Abstract
Background
Acute lung injury (ALI) is characterized by neutrophilic inflammation and increased lung permeability. Thiosulfate is a stable metabolite of hydrogen sulfide, a gaseous mediator that exerts anti-inflammatory effects. While sodium thiosulfate (STS) has been used as an antidote, the effect of STS in ALI is unknown. We assessed the effects of STS in mice lung and vascular endothelial cells subjected to acute inflammation.
Methods
Lung injury was assessed in mice challenged with intratracheal lipopolysaccharide or subjected to cecal ligation and puncture with or without STS. Effects of STS on endothelial permeability, and the production of inflammatory cytokines and reactive oxygen species were examined in cultured endothelial cells incubated with lipopolysaccharide or tumor necrosis factor alpha (TNFα). Levels of sulfide and sulfane sulfur were measured using novel fluorescence probes.
Results
STS inhibited lipopolysaccharide-induced production of cytokines (Interleukin-6 (pg/ml); 313±164, lipopolysaccharide; 79±27, lipopolysaccharide + STS (n=10)), lung permeability, histological lung injury, and nuclear factor-κB activation in the lung. STS also prevented upregulation of Interleukin-6 in the mouse lung subjected to cecal ligation and puncture. In endothelial cells, STS increased intracellular levels of sulfide and sulfane sulfur, inhibited lipopolysaccharide or TNFα-induced production of cytokines and reactive oxygen species. The beneficial effects of STS were associated with attenuation of the lipopolysaccharide-induced nuclear factor-κB activation through the inhibition of TNF receptor-associated factor 6 ubiquitination.
Conclusions
STS exerts robust anti-inflammatory effects in mice lung and vascular endothelium. Our results suggest a therapeutic potential of STS in ALI.
Introduction
Acute lung injury (ALI) is characterized by lung inflammation and increased pulmonary vascular permeability.1 Sepsis is a major cause of ALI,2 and lipopolysaccharide, a cell wall component of gram-negative bacteria, can reproduce the features of human ALI in mice.3 Studies have revealed that vascular endothelium plays a crucial role in mediating inflammatory response in the lung.4,5 Therefore, the pulmonary vascular endothelium represents one of the major targets of therapy.6
Hydrogen sulfide (H2S) is a reactive gaseous mediator. In mammalian tissues, H2S is serially oxidized to persulfide, sulfite (SO32-), thiosulfate (S2O32-7, and sulfate (SO42-). In addition, H2S may be stored as sulfane sulfur-containing polysulfides in cells.8 Although H2S can exert a host of biological effects on various targets,9 it is currently unknown whether the biological effects of H2S are mediated directly by H2S itself or its metabolites.8 In circulation, reaction with plasma proteins and or oxidation maintains free plasma H2S levels very low.10 Free H2S levels only transiently increase and quickly return to its baseline after systemic administration of H2S donor compounds (e.g., Na2S or NaHS).
We recently reported that the protective effects of inhaled H2S in mice subjected to lethal ipopolysaccharide challenge are associated with increased plasma thiosulfate levels.11 Furthermore, intraperitoneal administration of sodium thiosulfate (STS) improved survival after endoxemia11 and acute liver failure.12 These observations suggest that thiosulfate may be one of the “carrier” molecules that mediate anti-inflammatory effects of H2S. Sodium thiosulfate has been used for decades as an antidote against cyanide poisoning.13 STS has been also used for the treatment of calciphylaxis,14 vascular calcifications,15,16 and cisplatin-induced cytotoxicity.17 Therefore, if anti-inflammatory effects of STS are confirmed, it is highly clinically relevant and readily translatable. While H2S has been shown to mitigate lung injury18,19 and vascular endothelial dysfunction in a variety of animal models,20,21 effects of STS against ALI remains to be determined. Furthermore, mechanisms responsible for the anti-inflammatory effects of STS were not investigated in our previous studies.11,12 It is possible that thiosulfate protects vascular endothelium from inflammatory insults.
H2S appears to exert anti-inflammatory effects at least in part via inhibition of nuclear factor-κB (NFκB) dependent signaling.11,22 Upon binding of lipopolysaccharide to the toll-like receptor 4, tumor necrosis factor receptor-associated factor 6 (TRAF6) are recruited to the receptor complex, which facilitates lysine 63 (K63)-linked polyubiquitination of TRAF6.23,24 Polyubiquitinated TRAF6 induces phosphorylation and activation of transforming growth factor-β-activated kinase 1 (TAK1). TAK1 then activates inhibitor of nuclear factor kappa B kinase (IKK), resulting NFκB activation.24 Thus, inhibition of TRAF6 ubiquitination has been suggested as a target to modulate NFκB signaling.25,26
The objective of the current study was to examine the effects of STS in ALI. We hypothesized that STS prevents acute lung injury via inhibition of NFκB signaling in pulmonary vascular endothelium. We observed that STS inhibited acute lung injury and inflammation after intratracheal lipopolysaccharide challenge and cecal ligation and puncture (CLP). These results highlight the therapeutic potential of STS in the treatment of acute lung injury.
Materials and Methods
Animals
Male C57BL6J mice (Jackson Laboratories, Bar Harbor, ME), 8 to 10-weeks old, 19-27g body weight, were used in this study. Protocols for animal use were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care (Boston, MA). All animal experiments were performed in accordance with the guidelines of the National Institutes of Health (Bethesda, MD).
Lipopolysaccharide-induced lung injury
Mice were challenged by 2 mg/kg of lipopolysaccharide (O111: B4; Sigma, St. Louis, MO) in 50 μl saline via intratracheal route as an aerosol using a microsprayer (Penn-Century, Philadelphia, PA) with or without intraperitoneal administration of 2 g/kg STS at 0 and 12 h after intratracheal lipopolysaccharide. Control mice received 50μl intratracheal saline. Mice breathed spontaneously in ambient air and bronchoalveolar lavage fluid (BALF) was collected 24 h after saline or lipopolysaccharide challenge. We chose this time point because we found that inflammatory reaction peaks at 24 h after lipopolysaccharide challenge at this dose in pilot studies. Total number of leukocytes in BALF was counted using a hemocytometer. The BALF samples were subsequently centrifuged and cytospin samples were prepared from the cell pellet. Cytoslides were stained with Kwik-Diff stain kit (Thermo Shandon, Pittsburgh, PA). The number of polymorphonuclear neutrophils (PMNs) was determined by the cell counting of neutrophil fraction. Supernatant samples were used to quantify protein levels using Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL) or stored at −80°C for cytokine and myeloperoxidase activity analysis.
Cecal ligation and puncture
Mice were subjected to CLP as previously described.27 Mice were anesthetized and a midline abdominal incision was made to expose cecum. The cecum was ligated at 1.0 cm from the tip then punctured twice with an 18-gauge needle. A small amount of its contents was extruded before the cecum was returned into the abdominal cavity then the abdominal incision was closed in layers. Sham-operated controls underwent laparotomy without CLP. All mice were given fluid resuscitation with pre-warmed sterile saline (50ml/kg) subcutaneously. STS (0.5g/kg) or saline were intravenously injected via tail vein 10 min after CLP. Lungs were harvested 8 h after CLP, homogenized with 0.5% Triton X-100/phosphate buffered saline and centrifuged then the supernatants were subjected to cytokine measurement after equalization by the protein concentration.
Measurement of BALF cytokines and myeloperoxidase activity
Enzyme-Linked Immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) were used for measurement of mouse tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6) levels and myeloperoxidase activity in BALF according to the manufacturer's instruction.
Lung wet dry ratio
Mouse lungs were harvested 24 h after lipopolysaccharide challenge, blotted dry and weighed immediately to obtain the wet lung weight. Lungs were dried in a 70°C incubator for three days to obtain dry lung weight, then wet dry lung ratio was calculated.
Lung histology
Mouse lungs were inflated under a pressure of 23 cmH2O with 4% Paraformaldehyde (Boston Bio Products, Ashland, MA) for histological evaluation by hematoxylin and eosin staining as previously described.28 Twenty high-power fields (×400 magnification) were taken per mouse, and lung injury was graded using a modified ALI score in each high-power field. Each of three categories, (1) thickness of the alveolar walls, (2) infiltration of inflammatory cells and (3) hemorrhage was graded in a blinded fashion according to the following scale: 0 = minimal damage; 1 = mild damage; 2 = moderate damage; 3 = severe damage; 4 = maximal damage. The degree of lung damage was assessed by the total of scores ranging from 0 to 12.
Quantitative polymerase chain reaction
Mouse lungs were harvested 24 h after lipopolysaccharide or saline challenge, and total RNA was extracted using the RNAspin mini kit (GE Healthcare, Piscataway, NJ). Complementary DNA was synthesized with MMLV-RT (Promega, Madison, WI) and RNA transcript levels were measured using a Mastercycler Realplex system (Eppendorf North America, Westbury, NY). The primer sequences are listed in table1. Gene expression was normalized to 18S ribosomal RNA level. The mean value of control mice was set as 1.
Table 1.
List of primer sequences for quantitative polymerase chain reaction (5′ - 3′)
| TNF α | Forward | CAG CCT CTT CTC ATT CCT GC |
| Reverse | GGT CTG GGC CAT AGA ACT GA | |
| IL-6 | Forward | CCG GAG AGG AGA CTT CAC AGA |
| Reverse | CAG AAT TGC CAT TGC ACA AC | |
| ICAM-1 | Forward | TCC GCT GTG CTT TGA GAA CT |
| Reverse | AGG GTG AGG TCC TTG CCT AC | |
| KC | Forward | CTG GGA TTC ACC TCA AGA ACA TC |
| Reverse | CAG GGT CAA GGC AAG CCT C | |
| MCP-1 | Forward | TGT TCA CAG TTG CCG GCT GGA G |
| Reverse | AGC TTC TTT GGG ACA CCT GCT GC | |
| NOS2 | Forward | GTT CTC AGC CCA ACA ATA CAA GA |
| Reverse | GTG GAC GGG TCG ATG TCA C | |
| MMP9 | Forward | GCC GAC TTT TGT GGT CTT CC |
| Reverse | CGG CCG TAG AGA CTG CTT CT | |
| MMP2 | Forward | CAA GTT CCC CGG CGA TGT C |
| Reverse | TTC TGG TCA AGG TCA CCT GTC | |
| 18S rRNA | Forward | CGG CTA CCA CAT CCA AGG AA |
| Reverse | GCT GGA ATT ACC GCG GCT | |
| IL-1β | TaqMan Mm01336189_m1 (Applied Biosystems, Foster City, CA) | |
ICAM-1 = intercellular adhesion molecule 1; IL-1β = interleukin-1β; IL-6 = interleukin-6; KC = keratinocyte-derived chemokine; MCP-1 = monocyte chemoattractant protein-1; MMP = matrix metalloproteinase; NOS2 = nitric oxide synthase 2; rRNA = ribosomal RNA; TNFα, Tumor necrosis factor alpha
High performance liquid chromatography (HPLC)
To determine the impact of intraperitoneal administration of STS, concentrations of hydrogen sulfide and thiosulfate in lung and plasma were measured by HPLC as previously described.11 At 2 h after lipopolysaccharide challenge, blood was drawn and lung was dissected. Plasma and lung homogenates were added to 70 μl of 10 mM Tris-HCl buffer (pH9.5, 0.1 mM diethylenetriamine pentaacetic acid), followed by addition of 50 μl of 200 mM 5-sulfosalicylic acid after 30 min. The mixture was centrifuged and supernatant was analyzed by HPLC with a fluorescence detector (Waters, Milford, MA).
Cell culture
Human Umbilical Vein Endothelial Cells (HUVEC) and Human Lung Microvascular Endothelial Cells (HMVEC-L) were purchased from Lonza (Walkersville, MD). The cells were cultured in EGM-2 or EGM-2 MV (Lonza) supplemented with 2 or 5% fetal bovine serum and endothelial cell growth factors and used between passages 3 to 5 for all experiments.
Measurement of cytokine production in cell culture supernatants
HUVEC or HMVEC-L were seeded in 96-well plates at 1×104 cells per well and incubated with lipopolysaccharide (10 μg/ml) or recombinant human TNFα (10 ng/ml) (R&D Systems) with or without STS. Cell culture supernatants were collected after incubation for 20 h with lipopolysaccharide or TNFα. Human IL-6 and IL-8 levels were measured by ELISA (R&D Systems) according to the manufacturer's instruction.
Measurement of reactive oxygen species (ROS) production in HUVEC
Chloromethyl-2', 7'-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; Invitrogen, Eugene, OR) was used to measure intracellular production of general reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyradical, and peroxides. HUVEC were seeded at 1×104 cells per well in 96-well plate and cultured to confluent. Cells were loaded with 10μM of CM-H2DCFDA for 30 min. Then cells were washed twice, lipopolysaccharide (10μg/ml) or TNFα (10ng/ml) was added with or without STS for 30 min, followed by fluorescence measurement at excitation and emission wavelengths of 480 nm and 530 nm. The Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen) was also used to measure H2O2 and peroxide production. After treatment with lipopolysaccharide or TNFα, 100μM of Amplex Red reagent solution (Invitrogen) was added to each well. The cells were treated for 30 min, and the fluorescence intensity was measured at excitation and emission wavelengths of 560 nm and 590 nm.
Western Blotting
Protein extracts were prepared by lysing cells or homogenizing lung tissue in RIPA buffer (Boston BioProducts) supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN) and phosphatase inhibitor cocktail (Sigma). The samples were subjected to sodium dodecyl sulfate polyaclylamidegel electrophoresis, transferred to polyvinylidene fluoride membrane (Millipore, Billerica, MA) and immunoblotted. Antibodies used were: TRAF6 (H274), TAK1 (M579) from Santa Cruz (Dallas, TX), K63-linkage specific ubiquitin (HWA4C4) from Millipore, phospho-TAK1 (4531), phospho-IKKα/β (2697), IKKβ (2678), phospho-NFκB p65 (3033), NFκB p65 (8242), inhibitor of NF-κBα (IκBα) (4812), phospho-IκBα (2859), GAPDH (5174), β-tubulin (2146) from Cell Signaling (Danvers, MA). In densitometric analysis, the mean value of control group was set as 1.
Immunoprecipitation
Cell lysates were incubated with TAK1 or TRAF6 antibodies with Protein G Mag Sepharose (GE Healthcare) at 4°C for overnight. The complexes were washed four times with Tris buffered saline with Tween20 and eluted in SDS-sample buffer (Boston Bioproducts) at 90°C for 2 min. For TRAF6 immunoprecipitation, cells were pretreated with 10μM MG132 (Sigma) for 4 h before treatment with lipopolysaccharide and STS. Cells were lysed in RIPA buffer supplemented with protease inhibitor cocktail (Roche) and 10mM N-ethylmaleimide.
Measurement of hydrogen sulfide and sulfane sulfur levels
Two novel fluorescent probes, HSip-1DA29 and SSP4, an improved version of SSP230 were used for the detection of H2S and sulfane sulfur, respectively. SSP4 was prepared using the same method reported previously.30 1H NMR (300 MHz, DMSO-d6) δ 5.49 (s, 2H), 6.97 (d, J = 9.0 Hz, 2H), 7.12 (d, J = 9.0 Hz, 2H), 7.32 (t, J = 6.0 Hz, 2H), 7.44–7.55 (m, 5H), 7.66 (d, J = 9.0 Hz, 2H), 7.76–7.88 (m, 2H), 8.09 (d, J = 9.0 Hz, 1H), 8.19 (d, J = 6.0 Hz, 2H). 13C NMR (75 MHz, CD3Cl) δ 81.9, 110.9, 116.9, 118.2, 124.3, 124.7, 125.2, 125.5, 129.3, 131.4, 132.5, 133.7, 140.1, 151.8, 152.2, 153.2, 164.9, 169.4; MS (ESI+) m/z 627.6 (M+Na+). HUVEC were seeded at 1×104 cells per well in 96-well plate and cultured to confluent. Cells were loaded with 30μM of HSip-1DA, a cell membrane-permeable derivative of HSip-1, or 50μM of SSP4 for 30 min. Then cells were washed twice and treated with or without lipopolysaccharide and STS for 20 h, followed by fluorescence measurement at excitation and emission wavelengths of 490 nm and 515 nm. Intracellular H2S and sulfane sulfur levels were determined by relative fluorescence intensities normalized to levels of untreated control at 1 h after treatment.
Statistical Analysis
All data are presented as means ± SD. We did not conduct an a priori statistical power calculation. We estimated our sample size based on our previous studies in which effects of STS were examined in sepsis.11 No randomization methods were used to assign animals/cells to each treatment. Treatment conditions were alternated between samples in an effort to maintain experimental conditions as constant as possible among groups. Two-tailed hypothesis testing method was used throughout this study. Data were analyzed by one-way or two-way analysis of variance followed by Bonferroni's post-hoc comparisons tests as required using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Thiosulfate and sulfide levels of plasma and lung were analyzed by Mann-Whitney U test because the values were not normally distributed. P values of <0.05 were considered significant.
Results
Sodium thiosulfate attenuates lipopolysaccharide-induced lung injury and enhanced permeability
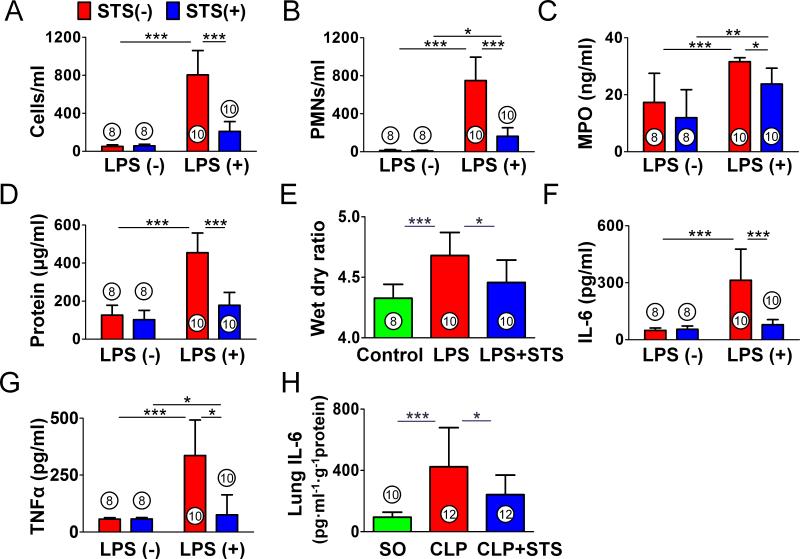
The number of cells in BALF was evaluated to examine the effects of STS on lipopolysaccharide-induced lung inflammation and permeability. The recovery rate of BALF was >90% in all groups. In lipopolysaccharide challenged mice, a marked increase in the number of total cells and PMNs in BALF was observed (figs. 1A and B). However, STS significantly decreased the influx of cells into the alveolar space after lipopolysaccharide challenge. STS treatment alone did not affect the number of cells in BALF. Myeloperoxidase levels, a marker of PMN infiltration was also measured by ELISA. In accordance with the result of PMNs influx into the lung, myeloperoxidase levels were significantly elevated in lipopolysaccharide-challenged mice, while it was attenuated by STS administration (fig. 1C). We also evaluated the effect of STS in lung vascular leak by measuring BALF protein and lung wet dry weight ratio. Lipopolysaccharide challenge induced a significant increase in BALF protein concentrations and wet dry ratio (figs. 1D and E). STS attenuated the pulmonary vascular leakage and lung edema in lipopolysaccharide-challenged mice. To evaluate inflammatory mediators recruiting PMNs to the lung, we measured cytokine levels in BALF by ELISA. STS significantly decreased the lipopolysaccharide-induced IL-6 and TNFα elevation in the BALF (IL-6, TNFα (pg/ml); 49±12, 57±6, control (n=8); 313±164, 336±156, lipopolysaccharide (n=10); 79±27, 76±88, lipopolysaccharide + STS (n=10); figs. 1F and G). Furthermore, intravenous STS attenuated IL-6 induction in the lung 8 h after CLP (pg · ml−1 · g−1 protein; 95±33, control (n=8); 424±256, CLP (n=12); 241±128, CLP + STS (n=12); fig. 1H). These results suggest that STS exerts potent anti-inflammatory effects and prevents the increase in lung permeability after lipopolysaccharide challenge or polymicrobial sepsis.
Figure 1. Effects of sodium thiosulfate on lipopolysaccharide- or polymicrobial sepsis-induced lung injury.
(A) Total number of leukocytes and (B) polymorphonuclear neutrophils (PMNs) in bronchoalveolar lavage fluid (BALF). (C) Myeloperoxidase (MPO) activity and (D) total protein concentration in BALF. (E) Mouse lung wet dry ratio. (F, G). Inflammatory cytokine levels in BALF. (H) Interleukin-6 (IL-6) levels in the mouse lung subjected to cecal ligation and puncture (CLP). ***p<0.001, **p<0.01, *p<0.05; (A-D, F, G) Two-way ANOVA Bonferroni post-test, (E, H) One-way ANOVA Bonferroni post-test, mean ± SD. Numbers in bars represent the sample size. LPS = lipopolysaccharide; SO = sham operation; STS = sodium thiosulfate; TNFα = Tumor Necrosis Factor alpha.
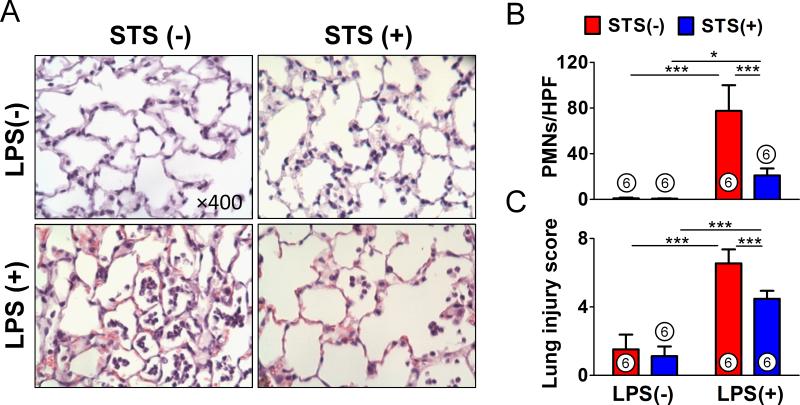
STS prevents lipopolysaccharide-induced lung injury
Histological assessment revealed that lipopolysaccharide stimulated a marked influx of PMNs to the alveolar space. Administration of STS attenuated the PMN infiltration into the lung (figs 2A and B). Semiquantitative analysis of lung sections by lung injury score demonstrated that lipopolysaccharide-induced lung injury was attenuated by STS treatment (fig. 2C).
Figure 2. Histological evaluation of the effects of sodium thiosulfate on lipopolysaccharide-induced lung injury.
(A) Representative microscope images of hematoxylin and eosin stained lung sections from each group (magnification ×400). (B) The number of polymorphonuclear neutrophils (PMNs) in each high-power field (HPF). (C) Semiquantitative analysis of lung sections by lung injury score. ***p<0.001, *p<0.05; Two-way ANOVA Bonferroni post-test, mean ± SD. Numbers in bars represent the sample size. LPS = lipopolysaccharide; STS = sodium thiosulfate.
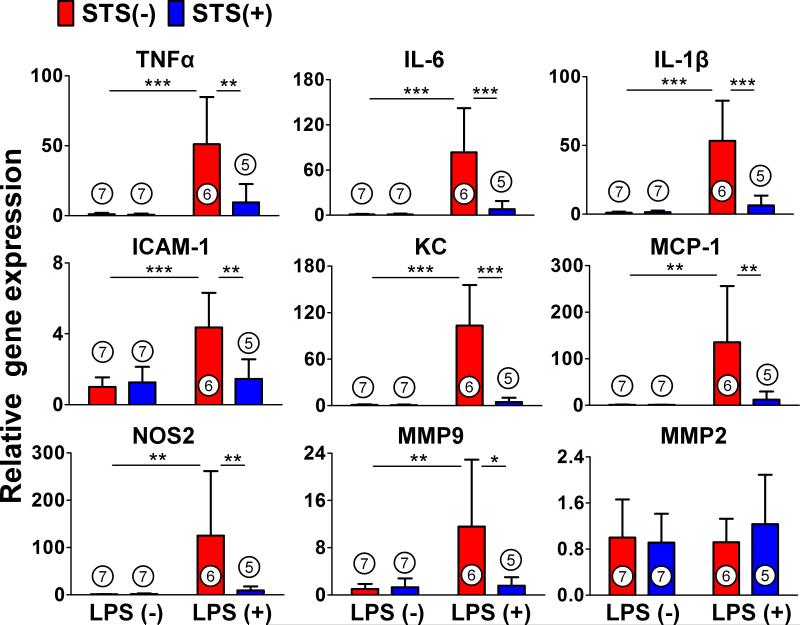
STS attenuates upregulation of proinflammatory mediators in the lung after lipopolysaccharide challenge
We measured the messenger RNA expression levels of proinflammatory mediators including cytokines, chemokines, and adhesion molecules in the whole lung of mice after lipopolysaccharide challenge (fig. 3.). STS attenuated lipopolysaccharide-induced upregulation of cytokines (TNFα, IL-6 and Interleukin-1β), intracellular adhesion molecule-1, chemokines, and nitric oxide synthase 2. STS also attenuated the lipopolysaccharide-induced upregulation of Matrix metalloproteinase 9, a family of proteinases that remodel extracellular matrix components, but not Matrix metalloproteinase 2.
Figure 3. Relative messenger RNA expression of proinflammatory mediators in lipopolysaccharide-induced lung injury.
Relative gene expression levels of inflammatory mediators in the lung 24 h after challenge with saline or lipopolysaccharide (LPS). Gene expression was normalized to 18S ribosomal RNA expression level, and the mean value for control mice challenged with saline was set to 1. ***p<0.001, **p<0.01, *p<0.05; Two-way ANOVA Bonferroni post-test, mean ± SD. Numbers in bars represent the sample size. ICAM-1 = intracellular adhesion molecule-1; IL-1β = Interleukin-1β; IL-6 = Interleukin-6; KC = keratinocyte-derived chemokine; MCP-1 = monocyte chemotactic protein 1; MMP = matrix metalloproteinase; NOS2 = nitric oxide synthase 2; STS = sodium thiosulfate; TNFα = Tumor Necrosis Factor alpha.
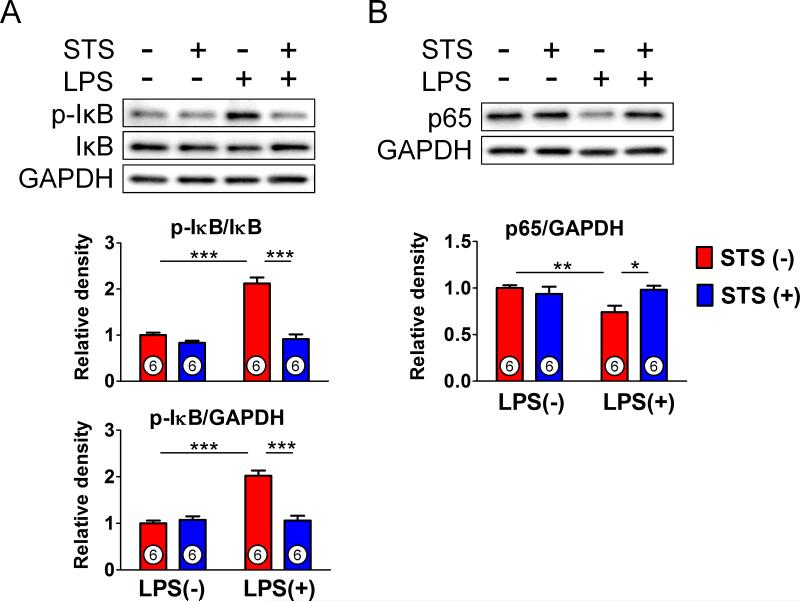
STS inhibits lipopolysaccharide-induced activation of IκB/NFκB signaling pathway in mice lung
Activation of IKK by lipopolysaccharide induces phosphorylation and degradation of IκB, leading to the nuclear translocation of NFκB and transcriptional activation.23 To elucidate the molecular mechanisms involved in the attenuated inflammatory responses by STS, we examined the effects of STS on IκB/ NFκB p65 pathway. STS inhibited lipopolysaccharide-induced IκB phosphorylation and p65 nuclear translocation in mice lung 24 h after lipopolysaccharide challenge (figs. 4A and B). These results suggest that STS inhibits lipopolysaccharide-induced activation of NFκB signaling by inhibiting IκB phosphorylation.
Figure 4. Effects of sodium thiosulfate on IκB/NFκB signaling pathway.
Levels of total and phosphorylated inhibitor of nuclear factor kappa B (p-IκB) and p65 subunit of nuclear factor kappa B (NFκB) nomalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in lung tissue homogenates 24 h after challenge with saline or lipopolysaccharide (LPS). ***p<0.001, **p<0.01, *p<0.05; Two-way ANOVA Bonferroni post-test, mean ± SD. Numbers in bars represent the sample size. IκB = inhibitor of NFκB; p65 = p65 subunit of NFκB; STS = sodium thiosulfate.
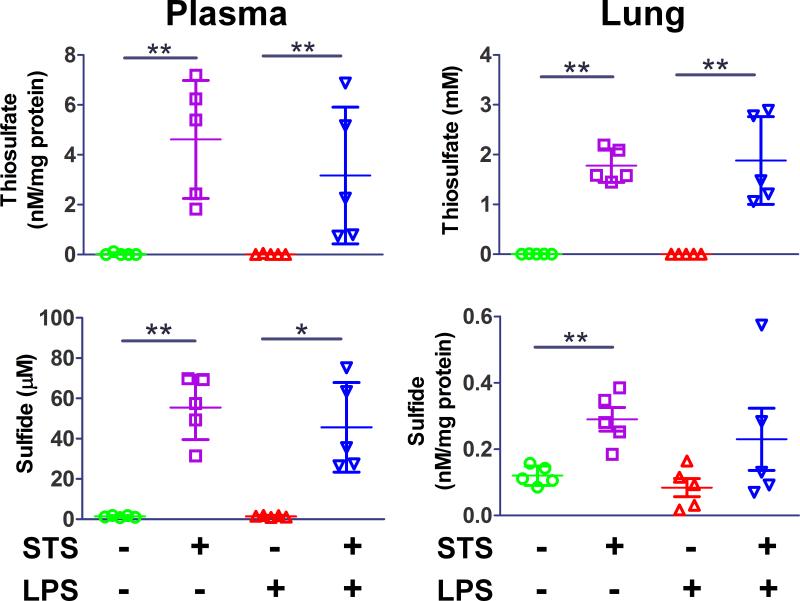
STS augments thiosulfate and sulfide levels of plasma and lung in mice
Administration of STS markedly increased thiosulfate levels in plasma and lung with or without lipopolysaccharide challenge (fig. 5). Plasma thiosulfate concentrations reached 1.8 ± 0.3 mM and 1.8 ± 0.9 mM in mice challenged with saline or lipopolysaccharide, respectively, at 2 h after STS administration. Plasma sulfide levels were also augmented to 56 ± 16 μM and 46 ± 22 μM by STS administration in mice challenged with saline or lipopolysaccharide, respectively. Administration of STS markedly increased the levels of thiosulfate in the lung with or without lipopolysaccharide challenge. Similarly, sulfide levels doubled in the lungs of saline-challenged mice and tended to increase in lipopolysaccharide-challenged mice lungs after STS administration.
Figure 5. Levels of thiosulfate and sulfide in plasma and lung 2 h after intratracheal lipopolysaccharide challenge.
Levels of thiosulfate and sulfide in plasma and lung were measured with high performance liquid chromatography at 2 h after intratracheal lipopolysaccharide (LPS) challenge with or without sodium thiosulfate (STS). **p<0.01, *p<0.05; Mann-Whitney U test, n=5 in each group.
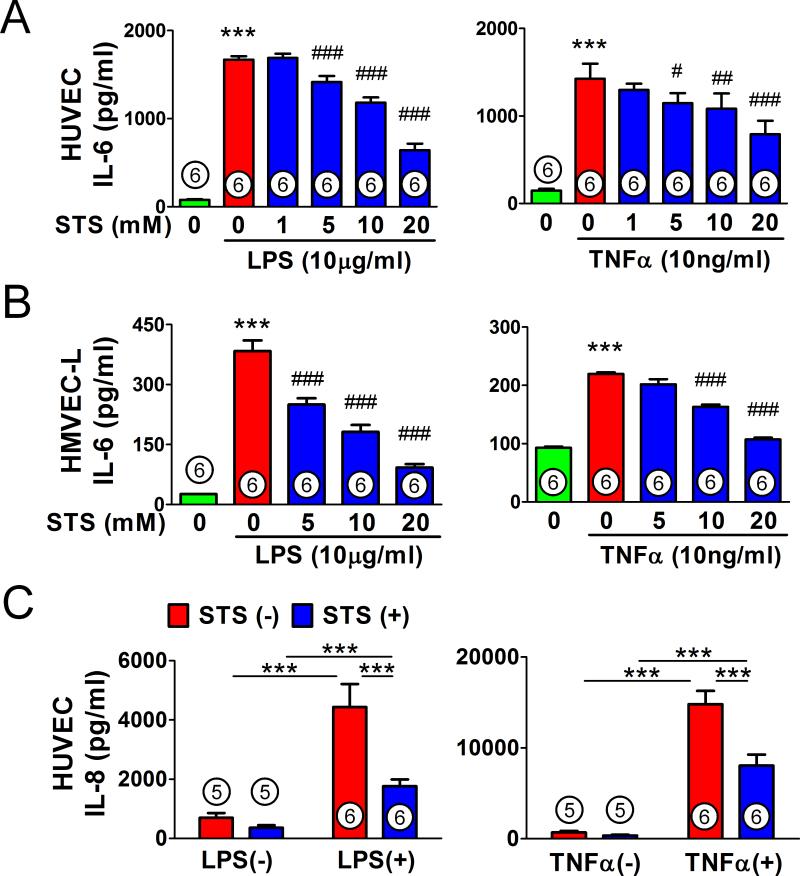
STS attenuates lipopolysaccharide or TNFα-induced proinflammatory mediator production in HUVEC and HMVEC-L
To examine the effects of STS on endothelium, we next evaluated the effects of STS on lipopolysaccharide or TNFα-induced cytokine production in HUVEC and HMVEC-L. STS (20 mM) per se had no significant cytotoxicity on HUVEC (data not shown). STS inhibited the lipopolysaccharide or TNFα-induced IL-6 production in HUVEC (fig. 6A) and HMVEC-L (fig. 6B) in a dose-dependent manner. Similarly, STS attenuated lipopolysaccharide or TNFα-induced IL-8 production in HUVEC (fig. 6C).
Figure 6. Effects of sodium thiosulfate on lipopolysaccharide or tumor necrosis factor alpha-induced cytokine production in human umbilical vein endothelial cells and human lung microvascular endothelial cells.
Interleukin-6 (IL-6) levels in human umbilical vein endothelial cells (HUVEC; A), and human lung microvascular endothelial cells (HMVEC-L; B) culture medium incubated with lipopolysaccharide (LPS) or Tumor Necrosis Factor alpha (TNFα) for 20 h with or without varying concentrations of sodium thiosulfate (STS). ***p<0.001 vs. control. ###p<0.001, ##p<0.01, #p<0.05 vs. LPS or TNFα; One-way ANOVA Bonferroni post-test, mean ± SD. (C) Interleukin-8 (IL-8) levels in HUVEC culture medium incubated with LPS or TNFα for 20 h with or without 20mM STS. ***p<0.001; Two-way ANOVA Bonferroni post-test, mean ± SD. Numbers in bars represent the sample size.
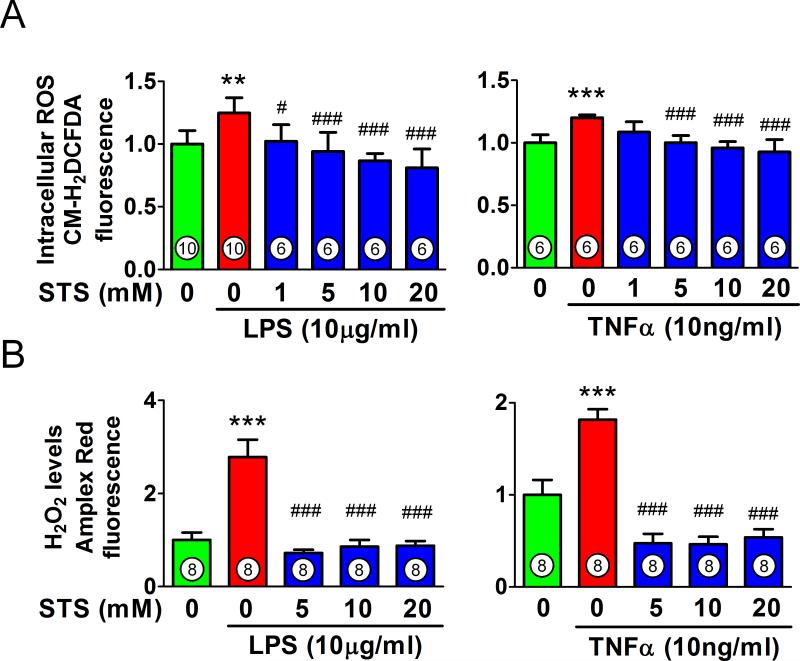
STS inhibits lipopolysaccharide or TNFα-stimulated ROS production in HUVEC
We assessed whether or not STS affects lipopolysaccharide or TNFα-induced production of ROS, which can lead further inflammatory response in endothelium.23 Lipopolysaccharide (10μg/ml) or TNFα (10ng/ml) significantly increased the fluorescence intensity of CM-H2DCFDA, an intracellular probe of ROS in HUVEC (fig. 7A). STS inhibited the intracellular ROS production induced by lipopolysaccharide or TNFα. The H2O2/peroxidase levels in HUVEC measured by fluorescent intensity of Amplex red (Invitrogen) were significantly increased with lipopolysaccharide or TNFα, which were markedly inhibited by STS (fig. 7B).
Figure 7. Effect of sodium thiosulfate on lipopolysaccharide or tumor necrosis factor alpha-stimulated reactive oxygen species production in human umbilical vein endothelial cells.
(A) Intracellular levels of reactive oxygen species (ROS) measured by chloromethyl-2'7'-dichlorofluorescein diacetate (CM-H2DCFDA) in human umbilical vein endothelial cells stimulated with lipopolysaccharide (LPS) or Tumor Necrosis Factor alpha (TNFα) for 30 min with or without varying concentrations of sodium thiosulfate (STS). (B) Levels of hydrogen peroxide (H2O2) measured by Amplex Red (Invitrogen, Eugene, OR) in human umbilical vein endothelial cells stimulated with LPS or TNFα for 30 min with or without varying concentrations of STS. ***p<0.001 vs. control, ###p<0.001, #p<0.05 vs. LPS or TNFα treatment; One-way ANOVA Bonferroni post-test, mean ± SD. Numbers in bars represent the sample size.
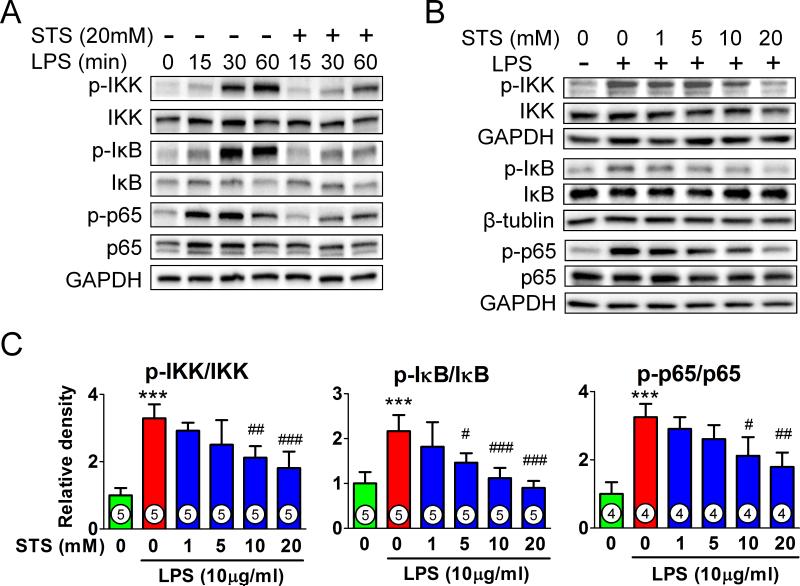
STS inhibits lipopolysaccharide-induced IKK/NFκB activation in HUVEC
To determine whether or not STS attenuates lipopolysaccharide-induced activation of IKK/NFκB pathway in endothelial cells, we analyzed IKKα/β, IκB and p65 in HUVEC. STS inhibited lipopolysaccharide-induced phosphorylation of IKKα/β, IκB and p65 in a dose-dependent manner (figs. 8A-C).
Figure 8. Effects of sodium thiosulfate on lipopolysaccharide-induced IκB/NFκB signaling in human umbilical vein endothelial cells.
(A) Representative immunoblots of total and phosphorylated IκB kinase (IKK), p65 subunit of nuclear factor kappa B (p65), and inhibitor of nuclear factor kappa B (IκB) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in human umbilical vein endothelial cells incubated with lipopolysaccharide (LPS; 10μg/ml) with or without sodium thiosulfate (STS; 20mM) for the indicated times. Representative immunoblots (B) and densitometric analyses (C) of phosphorylated/total IKK, p65, and IκB in human umbilical vein endothelial cells stimulated with LPS for 30 min with or without varying concentration of STS. ***p<0.001 vs. control, ###p<0.001, ##p<0.01, #p<0.05 vs. LPS treatment; One-way ANOVA Bonferroni post-test, mean ± SD. Numbers in bars represent the sample size.
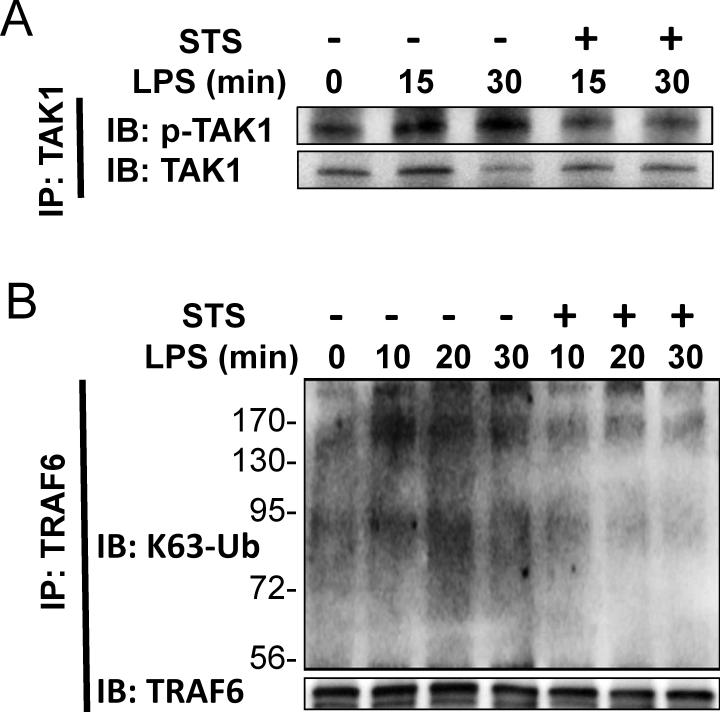
STS inhibits lipopolysaccharide-induced TAK1 activation and TRAF6 polyubiquitination in HUVEC
We examined the effect of STS on the activation of TAK1 and polyubiquitination of TRAF6 as upstream modulators of NFκB.24 Cell lysates were immunoprecipitated with TAK1 antibody and phosphorylated TAK1 was detected by immunoblot. STS inhibited lipopolysaccharide-induced TAK1 phosphorylation (fig. 9A). Next, TRAF6 was immunoprecipitated and probed with anti-K63-specific ubiquitin antibody. STS inhibited lipopolysaccharide-induced K63-linked polyubiquitination of TRAF6 (fig. 9B). These results suggest that STS attenuates lipopolysaccharide-induced IKK/NFκB activation through inhibiting the polyubiquitination of TRAF6 and activation of TAK1.
Figure 9. Effects of sodium thiosulfate on lipopolysaccharide-induced transforming growth factor-β-activated kinase 1 activation and tumor necrosis factor receptor-associated factor 6 ubiquitination.
(A) Representative immunoblots of total and phosphorylated transforming growth factor-β-activated kinase 1 (TAK1) in human umbilical vein endothelial cells incubated with or without lipopolysaccharide (LPS; 10μg/ml) and sodium thiosulfate (STS; 20mM) for indicated times. Cell lysates were immunoprecipitated (IP) with anti- TAK1 antibody and then immunoblotted (IB) with anti phospho-TAK1 antibody. (B) Representative immunoblots of lysine 63 (K63)-polyubiquitinated tumor necrosis factor receptor-associated factor 6 (TRAF6) of the cell lysates of human umbilical vein endothelial cells incubated with LPS with or without STS for indicated times. Cell lysates were immunoprecipitated with anti-TRAF6 antibody and then immunoblotted with anti K63-specific ubiquitin (K63-Ub) antibody.
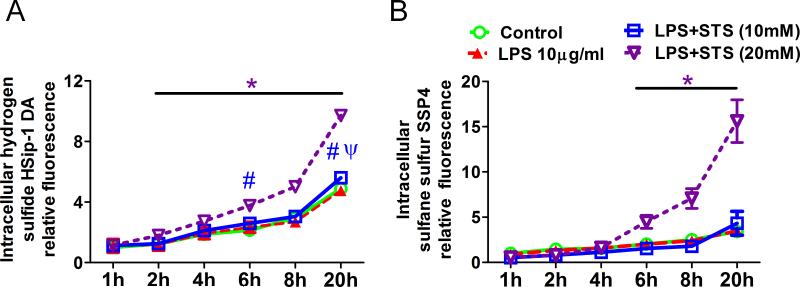
STS augments intracellular H2S and sulfane sulfur levels
To identify the sulfide metabolites increased by STS in the cells challenged with lipopolysaccharide, intracellular levels of free H2S/HS− and sulfane sulfur were evaluated using fluorescent probes HSip-1DA and SSP4, respectively. We performed kinetic measurement of fluorescence up to 20 h after treatment with lipopolysaccharide and STS. HSip-1 and SSP4 fluorescent intensity gradually increased over 20 h in control cells that are loaded with HSip-1DA or SSP4 but without lipopolysaccharide and STS. lipopolysaccharide alone or lipopolysaccharide with STS at concentrations less than 5 mM did not affect fluorescence intensity of HSip-1 and SSP4 compared to control at all times. Intracellular sulfide levels were augmented by 20 mM of STS between 2 and 20 h after treatment in cells incubated with lipopolysaccharide (fig. 10A). STS at 10 mM augmented intracellular sulfide levels at 6 and 20 h after treatment in lipopolysaccharide-treated cells. Intracellular sulfane sulfur levels were augmented by 20 mM of STS between 6 and 20 h after treatment (fig. 10B). These results suggest that STS increases intracellular levels of sulfide and sulfane sulfur in lipopolysaccharide-treated cells, with the former increases faster than the latter.
Figure 10. Effect of sodium thiosulfate on hydrogen sulfide and sulfane sulfur levels in human umbilical vein endothelial cells challenged with lipopolysaccharide.
Kinetic measurement of HSip-1DA (A) and SSP4 (B) fluorescence was performed to evaluate the impact of lipopolysaccharide (LPS) and sodium thiosulfate (STS) on sulfide metabolism up to 20 h after treatment. The relative fluorescence at each time point was normalized to the values of untreated control at baseline (1 h). *p<0.05, LPS+STS (20mM) vs. Control, LPS and LPS+STS (10mM); #p<0.05, LPS+STS (10mM) vs. Control; ψp<0.05, LPS+STS (10mM) vs. LPS; Two-way ANOVA Bonferroni post-test, n= 6 in each group.
Discussion
In the current study, we demonstrated that intraperitoneal administration of STS attenuated ALI in mice. STS inhibited the increase of lung permeability, influx of PMN, and expression of proinflammatory mediators in mice lung subjected to intratracheal lipopolysaccharide challenge. STS also attenuated lung tissue inflammation after polymicrobial sepsis. Administration of STS markedly increased levels of sulfide and thiosulfate in lung and plasma of mice. We also observed that STS markedly attenuated lipopolysaccharide or TNFα-induced cytokine/ROS production in cultured endothelial cells and prevented the increase in endothelial permeability in vascular endothelial monolayer. The beneficial effects of STS were associated with down-regulation of IKK/NFκB signaling pathways. Our results also revealed that STS markedly inhibited the lipopolysaccharide-induced activation of TAK1 and TRAF6 polyubiquitination, suggesting a novel regulatory mechanism responsible for the inhibitory effects of sulfide on NFκB signaling. Lastly, the current results suggest that STS exerts its beneficial effects at least in part by increasing intracellular sulfide and sulfane sulfur levels in vascular endothelium. Taken together, these observations suggest a therapeutic potential of STS against ALI.
Role of sulfide and sulfide metabolites in inflammatory organ injury remains incompletely defined. While acute administration of high doses of H2S donor compounds appears to be invariably toxic, lower and steady levels of H2S may be cytoprotective against systemic inflammation.11,22 Along these lines, we have recently reported that breathing low concentration of H2S prevents lethal endotoxemia and lipopolysaccharide-induced lung and liver injury in mice at least in part by increasing thiosulfate.11 We also observed that administration of STS dose-dependently prevents death from endotoxin shock in the previous study.11 These studies prompted us to further examine the lung protective effects of STS in the current study.
Thiosulfate is a potent antioxidant and STS has been used for the treatment of cyanide poisoning and calciphylaxis with a remarkable safety track record.13,14 In the current study, we observed that STS markedly inhibited ROS production induced by lipopolysaccharide in endothelial cells. While we used relatively high doses of STS, previous clinical studies have shown extremely low cytotoxicity and effectiveness of STS at similar doses in patients. For example, intravenous administration of STS at dose of 4 or 12 g/m2, showed no evidence of neuro- or nephrotoxicity in humans.31,32 Further, Neuwelt et al. used as much as 16 and 20 g/m2 doses of intravenous STS against Carboplatin-induced ototoxicity in human.17 In their study, after intravenous administration of 16 or 20 g/m2 of STS, serum thiosulfate levels reached 308 mg/dl (12.3 mM) and 330.8 mg/dl (13.2 mM), respectively, immediately after bolus infusion, with no signs of toxicity.17 Given the weight and height of the patient are 50 kg and 160 cm (1.5 m2 surface area), the doses of 16-20 g/m2 STS are assumed to be 0.48-0.6 g/kg, which correspond to the effective intravenous STS dose in mouse subjected to CLP in the current study. Therefore, the doses of STS used in the current study and the resultant plasma concentrations of thiosulfate fall within the doses of STS and plasma levels of thiosulfate that have been observed in patients. These observations suggest clinical relevance of our findings.
It is well established that lipopolysaccharide activates toll-like receptor 4-dependent signaling cascade. Binding of lipopolysaccharide to toll-like receptor 4 in endothelial cells up-regulates production of proinflammatory cytokines, chemokines, and adhesion molecules, predominantly via the transcription factor NFκB pathway.23 These actions lead to cell adhesion or increased vascular permeability that causes neutrophil migration and edema in the lung.23 STS markedly inhibited lipopolysaccharide-induced phosphorylation of IκBα and nuclear translocation of NFκB p65 in mice lung. STS inhibited the rapid phosphorylation of IKK, IκBα and p65 in HUVEC that occurred within 1 h after exposure to lipopolysaccharide. While these observations are consistent with the hypothesis that sulfide exerts anti-inflammatory effects via inhibition of NFκB,11,22 impact of sulfide or thiosulfate on NFκB-dependent signaling is incompletely defined. For example, while the majority of studies reported that H2S inhibits NFκB,22,33 some studies suggested that NFκB can be activated by sulfide.34 To further characterize the molecular mechanisms responsible for the inhibitory effects of STS on toll-like receptor 4-NFκB signaling, we examined the impact of STS on lipopolysaccharide-induced TRAF6 ubiquitination and TAK1 activation that are upstream of NFκB.
The protein ubiquitination is carried out through a three stepwise enzymatic reactions, involving E1 (ubiquitin activating enzyme), E2 (ubiquitin conjugating enzyme) and E3 (ubiquitin ligase).24 TRAF6 functions as an E3, which catalyzes K63 polyubiquitination. K63-linked autoubiquitination of TRAF6 is required for activation of TAK1 and subsequent NFκB activation.24 A crucial role of ubiquitinated TRAF6 on NFkB signaling has been demonstrated by inhibiting TRAF6 polyubiquitination.25,26 The inhibitory effects of STS on lipopolysaccharide-induced TRAF6 ubiquitination revealed in this study shed light on the novel mechanisms responsible for the anti-inflammatory effects of STS and sulfide. Additionally, STS inhibited TNFα-induced cytokine production in HUVEC. Of the six TRAF family members, TRAF6 is the only TRAF that mediates both of the TNF receptor and toll-like receptor signaling.35 While this unique property of TRAF6 may explain the inhibitory effects of STS on TNFα-induced cytokine production, the effects of STS on TNFα-induced signaling remain to be further elucidated in future studies.
It has been proposed that some of the effects of H2S are mediated via the properties of sulfide metabolites containing reactive sulfane sulfur (S0), a labile, highly reactive sulfur atom.8,10 Although thiosulfate is one of the sulfide metabolites that contain sulfane sulfur,8 thiosulfate itself appears to have limited reactivity. We therefore hypothesized that STS is converted to other sulfide metabolites that exert beneficial effects after lung injury. To determine the sulfide metabolites that are responsible for the beneficial effects of STS, we measured levels of sulfide and reactive sulfane sulfur in HUVEC using novel fluorescent probes HSip-129 and SSP4,30 respectively. Gradual increase of the HSip-1 and SSP4 fluorescence intensity in control cells may reflect endogenous production of sulfide or sulfane sulfur and or leakage of the fluorescence probes to extracellular spaces where the probes can react with sulfide metabolites in culture media. We observed that STS increased intracellular H2S levels within 2 h after the start of incubation of HUVEC with lipopolysaccharide and STS. In contrast, SSP4-reactive sulfane sulfur levels did not increase in HUVEC treated with lipopolysaccharide and STS until 6 h after the treatment. Since lipopolysaccharide triggered FκB activation within 1 h and STS markedly inhibited NFκB activation, our data suggest that the anti-inflammatory effects of STS are primarily mediated by intracellular sulfide that is converted from STS. It has been reported that thiosulfate can be converted to H2S via 3-mercaptopyruvate sulfurtransferase that is expressed in vascular endothelium.36,37 On the other hand, it has been suggested that H2S converted from thiosulfate is stored as sulfane sulfur.37 It is likely that levels of sulfide and sulfane sulfur are dynamically regulated in cells.
In summary, our results revealed that sodium thiosulfate exhibits robust anti-inflammatory effects in the lung. Taken together with our recent studies,11,12 results of this study suggest a role of STS in inflammatory ALI in addition to its established role as a therapeutic agent for cyanide toxicity, calciphylaxis, and chemotoxicity. Our results also revealed for the first time that the beneficial effects of STS are associated with the inhibition of TRAF6 ubiquitination, suggesting a novel regulatory mechanism of NFkB signaling by sulfide metabolites. Considering the clinical availability and established safety track record of STS and the critical role of NFkB signaling in cellular survival, further studies examining beneficial effects of STS in other diverse forms of organ injury are warranted.
MS #201403017 Final Boxed Summary Statement.
What we already know about this topic:
Acute lung injury (ALI) is characterized by neutrophilic inflammation and increased lung permeability. Thiosulfate is a stable metabolite of hydrogen sulfide, a gaseous mediator that exerts anti-inflammatory effects, but its role in ALI is unknown
What this study tells us that is new:
Using an experimental model of ALI in mice challenged with intratracheal LPS or subjected to cecal ligation and puncture (CLP) with or without sodium thiosulfate, it was shown that STS exerts robust anti-inflammatory effects in mice lung and vascular endothelium
Acknowledgments
Funding: This study was supported by a National Institutes of Health (Bethesda, Maryland) grant R01HL101930 to Dr. Ichinose.
Footnotes
Declaration of conflict of interests: The authors declare no competing interests.
References
- 1.Bernard GR. Acute respiratory distress syndrome: A historical perspective. Am J Respir Crit Care Med. 2005;172:798–806. doi: 10.1164/rccm.200504-663OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and Outcomes of Acute Lung Injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy AT, Lakshmi SP, Kleinhenz JM, Sutliff RL, Hart CM, Reddy RC. Endothelial cell peroxisome proliferator-activated receptor γ reduces endotoxemic pulmonary inflammation and injury. J Immunol. 2012;189:5411–20. doi: 10.4049/jimmunol.1201487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menden H, Tate E, Hogg N, Sampath V. LPS-mediated endothelial activation in pulmonary endothelial cells: Role of Nox2-dependent IKK-β phosphorylation. Am J Physiol Lung Cell Mol Physiol. 2013;304:L445–55. doi: 10.1152/ajplung.00261.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh H, Tasaka S, Hasegawa N, Yamada W, Shimizu M, Nakamura M, Yonemaru M, Ikeda E, Adachi Y, Fujishima S, Yamaguchi K, Ishizaka A. Protective role of vascular endothelial growth factor in endotoxin-induced acute lung injury in mice. Respir Res. 2007;8:60. doi: 10.1186/1465-9921-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson KR. The therapeutic potential of hydrogen sulfide: Separating hype from hope. Am J Physiol Regul Integr Comp Physiol. 2011;301:R297–R312. doi: 10.1152/ajpregu.00045.2011. [DOI] [PubMed] [Google Scholar]
- 8.Toohey JI. Sulfur signaling: Is the agent sulfide or sulfane? Anal Biochem. 2011;413:1–7. doi: 10.1016/j.ab.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 9.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–35. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 10.Kimura H, Shibuya N, Kimura Y. Hydrogen Sulfide is a Signaling Molecule and a Cytoprotectant. Antioxid Redox Signal. 2012;17:45–57. doi: 10.1089/ars.2011.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokuda K, Kida K, Marutani E, Crimi E, Bougaki M, Khatri A, Kimura H, Ichinose F. Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid Redox Signal. 2012;17:11–21. doi: 10.1089/ars.2011.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirozu K, Tokuda K, Marutani E, Lefer D, Wang R, Ichinose F. Cystathionine γ-Lyase Deficiency Protects Mice from Galactosamine/Lipopolysaccharide-Induced Acute Liver Failure. Antioxid Redox Signal. 2014;20:204–16. doi: 10.1089/ars.2013.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pettersen JC, Cohen SD. Antagonism of cyanide poisoning by chlorpromazine and sodium thiosulfate. Toxicol Appl Pharmacol. 1985;81:265–73. doi: 10.1016/0041-008x(85)90163-2. [DOI] [PubMed] [Google Scholar]
- 14.Cicone JS, Petronis JB, Embert CD, Spector DA. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104–8. doi: 10.1053/j.ajkd.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Pasch A, Schaffner T, Huynh-Do U, Frey BM, Frey FJ, Farese S. Sodium thiosulfate prevents vascular calcifications in uremic rats. Kidney Int. 2008;74:1444–53. doi: 10.1038/ki.2008.455. [DOI] [PubMed] [Google Scholar]
- 16.Adirekkiat S, Sumethkul V, Ingsathit A, Domrongkitchaiporn S, Phakdeekitcharoen B, Kantachuvesiri S, Kitiyakara C, Klyprayong P, Disthabanchong S. Sodium thiosulfate delays the progression of coronary artery calcification in haemodialysis patients. Nephrol Dial Transplant. 2010;25:1923–9. doi: 10.1093/ndt/gfp755. [DOI] [PubMed] [Google Scholar]
- 17.Neuwelt EA, Brummett RE, Doolittle ND, Muldoon LL, Kroll RA, Pagel MA, Dojan R, Church V, Remsen LG, Bubalo JS, Neuwelt EA. First evidence of otoprotection against carboplatin-induced hearing loss with a two-compartment system in patients with central nervous system malignancy using sodium thiosulfate. J Pharmacol Exp Ther. 1998;286:77–84. [PubMed] [Google Scholar]
- 18.Faller S, Zimmermann KK, Strosing KM, Engelstaedter H, Buerkle H, Schmidt R, Spassov SG, Hoetzel A. Inhaled hydrogen sulfide protects against lipopolysaccharide-induced acute lung injury in mice. Med Gas Res. 2012;2:26. doi: 10.1186/2045-9912-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis RC, Vaporidi K, Bloch KD, Ichinose F, Zapol WM. Protective and Detrimental Effects of Sodium Sulfide and Hydrogen Sulfide in Murine Ventilator-induced Lung Injury. Anesthesiology. 2011;115:1012–21. doi: 10.1097/ALN.0b013e31823306cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gerö D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci U S A. 2011;108:13829–34. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G, Sr, Gojon G, Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–27. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, Moore PK. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal. 2010;12:1147–54. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters K, Unger RE, Brunner J, Kirkpatrick CJ. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res. 2003;60:49–57. doi: 10.1016/s0008-6363(03)00397-3. [DOI] [PubMed] [Google Scholar]
- 24.Siqi Liu, Zhijian J Chen. Expanding role of ubiquitination in NF-κB signaling. Cell Res. 2011;21:6–21. doi: 10.1038/cr.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Zhang J, Zhang L, van Dam H, ten Dijke P. UBE2O negatively regulates TRAF6-mediated NF-κB activation by inhibiting TRAF6 polyubiquitination. Cell Res. 2013;23:366–77. doi: 10.1038/cr.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shembade N, Ma A, Harhaj EW. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327:1135–9. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou L, Feng Y, Zhang M, Li Y, Chao W. Nonhematopoietic toll-like receptor 2 contributes to neutrophil and cardiac function impairment during polymicrobial sepsis. Shock. 2011;36:370–80. doi: 10.1097/SHK.0b013e3182279868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichinose F, Zapol WM, Sapirstein A, Ullrich R, Tager AM, Coggins K, Jones R, Bloch KD. Attenuation of hypoxic pulmonary vasoconstriction by endotoxemia requires 5-lipoxygenase in mice. Circ Res. 2001;88:832–8. doi: 10.1161/hh0801.089177. [DOI] [PubMed] [Google Scholar]
- 29.Sasakura K, Hanaoka K, Shibuya N, Mikami Y, Kimura Y, Komatsu T, Ueno T, Terai T, Kimura H, Nagano T. Development of a highly selective fluorescence probe for hydrogen sulfide. J Am Chem Soc. 2011;133:18003–5. doi: 10.1021/ja207851s. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Liu C, Peng B, Zhao Y, Pacheco A, Xian M. New fluorescent probes for sulfane sulfurs and the application in bioimaging. Chem Sci. 2013;4:2892–6. doi: 10.1039/C3SC50754H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markman M, Cleary S, Pfeifle CE, Howell SB. High-dose intracavitary cisplatin with intravenous thiosulfate. Low incidence of serious neurotoxicity. Cancer. 1985;56:2364–8. doi: 10.1002/1097-0142(19851115)56:10<2364::aid-cncr2820561003>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Goel R, Cleary SM, Horton C, Kirmani S, Abramson I, Kelly C, Howell SB. Effect of sodium thiosulfate on the pharmacokinetics and toxicity of cisplatin. J Natl Cancer Inst. 1989;81:1552–60. doi: 10.1093/jnci/81.20.1552. [DOI] [PubMed] [Google Scholar]
- 33.Pan LL, Liu XH, Gong QH, Wu D, Zhu YZ. Hydrogen sulfide attenuated tumor necrosis factor-α-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS One. 2011;6:e19766. doi: 10.1371/journal.pone.0019766. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung JY, Park YC, Ye H, Wu H. All TRAFs are not created equal: Common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci. 2002;115:679–88. doi: 10.1242/jcs.115.4.679. [DOI] [PubMed] [Google Scholar]
- 36.Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–6. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 37.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–14. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]