Abstract
We evaluated a cocktail of HLA-A2-specific peptides including heteroclitic XBP1 US184-192 (YISPWILAV), heteroclitic XBP1 SP367-375 (YLFPQLISV), native CD138260-268 (GLVGLIFAV) and native CS1239-247 (SLFVLGLFL), for their ability to elicit multipeptide specific cytotoxic T lymphocytes (MP-CTL) using T cells from smoldering multiple myeloma (SMM) patients. Our results demonstrate that MP-CTL generated from SMM patients’ T cells show effective anti-MM responses including CD137 (4-1BB) upregulation, CTL proliferation, IFN-γ production, and degranulation (CD107a) in an HLA-A2-restricted and peptide-specific manner. Phenotypically, we observed increased total CD3+CD8+ T cells (>80%) and cellular activation (CD69+) within the memory SMM MP-CTL (CD45RO+/CD3+CD8+) subset after repeated multipeptide stimulation. Importantly, SMM patients could be categorized into distinct groups by their level of MP-CTL expansion and anti-tumor activity. In high responders, the effector memory (CCR7-CD45RO+/CD3+CD8+) T cell subset was enriched, while the remaining responders’ CTL contained a higher frequency of the terminal effector (CCR7-CD45RO-/CD3+CD8+) subset. These results suggest that this multipeptide cocktail has the potential to induce effective and durable memory MP-CTL in SMM patients. Therefore, our findings provide the rationale for clinical evaluation of a therapeutic vaccine to prevent or delay progression of SMM to active disease.
Keywords: Smoldering Multiple Myeloma, Multipeptide Vaccine, XBP1, CD138, CS1
INTRODUCTION
Smoldering myeloma (SMM) is a precursor to multiple myeloma (MM) in which the patient does not display any of the typical myeloma-related symptoms, such as elevated calcium levels, kidney damage, anemia or bone lesions.1,2 Early treatment strategies for SMM are particularly attractive, since the rate of progression to MM is substantially greater (10%) as compared to monoclonal gammopathy of undetermined significance (MGUS, 1%). Currently, the standard of care for most SMM patients remains vigilant observation while withholding standard therapy, due to chemotherapy-related toxicity and the challenge of identifying high-risk SMM patients at diagnosis.3-5 There have been several clinical trials to treat SMM patients; however, no significant clinical benefits have been observed with different therapeutic options including melphalan-prednisone, zoledronic acid or thalidomide and pamidronate, either in a single-arm trial or randomized-controlled trials.6-8
Recent findings indicate that development of active MM from asymptomatic precursor states is associated with worsening immune dysfunction. Defects in T-cell function, including loss of tumor-specific effector T cell activity and induction of Treg cells, along with cytokines and growth factors including interleukin-6 (IL-6), macrophage inflammatory protein (MIP)-1α, insulin-like growth factor (IGF)-I, VEGF, and hepatocyte growth factor (HGF), contribute to myeloma pathogenesis.9,10 The expression of PD-L1, which is detected on MM cells but not in healthy donors, may also be associated with reduced susceptibility to tumor cell lysis by CTL.11,12 MM has the unique ability to elude immunosurveillance through various mechanisms including reduced T-cell cytotoxic activity, induction of dendritic cell dysfunction, expansion of myeloid-derived suppressor cells, decreased responsiveness to IL-2, and defects in B-cell immunity.13-15 These immune defects associated with MM pathogenesis account at least in part, for the failure of recent immunotherapy trials in patients with MM.16 Thus, we hypothesize that early immunotherapeutic intervention in SMM patients with retained immune function may offer an opportunity to prevent or delay disease progression to active MM.
Antigen-specific cancer vaccines targeting various tumor-associated antigens (TAA) have been shown to elicit tumor-suppressive responses in the clinical setting. A large number of clinical trials have been carried out using different TAA with minimal toxicities and side effects.17, 18 XBP1 (X-box binding protein 1), CD138 (Syndecan-1) and CS1 (CD2 subset 1, CRACC, SLAMF7, CD319) antigens are highly expressed on MM with therapeutic potential, as demonstrated in preclinical and clinical studies.19-24 In previous studies, we identified immunogenic HLA-A2-specific peptides derived from each of these target antigens including heteroclitic XBP1 unspliced (US)184-192 (YISPWILAV),25 heteroclitic XBP1 spliced (SP)367-375 (YLFPQLISV),25 native CD138260-268 (GLVGLIFAV)26, and native CS1239-247 (SLFVLGLFL)27 peptides. These selected immunogenic peptides, either individually or combined as a four-peptide cocktail, induced antigen-specific CTL with functional activities against HLA-A2+ MM cells.21,25-27 Expanding on our previous studies, we demonstrate here that these HLA-A2-specific XBP1 US, XBP1 SP, CD138 and CS1 peptides are highly immunogenic to SMM patients’ T cells and induce highly effective anti-MM immunity. The capacity for SMM patients’ T cells to respond to each of these peptides and trigger expansion of CD3+CD8+ CTL with functional activities against the respective epitopes on MM cells provides the framework for clinical evaluation of vaccination with these peptides in patients with SMM to prevent progression to active disease.
MATERIALS AND METHODS
Cell lines
MM cell lines including HLA-A2+ McCAR, HLA-A2+ U266, HLA-A2- MM1S, and HLA-A2- RPMI along with the chronic myelogenous leukemia (CML) K562 cell line, were obtained from the ATCC. The K562 cell line transduced with HLA-A*0201 cDNA (K562-A*0201) was provided by Dr. P. Cresswell (Yale University). The T2 cell line, a human B and T cell hybrid expressing HLA-A2 molecules, was provided by Dr. J. Molldrem (University of Texas M. D. Anderson Cancer Center). All cell lines were cultured in RPMI-1640 medium (Gibco-Life Technologies) supplemented with 10% fetal calf serum (FCS; BioWhittaker), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Gibco-Life Technologies).
Synthetic peptides
Heteroclitic XBP1 US184-192 (YISPWILAV), heteroclitic XBP1 SP367-375 (YLFPQLISV), native CD138260-268 (GLVGLIFAV), and native CS1239-247 (SLFVLGLFL), along with an HLA-A2-specific control MAGE3271-279 (FLWGPRALV) peptide, were synthesized by standard fmoc (9-fluorenylmethyl-oxycarbonyl) chemistry, purified to >90% using reverse-phase chromatography, and validated by mass-spectrometry for molecular weight (Biosynthesis). Lyophilized peptides were dissolved in DMSO (Sigma), diluted in AIM-V medium (Gibco-Life Technologies), and stored at -140°C.
SMM patient samples
Heparinized venous blood samples were collected from SMM patients after informed consent, in accordance with the Declaration of Helsinki and approval by the institutional review board at Dana-Farber Cancer Institute (Boston, MA). Clinical characteristics of the SMM patients are shown in Table 1.
Table 1.
Clinical Characteristics of Eight SMM Patients Evaluated
| ID | Gender | Age | HLA-A2 | Treatment | Diagnosis | Cytogenetics |
|---|---|---|---|---|---|---|
| 1 | Female | 56 | Positive | Zoledronic Acid, IPH2101, MLN9708, Lenalidomide, Dexamethasone | IgGκ SMM | Trisomy 7, 9, 11 and 15 |
| 2 | Male | 60 | Positive | IPH2101 | IgGλ SMM | Deletion 17p, Translocation (4:14) |
| 3 | Male | 80 | Positive | Zoledronic Acid, IPH2101 | IgGκ SMM | Unknown |
| 4 | Female | 72 | Positive | Zoledronic Acid, Lenalidomide/Placebo, Hepatitis B Vaccine, IPH2101 | IgGλ SMM | 13q deletion, IGH/c-MAF fusion, Translocation (14;16) |
| 5 | Male | 55 | Positive | Zoledronic Acid, Elotuzumab | IgGκ SMM | Monosomy 13 |
| 6 | Female | 71 | Positive | Zoledronic Acid, Elotuzumab | λ Light Chain SMM | No Abnormalities |
| 7 | Female | 67 | Positive | Zoledronic Acid | IgAλ SMM | No Abnormalities |
| 8 | Male | 43 | Positive | BHQ880A | k Light Chain SMM | No Abnormalities |
Peripheral blood mononuclear cell isolation and generation of monocyte-derived dendritic cells
Peripheral blood mononuclear cells (PBMC) were isolated by standard density gradient centrifugation over Ficoll-Paque™ Plus (Amersham Pharmacia Biotech AB) from the blood obtained from HLA-A2+ SMM patients. Dendritic cells (DC) generated from autologous adherent monocytes were used as antigen-presenting cells (APCs).25,28
Isolation of CD3+ T cells from non-adherent cells of SMM patients
SMM patients’ CD3+ T cells were obtained by negative selection from the non-adherent cell fraction post-monocyte adherence using the EasySep® magnet and Robosep® (StemCell Technologies). The enriched CD3+ T cells (>99% purity) were used to generate MP-CTL.
Induction of MP-CTL generated from SMM patients’ T lymphoctyes
MP-CTL were generated ex vivo by repeated stimulation of CD3+ T lymphocytes obtained from HLA-A2+ SMM patients with a cocktail of heteroclitic XBP1 US184-192 (YISPWILAV), heteroclitic XBP1 SP367-375 (YLFPQLISV), native CD138260-268 (GLVGLIFAV), and native CS1239-247 (SLFVLGLFL) peptides. In brief, APCs (autologous mature DC, T2 cells) pulsed overnight with a cocktail containing the four peptides (25 μg/ml total; 6.25 μg/ml/peptide) were irradiated at 20 Gy and then used to stimulate autologous CD3+ T cells at a 1:20 APCs-to-CD3+ T cell ratio in AIM-V medium supplemented with 10% human AB serum. T cell cultures were restimulated every seven days with irradiated APCs pulsed with the multipeptide cocktail. IL-2 (50 units/ml) was added to the cultures two days after the second stimulation, and was replenished weekly until the cultures were completed.
Phenotypic analysis of SMM MP-CTL
One week after the last stimulation, MP-CTL and control T cells were harvested, washed in FACS buffer, and incubated with fluorochrome conjugated anti-human monoclonal antibodies (mAb) (BD Biosciences). After staining, the cells were washed, fixed in 2% paraformaldehyde-PBS, and analyzed by flow cytometry.
SMM MP-CTL proliferation in response to MM cell lines
To measure proliferation, SMM MP-CTL were labeled with CFSE (Molecular Probes), washed extensively, and co-incubated with irradiated (20 Gy) HLA-A2+ or HLA-A2- MM cell lines or control K562 cells in the presence of IL-2 (10 units/ml). As a control, CFSE-labeled SMM MP-CTL were cultured in media alone with IL-2. On days 5-7, cells were harvested and stained with anti-CD3/CD8 mAbs; the level of cell proliferation was evaluated by flow cytometry.
SMM MP-CTL degranulation and intracellular IFN-γ production in response to MM cells
CD107a degranulation and IFN-γ producing CD3+CD8+ T cells were identified within SMM MP-CTL by flow cytometry. Briefly, SMM MP-CTL were stimulated with HLA-A2+ or HLA-A2- MM cell lines, K562 cells, K562-A*0201 cells pulsed with respective peptide or K562-A*0201 cells alone in the presence of CD107a anti-human mAb. SMM MP-CTL alone served as a negative control. After 1 hour incubation, CD28/CD49d mAb (BD), as well as protein transport inhibitors Brefeldin A and Monensin (BD), were added for an additional 5 hours. Cells were harvested, washed in FACS buffer, and incubated with mAbs specific to CD3, CD8, CCR7, CD45RO, CD69 and/or CD137 antigens. After surface staining, cells were washed, fixed/permeabilized, stained with anti-IFN-γ mAb (BD), washed with Perm/Wash solution (BD), fixed in 2% paraformaldehyde, and analyzed by flow cytometry.
Analysis of SMM MP-CTL post-lenalidomide treatment
One week after the fourth stimulation, SMM MP-CTL were harvested and treated with Lenalidomide (5 μm, Celgene). Following an additional 4 days incubation, MP-CTL were evaluated for CD107a upregulation and IFN-γ production upon stimulation with MM cells, as described above. In addition, MP-CTL were evaluated for their phenotype by staining with mAbs specific to CD3, CD8, CD28 and/or CD137 antigens. The cells were washed, fixed in 2% paraformaldehyde, and analyzed by flow cytometry.
Statistical Analysis
Results are presented as mean ± SE. Groups were compared using unpaired Student’s t-test. Differences were considered significant when *p < 0.05.
RESULTS
A cocktail of HLA-2 specific XBP1 US/XBP1 SP/CD138/CS1 peptides effectively induces and expands CD3+CD8+ CTL from T cells of SMM patients, and the MP-CTL demonstrate HLA-A2 restricted cell proliferation in response to MM cell lines
A cocktail of HLA-A2 specific XBP1 unspliced, XBP1 spliced, CD138, and CS1 peptides was evaluated for its ability to induce antigen-specific CTL from enriched CD3+ T cells of SMM patients (n=4). One week after the first, third, and fourth MP-cocktail stimulation, cultures were evaluated for frequency of CD3+CD8+ T cells and CD3+CD4+ T cells by flow cytometry. An increase in the proportion of CD3+CD8+ T cells (Supplemental Figure 1A) and a corresponding decrease in CD3+CD4+ T cells (Supplemental Figure 1B) were detected following each round of multipeptide stimulation. The highest level of CD3+CD8+ (> 80%) and lowest level of CD3+CD4+ (< 20%) T cells were reached following the fourth stimulation (Figures 1A, 1B). These results demonstrate that stimulation with XBP1 US/XBP1 SP/CD138/CS1 multipeptide induces and expands CD3+CD8+ CTL from T cells of SMM patients.
Figure 1. HLA-A2 restricted cell proliferation of SMM MP-CTL against MM cells.
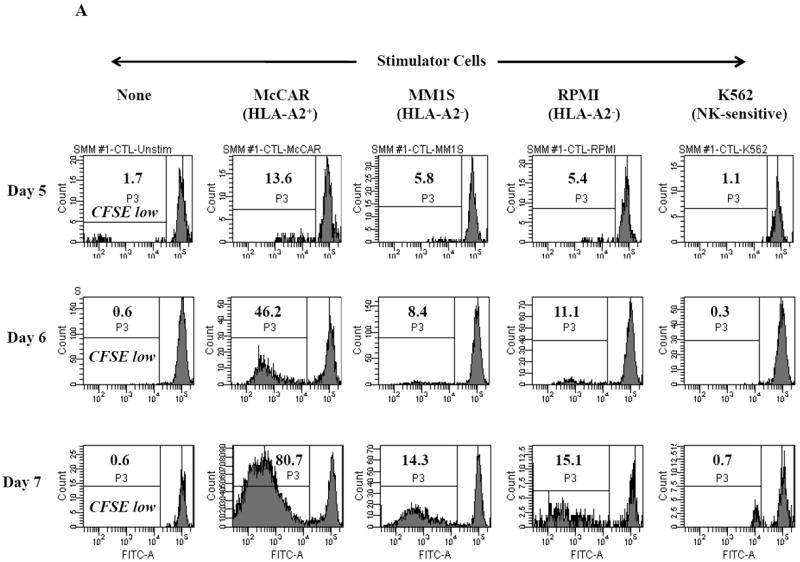
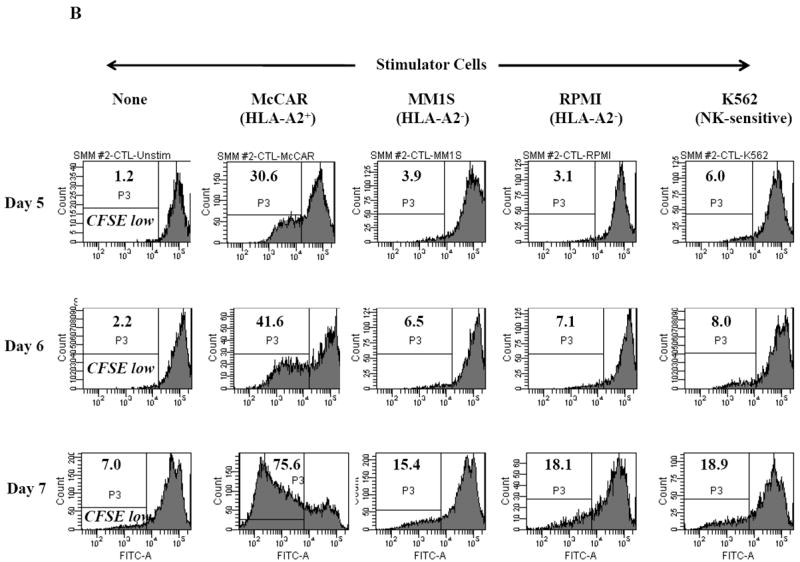
One week after the fourth stimulation, SMM MP-CTL (n=2) were evaluated for cell proliferation in response to various MM cell lines. MP-CTL proliferation is shown as the percent decrease in CFSE expression (P3-gated) on day 5, 6 or 7 of culture. MP-CTL in media alone was used to establish background cell proliferation. MP-CTL generated from SMM patient #1 (Figure 1A) or SMM patient #2 (Figure 1B) showed HLA-A2 restricted cell proliferation on days 5, 6, and 7 in response to HLA-A2+ MM cells (McCAR), but not to HLA-A2- MM cells (MM1S, RPMI) or NK-sensitive K562 cells.
Cell proliferation was measured, one week after the fourth peptides stimulation, by the decrease in fluorescence intensity (P3 gate) of CFSE labeled cells upon incubation with tumor cells. MP-CTL generated from SMM patient #1 (Figure 1A) showed increased CD3+CD8+ T cell proliferation on days 5 (13.6%), 6 (46.2%), and 7 (80.7%) in response to HLA-A2+ MM cells (McCAR). Similarly, MP-CTL from SMM patient #2 (Figure 1B) showed increased cell proliferation, starting on day 5 (30.6%) and peaking at day 7 (75.6%) in response to McCAR cells. However, the SMM MP-CTL did not proliferate to HLA-A2- MM cells (MM1S, RPMI) or NK-sensitive K562 cells (Figures 1A, 1B), thereby demonstrating HLA-A2-restricted cell proliferation.
Multipeptide-CTL generated from SMM patients demonstrate HLA-A2 restricted IFN-γ production and CD107a degranulation in response to MM cell lines
We further analyzed SMM MP-CTL for their functional anti-MM activities, including IFN-γ production and CD107a degranulation, following incubation with various MM cell lines or K562 cells. Representative flow cytometric analyses demonstrate that SMM MP-CTL had specific IFN-γ production (Figure 2A) against HLA-A2+ MM cells (McCAR), but not against HLA-A2- MM cells (MM1S, RPMI) or K562 cells. These observations were confirmed in further analyses of MP-CTL generated from additional HLA-A2+ SMM patients (n=4), which displayed IFN-γ production (Figure 2B) in an HLA-A2-restricted manner, and were not mediated by NK cells.
Figure 2. HLA-A2 restricted IFN-γ production by SMM MP-CTL against MM cells.
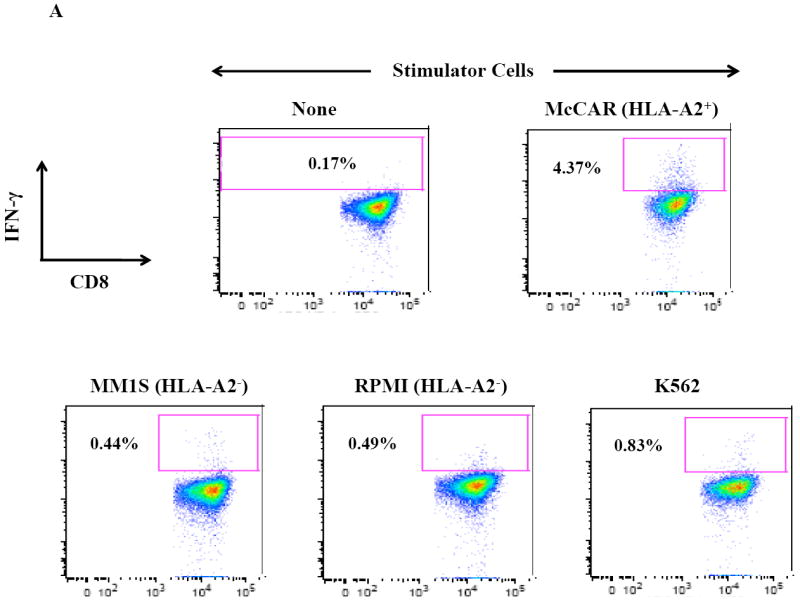
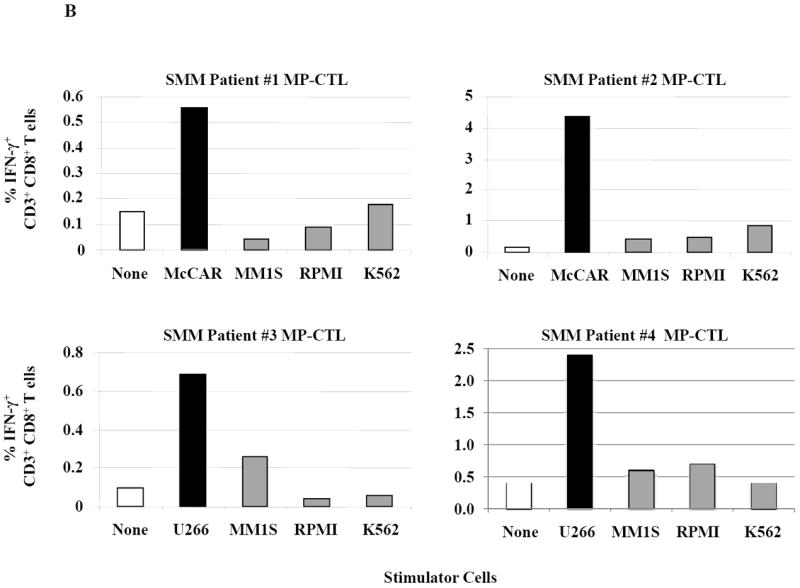
One week after the fourth stimulation, SMM MP-CTL were analyzed for IFN-γ production upon stimulation with various MM cell lines. Representative flow cytometric analysis demonstrates increased IFN-γ production by SMM MP-CTL in response to HLA-A2+ MM cells (McCAR), but not HLA-A2- MM cells (MM1S, RPMI) or NK-sensitive K562 cells (Figure 2A). MP-CTL generated from additional HLA-A2+ SMM patients (n=4) confirmed HLA-A2-restricted IFN-γ production to MM cells, but not to NK sensitive K562 cells (Figure 2B).
Next, further flow cytometric analyses demonstrate that the SMM MP-CTL had specific CD107a upregulation (Figure 3A) against HLA-A2+ MM cells (McCAR, U266), but not against HLA-A2- MM cells (MM1S, RPMI) or K562 cells. The MP-CTL generated from other HLA-A2+ SMM patients (n=4) displayed the same pattern of response in CD107a degranulation (Figure 3B) in an HLA-A2-restricted manner without NK cell activity. Interestingly, we detected higher levels of these anti-tumor activities against either HLA-A2+ MM cell line by MP-CTL generated from SMM patients #2 and #4 as compared to SMM patients #1 and #3 (Figures 2B, 3B).
Figure 3. HLA-A2 restricted CD107a degranulation by SMM MP-CTL against MM cells.
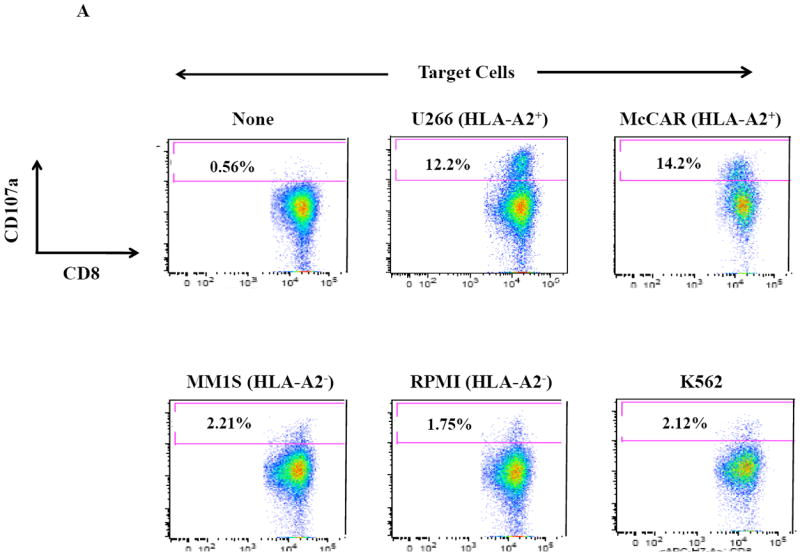
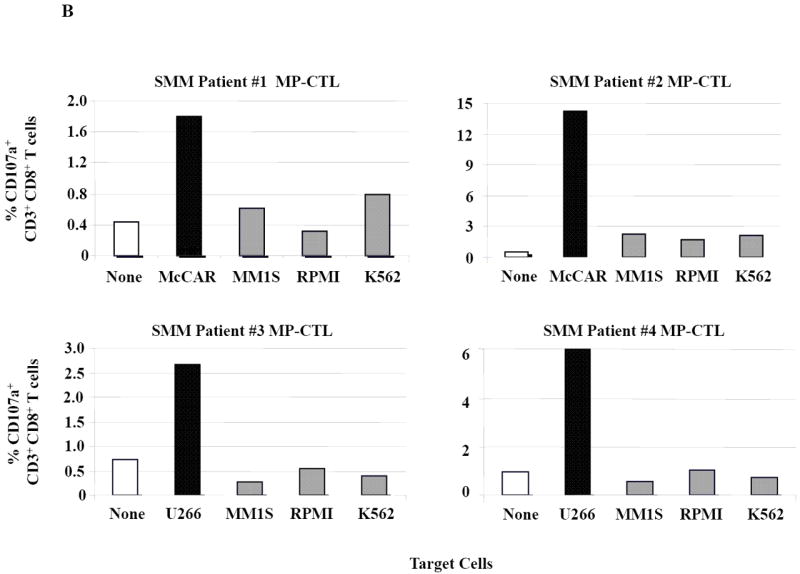
One week after the fourth stimulation, SMM MP-CTL were analyzed for CD107a degranulation upon stimulation with various MM cell lines. Representative flow cytometric analysis demonstrates increased CD107a degranulation by SMM MP-CTL in response to HLA-A2+ MM cells (McCAR, U266), but not HLA-A2- MM cells (MM1S, RPMI) or NK-sensitive K562 cells (Figure 3A). MP-CTL generated from additional HLA-A2+ SMM patients (n=4) confirmed HLA-A2-restricted CD107a degranulation to MM cells, but not to NK sensitive K562 cells (Figure 3B).
Multipeptide-CTL from SMM “high” responders are distinct in phenotype and functional activities against MM cells
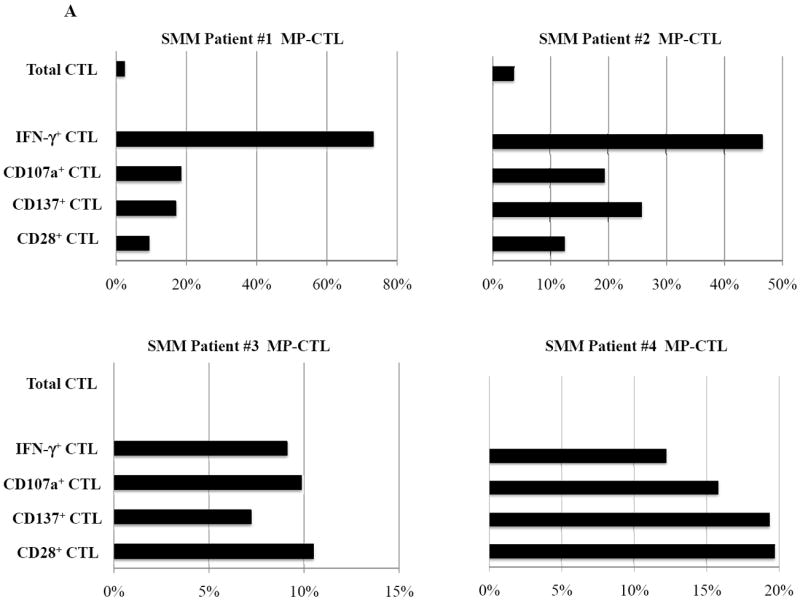
SMM MP-CTL (n=8) were evaluated for total CD3+CD8+ T cells, naïve:memory phenotypes and immune function. Results demonstrate that frequencies of CD3+CD8+ T cells in the MP-CTL generated from eight individual SMM patients were similar after four rounds of multipeptide stimulation (Figure 4A). However, total MP-CTL yields were distinctively higher from SMM patients #2 and #4 (1.2–1.3×109 cells) as compared to those from the remaining six SMM patients (6–15×106 cells). In addition, the highest level of IFN-γ production and CD107a degranulation in response to target cells (HLA-A2+ U266) was from MP-CTL generated from SMM patients #2 and #4, as compared to those from the other SMM patients (Figure 4A).
Figure 4. Characterization of SMM MP-CTL reveals a subgroup of SMM “high” responders with increased cell yield and immune functional activities.
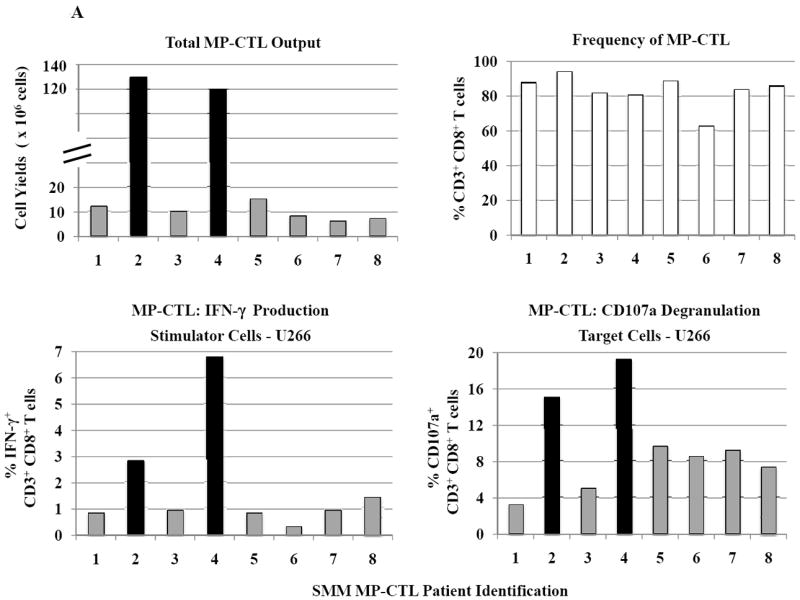

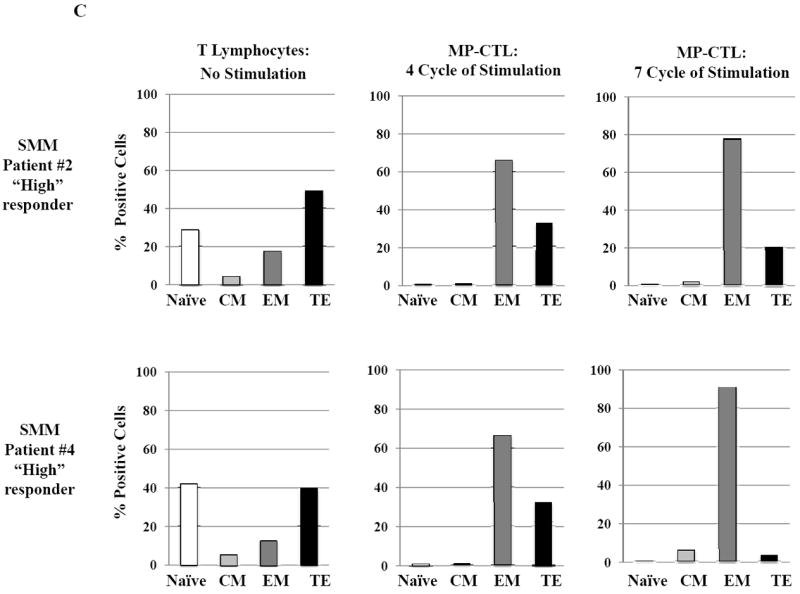
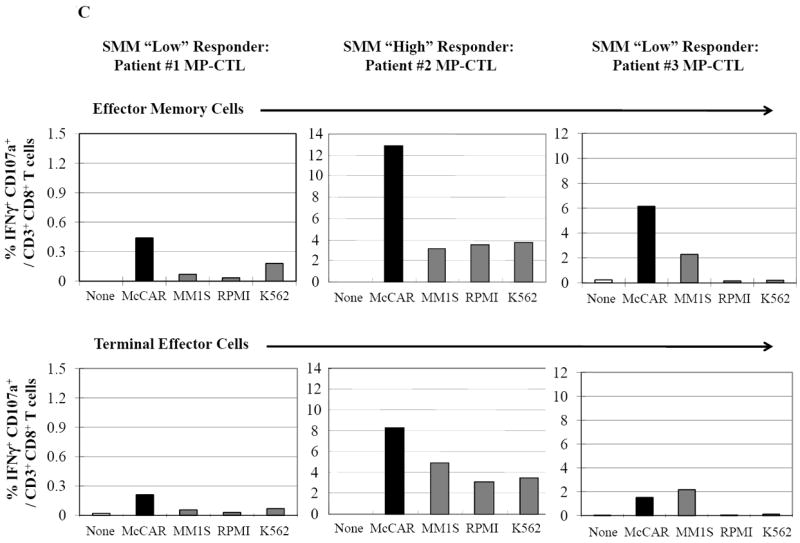
SMM MP-CTL (n=8) were analyzed one week after their fourth MP-cocktal stimulation. Total cell yield, % CD3+CD8+ T cells, % IFN-γ+ production, and % CD107a+ degranulation are shown for all MP-CTL in response to HLA-A2+ U266 MM cells (Figure 4A). A subgroup of MP-CTL (n=2) termed SMM “high” responders (#2, #4) displayed higher levels of total cell yield, % IFN-γ+ cells and % CD107a+ cells than MP-CTL generated from other SMM patients. Representative naïve:memory subsets analyses of SMM “high” and “normal” responders are demonstrated (Figure 4B). The percentage of effector memory cells was increased in the MP-CTL from SMM “high” responders, while MP-CTL from SMM “normal” responders contained a higher percentage of terminal effector cells. The MP-CTL from SMM “high” responders were cultured for an additional 3 rounds of weekly MP stimulation (total 7 stimulations). Continuous expansion of the effector memory cell subset was detected within the MP-CTL from SMM “high” responders (patients #2, #4) without a further differentiation into terminal effector cell subset (Figure 4C).
Next, we evaluated MP-CTL from the SMM “high” responders (patients #2 and #4) and two SMM “normal” responders (patients #1 and #3) for their naïve:memory phenotypic profile. CD3+CD8+ T lymphocytes responding to an antigen differentiate from naïve to antigen-specific CTL with various effector and memory functions.29,30 In these studies, SMM MP-CTL contained mainly effector memory (EM; CD45RO+CCR7-) and terminal effector (TE; CD45RO-CCR7-) along with a low frequency (<5%) of naïve (CD45RO-CCR7+) and central memory (CM; CD45RO+CCR7+) CD3+CD8+ T cells (Figure 4B). Importantly, a higher frequency of EM cells was detected in the MP-CTL from “high” responders (SMM #2: 66.4%, SMM #4: 66.5%) as compared to the “normal” responders (SMM #1: 20.5%, SMM #3: 45.3%). In contrast, a higher level of differentiated TE subset was seen in the “normal” responders as compared to the “high” responders.
Based on these observations, we continued to culture the MP-CTL from the “high” responders for 7 weeks with weekly MP-cocktail stimulation. Overall, we observed increased frequency of the EM subset in MP-CTL after 7 stimulations (SMM #2: 79.1%, SMM #4: 92.2%), without further differentiation into TE subset (Figure 4C). These results suggest that maintenance of an EM CTL subset might be critical for a long-term anti-tumor response, and may serve as a valuable biomarker when defining the efficacy of the multipeptide vaccine in future clinical studies.
Multipeptide-CTL generated from SMM patients include a high proportion of EM subset producing IFN-γ and expressing CD107a in response to MM cells with the highest levels detected in MP-CTL from SMM “high” responders
Next, we investigated the level of specific immune activity within different CD3+CD8+ T cell naïve:memory subsets of SMM MP-CTL in response to MM cells. Results (“% Total Adjusted”) were calculated as the percent contribution of each specific subset to total IFN-γ production or CD107a degranulation. The highest IFN-γ production was detected in the EM subset (67.25±16.88%), followed by TE (20.25±5.11), CM (8.25±3.15) and naïve (4.0±1.78) subsets, in response to HLA-A2+ McCAR or U266 MM cells (Figure 5A). A similar pattern of immune activity was observed for CD107a degranulation in the SMM MP-CTL (EM 69.50±6.36, TE 21.00±3.54, CM 7.50±3.43, naïve 1.50±1.19) to the HLA-A2+ MM cells (Figure 5B). In addition, the EM subset had a higher level of single response, IFN-γ production and CD107a degranulation, as compared to the TE subset (Figures 5A, 5B). We also observed that the highest proportion of dual functional cells (IFN-γ+/CD107a+) were from MP-CTL of a SMM “high” responder (patient #2) as compared to those of “normal” responders (patients #1 and #3) (Figure 5C). The dual functional activities were confirmed as HLA-A2-restricted in either EM or TE subsets of SMM MP-CTL.
Figure 5. Characterization of immune function in the naïve:memory cell subsets of SMM MP-CTL.
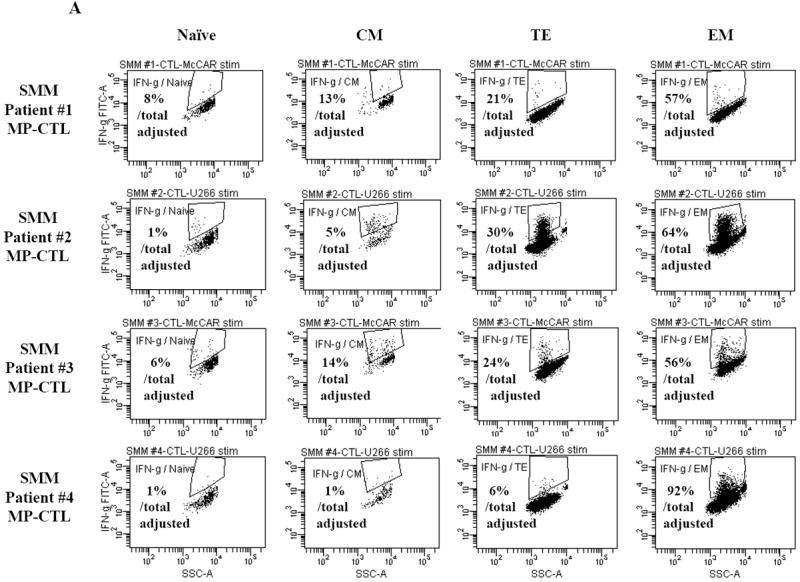
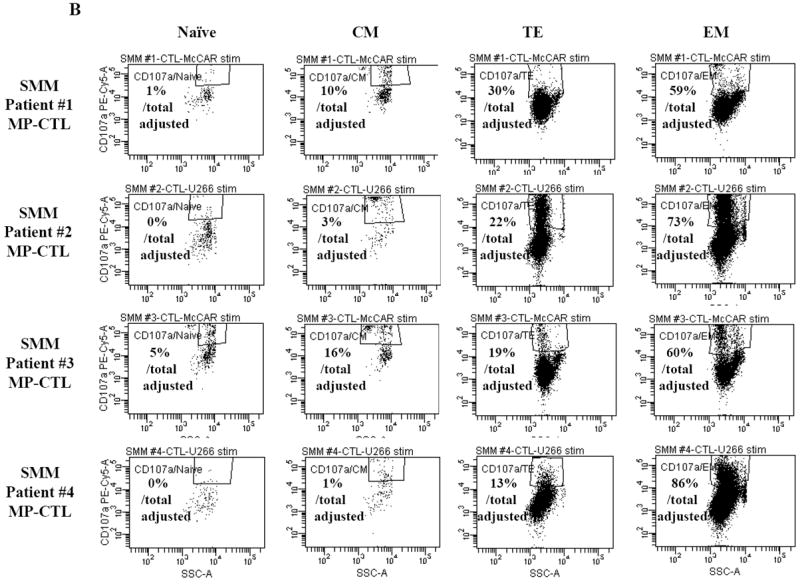
A and B. The specific immune function of SMM MP-CTL (n=4) was evaluated within the naïve:memory CD3+CD8+ T cell subsets in response to MM cells. The proportion (%) of each naïve:memory cell subset contribution to total immune activity is expressed as “% Total Adjusted”. The highest level of IFN-γ production (Figure 5A) or CD107a degranulation (Figure 5B) was detected in the effector memory cell subset, followed by the terminal effector, central memory, and naïve cell subsets within the SMM MP-CTL in response to HLA-A2+ MM cells. The effector memory cells from SMM “high” responders’ MP-CTL demonstrate increased IFN-γ production and CD107a degranulation as compared to those from SMM “normal” responders’ MP-CTL.
C. SMM MP-CTL (n=3) stimulated with HLA-A2+ MM cells were analyzed for the % IFN-γ+/CD107a+ double positive cells within the effector memory or terminal effector cell subset. Effector memory cells demonstrated a higher level of both IFN-γ production and CD107a degranulation as compared to terminal effector cells. Effector memory cells in MP-CTL from the SMM “high” responders had the highest % IFN-γ+/CD107a+ cells. The IFN-γ+/CD107a+ responses in the SMM MP-CTL were HLA-A2 restricted, and the activities were not mediated by NK cells.
Finally, we analyzed the level of MM specific cellular activation (% CD69+ cells) within the CD3+CD8+ naïve:memory subsets of SMM MP-CTL. Cellular activation was higher in the CD45RO+ memory cell subset as compared to the CCR7+CD45RO- naïve cell counterpart (Supplemental Figure 2). MM-specific cell activation was detected in response to HLA-A2+ McCAR or U266 MM cells, but not to HLA-A2- MM1S or RPMI MM cells (Supplemental Figure 3). However, unlike the IFN-γ production and CD107a degranulation, the level of CD69 upregulation could not be used to classify SMM MP-CTL as “high” or “normal” SMM responders.
Multipeptide-specific CTL induced from SMM patients demonstrate both IFN-γ production and CD107a degranulation in response to each relevant peptide
SMM MP-CTL were further examined for their ability to specifically recognize and respond to each relevant peptide contained within the MP-cocktail. In these assays, we analyzed individual peptide-specific responses in the activation-induced CD137+CD3+CD8+ T cell population.31 A representative flow cytometric analysis demonstrates both IFN-γ production and CD107a degranulation by SMM MP-CTL in response to K562-A*0201 cells pulsed with the relevant peptide including heteroclitic XBP1 US184-192 (YISPWILAV), heteroclitic XBP1 SP367-375 (YLFPQLISV), native CD138260-268 (GLVGLIFAV), and native CS1239-247 (SLFVLGLFL) peptide, but not to cells pulsed with an irrelevant HLA-A2 specific MAGE-3271-279 (FLWGPRALV) peptide or cells alone pulsed with no peptide (Figure 6A). In addition, relevant peptide-specific responses were detected in SMM MP-CTL (n=3) for both IFN-γ production and CD107a degranulation (Figure 6B). However, there were differences in the magnitude of IFN-γ production and CD107a degranulation in response to each relevant peptide among the MP-CTL from different SMM patients. Overall, SMM MP-CTL (n=3) displayed the highest level of total IFN-γ production or CD107a degranulation against native CD138260-268 (GLVGLIFAV) followed by heteroclitic XBP1 US184-192 (YISPWILAV), native CS1239-247 (SLFVLGLFL), and heteroclitic XBP1 SP367-375 (YLFPQLISV) peptides (data not shown). In summary, SMM MP-CTL are capable of recognizing each relevant peptide in MP cocktail, and targeting multiple TAAs on MM cells.
Figure 6. Peptide-specific IFN-γ production and CD107a degranulation immune responses by SMM MP-CTL.
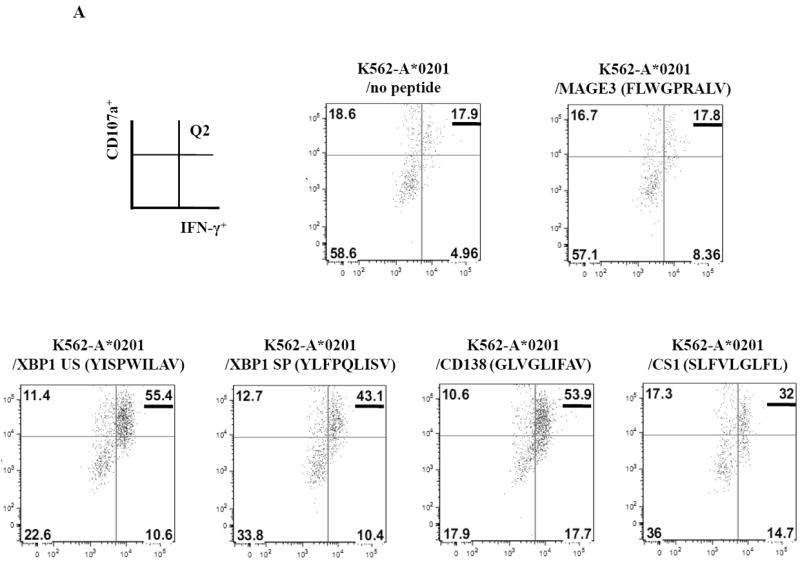
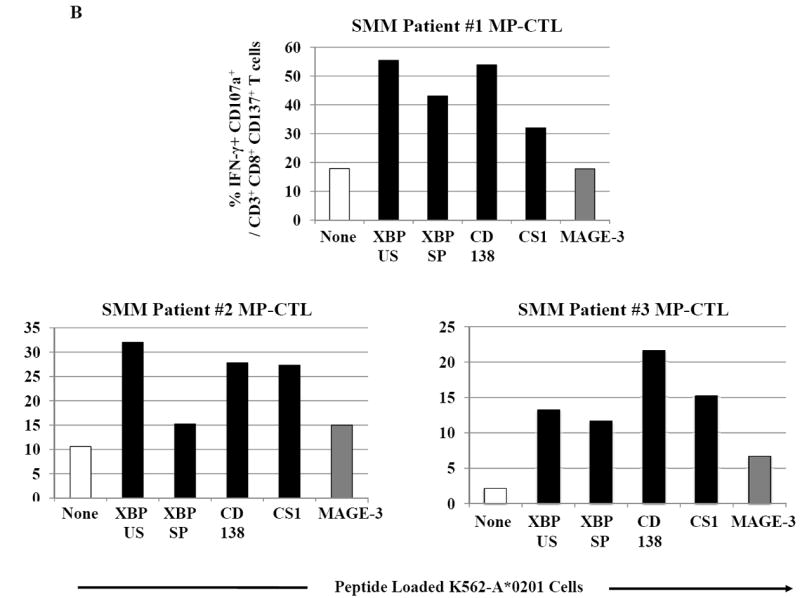
CD3+CD8+CD137+ cells from SMM MP-CTL were evaluated for their peptide-specific IFN-γ production or CD107a degranulation in response to individual peptide pulsed K562-A*0201 cells.
A. Representative flow cytometric analyses of MP-CTL generated from one SMM patient demonstrated the peptide-specific IFN-γ production and CD107a degranulation (Q2 gated) by CD3+CD8+CD137+ cells in response to HLA-A2-specific heteroclitic XBP1 US184-192, heteroclitic XBP1 SP367-375, native CD138260-268, and native CS1239-247 peptide presented by K562-A*0201 cells. Baseline level of IFN-γ production and CD107a degranulation was established in response to no peptide pulsed or irrelevant HLA-A2-specific MAGE-3271-279 peptide pulsed K562-A*0201 cells.
B. The peptide-specific immune responses were demonstrated in MP-CTL generated from three different SMM patients. The MP-CTL from each individual SMM patient (patient #1, patient #2, patient #3) demonstrated a high level of dual immune activities, % IFN-γ+CD107a+ cells, within the gated CD3+CD8+CD137+ T cell population, in response to each relevant HLA-A2-specific XBP1 US, XBP1 SP, CD138 or CS1 peptide, presented by K562-A*0201 cells.
Lenalidomide increases the expression of CD28 costimulatory molecule on SMM MP-CTL and augments their immune activities in response to MM cells
In these studies, we further investigated whether lenalidomide, an immunomodulatory drug, can increase cell yield, regulate expression of critical T cell molecules, or enhance immune function of SMM MP-CTL against MM cells. Short-term treatment of MP-CTL with lenalidomide (4 days, 5 uM) did not change the overall percentage of total CD3+CD8+ T cells as compared to the parent non-lenalidomide treated SMM MP-CTL. Lenalidomide treatment of SMM MP-CTL (n=4) enhanced their anti-myeloma activities against McCAR (Figure 7A) or U266 (Figure 7B) as shown by the “% increase” in IFN-γ production (9-73% increase to McCAR, 6-173% to U266), CD107a degranulation (10-20% increase to McCAR, 5-95% to U266), CD28 expression (9-20% increase to McCAR, 3-86% to U266) and CD137 upregulation (7-26% increase to McCAR, 0-91% to U266) as compared to baseline values of non-drug treated MP-CTL. Therefore, these results demonstrate that lenalidomide treatment augments immune function of SMM MP-CTL against MM cells, suggesting its potential role to enhance the CTL efficacy post vaccination.
Figure 7. Modulation of CD28 T cell co-stimulatory molecule expression and immune function of SMM MP-CTL by treatment with lenalidomide.
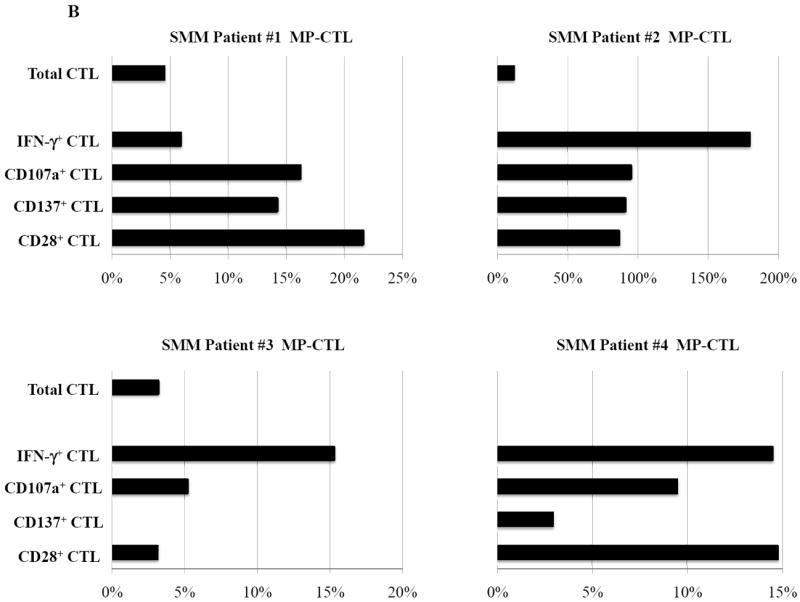
SMM MP-CTL (n=4) treated with lenalidomide (4 days, 5 uM) were analyzed for total CD3+CD8+ T cells, CD28 costimulatory molecule expression, and functional immune responses against MM cells. Lenalidomide treatment of SMM MP-CTL increased their anti-myeloma activities and specific antigen expression. The % increase in CD3+CD8+ T cells expressing IFN-γ, CD107a, CD137 or CD28 is shown for a respective SMM MP-CTL as compared to baseline values of non-drug treated MP-CTL, in response to McCAR cells (Figure 7A) or U266 cells (Figure 7B).
DISCUSSION
Design of an immunotherapeutic approach to induce broad CTL responses specific to multiple antigens may overcome antigen mutation or deletion by tumor cells or the variation or absence of the appropriate T-cell repertoire in the patient. Targeting multiple epitopes on MM cells using a multipeptide vaccine approach could allow a more robust immune response against tumor cells, as compared to vaccines specific to a single antigen. In these studies, we evaluated a cocktail of four immunogenic HLA-A2-specific peptides, heteroclitic XBP1 US184-192 (YISPWILAV), heteroclitic XBP1 SP367-375 (YLFPQLISV), native CD138260-268 (GLVGLIFAV) and native CS1239-247 (SLFVLGLFL), for its ability to evoke anti-MM immunity in T lymphocytes obtained from HLA-A2+ SMM patients. Consistent with our previous studies using “healthy” HLA-A2+ donors’ cells,21,25-27 MP-CTL generated from SMM patients’ cells displayed a gradual increase in CD3+CD8+ T cells upon multipeptide stimulation. In addition, the MP-CTL were predominantly CD45RO+ memory subset and demonstrated HLA-A2-restricted polyfunctional immune activities against MM cells.
Of potential concern during the development of a multipeptide vaccine is epitope dominance along with competition among the peptides for specific HLA-A2 molecules, which may impair the full spectrum of immune responses against all of the desired antigens. Due to these concerns, individual peptides were administered at different injection sites in previous multipeptide vaccine trials to avoid the possibility of competition among epitopes.32,33 However, this requirement might limit feasibility of an immunotherapeutic approach using multiple peptides from new antigens. In these studies, we demonstrate strong immune responses of SMM MP-CTL against each relevant peptide, suggesting that possible competition for HLA-A2 molecules among the multipeptide did not compromise the immunogenicity of peptides with differing affinities. This finding is consistent with our previous observations in MP-CTL generated from “healthy” donors’ T cells.21 Taken together, we conclude that simultaneous APC pulsing with our cocktail of four XBP1 US/XBP1 SP/CD138/CS1 peptides did not interfere with either the induction of MP-CTL or their functional capabilities, thereby validating our preclinical rationale for use of the multipeptide cocktail as an immunotherapeutic approach for SMM patients. This observation highlights the benefit for using the MP-cocktail to overcome differences in T-cell repertoire among individuals, which could result in the lack of peptide-specific CTL induction by a single antigen-based vaccine.
Combination therapies involving adjuvants, therapeutic drugs or immune modulators may prove critical for the long-term success of cancer vaccines. Among the potential candidates, lenalidomide has been shown to enhance immune function and may prove beneficial when used in combination with a therapeutic vaccine. In our studies, short-term exposure of MP-CTL to lenalidomide enhanced MP-CTL function by increasing CD28 expression, IFN-γ production, CD107a degranulation, and CD137 up-regulation, which are critical biomarkers to detect anti-tumor activity of CTL. These ex vivo results, along with others’ reports, support the use of lenalidomide as an effective combination therapy to increase vaccine efficacy in patients with MM.34-36
The efficacy of cancer vaccines requires induction of long-term “memory” CD8+ T cells with anti-tumor activities, which are critical for continuous control of disease. Optimally, cancer vaccine therapies would induce a “memory-like” CD8+ T cell population which can self-renew and respond quickly to tumor antigens, while providing effective and persistent long-term anti-tumor immunity.37,38 Clinical evidence has shown a correlation between high levels of “effector memory CTL (CD45RO+CCR7-/CD3+CD8+)” in tumor infiltrating lymphocytes with both the absence of early metastatic invasion and increased overall survival in cancer patients.39-42 In these studies, we generated MP-CTL having effective anti-MM activities in all HLA-A2+ SMM patients tested (n=8), thereby validating the immunogenicity of the XBP1 US/XBP1 SP/CD138/CS1 multipeptide and its potential as a vaccine for SMM patients. In addition, we identified a small group (n=2) of SMM MP-CTL, we termed as “high” responders, based on their increased levels of cell expansion and immune activities against MM cells. The other “normal” SMM MP-CTL (n=6) generated in these studies demonstrated characteristic phenotype and functional activities, as seen in our previous study using normal healthy donors’ cells to generate the MP-CTL.21 MP-CTL from SMM “high” responders demonstrated ~100 times greater CD3+CD8+ T cell expansion and increased levels of anti-MM immunity as compared to SMM “normal” responders. Importantly, enrichment in effector memory cell subset was detected in the MP-CTL from SMM “high” responders, while SMM “normal” responders contained a higher proportion of terminal effector subset. Comparison of anti-tumor activities revealed that the effector memory subset mediated greater anti-MM immunity than the counterpart terminal effector subset. Furthermore, effector memory CTL from SMM “high” responders were continuously maintained and expanded following 7 cycles of MP-stimulation, without differentiation into terminal effector CTL. Thus, these observations emphasize the potential to effectively induce strong and extended anti-tumor immunity upon MP-stimulation by maintaining the “effector memory” CTL in patients.
It has been hypothesized by others that self-renewal could be the basis for the continual generation of effector lymphocytes from the memory pool.43,44 Recently, studies have revealed dynamic roles for various transcription factors and other factors in effector and memory T cells. The transcriptional repressor Bcl6, transcriptional regulators Id2 and Id3, Wnt-β-catenin signaling pathway, and the metabolic status of T cells (i.e., fatty acid metabolism, mitochondrial functions) are known to be important for effector or memory cells generation.45-46 In contrast, high levels of T-bet and Blimp1 favor terminally differentiated effector cells. Additionally, sustained Akt activation is reported to evoke a transcriptional program that drives differentiation of effector cells and diminishes CTL’ potential to survive.47-48 The pathways and differences in hierarchical differentiation between “high” and “normal” SMM responders to the peptides present a unique challenge to developing optimal vaccine strategies. Our ongoing studies will elucidate the mechanism for arresting differentiation of long-lived and antigen-specific memory CTL to facilitate development of immunotherapeutic strategies.
In summary, we demonstrate ex vivo that a cocktail of HLA-A2 specific peptides, heteroclitic XBP1 US184-192, heteroclitic XBP1 SP367-375, native CD138260-268, and native CS1239-247, can elicit MP-CTL with anti-MM immunity from SMM patients’ T cells. These data provide evidence for induction of an effective and durable “effector memory” MP-CTL with strong anti-tumor activity in a subset of SMM patients (“high” responders). In addition, it would be of interest to know the impact of this MP vaccine in patients with symptomatic MM to see if there any of the known immune regulatory issues limit the clinical efficacy of the vaccine.49-50 Combining adjuvant therapies to enhance immune function in both myeloma and SMM will be explored in our future studies. These results provide the framework for a clinical trial with our multipeptide vaccination strategy targeting XBP1, CD138, and CS1 antigens to delay or prevent progression of SMM to active MM.
Supplementary Material
Key Points.
The multipeptide induces anti-myeloma specific T lymphocytes targeting XBP1, CD138 and CS1 antigens from smoldering myeloma patients.
Multipeptide cocktail has an immunotherapeutic potential as a vaccine in smoldering myeloma patients to control the disease progression.
Acknowledgments
The authors acknowledge the generous gift of K562-A*0201 cells from Dr. Peter Cresswell (Yale University; New Haven, Connecticut). We also thank Mr. John Daley and Ms. Suzan Lazo-Kallanian from the Flow Cytometry facility at Dana Farber Cancer Institute for providing flow cytometry assistance.
This work was supported in part by grants from the National Institutes of Health Grants RO1-124929 to Dr. Nikhil C. Munshi, P50-100007, PO1-78378 and PO1155258 to Drs. Kenneth C. Anderson and Nikhil C. Munshi, and RO1-50947 to Dr. Kenneth C. Anderson. This research was also supported in part by a kind donation from Mr. and Mrs. Stewart Nagler. Dr. Kenneth C. Anderson is an American Cancer Society Clinical Research Professor.
Footnotes
Supplementary information is available at Leukemia’s website.*
Conflict of Interest:
N.C. Munshi is an honorarium of Celgene, Millennium and Novartis; K.C. Anderson is an honorarium of Celgene, Millennium and Onyx; and P.G. Richardson is an honorarium of Celgene, Millennium and Johnson & Johnson. N.C. Munshi, K.C. Anderson and J. Bae have ownership interest and are advisory board members in OncoPep Inc. No relevant conflicts of interest were disclosed by the other authors.
References
- 1.Group TIMW. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 2.Korde N, Kristinsson SY, Landgren O. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM): novel biological insights and development of early treatment strategies. Blood. 2011;117:5573–5581. doi: 10.1182/blood-2011-01-270140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherry BM, Korde N, Kwok M, Manasanch EE, Bhutani M, Mulquin M, et al. Modeling progression risk for smoldering multiple myeloma: results from a prospective clinical study. Leuk Lymphoma. 2013;54:2215–2218. doi: 10.3109/10428194.2013.764419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Ghobrial IM. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: a review of the current understanding of epidemiology, biology, risk stratification, and management of myeloma precursor disease. Clin Cancer Res. 2013;19:985–994. doi: 10.1158/1078-0432.CCR-12-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajkumar SV, Merlini G, SanMiguel JF. Haematological cancer: Redefining myeloma. Nat Rev Clin Oncol. 2012;9:494–496. doi: 10.1038/nrclinonc.2012.128. [DOI] [PubMed] [Google Scholar]
- 6.Hjorth M, Hellquist L, Holmberg E, Magnusson B, Rödjer S, Westin J. Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I--a randomized study. Eur J Haematol. 1993;50:95–102. doi: 10.1111/j.1600-0609.1993.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 7.Musto P, Petrucci MT, Bringhen S, Guglielmelli T, Caravita T, Bongarzoni V, et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008;113:1588–1595. doi: 10.1002/cncr.23783. [DOI] [PubMed] [Google Scholar]
- 8.Barlogie B, van Rhee F, Shaughnessy JD, Jr, Epstein J, Yaccoby S, Pineda-Roman M, et al. Seven-year median time to progression with thalidomide for smoldering myeloma: partial response identifies subset requiring earlier salvage therapy for symptomatic disease. Blood. 2008;112:3122–3125. doi: 10.1182/blood-2008-06-164228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podar K, Richardson PG, Hideshima T, Chauhan D, Anderson KC. The malignant clone and the bone-marrow environment. Best Pract Res Clin Haematol. 2007;20:597–612. doi: 10.1016/j.beha.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Dhodapkar MV, Krasovsky J, Osman K, Geller MD. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med. 2003;198:1753–1757. doi: 10.1084/jem.20031030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuranda K, Berthon C, Dupont C, Wolowiec D, Leleu X, Polakowska R, et al. A subpopulation of malignant CD34+CD138+B7-H1+ plasma cells is present in multiple myeloma patients. Exp Hematol. 2010;38:124–131. doi: 10.1016/j.exphem.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 13.Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, et al. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+ HLA-DRlow myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. 2010;72:540–547. doi: 10.1111/j.1365-3083.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 14.Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107:3940–3949. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 15.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 16.Rutella S, Locatelli F. Targeting multiple-myeloma-induced immune dysfunction to improve immunotherapy outcomes. Clin Dev Immunol. 2012;2012:196063. doi: 10.1155/2012/196063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabaker K, Shell K, Kaufman HL. Vaccines for colorectal cancer and renal cell carcinoma. Cancer J. 2011;17:283–293. doi: 10.1097/PPO.0b013e318232ff44. [DOI] [PubMed] [Google Scholar]
- 18.Rezvani K, de Lavallade H. Vaccination strategies in lymphomas and leukaemias: recent progress. Drugs. 2011;71:1659–1674. doi: 10.2165/11593270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Rousseau C, Ferrer L, Supiot S, Bardiès M, Davodeau F, Faivre-Chauvet A, et al. Dosimetry results suggest feasibility of radioimmunotherapy using anti-CD138 (B-B4) antibody in multiple myeloma patients. Tumour Biol. 2012;33:679–688. doi: 10.1007/s13277-012-0362-y. [DOI] [PubMed] [Google Scholar]
- 20.Lonial S, Kaufman J, Laubach J, Richardson P. Elotuzumab: a novel anti-CS1 monoclonal antibody for the treatment of multiple myeloma. Expert Opin Biol Ther. 2013;13:1731–1740. doi: 10.1517/14712598.2013.847919. [DOI] [PubMed] [Google Scholar]
- 21.Bae J, Smith R, Daley J, Mimura N, Tai YT, Anderson KC, et al. Myeloma-specific multiple peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma and other plasma cell disorders. Clin Cancer Res. 2012;18:4850–4860. doi: 10.1158/1078-0432.CCR-11-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson PG, Lonial S, Jakubowiak AJ, Harousseau JL, Anderson KC. Monoclonal antibodies in the treatment of multiple myeloma. Br J Haematol. 2011 Jul 21; doi: 10.1111/j.1365-2141.2011.08790.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Jagannath S, Chanan-Khan AA, Heffner LT. BT062, an antibody-drug conjugate directed against CD138, shows clinical activity in a phase I study in patients with relapsed or relapsed/refractory multiple myeloma. Blood; ASH Annual Meeting Abstracts; 2010. p. 3060. [Google Scholar]
- 25.Bae J, Carrasco R, Lee AH, Prabhala R, Tai YT, Anderson KC, et al. Identification of novel myeloma-specific XBP1 peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma. Leukemia. 2011;25:1610–1619. doi: 10.1038/leu.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae J, Tai YT, Anderson KC, Munshi NC. Novel epitope evoking CD138 antigen-specific cytotoxic T lymphocytes targeting multiple myeloma and other plasma cell disorders. Br J Haematol. 2011;155:349–361. doi: 10.1111/j.1365-2141.2011.08850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae J, Song W, Smith R, Daley J, Tai YT, Anderson KC, et al. A novel immunogenic CS1-specific peptide inducing antigen-specific cytotoxic T lymphocytes targeting multiple myeloma. Br J Haematol. 2012;157:687–701. doi: 10.1111/j.1365-2141.2012.09111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Z, Takahashi M, Narita M, Toba K, Liu A, Furukawa T, et al. Generation of dendritic cells from adherent cells of cord blood by culture with granulocyte-macrophage colony-stimulating factor, interleukin-4, and tumor necrosis factor-alpha. J Hematother Stem Cell Res. 2000;9:453–464. doi: 10.1089/152581600419116. [DOI] [PubMed] [Google Scholar]
- 29.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 31.Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Mol Cancer Ther. 2012;11:1062–1070. doi: 10.1158/1535-7163.MCT-11-0677. [DOI] [PubMed] [Google Scholar]
- 32.Slingluff CL, Jr, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–7044. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14:169–177. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 34.Andhavarapu S, Roy V. Immunomodulatory drugs in multiple myeloma. Expert Rev Hematol. 2013;6:69–82. doi: 10.1586/ehm.12.62. [DOI] [PubMed] [Google Scholar]
- 35.van de Donk NW, Görgün G, Groen RW, Jakubikova J, Mitsiades CS, Hideshima T, et al. Lenalidomide for the treatment of relapsed and refractory multiple myeloma. Cancer Manag Res. 2012;4:253–268. doi: 10.2147/CMAR.S27087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noonan K, Rudraraju L, Ferguson A, Emerling A, Pasetti MF, Huff CA, et al. Lenalidomide-induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin Cancer Res. 2012;18:1426–1434. doi: 10.1158/1078-0432.CCR-11-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson JA, Jameson SC. Keeping STATs on memory CD8+ T cells. Immunity. 2011;35:663–665. doi: 10.1016/j.immuni.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol Rev. 2010;235:190–205. doi: 10.1111/j.0105-2896.2010.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Junker N, Kvistborg P, Køllgaard T, Straten Pt, Andersen MH, Svane IM. Tumor associated antigen specific T-cell populations identified in ex vivo expanded TIL cultures. Cell Immunol. 2012;273:1–9. doi: 10.1016/j.cellimm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Dobrzanski MJ, Reome JB, Hylind JC, Rewers-Felkins KA, Abdulsamad K, Adams SL. Ag-specific type 1 CD8 effector cells enhance methotrexate-mediated antitumor responses by modulating endogenous CD49b-expressing CD4 and CD8 T effector cell subpopulations producing IL-10. Immunol Invest. 2008;37:315–338. doi: 10.1080/08820130802083762. [DOI] [PubMed] [Google Scholar]
- 41.Ye SW, Wang Y, Valmori D, Ayyoub M, Han Y, Xu XL, et al. Ex-vivo analysis of CD8+ T cells infiltrating colorectal tumors identifies a major effector-memory subset with low perforin content. J Clin Immunol. 2006;26:447–456. doi: 10.1007/s10875-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 42.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 43.Viganò S, Perreau M, Pantaleo G, Harari A. Positive and negative regulation of cellular immune responses in physiologic conditions and diseases. Clin Dev Immunol. 2012;2012:485781. doi: 10.1155/2012/485781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001;293:248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 45.Pepper M, Pagán AJ, Igyártó BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 49.Rutella S, Locatelli F. Targeting multiple-myeloma-induced immune dysfunction to improve immunotherapy outcomes. Clin Dev Immunol. 2012;2012:196063. doi: 10.1155/2012/196063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tucci M, Stucci S, Strippoli S, Dammacco F, Silvestris F. Oncologist. 2011;16:1040–1048. doi: 10.1634/theoncologist.2010-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


