Abstract
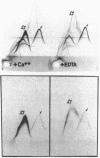
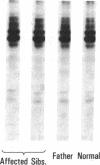
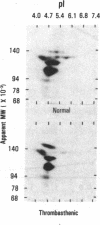
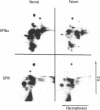
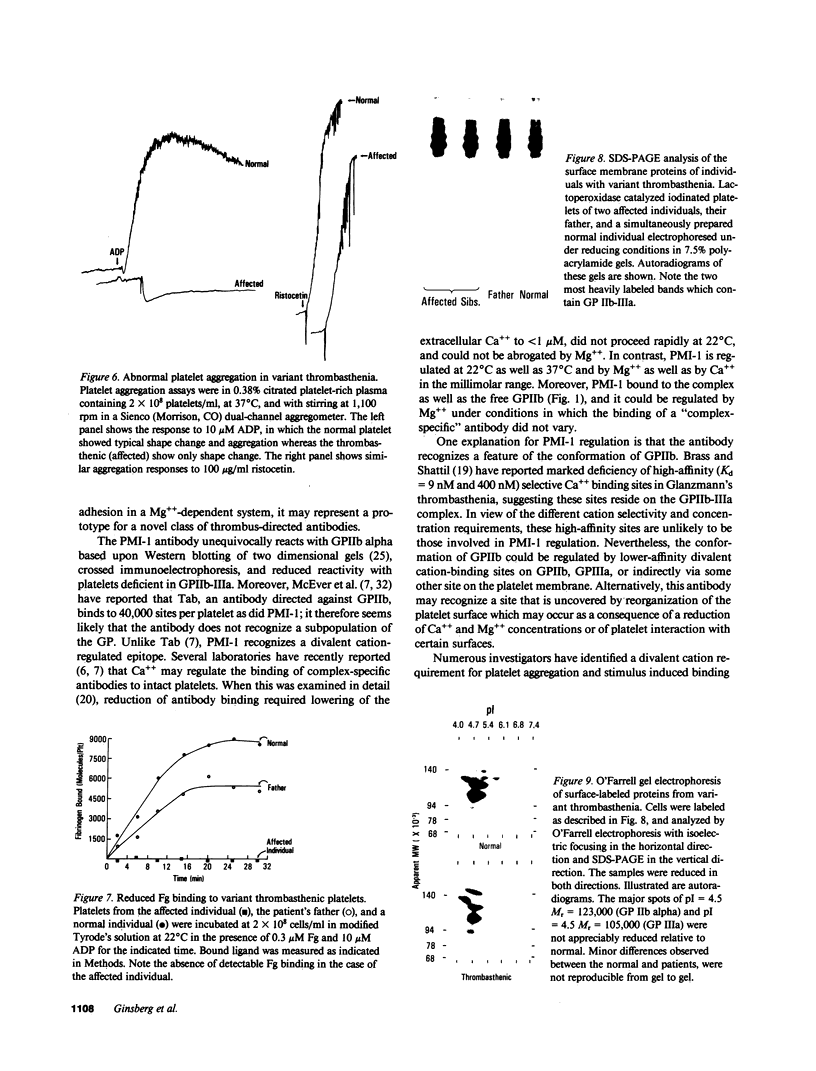
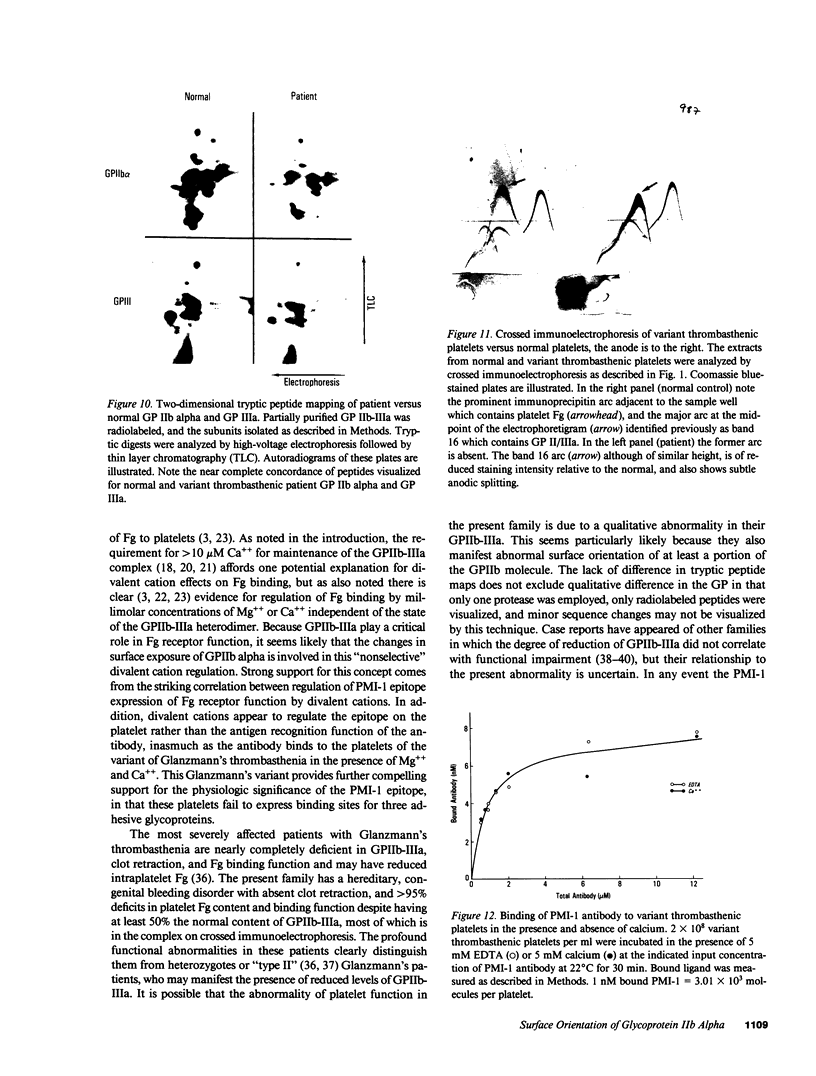
An antiplatelet monoclonal antibody, PMI-1, reacts with glycoproteins (GP) GPIIb, free GPIIb, and the GPIIb-IIIa complex. This antibody binds to 40,900 sites per platelet, with a Kd = 0.95 microM, and its binding is inhibited by the presence of magnesium or calcium in the suspending medium (50% suppression at approximately 0.5 mM divalent cation). Regulation of the PMI-1 epitope is independent of disassembly of the GPIIb-IIIa heterodimer, because it occurred at 22 degrees C and in response to mM magnesium as well as calcium. PMI-1 binding inversely correlated with fibrinogen binding. In addition, we identified a variant of Glanzmann's thrombasthenia with near-normal platelet content of the GPIIb-IIIa heterodimer as judged by crossed immunoelectrophoresis and surface labeling. Binding of PMI-1 to these patients' platelets was not dependent on reduction of the divalent cation concentration. These data suggest that the surface orientation of GPIIb is important in the capacity of platelets to bind fibrinogen.
Full text
PDF

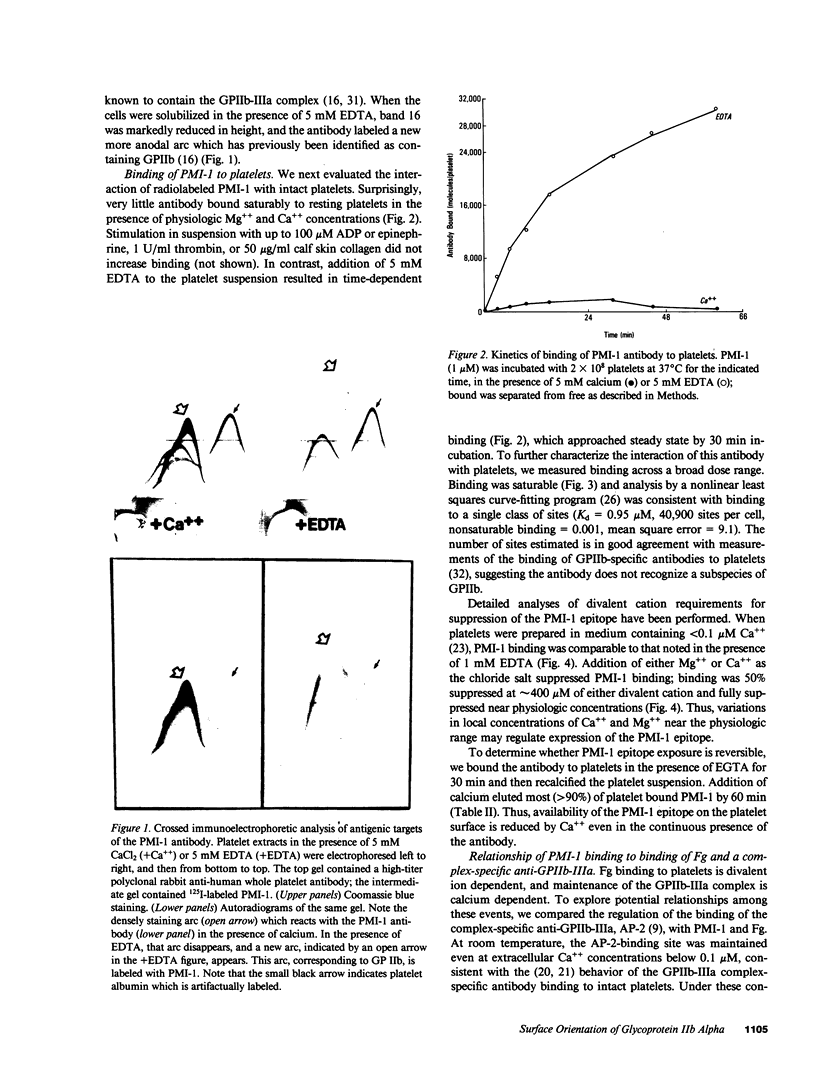
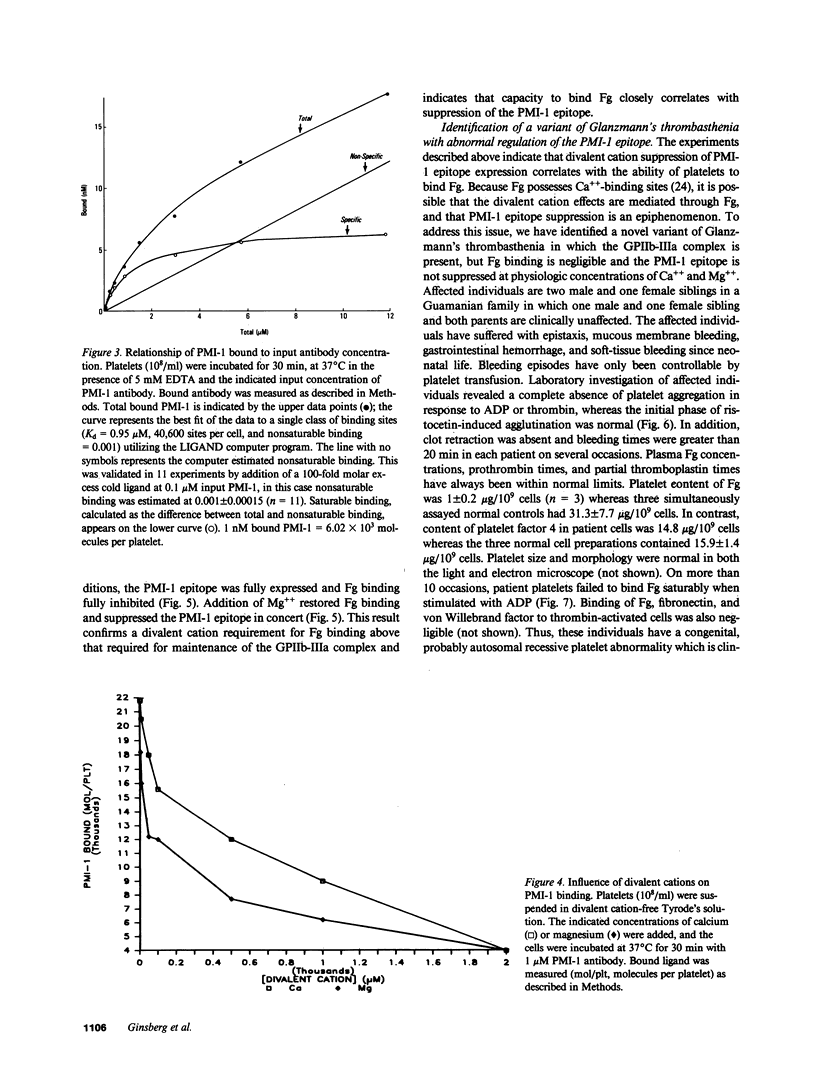
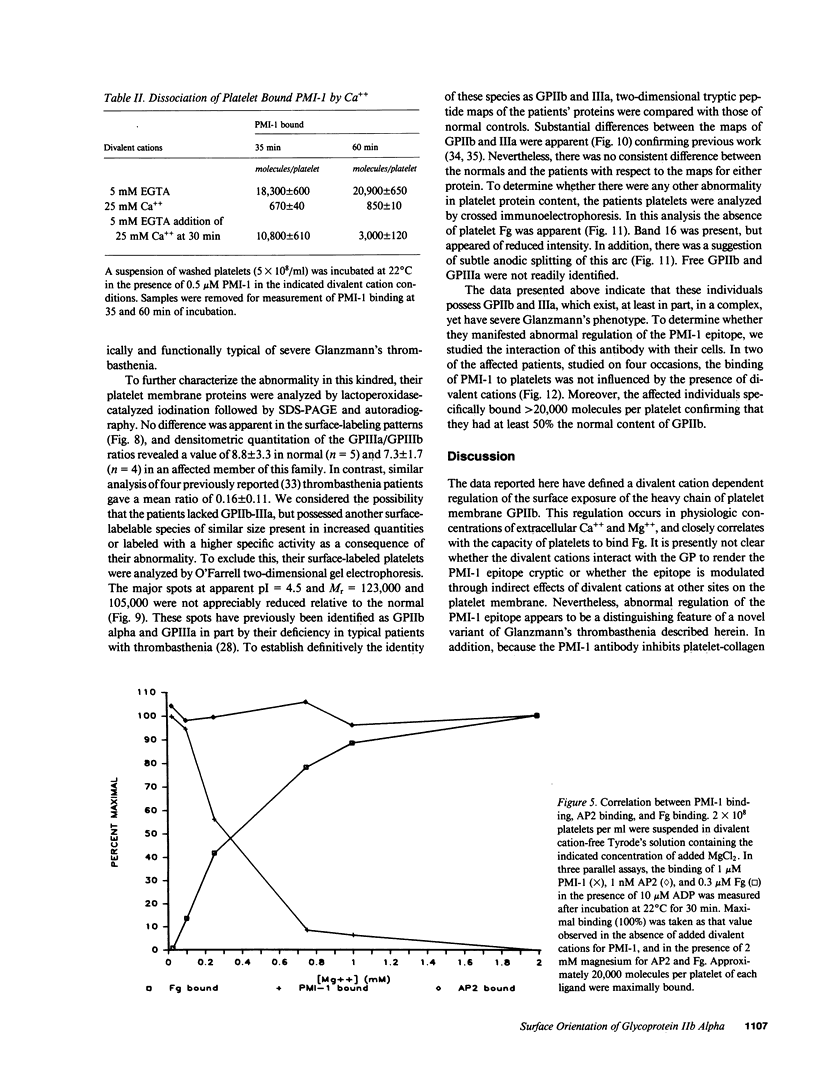


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldassare J. J., Kahn R. A., Knipp M. A., Newman P. J. Reconstruction of platelet proteins into phospholipid vesicles. Functional proteoliposomes. J Clin Invest. 1985 Jan;75(1):35–39. doi: 10.1172/JCI111693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Hoxie J. A., Leitman S. F., Vilaire G., Cines D. B. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(9):2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G., Cines D. B. Identification of the fibrinogen receptor on human platelets by photoaffinity labeling. J Biol Chem. 1982 Jul 25;257(14):8049–8054. [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass L. F., Shattil S. J. Identification and function of the high affinity binding sites for Ca2+ on the surface of platelets. J Clin Invest. 1984 Mar;73(3):626–632. doi: 10.1172/JCI111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Minno G., Thiagarajan P., Perussia B., Martinez J., Shapiro S., Trinchieri G., Murphy S. Exposure of platelet fibrinogen-binding sites by collagen, arachidonic acid, and ADP: inhibition by a monoclonal antibody to the glycoprotein IIb-IIIa complex. Blood. 1983 Jan;61(1):140–148. [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Fitzgerald L. A., Phillips D. R. Calcium regulation of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Sep 15;260(20):11366–11374. [PubMed] [Google Scholar]
- Fujimura K., Phillips D. R. Calcium cation regulation of glycoprotein IIb-IIIa complex formation in platelet plasma membranes. J Biol Chem. 1983 Sep 10;258(17):10247–10252. [PubMed] [Google Scholar]
- Ginsberg M. H., Forsyth J., Lightsey A., Chediak J., Plow E. F. Reduced surface expression and binding of fibronectin by thrombin-stimulated thrombasthenic platelets. J Clin Invest. 1983 Mar;71(3):619–624. doi: 10.1172/JCI110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Wencel J. D., White J. G., Plow E. F. Binding of fibronectin to alpha-granule-deficient platelets. J Cell Biol. 1983 Aug;97(2):571–573. doi: 10.1083/jcb.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogstad G. O., Brosstad F., Krutnes M. B., Hagen I., Solum N. O. Fibrinogen-binding properties of the human platelet glycoprotein IIb-=IIIa complex: a study using crossed-radioimmunoelectrophoresis. Blood. 1982 Sep;60(3):663–671. [PubMed] [Google Scholar]
- Hagen I., Nurden A., Bjerrum O. J., Solum N. O., Caen J. Immunochemical evidence for protein abnormalities in platelets from patients with Glanzmann's thrombasthenia and Bernard-Soulier syndrome. J Clin Invest. 1980 Mar;65(3):722–731. doi: 10.1172/JCI109719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan J. R., White G. C., 2nd Heterogeneity of membrane surface proteins in Glanzmann's thrombasthenia. Blood. 1981 Jan;57(1):174–181. [PubMed] [Google Scholar]
- Jaques B. C., Ginsberg M. H. The role of cell surface proteins in platelet stimulation by monosodium urate crystals. Arthritis Rheum. 1982 May;25(5):508–521. doi: 10.1002/art.1780250504. [DOI] [PubMed] [Google Scholar]
- Jennings L. K., Phillips D. R. Purification of glycoproteins IIb and III from human platelet plasma membranes and characterization of a calcium-dependent glycoprotein IIb-III complex. J Biol Chem. 1982 Sep 10;257(17):10458–10466. [PubMed] [Google Scholar]
- Kotite N. J., Staros J. V., Cunningham L. W. Interaction of specific platelet membrane proteins with collagen: evidence from chemical cross-linking. Biochemistry. 1984 Jun 19;23(13):3099–3104. doi: 10.1021/bi00308a038. [DOI] [PubMed] [Google Scholar]
- Kunicki T. J., Pidard D., Rosa J. P., Nurden A. T. The formation of Ca++-dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood. 1981 Aug;58(2):268–278. [PubMed] [Google Scholar]
- Lee H., Nurden A. T., Thomaidis A., Caen J. P. Relationship between fibrinogen binding and the platelet glycoprotein deficiencies in Glanzmann's thrombasthenia type I and type II. Br J Haematol. 1981 May;48(1):47–57. doi: 10.1111/j.1365-2141.1981.00047.x. [DOI] [PubMed] [Google Scholar]
- Leung L. L., Kinoshita T., Nachman R. L. Isolation, purification, and partial characterization of platelet membrane glycoproteins IIb and IIIa. J Biol Chem. 1981 Feb 25;256(4):1994–1997. [PubMed] [Google Scholar]
- Marguerie G. A., Edgington T. S., Plow E. F. Interaction of fibrinogen with its platelet receptor as part of a multistep reaction in ADP-induced platelet aggregation. J Biol Chem. 1980 Jan 10;255(1):154–161. [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- Marguerie G. A., Thomas-Maison N., Ginsberg M. H., Plow E. F. The platelet-fibrinogen interaction. Evidence for proximity of the A alpha chain of fibrinogen to platelet membrane glycoproteins IIb/III. Eur J Biochem. 1984 Feb 15;139(1):5–11. doi: 10.1111/j.1432-1033.1984.tb07968.x. [DOI] [PubMed] [Google Scholar]
- Marguerie G. The binding of calcium to fibrinogen: some structural features. Biochim Biophys Acta. 1977 Sep 27;494(1):172–181. doi: 10.1016/0005-2795(77)90145-3. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Baenziger J. U., Majerus P. W. Isolation and structural characterization of the polypeptide subunits of membrane glycoprotein IIb-IIIa from human platelets. Blood. 1982 Jan;59(1):80–85. [PubMed] [Google Scholar]
- McEver R. P., Baenziger N. L., Majerus P. W. Isolation and quantitation of the platelet membrane glycoprotein deficient in thrombasthenia using a monoclonal hybridoma antibody. J Clin Invest. 1980 Dec;66(6):1311–1318. doi: 10.1172/JCI109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Kinlough-Rathbone R. L., Packham M. A., Perry D. W., Harfenist E. J., Pai K. R. Comparison of fibrinogen association with normal and thrombasthenic platelets on exposure to ADP or chymotrypsin. Blood. 1979 Nov;54(5):987–993. [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Kinlough-Rathbone R. L., Packham M. A. Factors responsible for ADP-induced release reaction of human platelets. Am J Physiol. 1975 Jun;228(6):1757–1765. doi: 10.1152/ajplegacy.1975.228.6.1757. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Polley M., Leung L. L. Complex formation of platelet membrane glycoproteins IIb and IIIa: a model of platelet activation. Bibl Haematol. 1983;(49):127–130. doi: 10.1159/000408453. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. The different glycoprotein abnormalities in thrombasthenic and Bernard-Soulier platelets. Semin Hematol. 1979 Jul;16(3):234–250. [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B., Grant R. A., Egan J. J., Johnson M. M. Correlation between fibrinogen binding to human platelets and platelet aggregability. Blood. 1980 May;55(5):841–847. [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Phillips D. R., Baughan A. K. Fibrinogen binding to human platelet plasma membranes. Identification of two steps requiring divalent cations. J Biol Chem. 1983 Sep 10;258(17):10240–10246. [PubMed] [Google Scholar]
- Pidard D., Montgomery R. R., Bennett J. S., Kunicki T. J. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb-IIIa complex, with intact platelets. J Biol Chem. 1983 Oct 25;258(20):12582–12586. [PubMed] [Google Scholar]
- Shadle P. J., Barondes S. H. Platelet-collagen adhesion: evidence for participation of antigenically distinct entities. J Cell Biol. 1984 Dec;99(6):2048–2055. doi: 10.1083/jcb.99.6.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadle P. J., Ginsberg M. H., Plow E. F., Barondes S. H. Platelet-collagen adhesion: inhibition by a monoclonal antibody that binds glycoprotein IIb. J Cell Biol. 1984 Dec;99(6):2056–2060. doi: 10.1083/jcb.99.6.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil S. J., Brass L. F., Bennett J. S., Pandhi P. Biochemical and functional consequences of dissociation of the platelet membrane glycoprotein IIb-IIIa complex. Blood. 1985 Jul;66(1):92–98. [PubMed] [Google Scholar]
- Tse T. F., Clutter W. E., Shah S. D., Cryer P. E. Mechanisms of postprandial glucose counterregulation in man. Physiologic roles of glucagon and epinephrine vis-a-vis insulin in the prevention of hypoglycemia late after glucose ingestion. J Clin Invest. 1983 Jul;72(1):278–286. doi: 10.1172/JCI110967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunehisa S., Tsuji T., Tohyama H., Osawa T. Interaction of human platelet membrane glycoproteins with collagen and lectins. Biochim Biophys Acta. 1984 Jan 24;797(1):10–19. [PubMed] [Google Scholar]