Abstract
Background
CPX-351, a liposomal formulation of cytarabine and daunorubicin co-encapsulated at an optimized synergistic 5:1 molar ratio, has demonstrated improved clinical outcomes over conventional cytarabine/daunorubicin treatment in a randomized phase 2 trial in patients with AML as well as superior efficacy against preclinical leukemia models when compared to the free drugs in combination.
Procedures
Given the promising phase 2 data, limited toxicities observed, and the known clinical activities of cytarabine/daunorubicin, we assessed the efficacy of CPX-351 against a panel of childhood ALL xenograft models. Plasma pharmacokinetics of cytarabine and daunorubicin following CPX-351 treatment were determined by HPLC in order to correlate efficacy with drug exposure.
Results
CPX-351, at a dose of 5 units/kg (corresponding to 5 mg/kg cytarabine and 2.2 mg/kg daunorubicin), was highly efficacious against all xenografts tested, inducing complete responses in four B-lineage xenografts and partial response in one T-lineage xenograft. These therapeutic responses were achieved with CPX-351 doses that provided drug exposures (based on Cmax and AUC) comparable to those observed in patients with AML.
Conclusions
These results suggest that CPX-351 may be a promising chemotherapeutic to be utilized in the treatment of ALL and support its testing in pediatric patients with leukemia.
Keywords: acute leukemia, efficacy, pharmacokinetics, CPX-351, synergy
INTRODUCTION
Acute lymphoblastic leukemia (ALL) accounts for 80% of pediatric leukemias and for approximately 20% of adult leukemias [1]. It occurs at a frequency of ~1.5/100,000 people, with an early peak in children between 2 and 3 years of age (9–10/100,000 people) and a later peak in adults older than 50 years (2/100,000 people) [1–3].
Despite steady progress in the development of therapies targeting the disease in children [4], ALL in older adults remains among the most difficult-to-treat malignancies [5]. Although many children with ALL have a favorable outcome, those who experience relapse within 36 months of diagnosis (i.e., early relapse) also have a poor prognosis [6]. Therapies effective in pediatric ALL consist generally of a remission-induction phase, an intensified consolidation phase, a prolonged maintenance phase to eliminate residual disease, as well as a central nervous system-directed prophylactic component that accompanies induction and consolidation [1,2], and such regimens typically form the basis for treatment of adult patients with ALL.
Patients with ALL who relapse may be treated with chemotherapeutic agents similar to those used during the first round of remission-induction treatment. This approach is effective at inducing second remission in most children, with remission rates dependent upon the duration of first remission [7–9]. However, the likelihood of achieving a second CR is lower in adults, with rates being lowest for older adults [10–12]. The use of high dose cytarabine (CYT) in combination with anthracyclines (or their analogs) has shown similar or better activity against ALL compared to other reinduction regimens, particularly in patients relapsing on therapy or patients who did not respond to vincristine/prednisone induction [13–17]. In spite of the well-documented activity of these agents against lymphomas and leukemias [18,19], combination chemotherapy consisting of CYT and anthracyclines such as daunorubicin (DNR) has not been as extensively utilized for the treatment of ALL as it has been for acute myeloid leukemia (AML) [19].
Conventional CYT plus anthracycline combination chemotherapy (e.g., ‘7+3’) continues to be the “standard of care” remission-induction treatment for newly diagnosed patients with AML [20,21]. Patients undergo 3 days of treatment with an anthracycline (such as DNR) and 7 days of continuous intravenous (i.v.) infusion of CYT. In an effort to reduce cardiotoxicity and improve efficacy, anthracyclines have been formulated in liposomes [22,23]. A combination of CYT with liposome-encapsulated DNR was also highly efficacious as re-induction therapy for patients with ALL suffering from relapse [14].
Combination drugs administered in vivo often differ in their pharmacokinetic parameters, causing fluctuations of the drug ratios that may range from being ideally synergistic, to clearly antagonistic [24]. In vitro cytotoxicity testing of the CYT and DNR combination revealed that a molar ratio of 5:1 CYT:DNR maximized their synergy, and delivery of this ratio in vivo utilizing liposomes (in a formulation referred to as CPX-351) has resulted in dramatic efficacy improvements in preclinical leukemia models [24,25] as well as promising evidence of enhanced clinical anti-leukemic activity in patients with AML [26,27]. CPX-351 has also shown potent anti-tumor activity against a wide range of leukemia cell types freshly isolated from leukemic patients’ biopsies, including a number of ALL samples [28].
Given the wide therapeutic window, favorable toxicity profile, and improved outcome associated with CPX-351 treatment in the clinic, this drug formulation may be suitable for use in induction or re-induction therapy not only against AML but against other leukemic indications as well. In light of the documented successes involving CYT and anthracyclines in the treatment of ALL [14], we investigated the efficacy of CPX-351 against a panel of pediatric preclinical ALL models. We report here results on efficacy and pharmacokinetic parameters in preclinical models that point to the potential of this drug formulation as an alternative chemotherapeutic tool in ALL and that support conduct of an ongoing phase 1 trial in children with relapsed ALL and AML.
METHODS
CPX-351 Drug and Treatments
CPX-351, supplied by Celator Pharmaceuticals (Princeton, NJ, USA), is a liposome encapsulation of CYT and DNR at a chosen molar ratio of 5:1. One unit of CPX-351 is defined as the amount of liposomes containing 1 mg of CYT and 0.44 mg of DNR.
In preclinical studies, mice received CPX-351 by i.v. injection into the lateral tail vein, either as a single bolus dose (for pharmacokinetic studies) or administered three times on a Q2Dx3 schedule (for efficacy studies).
All animal experimentation was carried out according to the Canadian Council on Animal Care (CCAC) or the UNSW Animal Care and Ethics Committee guidelines, using protocols and conditions adhering to the corresponding institutional policies. Mice were housed in micro-isolator cages, received sterile food and water ad libitum, and were monitored daily for signs of stress and morbidity, including changes in hydration level, coat appearance, movement, and behavior.
Maximum Tolerated Dose Assessment
We assessed the maximum tolerated dose (MTD) of CPX-351 in female adult tumor-free NOD/SCID mice. The MTD was defined as the highest dose that did not cause any deaths or induced ≥15% mean body weight loss for more than two consecutive days. In two independent studies, CPX-351 was administered i.v. into the lateral tail vein at doses of up to 10 units/kg CPX-351, on a Q2Dx3 schedule. Individual body weights were recorded three times weekly; animals showing cumulative signs meeting the criteria of a moribund state and/or humane endpoint were euthanized.
Pharmacokinetics
For preclinical pharmacokinetic studies, female CD-1 mice (Charles River, Wilmington, MA, USA) aged 8–9 weeks were used. Animals experiencing weight loss equal to or greater than 15%, or showing cumulative signs meeting the criteria of a moribund state, were euthanized. Mice were administered a single bolus i.v. dose of 5 units/kg CPX-351. Blood plasma samples were collected over a 48-hour period and analyzed by HPLC for CYT and DNR content using validated methodologies as previously described [25]. Total plasma CYT and DNR levels (sum of encapsulated and non-encapsulated drug) were measured, and elimination kinetics were analyzed using “PK Solutions” pharmacokinetic software from (SummitPK, Montrose, CO, USA) to determine pharmacokinetic parameters including maximum plasma concentration (Cmax), area under the concentration-time curve between 0 and 48 hours (AUC0–48), and terminal elimination half-life (t1/2, Z). Comparisons of preclinical and clinical CPX-351 pharmacokinetics were based on data reported from patients treated with CPX-351 at a dose of 101 units/m2 [26].
Xenograft Models and In Vivo Efficacy Assessment
Murine xenograft models of childhood ALL, generated at the Children’s Cancer Institute Australia for Medical Research, are an integral part of the in vivo preclinical xenograft panel of the Pediatric Preclinical Testing Program [29] and have been previously described [30,31].
Briefly, female adult non-obese diabetic/scid−/− (NOD.CB17-Prkdcscid/J, NOD/SCID) mice were inoculated with human leukemia cells (originally derived from patient biopsy samples, whose clinical features are listed in Table I) propagated as xenografts in mice. A total of up to 18 mice were inoculated with viable cells via lateral tail vein injection, and animals were randomly assigned to one of two treatment arms for each xenograft. Engraftment was assessed by monitoring the proportion of human leukemia cells (hCD45+) in the total (human plus murine) leukocyte population in circulating peripheral blood (PB), by multi-parameter flow cytometry using species-specific APC-anti-human and FITC-anti-mouse CD45 antibodies, as described previously [31].
TABLE I.
Clinical data of ALL patients from whom xenograft lines were derived (from [30])
| Xenograft | Age at Diagnosis (mo) / Gender |
ALL Lineage1 | Disease Status at Biopsy |
Length of CR12 (mo) |
Current Clinical Status3 |
|---|---|---|---|---|---|
| ALL-2 | 65 / F | B | Relapse 3 | 30 | DOD |
| ALL-4 | 105 / M | Ph+, B | Diagnosis | 10 | DOD |
| ALL-7 | 88 / M | Biphen | Diagnosis | 7 | DOD |
| ALL-8 | 152 / M | T | Relapse 1 | 17 | DOD |
| ALL-19 | 194 / M | B | Relapse 1 | 3 | DOD |
Ph+, Philadelphia chromosome-positive ALL; Biphen, biphenotypic ALL;
CR1 indicates first complete remission;
DOD, dead of disease.
Treatment for each xenograft was initiated when the median %hCD45+ cells in PB was greater than 1% for the complete cohort as determined by flow cytometry. Mice in the treatment group received 5 units/kg CPX-351 (on a Q2Dx3 schedule) while controls received vehicle only by i.v. injection on the same days. The experimental endpoint for each mouse was defined a priori to be when %hCD45+ cells in PB reached 25% (an event), or 42 days post-treatment initiation. Mice were euthanized if indications of morbidity were evident or if weight loss was 20% or more.
Time-to-event was assessed as a measure of efficacy. Individual and median event-free survival (EFS) of treated and control animals were calculated from the initiation of treatment, and the corresponding leukemia growth delay (LGD) calculated as the difference in median EFS between CPX-351-treated and control arms for each xenograft. The ratio of the median EFS for the treated arm over that of the control arm was calculated (EFS T/C). All mice evaluable on day 42 were assigned objective response measure (ORM) scores based on the criteria outlined in Supplemental Table I, modeled after the clinical setting, and a median ORM score for each xenograft was obtained. An in-depth description of the analysis methods is included in the Supplemental Response Definitions section.
Statistical Analysis
EFS distributions between treatment and control groups were compared and significance of the differences determined by the log-rank test with a p value of 0.05 as the upper limit.
RESULTS
Maximum Tolerated Dose
The MTD of CPX-351 in NOD/SCID mice was evaluated using a Q2Dx3 (every other day for 3 doses) schedule of administration to mimic the schedule utilized in the clinic [26]. Mice receiving the highest dose of 10 units/kg CPX-351 experienced ~30% mean body weight loss, and all mice in this group reached humane endpoint (100% morbidity), while 33% of mice dosed at 7.5 units/kg reached endpoint. Based on results from two independent studies, the MTD of CPX-351 administered on a Q2Dx3 schedule in non-tumor-bearing NOD/SCID mice was estimated to be 5 units/kg, in contrast to immune-competent mice which can tolerate CPX-351 at doses of up to 12 units/kg on a Q2Dx3 schedule [25,32]. This can be attributable to the increased sensitivity of these mice to anthracyclines [33].
Pharmacokinetics
The pharmacokinetics of CPX-351 were evaluated at 5 units/kg in order to determine whether or not the dose used in our preclinical efficacy studies produced plasma drug exposure levels that were similar to those observed in patients at the efficacious recommended dose. Peak plasma drug concentrations (Cmax) initially observed following bolus i.v. injection were 52 and 24 µg/mL for CYT and DNR, respectively. CPX-351 exhibits essentially no distribution phase, where early time points reflect nearly all of the injected dose remaining in the plasma compartment [25] with a single, mono-exponential drug elimination curve having an elimination half-life (t1/2, Z) of 6.2 and 5.9 hours for CYT and DNR, respectively (Table II). For comparison, Cmax values of 43 and 25 µg/mL were obtained for CYT and DNR, respectively, in blood plasma samples from patients with AML following CPX-351 treatment at the recommended dose [26]. In addition, AUC values were determined for both drugs and compared to reported values in patients with AML. As shown in Table II, AUC values for both CYT and DNR in mice were approximately 3.5-fold lower than those previously reported in humans, correlating with shorter elimination half-lives for both drugs in mice compared to humans.
TABLE II.
Blood plasma pharmacokinetic parameters for cytarabine and daunorubicin following CPX-351 administration in mice (preclinical) versus humans (clinical)
| Cytarabine | Daunorubicin | |||
|---|---|---|---|---|
| Preclinical1 | Clinical2 | Preclinical | Clinical | |
| Cmax (µg/mL)3 | 52 ± 164 | 43 ± 8 | 24 ± 5 | 25 ± 5 |
| AUC0–48 (µg*hr/mL)5 | 322 | 1158 ± 385 | 160 | 553 ± 152 |
| t1/2, Z (hr)6 | 6.2 | 42.5 ± 26.4 | 5.9 | 22.1 ± 6.9 |
For preclinical pharmacokinetic analyses, female CD-1 mice were administered a single dose of CPX-351 at 5 units/kg;
For the clinical dataset, blood plasma was obtained from Phase I study patients with advanced hematologic malignancies following administration of single-dose CPX-351 at 101 units/m2 by i.v. infusion over 90 minutes;
Cmax, initial observed peak plasma concentration (preclinical) or observed maximum plasma concentration (clinical). Preclinical Cmax based on observed data at 1hr;
Values are reported as mean ± SD;
AUC0–48 based on trapezoidal summation;
t1/2, Z calculated as ln2/λ where λ is the terminal elimination rate constant estimated from negative slope of log C×T curve between t=6 and t=16 hr time points.
Efficacy In Vivo
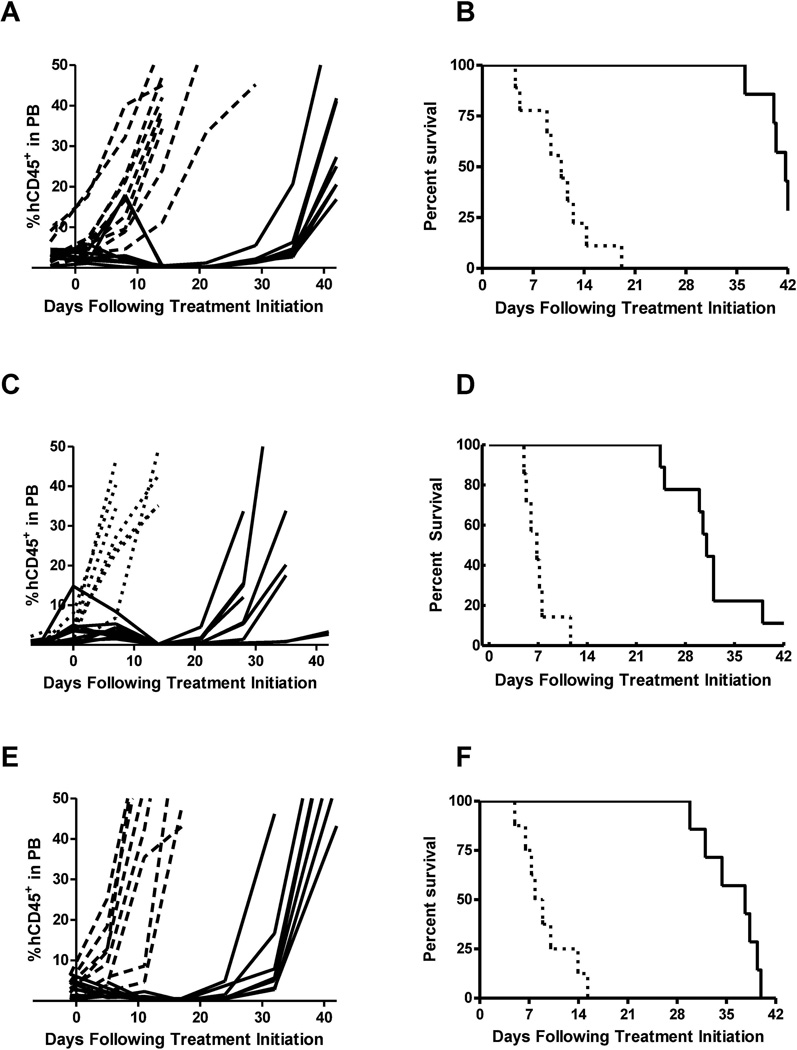
CPX-351 treatment of mice bearing B-lineage subtype ALL xenografts (ALL-2, ALL-4, and ALL-19) significantly delayed tumor progression (Fig. 1; Table III). Within this group, the longest LGD was achieved for the ALL-2 xenograft, for which the median EFS was delayed by 31.1 days (p=0.0001) in the treated group as compared to controls, resulting in a median ORM score of CR. In the ALL-4 xenograft model, treatment of mice led to 3 out of 7 evaluable animals scoring PRs and 4 animals achieving CRs, resulting in a median ORM score of CR. A statistically significant LGD of 28.2 days (p=0.024) was observed. In the case of the ALL-19 xenograft, CPX-351 treatment extended the median EFS from 8.4 days to 35.4 days, corresponding to a statistically significant LGD of 27.0 days (p=0.0001) and resulted in a median ORM score of CR.
Fig. 1.
Leukemia progression plots (A, C, E) showing %hCD45+ cells in PB of individual mice engrafted with B-lineage subtype ALL (ALL-2, ALL-4, ALL-19) over time. Kaplan-Meier plots (B, D, F) of EFS over time for B-lineage subtype ALL-engrafted mice. Dotted lines indicate vehicle-treated control animals while solid lines indicate CPX-351-treated mice.
TABLE III.
Median time to event, LGD, median group responses, and activity based on event-free survival (EFS) or objective response measure of control versus CPX-351-treated mice bearing ALL xenografts
| Line Description | Tumor Type | Median Time to Event (days) |
LGD (days) |
P-value | EFS T/C | Median hCD45+ at End of Study |
Median Group Response |
EFS Activity |
Response Activity |
|
|---|---|---|---|---|---|---|---|---|---|---|
| control | treated | |||||||||
| ALL-2 | ALL B-precursor | 10.8 | 41.9 | 31.1 | <0.001 | 3.9 | >25 | CR | Int | High |
| ALL-4 | ALL B-precursor | 5.6 | 33.8 | 28.2 | 0.024 | 6.0 | >25 | CR | Int | High |
| ALL-7 | ALL B-precursor | 2.4 | 34.3 | 31.9 | <0.001 | 14.1 | >25 | CR | Int | High |
| ALL-8 | ALL T-cell | 10.8 | 32.8 | 22.0 | <0.001 | 3.0 | >25 | PR | Int | High |
| ALL-19 | ALL B-precursor | 8.4 | 35.4 | 27.0 | <0.001 | 4.2 | >25 | CR | Int | High |
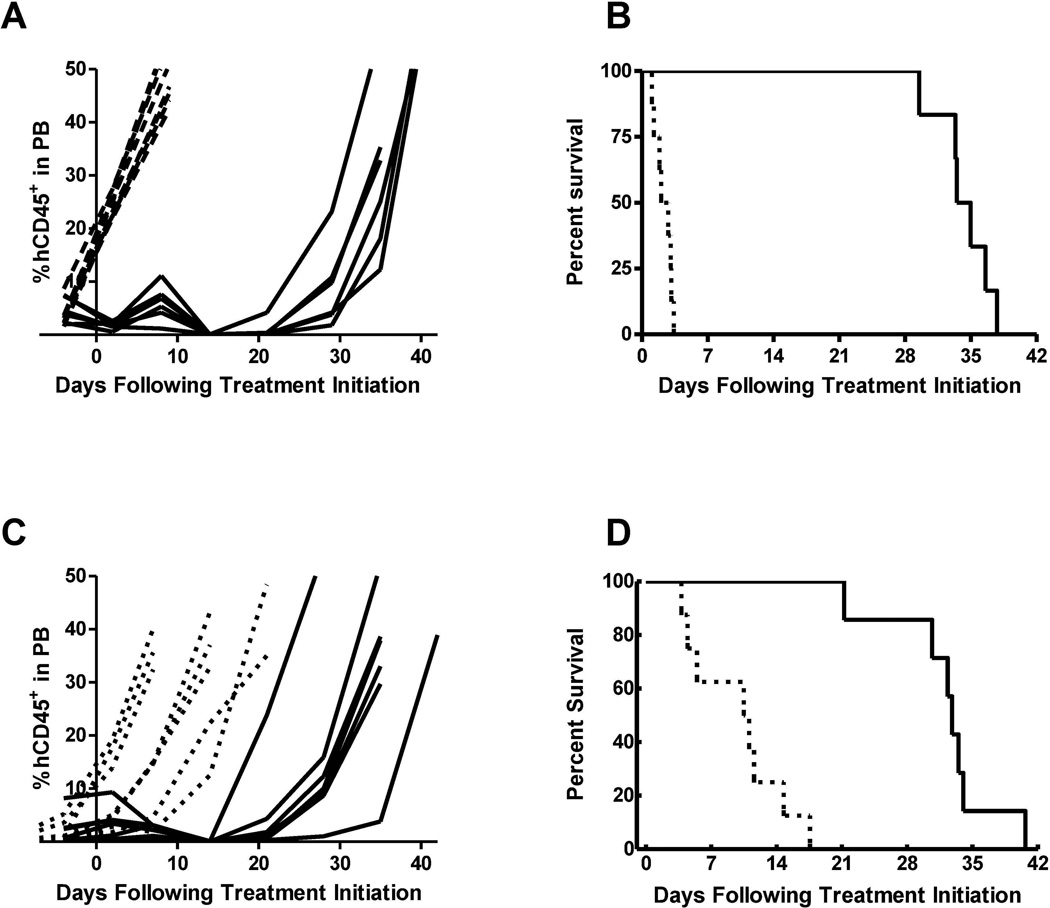
Similarly, leukemia progression was delayed by CPX-351 treatment in mice bearing the ALL-7 xenograft (Fig. 2 and Table III). All mice in the CPX-351-treated group reached an event before the end of the 42-day study period. However, the median EFS was extended by CPX-351 treatment from 2.4 days in control untreated animals to 34.3 days in treated animals, corresponding to a statistically significant LGD of 31.9 days (p=0.0001) and resulting in a median ORM score of CR.
Fig. 2.
(A) Leukemia progression plots showing %huCD45+ cells in PB of individual mice engrafted with ALL-7 over time. (B) Kaplan-Meier plot of EFS over time for ALL-7-engrafted mice. (C) Leukemia progression plots showing %hCD45+ cells in PB of individual mice engrafted with T-lineage subtype ALL-8 over time. (D) Kaplan-Meier plot of EFS over time for T-lineage subtype ALL-8-engrafted mice. Dotted lines indicate vehicle-treated control animals while solid lines indicate CPX-351-treated mice.
In mice bearing the T-lineage subtype ALL xenograft (ALL-8), CPX-351 was also efficacious and demonstrated anti-leukemic activity (Fig. 2; Table III). ALL-8-bearing mice treated with CPX-351 displayed a median EFS of 32.8 days compared to 10.8 days for the control group, corresponding to an LGD of 22.0 days which was the shortest among the 5 ALL models tested, although the difference in EFS distribution was still statistically significant (p=0.0001). CPX-351 treatment resulted in 4 out of 7 evaluable mice being scored as PR and 3 mice achieving CRs, leading to a median ORM score of PR in this xenograft model.
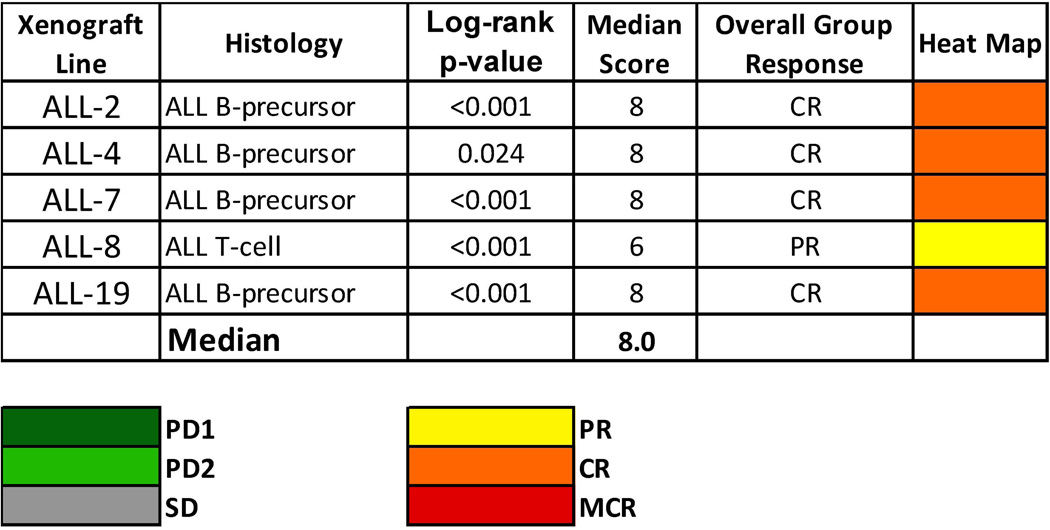
Figure 3 shows the ORM for the five xenografts tested both as a heat map and in a “COMPARE”-like graph. A complete summary of results is provided in Supplemental Table II, including total numbers of mice, numbers of mice that died (or were otherwise excluded), numbers of mice with events and average times to event, leukemia growth delay, as well as numbers of responses and T/C values.
Fig. 3.
CPX-351 in vivo objective response activity: colored heat map depicts group response scores. A high level of activity is indicated by a score of ≥6, intermediate activity by a score of ≥2 but <6, and low activity by a score of <2.
DISCUSSION
The ALL models utilized in the studies presented here were developed and characterized at the Children’s Cancer Institute Australia for Medical Research to provide relevant preclinical experimental systems for testing novel therapies against ALL [30,31]. They have been included as the ALL component of the xenograft testing panel of the Pediatric Preclinical Testing Program (PPTP) [29]. The cells engrafted into NOD/SCID mice possess similar phenotypic and genotypic characteristics as the original patient sample, and xenograft responses to drug treatment have been found to correlate with patient clinical outcome. The xenografts selected for this study were all generated from leukemic cells of patients that succumbed to the disease and in all cases were considered relatively chemoresistant based on previous testing results [31]. Using these pediatric ALL models, we evaluated the anti-leukemic activity of CPX-351 in vivo, strictly following the methodology applied by the PPTP for single agent testing (see response definitions in Supplementary Material).
As individual agents, anthracyclines and CYT are widely used in different phases of ALL treatment. Investigation of the therapeutic efficacy of CPX-351 against non-AML indications such as pediatric ALL was warranted based on several observations. First, CPX-351 provided promising evidence of anti-leukemic activity in patients with relapsed ALL as part of a phase 1 clinical trial [26]. Second, CPX-351 has demonstrated markedly superior efficacy of CPX-351 over conventional (free) CYT and DNR combination in preclinical studies [25]. Third, in a randomized, controlled phase 2 study in newly diagnosed patients with AML (ClinicalTrials.gov Identifier: NCT00788892), CPX-351 provided increased CR and survival rates compared to conventional 7+3 treatment despite administering roughly 60% less cytarabine and 30% less daunorubicin per induction course [27].
The results to date from PPTP testing in the NOD/SCID models of childhood ALL suggest that, because these xenografts retain the fundamental biologic characteristics of the human disease, they can be highly valuable in guiding therapeutic efforts in human patients. However, in order for the xenograft preclinical models to provide clinically relevant information about a given regimen or therapy, it is essential to dose test animals with an amount of drug that produces systemic exposures comparable to those achieved in humans under clinical conditions. Analysis and comparison of systemic exposures between species can often be complicated, however, due to differences in the route, duration, and frequency with which the test compound is administered in preclinical models versus patients in the clinic. Data from clinical trials of CPX-351 indicate that an i.v. infusion dose of 101 units/m2 is safe and therapeutically effective in patients with leukemia, leading to maximal plasma concentrations of 43 µg/mL CYT and 25 µg/mL DNR. It is important to note that the PK parameters for CPX-351 have been shown to be dose-independent clinically as well as in preclinical models, and these parameters were comparable on day 1 as well as day 5 of a day 1,3,5 dosing schedule [34].
Comparable levels of drug exposure (with observed Cmax values of 52 and 24 µg/mL CYT and DNR, respectively) were achieved in mice dosed i.v. with CPX-351 at 5 units/kg, a dose that exhibited significant anti-leukemic activity in all of the ALL xenograft models tested. These data therefore suggest that CPX-351 may also be used safely and effectively to treat patients with ALL in the clinic. In this context, it should be noted that in the phase 1 study of CPX-351, three adult patients with relapsed ALL were entered during the dose escalation phase and of these three patients, one achieved a CR with a single induction treatment [26].
In all five models tested, treatment with CPX-351 proved to be highly efficacious, yielding prolonged objective responses (ORs) (Fig. 3). CPX-351 treatment extended the median EFS from 2–11 days in untreated control animals to 33–42 days in CPX-351-treated animals, and the EFS distributions of treatment and control groups in each xenograft were found to be significantly different by log-rank test. In fact, all four B-lineage models tested (ALL-2, ALL-4, ALL-7, and ALL-19) achieved median ORM scores of CR, while the one T-lineage model (ALL-8) obtained an overall PR following CPX-351 treatment.
One of the B-cell subtype xenograft models, ALL-4, was established from Philadelphia chromosome-positive (Ph+) lymphoblastic leukemia cells. As this genetic alteration would generally predict a poor response to conventional ALL-targeted chemotherapy [1,2], the positive efficacy results obtained for this xenograft are especially encouraging.
It is likely that the differences in response of T- and B-lineage ALL xenografts to CPX-351 treatment observed in this study may be attributable to inter-patient variation rather than lineage-specific properties. However, our results are consistent with observations in the literature which suggest that patients with T-lineage ALL tend to have a poorer clinical outcome with increased risk of relapse following conventional chemotherapy, compared to patients with B-lineage ALL [35,36]. It is nevertheless encouraging that, while the ALL-8 xenograft has been previously reported to be resistant to agents such as methotrexate [31], cisplatin [37], and vorinostat [38], we observed here that CPX-351 treatment resulted in significant anti-leukemic activity with objective responses in 7 out of 7 treated mice (4 mice scored as PR and 3 scored as CR).
In conclusion, in these studies CPX-351 has demonstrated potent anti-leukemic activity in vivo against five childhood ALL xenograft models tested at a dose that provides clinically relevant plasma drug exposure. There was evidence of induction of significant delay in leukemia progression in all cases with ORs obtained for all of them. CPX-351 was highly effective against both T- and B-lineage ALL xenografts (including a Ph+ model) and our results provided support for an ongoing phase 1 trial of CPX-351 in pediatric patients with relapsed ALL and AML.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NO1-CM-42216,CA21765 and CA108786 from the National Cancer Institute. The authors wish to thank C. Boland and M. Dolotin for technical assistance. Children’s Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and Sydney Children’s Hospitals Network.
Footnotes
POTENTIAL CONFLICTS OF INTERESTS
MMYF, TOH and LDM are paid employees of Celator Pharmaceuticals Corp.
REFERENCES
- 1.Fullmer A, O'Brien S, Kantarjian H, et al. Emerging therapy for the treatment of acute lymphoblastic leukemia. Expert Opin Emerg Drugs. 2010;15(1):1–11. doi: 10.1517/14728210903456026. [DOI] [PubMed] [Google Scholar]
- 2.Faderl S, O'Brien S, Pui C-H, et al. Adult acute lymphoblastic leukemia. Cancer. 2010;116:1165–1176. doi: 10.1002/cncr.24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al., editors. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975–2010. http://seercancergov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. [Google Scholar]
- 4.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sive JI, Buck G, Fielding A, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol. 2012;157(4):463–471. doi: 10.1111/j.1365-2141.2012.09095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freyer DR, Devidas M, La M, et al. Postrelapse survival in childhood acute lymphoblastic leukemia is independent of initial treatment intensity: a report from the Children's Oncology Group. Blood. 2011;117(11):3010–3015. doi: 10.1182/blood-2010-07-294678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abshire TC, Pollock BH, Billett AL, et al. Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: a Pediatric Oncology Group Study. Blood. 2000;96(5):1709–1715. [PubMed] [Google Scholar]
- 8.Kelly ME, Lu X, Devidas M, et al. Treatment of relapsed precursor-B acute lymphoblastic leukemia with intensive chemotherapy: POG (Pediatric Oncology Group) study 9411 (SIMAL 9) J Pediatr Hematol Oncol. 2013;35(7):509–513. doi: 10.1097/MPH.0b013e31829f3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children's Oncology Group Study[corrected] J Clin Oncol. 2008;26(24):3971–3978. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oriol A, Vives S, Hernandez-Rivas JM, et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95(4):589–596. doi: 10.3324/haematol.2009.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21(9):1907–1914. doi: 10.1038/sj.leu.2404824. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Manero G, Thomas DA. Salvage therapy for refractory or relapsed acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2001;15(1):163–205. doi: 10.1016/s0889-8588(05)70204-5. [DOI] [PubMed] [Google Scholar]
- 13.Feldman E, Alberts D, Arlin Z, et al. Phase I clinical and pharmacokinetic evaluation of high-dose mitoxantrone in combination with cytarabine in patients with acute leukemia. J Clin Oncol. 1993;11(10):2002–2009. doi: 10.1200/JCO.1993.11.10.2002. [DOI] [PubMed] [Google Scholar]
- 14.Candoni A, Michelutti A, Simeone E, et al. Efficacy of liposomal daunorubicin and cytarabine as reinduction chemotherapy in relapsed acute lymphoblastic leukaemia despite expression of multidrug resistance-related proteins. Eur J Haematol. 2006;77:293–299. doi: 10.1111/j.1600-0609.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 15.Testi AM, Del Giudice I, Arcese W, et al. A single high dose of idarubicin combined with high-dose ARA-C for treatment of first relapse in childhood ‘high-risk’ acute lymphoblastic leukaemia: a study of the AIEOP group. Br J Haematol. 2002;118(3):741–747. doi: 10.1046/j.1365-2141.2002.03706.x. [DOI] [PubMed] [Google Scholar]
- 16.Tedeschi A, Montillo M, Strocchi E, et al. High-dose idarubicin in combination with Ara-C in patients with relapsed or refractory acute lymphoblastic leukemia: a pharmacokinetic and clinical study. Cancer Chemother Pharmacol. 2007;59(6):771–779. doi: 10.1007/s00280-006-0332-4. [DOI] [PubMed] [Google Scholar]
- 17.Weiss M. Induction therapy of adult acute lymphocytic leukemia without the use of vincristine or prednisone. Hematol Oncol Clin North Am. 2001;15(1):1–7. doi: 10.1016/s0889-8588(05)70196-9. [DOI] [PubMed] [Google Scholar]
- 18.Hallböök H, Simonsson B, Ahlgren T, et al. High-dose cytarabine in upfront therapy for adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2002;118(3):748–754. doi: 10.1046/j.1365-2141.2002.03685.x. [DOI] [PubMed] [Google Scholar]
- 19.Rowe JM. Optimal management of adults with ALL. Br J Haematol. 2009;144(4):468–483. doi: 10.1111/j.1365-2141.2008.07513.x. [DOI] [PubMed] [Google Scholar]
- 20.Robak T, Wierzbowska A. Current and emerging therapies for acute myeloid leukemia. Clin Ther. 2009;31(Part 2):2349–2370. doi: 10.1016/j.clinthera.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 22.Tardi P, Boman N, Cullis P. Liposomal Doxorubicin. J Drug Target. 1996;4(3):129–140. doi: 10.3109/10611869609015970. [DOI] [PubMed] [Google Scholar]
- 23.Fassas A, Anagnostopoulos A. The use of liposomal daunorubicin (DaunoXome) in acute myeloid leukemia. Leuk Lymphoma. 2005;46(6):795–802. doi: 10.1080/10428190500052438. [DOI] [PubMed] [Google Scholar]
- 24.Mayer LD, Harasym TO, Tardi PG, et al. Ratiometric dosing of anticancer drug combinations: Controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5(7):1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 25.Tardi P, Johnstone S, Harasym N, et al. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33(1):129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Feldman EJ, Lancet JE, Kolitz JE, et al. First-In-Man Study of CPX-351: A Liposomal Carrier Containing Cytarabine and Daunorubicin in a Fixed 5:1 Molar Ratio for the Treatment of Relapsed and Refractory Acute Myeloid Leukemia. J Clin Oncol. 2011;29(8):979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lancet JE, Cortes JE, Hogge DE, et al. Phase 2B Randomized Study of CPX-351, Vs, Cytarabine (CYT) + Daunorubicin (DNR) (7+3 Regimen) In Newly Diagnosed AML Patients Aged 60–75. Blood (ASH Annual Meeting Abstracts) 2010;116(21):655. [Google Scholar]
- 28.Tyner JW, Tardi P, Mayer L, et al. Evaluation of CPX-351 (cytarabine:daunorubicin) Liposome Injection Anti-Leukemic Activity Against Primary Patient Leukemia Cells. Blood (ASH Annual Meeting Abstracts) 2010;116(21):2886. [Google Scholar]
- 29.Houghton PJ, Morton CL, Tucker C, et al. The Pediatric Preclinical Testing Program: description of models and early testing results. Pediatr Blood Cancer. 2007;49(7):928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 30.Lock RB, Liem N, Farnsworth ML, et al. The nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model of childhood acute lymphoblastic leukemia reveals intrinsic differences in biologic characteristics at diagnosis and relapse. Blood. 2002;99(11):4100–4108. doi: 10.1182/blood.v99.11.4100. [DOI] [PubMed] [Google Scholar]
- 31.Liem NL, Papa RA, Milross CG, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103(10):3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 32.Lim WS, Tardi PG, Dos Santos N, et al. Leukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine:daunorubicin formulation, in bone marrow xenografts. Leuk Res. 2010;34(9):1214–1223. doi: 10.1016/j.leukres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Lee VW, Wang Y, Qin X, et al. Adriamycin nephropathy in severe combined immunodeficient (SCID) mice. Nephrol Dial Transplant. 2006;21(11):3293–3298. doi: 10.1093/ndt/gfl413. [DOI] [PubMed] [Google Scholar]
- 34.Feldman EJ, Kolitz JE, Trang JM, et al. Pharmacokinetics of CPX-351, a nano-scale liposomal fixed molar ratio formulation of cytarabine:daunorubicin, in patients with advanced leukemia. Leuk Res. 2012;36(10):1283–1289. doi: 10.1016/j.leukres.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Pui C-H, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371(9617):1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 36.Uckun FM, Sensel MG, Sun L, et al. Biology and treatment of childhood T-lineage acute lymphoblastic leukemia. Blood. 1998;91(3):735–746. [PubMed] [Google Scholar]
- 37.Tajbakhsh M, Houghton PJ, Morton CL, et al. Initial testing of cisplatin by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2008;50(5):992–1000. doi: 10.1002/pbc.21263. [DOI] [PubMed] [Google Scholar]
- 38.Keshelava N, Houghton PJ, Morton CL, et al. Initial testing (stage 1) of vorinostat (SAHA) by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2009;53(3):505–508. doi: 10.1002/pbc.21988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.