA, Kelsey J, Korenman S, Luborsky JL, Matthews K, Midgley R, Powell L, Sabatine J, Schocken M, Sowers MF, Weiss G. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women's Health across the Nation (SWAN) Daily Hormone Study. J Cli nEndocrinol Metab. 2004;89(6):2622–2631. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 51.Randolph JF, Jr, Zheng H, Sowers MR, Crandall C, Crawford S, Gold EB, Vuga M. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96(3):746–754. doi: 10.1210/jc.2010-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Touzet S, Rabilloud M, Boehringer H, Barranco E, Ecochard R. Relationship between sleep and secretion of gonadotropin and ovarian hormones in women with normal cycles. Fertil Steril. 2002;77(4):738–744. doi: 10.1016/s0015-0282(01)03254-x. [DOI] [PubMed] [Google Scholar]
- 53.Efron B, Tibshirani R. Bootstrap Methods for Standard Errors, Confidence Intervals, and Other Measures of Statistical Accuracy. Statist. Sci. 1986;1(1):54–75. [Google Scholar]
- 54.Claeskens G, Keilegom I. Bootstrap confidence bands for regression curves and their derivatives. Ann. Statist. 2003;31(6):1852–1884. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.