Abstract
Previous reports have shown that a high protein diet improves weight gain and decreases expression of inflammatory markers in weanling Berkeley transgenic sickle cell mice. The effect of this diet on the underlying histopathology, however, has not been studied. Age-matched, male C57BL/6 controls (n=24), Berkley sickle mice (n=31) and Townes sickle mice (n=14) were randomized in a terminal experiment at weaning to isoenergetic diets, with either normal (20%) or high (35%) amount of energy from protein, by replacing dextrin. Tissue sampling for blinded histologic study and scoring of changes at baseline and after 3 months of feedings showed progressive siderosis and infarcts in spleen, kidney, and liver in all sickle groups, and no significant changes in age- and sex-matched normal controls. High-protein (35%) fed Berkeley sickle mice had significantly fewer (p<0.01) infarcts in spleen (35.7% less), liver (12.5% less), and kidney (28.6% less) and lower histopathologic scores (p<0.01) for chronic tissue injury in liver and spleen than matched normal-protein (20%) fed Berkeley sickle mice. In addition, high-protein fed Townes sickle mice had less vascular leakage (~36%) in the heart, lungs, and brain and a better survival rate (21%) than matched normal-protein Townes sickle mice. This is the first report of histopathologic evidence that a high protein:calorie diet attenuates sickle cell related chronic organ injury in transgenic sickle cell mouse models.
Keywords: Transgenic mice, sickle cell anemia, nutrition, histopathology, vascular dysfunction
Introduction
Animal models offer valuable opportunities to test therapeutic interventions and management regimens; each model having its own strengths and weaknesses.1 Since its introduction in 1997, the Berkeley sickle cell transgenic murine model (S) has served well for the study of some features of human sickle cell disease, especially chronic erythrocytic sickling, tissue hypoxia, hemolytic anemia, and extramedullary hematopoiesis.2 It has been less well suited, however, for studies of sickle cell chronic lung disease, vasculopathy, bone marrow infarction or embolization, retinopathy, renal papillary necrosis, or splenic atrophy.3 The histopathological changes of chronic tissue hypoxia and hemolytic anemia in the Berkeley model include infarcts and siderosis in spleen, kidneys, and liver, as well as, splenomegaly secondary to exuberant hematopoiesis.
In homozygous sickle cell disease (SCD), the substitution of valine for glutamic acid in the beta chain of the globin portion of the hemoglobin molecule forms sickle hemoglobin.4 Sickle hemoglobin crystallizes during conditions of hypoxemia, leading to defective erythrocytes that are fragile and easily lysed.5 The resulting chronic hemolysis and anemia are compensated, not only by exuberant erythropoiesis with increased catabolism and protein synthesis,6 but also by chronic inflammation, secondary to ongoing vascular endothelial injury and recurrent vaso-occlusive events.7,8 The net result is a hypermetabolic state, which causes increased utilization of protein/energy substrates, chronic nutritional deficiencies, and impaired growth and development.8,9 It follows that nutritional supplementation could compensate for some of these deficiencies, protect tissues fromchronic injury, and prevent impairment of growth and development.
In a recent study, introduction of a high protein/energy% diet at weaning in the Berkeley sickle cell transgenic murine model (S), not only improved weight gain, but also had anti-inflammatory effects, as evidenced by reduction of both C-reactive protein and the cytokine protein interleukin-6.10 These findings support the hypothesis that supplementation of protein substrates can compensate for the extraordinary energy and nutrient demands caused by the chronic anemia and hemolysis in sickle cell mice. In another recently published study, it was shown that plasma levels of mediators of immune activity such as interferon gamma, reported to be low in children or animal models with sickle cell disease, were elevated in Berkeley transgenic sickle cell mice receiving a high protein (35% protein) diet, compared to the same mice fed a normal (20% protein) diet.11
The effects of protein/energy supplementation (high protein) on the underlying histopathology in transgenic sickle cell (S) mice have not been investigated. Further evidence from changes in histology could support the suggestion that a readily available and easily administered dietary measure can benefit patients with sickle cell disease. This study was initiated to test the hypothesis that high dietary protein:energy ratio protects tissues in S mice.
Materials and methods
Berkeley (S) transgenic and Townes sickle cell (TS) murine male mice were obtained from colonies established at Emory University School of Medicine and maintained according to recommendations of the Institutional Animal Care and Use Committee. The Berkeley sickle mice were originally supplied by Dr C. Paszty, who at that time was at the Lawrence Berkeley National Laboratory.2 The Townes sickle mice were supplied by Dr T. Townes from the University of Alabama.12 Both murine models express exclusively human α- and sickle β-globin. The Townes model was used for the second experiment because our collaborators were switching over their colony from the Berkeley to the Townes model at the time of that experiment. Expression of exclusively sickle β-globin in S mice was confirmed by differential hemoglobin electrophoresis performed with the Helena Titan III electrophoresis system (Helena Laboratories, Beaumont, TX). Control (C) mice were male C57/BL/6 for the Berkeley model. There was no use of HbAA controls for the Townes model because our experiment with the Berkeley model showed that they were not necessary, since there was no significant difference between baseline and 3 months.
We used a longitudinal cross-sectional (terminal) study design with two major time points for organ harvesting: at weaning for baseline and at 3 months when the feeding ended. The C, S, and TS mice were randomized at weaning to receive isoenergetic diets containing either 20% (normal mouse diet) or 35% (high protein test mouse diet) of energy from protein, by replacing dextrin in the normal mouse diet (See supplemental 1 and 2). Therefore, six groups were studied C20, C35, S20, S35, TS20, and TS35, representing the mouse type and feed randomization. C and S mice were matched for age and TS20 and TS35 mice were also matched for age. The feeding protocol included 1 week of acclimatization to each test diet prior to the feeding period. Therefore, baseline animals were fed for 1 week before tissues were harvested. Similarly, test mice were fed for 1 week of acclimatization prior to the 3-month test period. Tissues were harvested at the end of the feeding period. The sample sizes were eight mice for all six groups at the baseline and 4, 4, 7, 8, 7, and 7 mice for C20, C35, S20, S35, TS20, and TS35 groups, respectively, at 3 months. In the sickle cell group with seven mice, one mouse from each group died soon after the feeding period began. The sample size for the control mice was halved after the discovery from a separate experiment (data not presented here) that there was no significant difference in the degree of organ damage between C20 and C35, even after 3 months of feeding.
The effects of the test diet on the weight and growth of the mice have been reported elsewhere.13 At tissue harvest for the S mice, the body weight and intact wet weights of the heart, spleen, and liver were recorded as previously reported.3 Kidneys were weighed together and reported as combined weights for each mouse. The organ weight per body weight for each mouse was calculated to compensate for growth variations. Samples of heart, spleen, liver, and kidney were identified with the Morehouse animal accession number, which was maintained during the microscopic studies. Tissue was fixed in buffered formalin, processed routinely, and embedded in paraffin at the Department of Neurobiology/Anatomy of Morehouse School of Medicine and then sent to the Centralized Pathology Unit for Sickle Cell Disease at the University of South Alabama. The blocks were sectioned at 5–7 µm for hematoxylin and eosin (H&E) stains and, as indicated, Masson trichrome and Gomori-modified Prussian blue stains. Appropriate controls accompanied each batch of special stains and were approved before study of the test tissues. For uniformity of diagnoses, all samples were studied and graded by one pathologist (EAM), who was unaware of the details of the study design, animal groups, diets, or controls. Histologic sections were studied by routine light microscopy for significant changes and were graded subjectively on a scale of 0 (absent, none) to 10 (maximal, severe, abundant). Histopathological features of special interest included vascular ectasia (E), congestion, muscular hypertrophy, thrombosis (T), vasculitis, pyknosis, necrosis, fibrosis, infarcts (I), calcifications, siderosis, inflammatory infiltrates, and sickling of erythrocytes. The graded scores of microscopic findings were returned to Morehouse for statistical analysis.
The TS mice were used to investigate vascular barrier dysfunction in the organs, which we expected might be contributing to occurrence of tissue or organ damage and the life span. An a priori decision was made based on past experience14 to study the kidneys, brain, heart, and lungs. These mice were fed for approximately 7 months and prior to sacrifice were injected intravenously with 100 µL of 1% cell-impermeable Evans Blue dye (Sigma-Aldrich, St. Louis, MO) in Phosphate Buffered Saline (PBS).14 After 40 min, anesthesia was administered followed by perfusion with a PBS/ethylene diaminetetraacetic acid (EDTA) mixture to flush out intravascular Evans Blue dye. Lungs, kidneys, heart, and brain were then harvested, immersed in formamide, and incubated for 3 days to facilitate dye extraction. The optical density of the formamide extract was determined by spectrophotometry and considered equivalent to the severity of vascular permeability (dysfunction in endothelial barrier) via dye leakage.
To investigate a possible mechanism of organ protection, serum markers of organ damage (Aspartate transaminase [AST], Alanine transaminase [ALT], blood urea nitrogen, creatinine [Cr], and creatinine kinase [CK]) were measured at the Yale University mouse metabolic phenotyping center using the COBAS Mira Plus automated chemistry analyzer (Roche Inc., Bohemia, NY). Liver iron deposit was also measured by Molecular Diagnostic Services Inc., using the Ferene based method described by the iron panel of the International Committee for Standardization in Hematology.15,16 It was also decided a priori, not to assay lactate dehydrogenase level as a marker for tissue/organ damage because of possible confounding by background hemolysis from the sickle cell state.
Data handling and analysis
Data analysis was done using GraphPad® Prism. The graded scores for severity of microscopic findings, which were based on the number of harmful histological features seen in each organ section examined, were combined to give an overall tissue injury score for each organ in each mouse. The heart and spleen were scored on three criteria, while the liver and kidneys were scored on five and six criteria, respectively. These criteria and scoring system have been previously published.3 With a maximum score of 10 per criteria, the maximum score obtainable for the heart and spleen was 30, while it was 50 and 60 for the kidneys and liver, respectively. The total overall score obtained by each animal per organ was then calculated and plotted using a bar graph as means with standard deviations. The frequency and severity of each of the three most significant histological changes (congestion, infarcts, and siderosis) were estimated. The effect of the dietary intervention was based on organ damage scores for these changes (see Tables 2 and 3). An Analysis of Variance (ANOVA) test with multiple comparisons (for more than two groups) was used to evaluate the differences in organ damage scores and t-test was used to compare the difference in means between two groups. Values are reported as means ± standard deviation.
Table 2.
Summary of frequency of histological findings at baseline and at 3 months in the spleen, liver, and kidney in S and matched Cmice by type of feed consumed
| Mouse groups | Histological feature |
Frequency e/n (%) at baseline | Frequency e/n (%) at 3 months | ||||
|---|---|---|---|---|---|---|---|
| Spleen | Liver | Kidneys | Spleen | Liver | Kidneys | ||
| C20 | Congestion | 1/8 (12.5) | 0/8 (0.0) | 4/8 (50.0) | 2/4 (50.0) | 0/4 (0.0) | 0/4 (0.0) |
| Infarct | 0/8 (0.0) | 1/8 (12.5) | 0/8 (0.0) | 0/4 (0.0) | 0/4 (0.0) | 0/4 (0.0) | |
| Siderosis | 2/8 (25.0) | 0/8 (0.0) | 0/8 (0.0) | 4/4 (100) | 0/4 (0.0) | 0/4 (0.0) | |
| C35 | Congestion | 2/8 (25.0) | 1/8 (12.5) | 1/8 (12.5) | 0/4 (0.0) | 0/4 (0.0) | 0/4 (0.0) |
| Infarct | 0/8 (0.0) | 0/8 (0.0) | 0/8 (0.0) | 0/4 (0.0) | 0/4 (0.0) | 0/4 (0.0) | |
| Siderosis | 1/8 (12.5) | 1/8 (12.5) | 0/8 (0.0) | 4/4 (100) | 0/4 (0.0) | 0/4 (0.0) | |
| S20 | Congestion | 8/8 (100) | 8/8 (100) | 8/8 (100) | 7/7 (100) | 7/7 (100) | 6/7 (85.7) |
| Infarct | 8/8 (100) | 8/8 (100) | 0/8 (0.0) | 6/7 (85.7) | 7/7 (100) | 2/7 (28.6) | |
| Siderosis | 8/8 (100) | 8/8 (100) | 7/8 (87.5) | 7/7 (100) | 7/7 (100) | 7/7 (100) | |
| S35 | Congestion | 8/8 (100) | 8/8 (100) | 7/7 (100) | 8/8 (100) | 8/8 (100) | 7/8 (87.5) |
| Infarct | 8/8 (100) | 7/8 (87.5) | 0/7 (0.0) | 4/8 (50.0) | 7/8 (87.5) | 0/8 (0.0) | |
| Siderosis | 8/8 (100) | 8/8 (100) | 5/7 (71.4) | 8/8 (100) | 7/8 (87.5) | 8/8 (100) | |
Shows frequency of congestion, infarction, and siderosis among groups for some of the organs examined at baseline and at 3 months, expressed as e/n which is number of events (e) in that group (in this case, congestion, infarction, or siderosis) per sample size with the percentage in bracket. Note that the number of events and percentages in this case do not take into account the severity of that event. Thus, lower number of infarcts in a group for that organ does not mean a lower severity of infarct in that organ for that group and vice versa.
Table 3.
Summary of severity of histological findings at baseline and at 3 months in the spleen, liver, and kidney in S matched and C mice by type of feed consumed
| Groups | Histological feature |
Mean organ damage score at baseline | Mean organ damage score at 3 months | ||||
|---|---|---|---|---|---|---|---|
| Spleen | Liver | Kidneys | Spleen | Liver | Kidneys | ||
| C20 | Congestion | 0.1 ± 0.4 | 0 | 0.9 ± 1.1 | 0.5 ± 0.6 | 0 | 0 |
| Infarct | 0 | 0.1 ± 0.4 | 0 | 0 | 0 | 0 | |
| Siderosis | 0.2 ± 0.5 | 0 | 0 | 1.5 ± 1.0 | 0 | 0 | |
| C35 | Congestion | 0.3 ± 0.5 | 0.6 ± 1.8 | 0.3 ± 0.7 | 0 | 0 | 0 |
| Infarct | 0 | 0 | 0 | 0 | 0 | 0 | |
| Siderosis | 0.1 ± 0.4 | 0.5 ± 1.4 | 0 | 2.5 ± 0.6 | 0 | 0 | |
| S20 | Congestion | 4.6 ± 1.1* | 3.9 ± 0.6* | 3.5 ± 0.9* | 8.1 ± 2.0*† | 6.1 ± 3.1* | 5.6 ± 3.8* |
| Infarct | 4.1 ± 1.4* | 4.4 ± 0.7* | 0 | 6.1 ± 3.4*† | 5.0 ± 2.9* | 1.0 ± 1.9* | |
| Siderosis | 4.5 ± 0.8* | 7.6 ± 3.3* | 2.5 ± 2.0* | 7.7 ± 1.4*† | 21.0 ± 5.4*† | 8.6 ± 1.1* | |
| S35 | Congestion | 4.9 ± 0.4* | 4.3 ± 0.7* | 2.4 ± 1.0* | 5.5 ± 2.4* | 3.9 ± 1.2* | 4.5 ± 3.6* |
| Infarct | 4.0 ± 1.1* | 3.5 ± 1.7* | 0 | 0.9 ± 1.0* | 5.1 ± 1.6* | 0 | |
| Siderosis | 3.0 ± 1.1* | 5.9 ± 3.7* | 1.7 ± 2.0* | 4.4 ± 1.5* | 10.4 ± 4.6* | 8.0 ± 2.1* | |
Indicates p-value for comparisons between transgenic sickle cell anemia mice and C57BL/6 mice fed the same diet and p < 0.001 in each case.
Indicates a significant p-value, with p < 0.05 for comparison of mean organ damage severity score between S20 and S35 per histological parameter.
Results
Comparison of body weights (Table 1) shows sickle groups (S20, S35) tend to have lower body weights than the age-, sex-, and feed-matched control groups (C20, C35), both at baseline and after 3 months of feeding the test diet.
Table 1.
Organ wet weights as a percentage of body weight in S and C mice at baseline and at 3 months by type of feeds
| N | Weight (g) | Liver (%) | Spleen (%) | Kidney (%) | Heart (%) | |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| C20 | 8 | 19.40 ± 1.88 | 5.6 ± 0.7 | 0.3 ± 0.1 | 1.3 ± 0.1 | 0.6 ± 0.1 |
| C35 | 8 | 18.25 ± 4.17 | 4.0 ± 0.4† | 0.7 ± 0.8 | 1.4 ± 0.1 | 0.6 ± 0.1 |
| S20 | 8 | 16.95 ± 1.53* | 6.2 ± 1.0 | 5.5 ± 1.4* | 1.7 ± 0.1* | 1.0 ± 0.1* |
| S35 | 8 | 17.58 ± 2.52 | 6.3 ± 0.5* | 5.6 ± 1.3* | 1.7 ± 0.2* | 0.9 ± 0.2* |
| Three months | ||||||
| C20 | 4 | 29.03 ± 3.24 | 4.8 ± 0.4 | 0.3 ± 0.1 | 1.4 ± 0.1 | 0.6 ± 0.1 |
| C35 | 4 | 34.3 ± 2.88 | 4.8 ± 0.3 | 0.2 ± 0.1 | 1.2 ± 0.1 | 0.5 ± 0.1 |
| S20 | 7 | 27.96 ± 3.09 | 6.9 ± 0.8* | 7.2 ± 1.4* | 1.9 ± 0.3* | 0.8 ± 0.2* |
| S35 | 8 | 27.55 ± 0.97* | 7.0 ± 0.4* | 7.0 ± 2.2* | 2.0 ± 0.4* | 0.9 ± 0.2* |
Indicates p<0.05 for comparison with control mice fed the same diet.
Indicates p<0.05 for comparison between mice (S or C) on 20% vs. 35% diet (organ weight/body weight).
All groups showed increased body weight over the study period, but total weight gain for mice fed for 3 months was greatest among C35 (13.36 g) followed by S35 (10.56 g), C20 (10.12 g), and S20 (9.39 g). Although unexpected, total weight gain for C35 mice was significantly higher than for C20 mice (p=0.043). The average total feed intake over the feeding period for C20, C35, S20, and S35 was 235.6 ± 35.5, 221.8 ± 25.6, 249.2 ± 28.3, and 226.4 ± 21.8 g, respectively, and the average daily feed intake was 2.62 ± 0.39, 2.46 ± 0.28, 2.77 ± 0.31, and 2.52 ± 0.24 g per day, respectively. There was no statistically significant difference between mouse groups for the total average amount of feed consumed per day. When converted to average total energy intake from protein and total energy intake per day, the results were
C20 intake=2.62 ± 0.39 g/d=0.52 gPr, 2.10 kcalPr and 9.56 kcal/d
C35 intake=2.46 ± 0.28 g/d=0.86 gPr, 3.44 kcalPr and 8.73 kcal/d
S20 intake=2.77 ± 0.31 g/d=0.55 gPr, 2.22 kcalPr and 10.11 kcal/d
S35 intake=2.52 ± 0.24 g/d=0.88 gPr, 3.53 kcalPr and 8.95 kcal/d.
Similarly, there was no statistically significant difference in the average amount of protein energy or total energy intake per day.
Microscopic organ changes and organ damage
As expected, comparison of organ weights as a percentage of body weight (Table 1) showed mice with sickle cell anemia (S20, S35) at baseline and at 3 months had higher organ weights than their matched controls (C20, C35), though the differences were not significant for S20 baseline liver, S20 and S35 baseline kidney, and S20 kidney and heart at 3 months. Comparison of organ weights between the sickle groups (S20, S35) showed no significant differences at baseline or at 3 months. Furthermore, organomegaly of the liver, spleen, and kidney was progressive with age in both sickle groups (S20, S35), but this observation was not present in their matched controls (C20, C35).
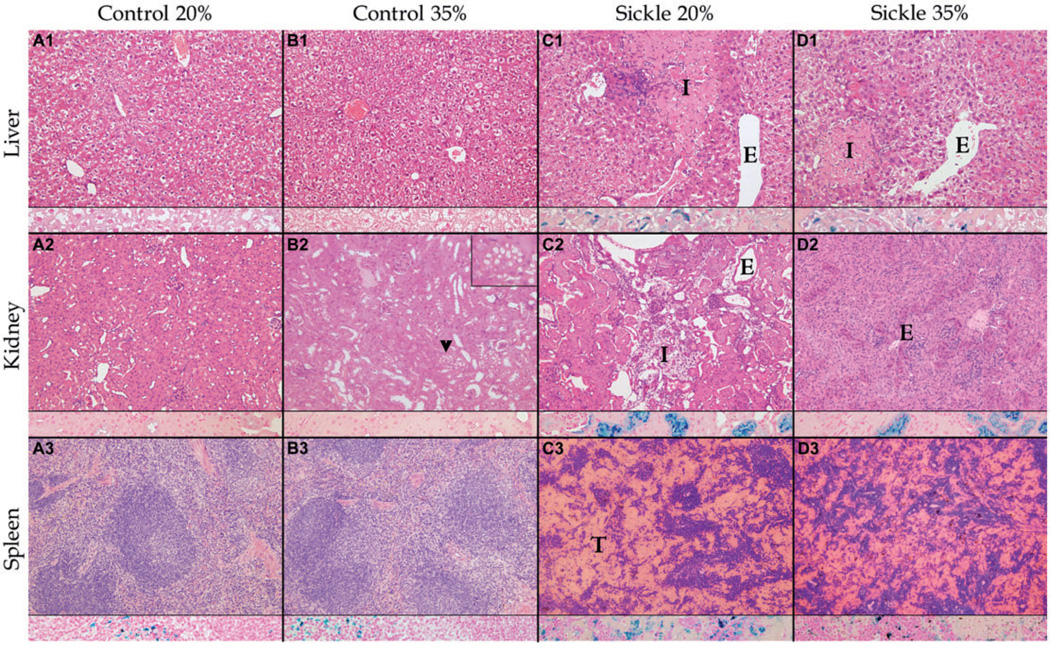
The most significant histopathological findings are summarized by representative photomicrographs in Figure 1. No significant microscopic differences were noted between C20 and C35, except that the kidneys for C35 had multiple foci of cytoplasmic vacuoles in the renal tubules (Figure 1, panel b2, arrow heads and inset), possibly from excessive activity and ischemia due to a high protein induced glomerular hyperfiltration activity.17 Histologically, S20 and S35 were significantly different fromtheir matched controls and from each other. Sickling of the erythrocytes was present in both sickle groups with dilation of vascular lumens (ectasias). Vascular ectasias were more pronounced in the S20 liver and kidneys compared with S35. In both sickle groups, splenic sinusoids were distended (congestion) but in S20 congestion was so severe it caused complete loss of architecture (thrombosis). Infarcts were seen in the liver, spleen, and kidneys of both sickle groups as loss of normal architecture with coagulative necrosis (acute ischemia) and/or fibrosis (chronic ischemia). The frequency and sizes of the infarcts were more pronounced in S20 than for S35 in all three organs. There were inflammatory infiltrates such as neutrophilic (acute) and/or mononuclear cells (chronic) in both sickle cell mouse groups. In both sickle groups, iron deposition (siderosis) was prominent in the spleen, liver, and kidney as golden brownish pigmented granules on H&E stains and was confirmed by Gomorimodified Prussian blue stains in macrophages, hepatic Kupffer cells, hepatocytes, and renal tubular epithelium. Based on quantitative liver iron deposit assay, here was a slightly higher iron deposit in S20 than for S35 but this was not statistically significant. At 3 months bilirubinate-like calculi were suspected in the biliary tract and, in one S20 liver, were associated with biliary-like cirrhosis.
Figure 1.
Comparison of histopathological findings after 3 months of feeding in weanling sickle mice (S) receiving normal (20%) or high (35%) protein feeds with age-, sex-, and feed-matched controls (C) shows less tissue injury in sickle mice on the high protein feed (S35) compared with mice on the 20% protein feed. Columns (from left to right) show representative findings in C20 (a), C35 (b), S20 (c), and S35 (d) mice. Each column shows a section of liver (row 1), kidney (row 2), and spleen (row 3), each with an inset showing iron staining (blue) at that site. No significant differences were noted between C20 and C35, except multifocal cytoplasmic vacuoles within renal tubular epithelium (arrow heads and inset in upper right-hand corner) in C35 (b2). Both S20 and S35 are significantly different from C20 and C35 with hepatic infarcts (I) in c1 and d1; renal infarcts (I) in c2; vascular ectasia (E) in c1, c2, and d1; and thrombosis (T) of splenic sinusoids in c3 and d3. Organ injury was more severe in S20 than in S35 mice. The representative photomicrograph shows a smaller sized infarct in the liver, absence of infarct in the kidney, less distention and thrombosis of splenic sinusoids in S35 compared with S20. Micrograph was randomly selected from a collection of micrographs of each organ (Zeiss AxioSkop; hematoxylin and eosin stains [H&E], original magnification × 200, with iron [blue] stain insets, original magnification × 400); Olympus DP70 Camera; Olympus DP2 TWAIN Software
Frequency and severity of organ damage
The combined average frequencies at baseline showed that all the groups were essentially similar for frequencies of congestion/thrombosis, infarcts, and siderosis. After 3 months of feeding, the frequency of splenic thrombosis was 35.7% lower and that of hepatic infarcts and hepatic siderosis were each 12.5% lower, for S35 mice compared with matched S20 mice. Similarly, the frequency of renal infarcts was 28.6% lower for S35 mice compared with S20 mice. The frequency of splenic congestion/thrombosis was 50% higher among the C20 versus C35 mice. The reason for this is unknown, as the 20% protein diet is optimal for the control mice (Table 2).
Comparison of severity of the histopathological scores is summarized in Table 3. C20 and C35 mice were similar at baseline and after 3 months of feedings. S20 and S35 mice were also similar at baseline. After 3 months of feeding, S35 mice had significantly lower histopathological scores for renal infarcts (p < 0.001), hepatic siderosis (p < 0.001), splenic congestion (p = 0.03), splenic thrombosis (p < 0.001), and splenic siderosis (p = 0.003) compared with S20 mice. At baseline and at 3 months, C20 and C35 scores were both significantly lower than the S20 and S35 for all histological features scored (p < 0.001), except for absence of renal infarcts in S35 mice.
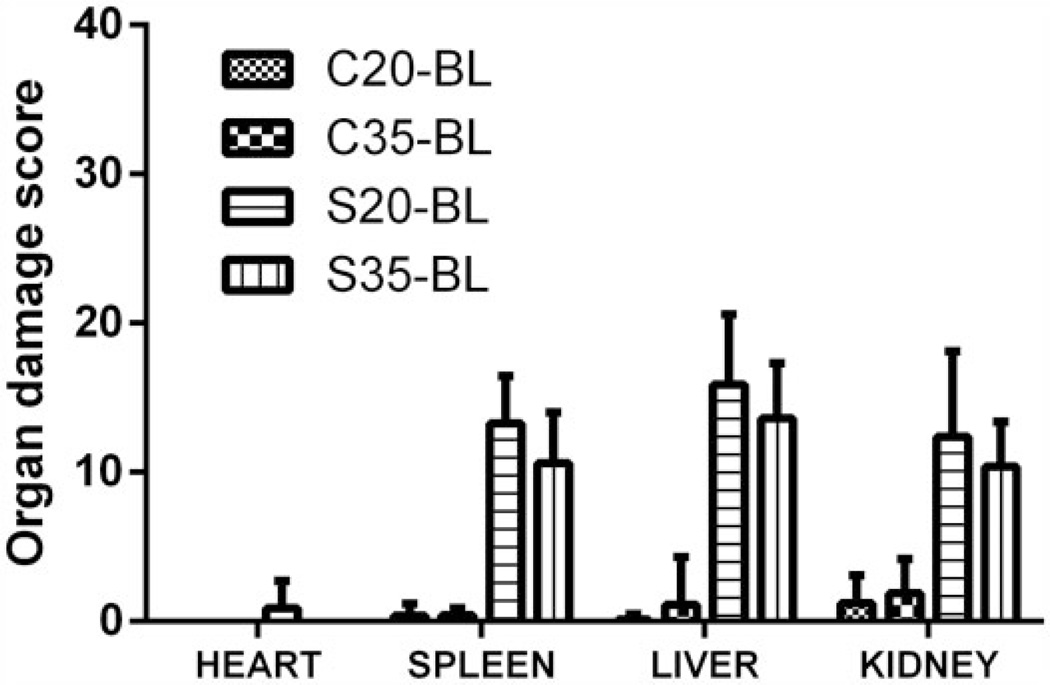
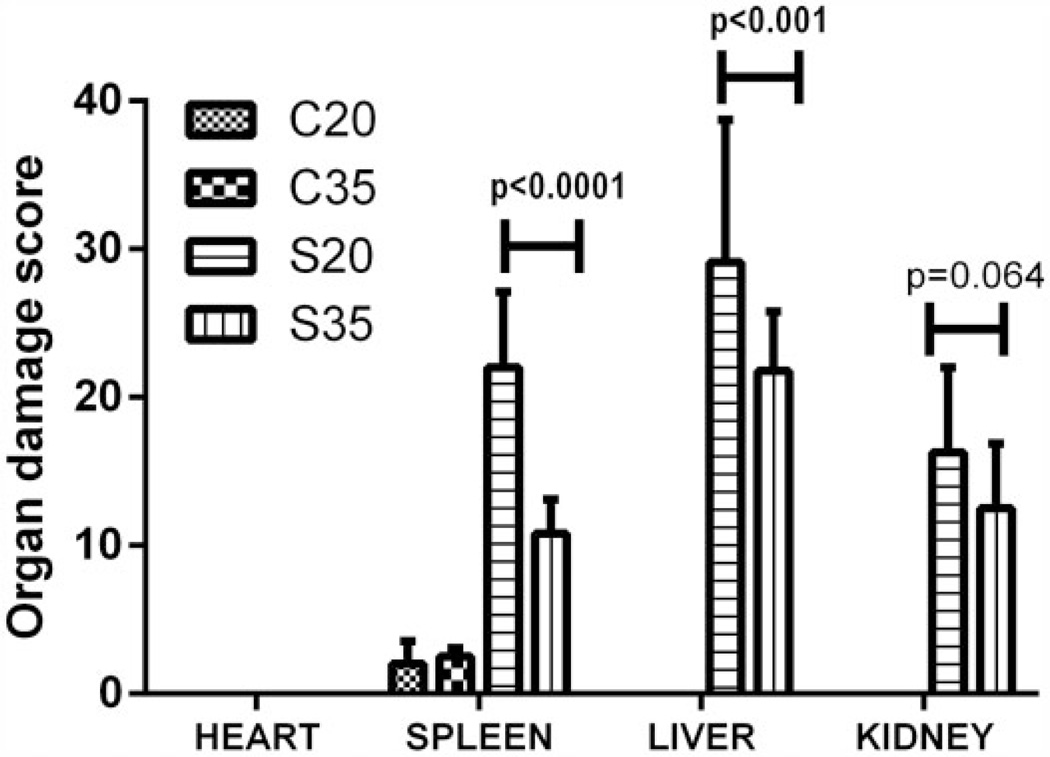
Figures 2 and 3 show comparison of the overall median organ damage score for each organ and experimental group. At baseline (Figure 2), the average organ damage scores were significantly greater in both S groups compared with their matched C groups (p < 0.001), but organ damage scores were not significantly different between the sickle groups in any of the organs evaluated. After 3 months of feeding (Figure 3), the organ damage score for the control groups (C20, C35) was not significantly different from each other, but the mean score for splenic damage in S35 was significantly lower than for S20 (p < 0.001). Similarly, the mean organ damage score for the liver in the S35 was significantly lower (p = 0.001) than for the S20 and the mean score for kidney damage among S35 tended to be lower than for the S20 mice, demonstrating a statistical trend (p = 0.064).
Figure 2.
Sickle cell mice had significantly higher organ injury score compared with controls but not from each other at baseline. Comparison of overall mean scores for organ injury by study group and by feed type shows no significant differences between control groups (C20, C35) or between sickle groups (S20, S35), but both sickle groups (irrespective of feed types) had greater organ injury than either of the control groups
Figure 3.
Sickle cell mice had significantly higher mean organ injury score than controls at 3 months, and S35 had significantly lower organ injury score than S20. Comparison of the overall mean organ injury score by study group and by feed type after 3 months of test feedings shows progressive injury in S20 but nearly stable injury in S35 which had significantly lower splenic and hepatic injury score compared with S20 mice. There were no significant differences in the scores for C20 and C35 (irrespective of the type of feed). There was a trend in lower kidney injury score among S35 compared with S20
Vascular barrier dysfunction (leakage)
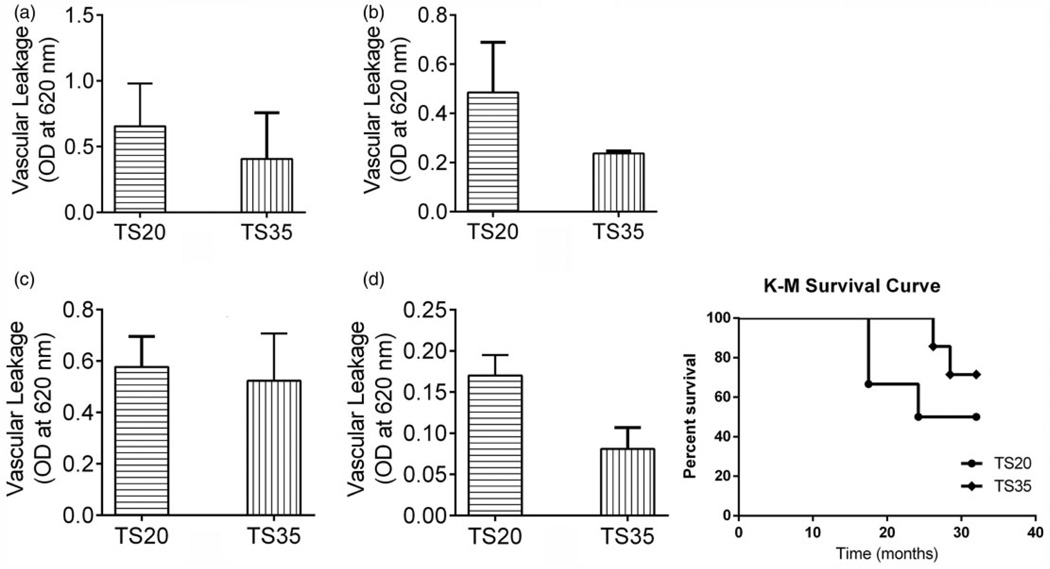
To demonstrate a possible mechanism of organ protection by the high protein diet, we evaluated the severity of vascular leakage using Townes transgenic sickle cell anemia (SCA) mice. The brain, lungs, and heart were evaluated; however, the kidneys were usually too damaged at weaning to benefit from intervention. A feeding period of ~7 months enabled evaluation of survival rate. After the feeding, TS35 mice had ~36% less vascular leakage in the lung, heart, and brain compared with the TS20 mice. Furthermore, the TS35 mice had a 21% higher survival rate at 32 weeks compared with TS20 mice (Figure 4). Hazard ratio based on log-rank test was 2.4 (0.4–15.9), but the difference was not statistically significant.
Figure 4.
High protein diet reduced vascular leakage and mortality in transgenic sickle mice. Analysis of vascular leakage in TS20 (Townes mice given a standard diet) and TS35 (Townes mice given high protein) showed that higher protein diet was associated with reduced vascular leakage in the lungs (a), heart (b), kidneys (c) and brain (d), and improved survival
Tissue damage and hematology
The plasma AST, ALT, and CK levels were not significantly different between S35 and S20 mice at either baseline or after 3 months of feeding. Tissue iron level was slightly lower (15%) per gram of tissue in the liver of S35 compared with S20, but this was not statistically significant. In addition, the S35 had a slightly higher mean red blood cell (RBC) count than the S20 mice (7.7 ± 0.59 × 106/µL versus 6.8 ± 0.77 × 106/µL, p=0.09), a slightly higher mean hemoglobin level compared with S20 (10.56 ± 1.09 g/dL versus 9.98 ± 0.49 g/dL, p=0.08), and a slightly lower mean reticulocyte percent (34.74 ± 8.80% versus 39.16 ± 7.13%) than the S20 mice.
Discussion
The findings in this study confirm previous studies of the pathology of the Berkeley sickle cell mouse model, which show good expression of severe erythrocytic sickling, splenomegaly, cardiomegaly, hepatomegaly, nephromegaly, infarcts (liver, spleen, kidney), fibrosis (liver, kidney), siderosis (liver, spleen, kidney), and generalized vascular ectasias and congestion.2,3 Likewise, the finding that visceral weights in all S study groups were greater than those in all C study groups is consistent with the histologic finding of increased vascular congestion and sinusoidal thrombosis causing increased blood content in all tissues. Similar findings have been recorded for the TS model.18
The most important finding is that after 3 months on high protein feeds, sickle mice (S35) had significantly less histologic evidence of tissue injury in the liver and spleen than the sickle mice on normal levels of protein feeds (S20). This result supports the hypothesis that the increased protein/calorie percentage of the high protein (35%) diet protects these organs from some of the characteristic chronic organ damage seen in sickle cell disease.5 Furthermore, the high protein diet improved endothelial integrity by reducing the severity of vascular leakage (by Evans blue dye method) in the lung, heart, and brain, supporting our hypothesis that vascular leakage in sickle mice would be reduced by a high protein diet. In general, among the sickle mice, the additional protective effect of increased protein calories seems to be multifactorial. Some features observed in this study include decreasing the occurrence of vasculopathy, including reduced vascular leakage, improved hematological profile and tissue oxygenation, and increased nutrient supply needed for growth and repair. All of these resulting in reduced infarction and siderosis (from delayed hemolysis and thus slowed iron related tissue damage), as shown in the lower portion of Table 2. An additional organ protective mechanism could be improved tissue oxygenation as a result of a slightly higher albeit, statistically nonsignificant, increase in hemoglobin (5%) level observed among the S35 mice compared with the S20 mice. The dramatic effect on organ injury demonstrated in our study supports findings in prior studies with a similar design.17 Kaul et al. demonstrated that supplementing the diet of transgenic SCA mice with 5% more arginine resulted in decreased expression of non-nitric oxide (NO) vasodilators, arteriolar dilation, oxidative stress, and hemolysis, while resulting in improve NO-mediated vessel reactivity and NO bioavailability.19 Other studies using arginine supplementation in mouse models of sickle cell disease have reported improved protection from oxidative stress,20 improvement in coordination and muscle strength,21 and reduction in red cell density via improvement in the regulation of the Gardos channel to limit K+ loss and or Ca2+ via this channel.22 Our 35% diet contained 1.8% calories from arginine. We believe that the dramatic effect from this small amount could be attributed to the presence of other amino acids in slightly higher amounts than in the regular mouse feed contained.
In humans with SCA, there is overwhelming evidence for a dysregulated metabolism of protein and specific amino acids.23–26 Morris et al. reported a decrease in the circulating level of arginine among individuals with SCD and reported an inverse relationship between circulating levels of arginine and several indices of severity of SCD.27 Ohnishi et al. were able to demonstrate that supplementation with a cocktail of dietary supplements rich in protein and conditionally essential amino acid resulted in reduced SCA-associated complications and moderately increased hematocrit compared with the non-supplemented group.28 Despite using animal models, our study supports these findings and provide additional evidence from histology, on the possible role of improved nutrition in the management of SCD.
Children with SCA have high resting energy expenditure (REE), possibly from excess demand from compensatory erythropoiesis and myocardial energy demand.6 Williams et al. reported that oral glutamate supplementation resulted in reduced REE among supplemented children with SCA, supporting our hypothesis of benefit from amino acid other than arginine, which is more widely reported in the literature. Translating our findings to humans given the difference in rate of metabolism between humans and mice (approximately 25 kcal/kg/day versus 600 kcal/kg/day) would have been challenging since mice tend to require more protein in their diet than humans. But our laboratory has developed a high calorie supplement that incorporates the required amino acids and avoids the high protein load.29
Recently, our laboratory in a small randomized placebo controlled pilot clinical trial (not yet published) demonstrated that using the high calorie supplement providing selected deficient amino acid and 50% extra calories to children with SCA resulted in increased circulating levels of arginine, glutamine, and other essential or conditionally essential amino acids. Furthermore, there were increased levels of reduced glutathione synthase and decreased levels of oxidized glutathione synthase. This supplement also resulted in a decrease in REE among the supplemented group (16.8% versus 0.2%) compared with the placebo group. Taken together with data from this current study these findings suggest an important role for nutritional therapy as an adjunct to currently available therapy (hydroxyurea and chronic packed RBC transfusion) in the management of SCD and its complications.
The exact mechanism for the benefits of nutritional or select nutrient supplementation is still unknown. For instance, in the current study, measurement of total hepatic iron deposit was not significantly different between S35 and S20 (491.0 ± 199.7 µg versus 536.1 ± 181.8 µg) per gram of tissue. This modest difference (9.0%) in tissue iron deposit may point to a possible mechanism of organ protection. The lower liver iron deposit was also reflected in a lower siderosis score on histology. This finding warrants further investigation as excess iron in the oxidized state forms a potent oxidative stress and can propagate a mechanism for organ damage.
In summary, this is the first study to show actual histological evidence that high protein feeds in transgenic murine models of sickle cell disease protect tissues from histological changes associated with chronic hypoxia and hemolytic anemia and improves survival. Feeding of a high protein diet decreased both the frequency and severity of the histopathological changes associated with chronic organ injury (infarcts). The diet also decreased vascular leakage. But changes associated with hemolysis (siderosis/splenomegaly) did not differ significantly between mice fed the high protein diet and those who received regular mouse chow. Further studies are needed to understand both the mechanism of this tissue sparing effect, the stage of feeding where it is most beneficial and the human correlates of this stage.
Supplementary Material
ACKNOWLEDGEMENTS
The authors want to thank Ms Shayla Cue from Dr. Hibbert’s lab at Morehouse School of Medicine, Ms Tatyana Vikulina and Ms Susane Roser-Paige both from Dr.Weizmann’s lab at Emory University, and Ms Prasanthi Chappa of Dr. Archer’s lab at Emory University. This work was supported by the following grants: 8U54MD007588 (to MSM), NIH/NHLBI-R21HL092358 (to JMH), NIH/NCRR-5P20RR0111044 (to JMH), NIH/HLBI-N01-HB-07086 (to EAM), and in part by MUSC/Department of Defense Cooperative Agreement (SE VIEW) number: W81XWH-10-2-0057.
Footnotes
Author contributions: EAM and HIH contributed equal effort to this manuscript and should be regarded as joint first authors. EAM provided study and grading of microscopic slides, interpretation of histologic findings, and writing of manuscript. HIH conducted the feeding experiments, performed sacrifice and organ extraction, data analysis and writing of manuscript, and critical review of final submitted version. PLC provided critical assistance in execution of experiments, feeding of mice, sacrifice, organ extraction, data analysis, and organization of data. SP did the tissue processing and blocking. JP provided critical supervision in tissue processing and blocking and his lab provided reagent needed for tissue preservation. DRA provided the Berkeley mice and housing for the experiment, logistics needed for sacrifice and transport of mice and tissues, and intellectual input regarding data interpretation. SG performed the vasculopathy investigations with the Townes mice. MET carried out tissue microtome sections, histochemical staining, and preparation of histologic glass slides. SFO designed the experiment, provided Townes mice and housing, and provided oversight of this experiment. JMH conceived and designed the experiments in addition to providing supervision, leadership, and direction for the entire project. She was also involved in critical manuscript review.
All authors have revised and approved this article.
REFERENCES
- 1.Fabry ME. Transgenic animal models of sickle cell disease. Cell Mol Life Sci. 1993;49:28–36. doi: 10.1007/BF01928785. [DOI] [PubMed] [Google Scholar]
- 2.Pászty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 3.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood. 2006;107:1651–1658. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauling L, Itano HA. Sickle cell anemia a molecular disease. Science. 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 5.Aster JC. Disease of organs systems: Red blood cells and bleeding disorders - sickle cell disease. In: Vinay K, Fausto N, Abbas AK, editors. Robbins and Cotran pathologic basis of disease. 7th ed. Philadelphia: Elsevier Saunders; 2005. pp. 628–632. [Google Scholar]
- 6.Hibbert JM, Creary MS, Gee BE, Buchanan I, Quarshie A, Hsu LL. Erythropoiesis and myocardial energy requirements contribute to the hypermetabolism of childhood sickle cell anemia. J Pediatr Gastroenterol Nutr. 2006;43:680–687. doi: 10.1097/01.mpg.0000228120.44606.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, Hebbel RP, Vercellotti GM. Transgenic sickle mice have vascular inflammation. Blood. 2003;101:3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- 8.Hibbert JM, Hsu LL, Bhathena SJ, Irune I, Sarfo B, Creary MS, Gee BE, Mohamed AI, Buchanan ID, Al-Mahmoud A, Stiles JK. Proinflammatory cytokines and the hypermetabolism of children with sickle cell disease. Exp Biol Med. 2005;230:68–74. doi: 10.1177/153537020523000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borel MJ, Buchowski MS, Turner EA, Goldstein RE, Flakoll PJ. Protein turnover and energy expenditure increase during exogenous nutrient availability in sickle cell disease. Am J Clin Nutr. 1998;68:607–614. doi: 10.1093/ajcn/68.3.607. [DOI] [PubMed] [Google Scholar]
- 10.Archer DR, Stiles JK, Newman GW, Quarshie A, Hsu LL, Sayavongsa P, Perry J, Jackson EM, Hibbert JM. C-reactive protein and interleukin-6 are decreased in transgenic sickle cell mice fed a high protein diet. J Nutr. 2008;138:1148–1152. doi: 10.1093/jn/138.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyacinth HI, Capers PL, Archer DR, Hibbert JM. TNF-α, IFN-γ, IL-10, and IL-4 levels were elevated in a murine model of human sickle cell anemia maintained on a high protein/calorie diet. Exp Biol Med. 2014;239:65–70. doi: 10.1177/1535370213508357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levasseur DN, Ryan TM, Pawlik KM, Townes TM. Correction of a mouse model of sickle cell disease: Lentiviral/antisickling beta-globin gene transduction of unmobilized, purified hematopoietic stem cells. Blood. 2003;102:4312–4319. doi: 10.1182/blood-2003-04-1251. [DOI] [PubMed] [Google Scholar]
- 13.Capers PL, Hyacinth H, Cue S, Chappa P, Archer D, Hibbert J. Effect of high protein diet on transgenic sickle mice. FASEB J. 2010;24:lb394. [Google Scholar]
- 14.Ghosh S, Tan F, Ofori-Acquah SF. Spatiotemporal dysfunction of the vascular permeability barrier in transgenic mice with sickle cell disease. Anemia. 2012;2012:6. doi: 10.1155/2012/582018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pieroni L, Khalil L, Charlotte F, Poynard T, Piton A, Hainque B, Imbert-Bismut F. Comparison of bathophenanthroline sulfonate and ferene as chromogens in colorimetric measurement of low hepatic iron concentration. Clin Chem. 2001;47:2059–2061. [PubMed] [Google Scholar]
- 16.Iron Panel of the International Committee for Standardization in H. Revised recommendations for the measurements of the serum iron in human blood. Br J Haematol. 1990;75:615–616. doi: 10.1111/j.1365-2141.1990.tb07808.x. [DOI] [PubMed] [Google Scholar]
- 17.Prasad AS, Kaplan J, Brewer GJ, Dardenne M. Immunological effects of zinc deficiency in sickle cell anemia (SCA) Prog Clin Biol Res. 1989;319:629–647. discussion 648–29. [PubMed] [Google Scholar]
- 18.Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278:873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 19.Kaul DK, Zhang X, Dasgupta T, Fabry ME. Arginine therapy of transgenic-knockout sickle mice improves microvascular function by reducing non-nitric oxide vasodilators, hemolysis, and oxidative stress. AJP Heart Circ Physiol. 2008;295:H39–H47. doi: 10.1152/ajpheart.00162.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dasgupta T, Hebbel RP, Kaul DK. Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radical Biol Med. 2006;41:1771–1780. doi: 10.1016/j.freeradbiomed.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fasipe FR, Ubawike AE, Eva R, Fabry ME. Arginine supplementation improves rotorod performance in sickle transgenic mice. Hematology. 2004;9:301–305. doi: 10.1080/10245330410001714185. [DOI] [PubMed] [Google Scholar]
- 22.Romero JR, Suzuka SM, Nagel RL, Fabry ME. Arginine supplementation of sickle transgenic mice reduces red cell density and Gardos channel activity. Blood. 2002;99:1103–1108. doi: 10.1182/blood.v99.4.1103. [DOI] [PubMed] [Google Scholar]
- 23.Schnog JJ, Jager E, Dijs F, Duits A, Moshage H, Muskiet F, Muskiet F. Evidence for a metabolic shift of arginine metabolism in sickle cell disease. Ann Hematol. 2004;83:371–375. doi: 10.1007/s00277-004-0856-9. [DOI] [PubMed] [Google Scholar]
- 24.Waugh WH. Arginine metabolism, pulmonary hypertension, and sickle cell disease. J Am Med Assoc. 2005;294:2432–2243. doi: 10.1001/jama.294.19.2432-c. [DOI] [PubMed] [Google Scholar]
- 25.Williams R, Olivi S, Li CS, Storm M, Cremer L, Mackert P, Wang W. Oral glutamine supplementation decreases resting energy expenditure in children and adolescents with sickle cell anemia. J Pediatr Hematol/Oncol. 2004;26:619–625. doi: 10.1097/01.mph.0000140651.65591.b8. [DOI] [PubMed] [Google Scholar]
- 26.Hyacinth HI, Gee BE, Hibbert JM. The role of nutrition in sickle cell disease. Nutr Metabol Insights. 2010;3:57–67. doi: 10.4137/NMI.S5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. J Am Med Assoc. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohnishi ST, Ohnishi T, Ogunmola GB. Sickle cell anemia: A potential nutritional approach for a molecular disease. Nutrition. 2000;16:330–338. doi: 10.1016/s0899-9007(00)00257-4. [DOI] [PubMed] [Google Scholar]
- 29.Hibbert JM, Stiles JK, Umeakunne K, Hyacinth HI. United States’ Patent Office, editor. Compositions of nutritional supplement for nutritional deficiencies and method of use therefore. 20110294727. United States’ Patent Office. 2011:1–6.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.