Summary
Objective
A meta-analysis was performed to compare mold-active triazoles or lipid amphotericin B plus an echinocandin to non-echinocandin monotherapy for acute invasive aspergillosis (IA).
Methods
We searched PubMed, EMBASE, and other databases through May 2013 unrestricted by language. We included observational and experimental studies wherein patients with proven or probable IA by EORTC/MSG criteria underwent our comparative intervention. PRISMA and MOOSE guidelines were followed and quality was assessed using the Jadad and Newcastle–Ottawa criteria. Meta-regression with fixed and random effects and sensitivity analyses were performed. The primary study outcome measure was 12-week overall mortality. The secondary outcome assessed was complete and partial response.
Results
Only observational studies of primary 12-week survival showed heterogeneity (I2 = 48.96%, p = 0.05). For salvage IA therapy, fixed effects models demonstrated improved 12-week survival (Peto odds ratio (OR) 1.80, 95% confidence interval (CI) 1.08–3.01) and success (Peto OR 2.17, 95% CI 1.21–3.91) of combination therapy. Significance remained after applying random effects as a sensitivity analysis (12-week survival: Peto OR 1.90, 95% CI 1.04–3.46, and unchanged value for success). Restriction to high quality studies and including echinocandins as the comparator for refractory IA revealed an adjusted OR of 1.72 (95% CI 0.96–3.09; p = 0.07) for global success, while the survival endpoint remained unaltered.
Conclusions
Combination antifungals for IA demonstrate improved outcomes over monotherapy in the salvage setting. Clinicians should consider this approach in certain situations.
Keywords: Invasive aspergillosis, Combination antifungal therapy, Outcomes, Salvage therapy, Epidemiology, Observational studies, Clinical trials
Introduction
Aspergillus species are ubiquitous fungi that can be inhaled and develop into angioinvasive forms. The results of a study using data from the Prospective Antifungal Therapy Alliance (PATH Alliance) registry reported in 2012, showed the most common species causing invasive disease in decreasing frequency to be A. fumigatus, A. flavus, A. niger, A. terreus, and A. versicolor.1 Acute invasive aspergillosis (IA) leads to high morbidity and mortality in the immunocompromised host. For instance, data from the Transplant Associated Infections Surveillance Network (TRANSNET) revealed 1-year survival from IA of 59% among solid organ transplant (SOT) recipients and 25.4% among hematopoietic stem cell transplant (HSCT) recipients, from 2001 to 2006.2,3 Despite improved care, IA-associated hospitalization costs remain exorbitant. According to data from the Healthcare Utilization Project (HCUP), the average length of hospitalization due to IA in 1996 was 17.3 ± 0.6 days, corresponding to a cost of $62 426 ± $4977; this dropped in 2004 only to 16.4 ± 0.5 days with a reduction in cost to $41 891 ± $1842 (p = 0.09), which is still high.4,5 While sinopulmonary involvement is most common, dissemination to the central nervous system, gastrointestinal tract, skin, or contiguously may occur amongst the severely immunosuppressed.
Effective therapeutic options are limited once infection is established, relying on the host’s immune status to improve outcomes. Historically, amphotericin B deoxycholate (AmB-d) – a polyene that forms pores in the fungal ergosterol-laden cell membrane – was deemed the ‘gold standard’ for treating IA, but dose-related nephrotoxicity limited its widespread use.6 To lessen the nephrotoxicity, lipid formulations were developed: liposomal amphotericin B (L-AmB), amphotericin B lipid complex (ABLC), and amphotericin B colloid dispersion (ABCD). However, infusion-related toxicity was not eliminated by such modifications and renal toxicity was found to persist at higher cumulative doses.
In May 2002, voriconazole – a triazole with high oral bioavailability that inhibits a step in fungal cell membrane ergosterol biosynthesis by blocking 14α-demethylase – received approval for the primary therapy of IA as a consequence of the clinical trial by Herbrecht et al.7 Voriconazole was found to be superior to AmB-d, given its 52.8% vs. 31.6% 12-week global response rate and 22% reduction in overall mortality (p = 0.02).7 In a subsequent analysis, Patterson et al. found that fewer patients receiving voriconazole switched to other antifungals due to disease progression or intolerance than patients in the AmB-d arm (24% vs. 70%, p < 0.001), and, despite the switch, success at 12 weeks was less common in the latter than in the former group (32% vs. 55%, p < 0.001).8
Since IA treatment responses were found to remain poor in certain populations (e.g., allogeneic HSCT) and in cases of extrapulmonary involvement, with a positive response rate of 32–42%, alternative strategies were considered. In 2001, an echinocandin – caspofungin – was approved as salvage therapy for IA; the favorable response rate was 45–56%, with better outcomes among those receiving it due to drug intolerance rather than disease progression.9,10 A similar successful response rate but lower 12-week survival (50%) was obtained when caspofungin was used as primary therapy.11 Of particular interest is the unique target of this class – β-1,3-glucan synthase, an enzyme that makes an important component of some fungal cell walls.
Subsequently, several investigators noted further improvements in outcome based upon in vitro and animal studies that demonstrated synergistic or additive effects when combining a mold-active triazole (itraconazole, voriconazole, or posaconazole) or an amphotericin B with an echinocandin (caspofungin, micafungin, or anidulafungin).12,13 These translational studies led practitioners to use such combination therapy routinely, with the hope of improving IA outcomes.
However, few human observational studies and small-scale clinical trials have been published to support this practice. In fact, logistical issues made recent completion of a randomized controlled trial to investigate this approach for primary IA challenging.14,15 Since investigators have found comparable 12-week survival for voriconazole (70.8%) and lipid amphotericin B (e.g., 72% using L-AmB 3 mg/kg) and comparable response rates (50.8% compared to 50%, respectively) in the treatment of IA among HSCT, SOT, and hematological malignancy patients when used as primary therapy for IA, and because these classes have largely similar salvage efficacy, it is cogent to combine these to minimize heterogeneity.7,16–18 Indeed, in a retrospective non-comparative salvage study, Maertens et al. found no significant difference in the favorable response rate at 84 days when caspofungin was combined with either an amphotericin B or triazole.19 We therefore conducted a systematic review and meta-analysis of studies that evaluated the efficacy of combining mold-active triazoles or a lipid amphotericin B product with an echinocandin compared to non-echinocandin monotherapy in order to determine the optimum treatment strategy for IA.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines were followed in describing our findings and standard methodology.20–22
Eligibility criteria
Inclusion criteria
Inclusion criteria encompassed any experimental or observational study in which mold-active triazoles or a lipid amphotericin B product was used in combination with an echinocandin for primary and/or salvage treatment of IA. Studies that investigated sequential mono or dual therapies in a comparative manner were permitted, given the prolonged antifungal drug half-life in the setting of hepatic and/or renal failure that is prevalent in the affected population. Salvage therapy was defined as the receipt of antifungal(s) due to prior antifungal intolerance (e.g., toxicity) or refractory disease (e.g., clinical or microbiological progression). In some studies, ‘other licensed antifungal therapies’ (OLAT) was used as an aggregate term to encompass a mixture of such triazoles and amphotericin B products. To enhance uniformity, we selected studies on immunocompromised human cases that compared this combined intervention to ‘monotherapy’ – a single antifungal drug with similar IA survival rates (i.e., mold-active triazoles or a lipid amphotericin B product in the primary and salvage settings) – to permit analysis of our pooled study population. Furthermore, only proven and probable IA cases were included, in accordance with the original and revised European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) guideline definitions.23,24 Studies needed to enable calculation of 12-week survival as our primary endpoint. Although most deaths attributable to IA occur within 6 weeks after therapy, we found a lack of consistency across studies in measuring this as an outcome and so we chose 12-week survival as our major endpoint.25 The secondary endpoint was composite clinical, microbiological, and radiographic success (‘complete’ or ‘partial’ response) ascertained at the end of treatment (EOT), according to previously published definitions.26 To minimize publication bias, we included studies in languages other than English.
Exclusion criteria
Exclusion criteria encompassed any case reports, case series, reviews, guidelines, and non-human studies that dealt with our research question. Any study that failed to have a comparator, did not include the desired combination, or did not include data to derive an effect measure of our endpoint was removed. In cases where multiple studies included the same study subjects, only one study was selected to avoid duplication.
Literature search
We conducted a systematic search of MEDLINE via PubMed (http://www.ncbi.nlm.nih.gov/PubMed), EMBASE (http://www.embase.com), BIOSIS/Web of Knowledge (http://apps.webofknowledge.com.ezproxy.nihlibrary.nih.gov/WOS_GeneralSearch_input.do?product=WOS&search_mode=GeneralSearch&SID=4CMPf38K95Aj2alO2bo&preferencesSaved=), the Cochrane Controlled Trials Register (http://www.cochrane.org/cochrane/hbook.htm), the National Institutes of Health (http://www.clinicaltrials.gov), and a meta-register of controlled trials (http://www.controlled-trials.com) using the following search terms: antifungal, combination, and/or aspergillosis. These medical subject headings were deemed most expansive, but were cross-checked with more specific terms such as aspergillosis, echinocandin, triazole, or amphotericin B. Unpublished studies were discovered using the British Library Index to Conference Proceedings (http://www.bl.uk) and other sources (e.g., Google Scholar and national and international meetings/abstracts).
Data extraction
Using a developed abstraction template, two investigators (AP and EP) independently extracted data from studies meeting our eligibility criteria. These investigators were blinded to the authors’ affiliated institutions, funding sources, and acknowledgments to minimize ascertainment bias. Any discrepancies were resolved via referencing the original source and group discussions. Piloting and revision of the instrument was done as needed. We acquired the following information: journal article citation, study type, mean age of participants, sex ratio, source population (e.g., HSCT), predominant IA treatment indication (primary or salvage – the latter defined as refractory or intolerant to treatment IA requiring a new regimen including a different antifungal class), antifungal combination intervention and monotherapy comparator, drug duration, and number of participants in each group, as well as the duration of follow-up, infection site, 12-week survival, and composite response. Data quality assessment with respect to the risk of bias was performed among experimental studies using allocation methodology, therapy concealment determination, outcome ascertainment (reliability and validity), and attrition based on the Jadad method.27 Similar component sources of bias were determined for observational studies using the Newcastle–Ottawa scale.28
Data analysis
We stratified by therapeutic indication (primary vs. salvage) and presented studies by design via forest plots. We assessed heterogeneity of the studies using I2 – a quantification of the degree of variation or inconsistency across studies.29 Fixed effects results were reported for all analyses with the Peto odds ratio (OR) as the conglomerate effect measure, which did not differ much from the Mantel–Haenszel test (data not shown); this was accompanied by a 95% confidence interval (CI). More conservative random effects confidence intervals were also displayed when the test of heterogeneity was significant and the number of studies in the group was greater than eight. These were based on DerSimonian and Laird weights in conjunction with a permutation method using a t reference distribution.30,31 Residual heterogeneity (τ2) was calculated and represented pictorially using Galbraith plots (Z-score vs. precision). To control for potential confounders, we then determined a quantitative summary estimate, using multivariate meta-regression by study quality components. A ‘high’ score was conferred among clinical trials for each Jadad criterion ≥1 and among observational studies for each Newcastle–Ottawa component: selection >2 (on a 0–4 scale), comparability >1 (on a 0–2 scale), and outcome >1 (on a 0–3 scale). Inappropriate control of such parameters will influence the magnitude and directionality of the effect estimate, which may create spurious results.32
A sensitivity analysis, including studies in which an echinocandin was the monotherapy comparator, was also performed. Moreover, since random effects analysis permits investigating the effect of changing the weights of the different studies, with larger studies being given less weight, this method was presented as an additional sensitivity analysis in certain instances.31 Publication bias was depicted using funnel plots of the inverse standard error – a marker of study sizes – against the effect measure of each study and was quantified by the Egger regression test for plot asymmetry.33 All analyses were performed initially using R version i386 3.0.1 (2013-05-16; The R Foundation for Statistical Computing: http://www.r-project.org/) with package ‘metafor’ (by AP) and then subsequently confirmed and presented in S-Plus (Tibco Software Inc.) (by MP).
Results
Figure 1 depicts the selection process for studies pertaining to combination versus monotherapy of IA. A total of 4331 citations were identified via our medical subject heading search and the majority (60%) were removed after excluding duplicates and focusing on our inclusion criteria. Of the remaining 90 screened, 55 full-text articles were assessed for eligibility. Thirty-three were reviewed in further detail, but nine were excluded from the quantitative meta-analysis, as these did not provide sufficient information to produce an outcome estimate. Of the remaining 24 articles, 16 (k = 16) were included in the initial analysis (Table 1)14,15,34–48 and eight (k = 8) were added (echinocandin comparator) in the sensitivity analysis (Table 2).49–56 Table 3 lists current or withdrawn clinical trials on the topic.
Figure 1.
Flow Diagram of the Selection Process for Study Inclusion in the Meta-analysis of Combination Antifungal Therapy for the Treatment of Acute Invasive Aspergillosis.
Table 1.
Observational and experimental studies oi combination antilungal therapy compared with monotherapy for the treatment oi invasive aspergillosis, meeting the inclusion criteria
| Reference | Year of publication |
Type of study | Study location |
Study population |
Predominant indication |
Combination treatment regimena |
Combination treatment duration (median (range) or mean ± SD days, or approximation) |
Monotherapy treatment regimena |
Monotherapy treatment duration (median (range) or mean ± SD days, or approximation) |
Follow- up |
Age (median (range) or mean ± SD years) |
Male to female ratio |
Organ involvement | Number in combination group |
Number in monotherapy group |
Data quality assessment
points: Experimental studiesc (max. 5 points) |
Data quality assessment
points: Observational studiesd (max. 9 points) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||
| Pulmonary | Extrapulmonary | Primary | Salvage | Primary | Salvage | Randomized | Concealment | Attrition | Selection | Comparability | Outcome | |||||||||||||
| Munoz et al. |
2004 | Retrospective cohort |
Boston, MA | Hematological malignancy |
Primary | Voriconazole + caspofungin |
N/A | Voriconazole | ≥14 | >100 days |
NR | NR | NR | NR | 10 | N/A | 24 | N/A | N/A | N/A | N/A | 2 | 1 | 0 |
| Steinbach et al. |
2004 | Retrospective cohort |
USA (multicenter) |
HSCT, SOT, hematological malignancy |
Primary | OLAT + caspofungin |
≥7 | Voriconazole | 14–28 | 12 weeks |
NR | 2.1 | 74 | 9 | 17 | N/A | 34 | N/A | N/A | N/A | N/A | 4 | 1 | 2 |
| Marr et al. | 2004 | Retrospective cohort |
Seattle, WA | HSCT | Salvage | Voriconazole + caspofungin |
68 (3–248) | Voriconazole | 33 (2–205) | 3 months |
47 (17– 66) |
0.62 | 40 | 7 | N/A | 16 | N/A | 31 | N/A | N/A | N/A | 4 | 2 | 3 |
| Trullas et al. |
2005 | Retrospective cohort |
Spain | HSCT, SOT | Primary | AmB-d + caspofungin |
NR | L-AmB or ABLC | NR | NR | 51.7 ± 14.1 |
1.81 | 25 | 6 | 8 | N/A | 13 | N/A | N/A | N/A | N/A | 2 | 0 | 0 |
| Diaz Pedroche et al. |
2005 | Retrospective cohort |
Spain | SOT, hematological malignancy |
Salvage | Voriconazole + caspofungin |
NR | Voriconazole | NR | 30 days | 51.5 (4.5– 75.5) |
1.29 | 15 | 3 | N/A | 12 | N/A | 12 | N/A | N/A | N/A | 3 | 1 | 1 |
| Singh et al. |
2006 | Prospective cohort |
International (multicenter) |
SOT | Primary | Voriconazole + caspofungin |
NR | L-AmB | <90 | 90 days | 50 (19– 68) |
mono: 0.9; combo: 2.1 |
78 | 30 | 40 | N/A | 47 | N/A | N/A | N/A | N/A | 3 | 2 | 2 |
| Caillot et al. |
2007 | Clinical trial | France | HSCT, hematological malignancy |
Primary | L-AmB + caspofunginb |
18(10–35) | L-AmB (10 mg/kg/day)b |
17(4–24) | 12 weeks |
53.6 (16–75) |
2.33 | 30 | 0 | 15 | N/A | 15 | N/A | 2 | 0 | 1 | N/A | N/A | N/A |
| Waala et al. |
2009 | Retrospective cohort |
Seattle, WA | HSCT, hematological malignancy |
Primary | Voriconazole + echinocandin |
16.2 ±10 | Voriconazole | 28.1 ±28.2 | 3 months |
NR | 0.8 | NR | NR | 19 | N/A | 38 | N/A | N/A | N/A | N/A | 3 | 1 | 2 |
| Pagano et al. |
2010 | Prospective cohort |
Italy (multicenter) |
AML | Primary | L-AmB or voriconazole or posaconazole + caspofungin |
20 (2–90) | L-AmB or voriconazole or posaconazole |
20 (2–90) | 120 days |
57(14– 79) |
1.8 | 126 | 14 | 14 | 3 | 77 | 4 | N/A | N/A | N/A | 4 | 2 | 2 |
| Mihu et al. | 2010 | Retrospective cohort |
Houston, TX | Hematological malignancy |
Salvage | L-AmB + echinocandin |
14(2–112) | L-AmB | 8(1–170) | 12 weeks |
50 (9– 79) |
1.6 | 115 | 26 | N/A | 71 | N/A | 70 | N/A | N/A | N/A | 4 | 2 | 3 |
| Schwartz et al. |
2011 | Retrospective cohort |
International (multicenter) |
HSCT, SOT, hematological malignancy |
Salvage | Voriconazole + caspofungin |
NR | Voriconazole | 48(1–1128) | 12 months |
44 (0.75– 81) |
1.35 | 0 | 120 | NR | 18 | NR | 102 | N/A | N/A | N/A | 4 | 2 | 2 |
| Lortholary etal. |
2011 | Prospective cohort |
France | HSCT, SOT, hematological malignancy |
Primary | Voriconazole + OLAT |
NR | Voriconazole | NR | 12 weeks |
56(18– 84) |
1.64 | 365 | 28 | 73 | N/A | 294 | N/A | N/A | N/A | N/A | 4 | 2 | 2 |
| Jacobs et al. |
2012 | Prospective cohort |
Belgium (multicenter) |
HSCT, SOT, hematological malignancy |
Primary | Voriconazole + OLAT |
49.5 (1–183) | Voriconazole | 32 (1–183) |
183 days |
55.6 (14–85) |
1.46 | NR | NR | 27 | N/A | 72 | N/A | N/A | N/A | N/A | 3 | 2 | 2 |
| Marr et al. | 2012 | Clinical trial | International (multicenter) |
HSCT, hematological malignancy |
Primary | Voriconazole + anidulafungin |
14–28 | Voriconazole | ≥42 | 12 weeks |
52 (18– 83) |
1.25 | 277 | 0 | 135 | N/A | 142 | N/A | 2 | 2 | 1 | N/A | N/A | N/A |
| Baddley et al. |
2013 | Retrospective cohort |
USA (multicenter) |
HSCT, SOT | Primary | Voriconazole or L-AmB + caspofungin |
Mean 115 | Voriconazole or L- AmB |
Mean 115 | 12 weeks |
49 ± 14.7 |
1.55 | 334e | 72e | 103 | NR | 141 | NR | N/A | N/A | N/A | 3 | 2 | 2 |
| Racil et al. | 2013 | Retrospective cohort |
Czech Republic (multicenter) |
HSCT, hematological malignancy |
Primary + salvage |
Voriconazole + caspofungin |
19 (5–159) | Voriconazole or L- AmB |
15(5–139) | 12 months |
56 (3– 77) |
1.44 | 165 | 11 | 41 | 7 | 76 | 12 | N/A | N/A | N/A | 4 | 0 | 2 |
ABLC, amphotericin B lipid complex; AmB-d, amphotericin B deoxycholate; AML, acute myeloid leukemia; EOT, end oi treatment; HSCT, hematopoietic stem cell transplant; IV, intravenous; L-AmB, liposomal amphotericin B; N/A, not applicable; NR, not reported; OLAT, other licensed antiiungal therapy; PO, oral; SD, standard deviation; SOT, solid organ transplant; TID, three times daily.
Antifungal drug doses were as follows unless indicated otherwise: itraconazole 200 mg IV or PO TID; voriconazole 6 mg/kg IV or PO every 12 h × 1 day followed by 4 mg/kg IV every 12 h; posaconazole 200 mg PO TID; L-AmB 3—5 mg/kg IV daily; ABLC 5 mg/kg IV daily; amphotericin B colloid dispersion (ABCD) 3—4 mg/kg IV daily; AmB-d 0.7 mg/kg IV daily; caspoiungin 70 mg IV × 1 day then 50 mg IV daily; micaiungin 100 mg IV daily; anidulaiungin 200 mg IV × 1 then 100 mg IV daily.
MITT (modified intention-to-treat).
Jadad quality assessment scale.
Newcastle–Ottawa quality assessment scale.
Number from total cohort.
Table 2.
Studies in which an echinocandin was used as a comparator to combination therapy with an echinocandin and a mold-active triazole or lipid amphotericin B product for the treatment of invasive aspergillosis. See text and Figure 2e for the sensitivity analysis
| Reference | Year of publication |
Type of study | Study location | Study population |
Predominant indication |
Combination treatment regimena |
Combination treatment duration (median (range) or mean ± SD days, or approximation) |
Monotherapy treatment regimena |
Monotherapy treatment duration (median (range) or mean ± SD days, or approximation) |
Follow- up |
Age (median (range) or mean ± SD years) |
Male to female ratio |
Pulmonary | Extrapulmonary | Primary combo |
Salvage combo |
Primary mono |
Salvage mono |
Randomizedb | Concealmentb | Attritionb | Selectionc | Comparabilityc | Outcomec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Denning et al. | 2006 | Clinical trial | International (multicenter) |
HSCT,
SOT, hematological malignancy, others |
Primary + salvage |
Micafungin + OLAT |
35 (7–284) | Micafungin | <90 | 6 weeks after Rx |
36 (0.2– 84) |
1.73 | 61 | 19 | 17 | 173 | 12 | 22 | 0 | 1 | 1 | N/A | N/A | NA |
| Izumikawa et al. | 2007 | Retrospective cohort |
Japan | non-HSCT, non- hematological malignancy |
Primary | OLAT + micafungin |
59.2 (29–96) | Micafungin | 59.2 (29–96) | EOT | 61.9 (20–83) |
3.5 | 9 | 0 | 5 | N/A | 4 | N/A | N/A | N/A | N/A | 2 | 1 | 1 |
| Kontoyiannis et al. | 2009 | Prospective cohort |
International (multicenter) |
HSCT | Primary + salvage |
OLAT + micafungin |
NR | Micafungin | NR | ≤90 days |
NR | NR | 81 | 17 | 7 | 83 | 2 | 4 | N/A | N/A | N/A | 4 | 1 | 2 |
| Lichtenstern et al. | 2010 | Retrospective cohort |
Multinational (Germany and other European countries) |
SOT | Primary | OLAT + caspofungin |
56 (22–228) | Caspofungin | 59 (9–398) | 7 days after EOT |
56 | 1.92 | NR | NR | 4 | N/A | 4 | N/A | N/A | N/A | N/A | 4 | 1 | 2 |
| Maertens et al. | 2010 | Prospective cohort |
International (multicenter) |
HSCT, hematological malignancy |
Primary + salvage |
OLAT + caspofungin |
NR | Caspofungin | NR | 7 days after EOT |
50.4 ± 16.09 |
1.64 | 87 | 16 | 5 | 11 | 15 | 70 | N/A | N/A | N/A | 4 | 2 | 2 |
| Winkler et al. | 2010 | Retrospective cohort |
International (multicenter) |
SOT | Primary | OLAT + caspofungin |
NR | Caspofungin | NR | 7 days after EOT |
NR | NR | NR | NR | 10 | N/A | 9 | N/A | N/A | N/A | N/A | 3 | 1 | 2 |
| Egerer et al. | 2012 | Retrospective cohort |
International (multicenter) |
HSCT, hematological malignancy, other |
Primary + salvage |
OLAT + caspofungin |
NR | Caspofungin | 13.7 (5–38) | 7 days after EOT |
57 (17– 75) |
1.33 | NR | NR | 1 | 5 | 10 | 26 | N/A | N/A | N/A | 4 | 1 | 1 |
| Jarque et al. | 2013 | Clinical trial | Spain | Hematologica l malignancy |
Salvage | Voriconazole or L-AmB + caspofungin |
19 (2–52) | Caspofungin | 19 (2–52) | 28 days | 52 (23– 78) |
1.5 | 34 | 0 | N/A | 20 | N/A | 14 | 0 | 0 | 1 | N/A | N/A | N/A |
EOT, end of treatment; HSCT, hematopoietic stem cell transplant; IV, intravenous; L-AmB, liposomal amphotericin B; N/A, not applicable; NR, not reported; OLAT, other licensed antifungal therapy; PO, oral; Rx,; SD, standard deviation; SOT, solid organ transplant; TID, three times daily.
Antifungal drug doses were as follows unless indicated otherwise: itraconazole 200 mg IV or PO TID; voriconazole 6 mg/kg IV or PO every 12 h × 1 day followed by 4 mg/kg IV every 12 h; posaconazole 200 mg PO TID; L-AmB 3–5 mg/kg IV daily; amphotericin B lipid complex (ABLC) 5 mg/kg IV daily; amphotericin B colloid dispersion (ABCD) 3–4 mg/kg IV daily; amphotericin B deoxycholate (AmB-d) 0.7 mg/kg IV daily; caspofungin 70 mg IV × 1 day then 50 mg IV daily; micafungin 100 mg IV daily; anidulafungin 200 mg IV × 1 then 100 mg IV daily.
Jadad quality assessment scale.
Newcastle–Ottawa quality assessment scale.
Table 3.
Current additional clinical trials underway or withdrawn addressing combination antifungal use versus monotherapy for invasive aspergillosis
| Start date | Study team, ClinicalTrials.gov protocol number |
Study design | Description |
|---|---|---|---|
| 2/2011 | Annasie et al., NCT01207128) |
Randomized controlled trial (phase II) |
Trial of Combination Antifungal Therapy (Vori+Mica vs.
Vori+Placebo) in Invasive Aspergillosis (http://clinicaltrials.gov/ct2/show/NCT01207128?term=aspergillosis+and+combination&rank=4) Primary outcome: Response at 6 weeks where response is defined as normalization of the serum Aspergillus galactomannan index (GMI) values (first normal GMI value is considered day of response) Withdrawn prior to enrollment |
| 5/2012 | Pfizer, NCT01188759 | Randomized controlled trial (phase III) |
Voriconazole And Anidulafungin Combination For Invasive
Aspergillosis In Pediatric Subjects (http://clinicaltrials.gov/ct2/show/NCT01188759?term=aspergillosis&rank=10) Primary outcome: Safety and tolerability of voriconazole and anidulafungin in combination versus voriconazole alone as determined by rates of adverse events at 12 weeks (EOT) Secondary outcomes: Rate of all-cause mortality at 6 weeks and at EOT Global response to therapy at 6 weeks and at EOT Withdrawn prior to enrollment |
| 2/2007 | Astellas, NCT00423163 |
Randomized controlled trial (phase IV) |
A Study to Evaluate the Effectiveness of Voriconazole +
Micafungin Versus Voriconazole Alone for Invasive Aspergillosis (http://clinicaltrials.gov/ct2/show/NCT00423163?term=aspergillosis&rank=12). Primary outcome: Global success (complete and partial response) at 6 weeks based on the combination of clinical, mycological, and radiological response Secondary outcomes: Global success at week 12, favorable response and survival at weeks 6 and 12. Global success and favorable response by infection site, overall frequency of emergent and recurrent fungal infections at weeks 6 and 12, duration of favorable response, and relationship of galactomannan to clinical outcome Estimated enrollment 350 Withdrawn prior to enrollment |
EOT, end of treatment.
In the initial analysis, the total number of subjects in the combination therapy arm was 629 (502 primary, 127 salvage) and that in the monotherapy arm was 1204 (973 primary, 231 salvage). Regarding sites of infection, 1644 were pulmonary (1309 primary, 170 salvage, and 165 both) and 326 were extrapulmonary (159 primary, 156 salvage, and 11 both). The age and male to female distribution was similar among the combination and monotherapy groups (age: median (interquartile range) 51.7 (50.0–55.6) years and 1.50 (1.32–1.52) years, respectively). The source population comprised HSCT patients (11 studies), SOT patients (eight studies), and patients with a hematological malignancy (12 studies). Similar measures of duration of therapy for combination and monotherapy were not easily estimable as there was variability in reporting and, when reported, the duration spanned a wide range.
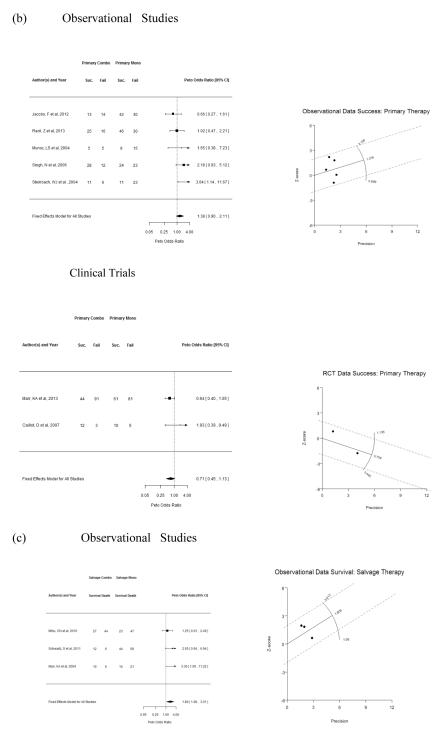
Figure 2 illustrates the summary effect measure across studies by primary or salvage therapeutic indication for IA. Observational studies of primary 12-week survival demonstrated heterogeneity (τ2 = 0.20 ± 0.22; I2 = 49.0%; p = 0.05). This was confirmed visually by the Galbraith plot (Figure 2a, right); under homogeneity, only about one in 20 trials should lie on or outside the two dotted lines. The fixed effects model produced a Peto OR of 1.36 (95% CI 1.02–1.80). The random effects model, however, failed to attain statistical significance, yielding an OR of 1.25 (95% CI 0.74–2.09). Nonetheless, the two clinical trials remained uniform (p = 0.26), with significantly improved survival with combination therapy compared to monotherapy (Peto OR 1.66, 95% CI 1.02–2.68) (Figure 2a). The composite success of primary therapy was relatively homogeneous across observational (p = 0.13) and clinical trials (p = 0.20), as noted in the Galbraith plot, but failed to attain statistical significance (Figure 2b). For salvage IA therapy, fixed effects models were used (test of heterogeneity p = 0.28 for 12-week survival and p = 0.76 for global success) and confirmed significantly improved 12-week survival (Peto OR 1.80, 95% CI 1.08– 3.01) (Figure 2c) and success (Peto OR 2.17, 95% CI 1.21–3.91) (Figure 2d) of combination therapy compared to monotherapy for IA. These effects remained significant after applying a random effects approach as a sensitivity analysis (12-week survival: Peto OR 1.90, 95% CI 1.04–3.46, and unchanged value for success).
Figure 2.
Forest (left column) and Galbraith (right column) plots of studies containing data of combination antifungal therapy versus monotherapy by therapeutic indication and primary and secondary outcomes (as defined in the text). (a) primary survival, (b) primary clinical response, (c) salvage survival, (d) salvage clinical response. Composite effect measure is expressed as Peto Odds Ratio. A fixed effects model was fitted for all analyses. If heterogeneity was present via the I2 test and Galbraith plots (x-axis = inverse standard error or precision; y-axis = z-score; slope is the model point estimate) and the number of studies (k) > 8, a random effects model using the DerSimonian-Laird estimator and an approximate t-test for confidence intervals are also reported (see text). (e.) When studies that included echinocandins as the monotherapy comparator for salvage were included, since this drug class has similar efficacy for refractory IA as the other single drug classes, combination therapy remained of significant benefit over monotherapy among observational studies; success endpoint is only listed as survival was not uniformly assessed in these studies. RCT = clinical trials and not necessarily method of allocation.
Since echinocandins have comparable efficacy to the newer mold-active triazoles and lipid amphotericin B formulations for refractory IA and are indicated for salvage IA monotherapy, we added the echinocandin comparator studies in this setting (k = 6; Table 2). Figure 2e illustrates that the aggregate effect measure for success remained significant – albeit less compared to Figure 2d – in favor of combination therapy (Peto OR 1.78, 95% CI 1.08–2.94) from observational studies. However, the combined clinical trials for salvage IA therapy, which were only available with echinocandins as a comparator, did not yield a significant overall composite success (Peto OR 0.98, 95% CI 0.44–2.21). Moreover, inclusion of this drug class, which has known lower efficacy compared to the newer triazoles and liposomal amphotericin B for primary IA (k = 3), was influential on the overall composite success of primary observational studies in an unexpected direction (i.e., no longer significant, with fixed effects Peto OR 1.29, 95% CI 0.87–1.92 compared to Figure 2a). Survival was not systematically assessed as an endpoint in these studies.
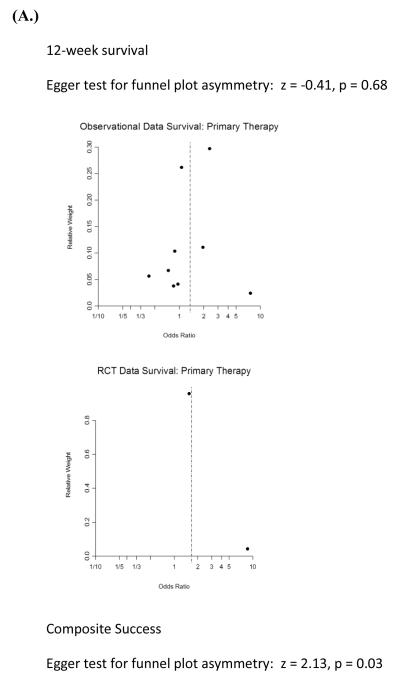
Funnel plots showed a predominant lack of publication bias except for studies that included primary composite success as an endpoint (Egger’s regression test under a mixed effects model for funnel plot asymmetry, p = 0.03), given the breadth of our literature search and inclusion of non-English language studies (Figure 3). However, with so few studies by indication and outcome, it is difficult to draw firm conclusions.
Figure 3.
Funnel plots of observational and experimental studies comparing combination to monotherapy of IA (as defined in text) for (A.) primary or (B.) refractory disease (i.e., salvage) illustrates statistically significant asymmetry around the null value only for primary composite success, suggesting the presence of publication bias in which smaller studies with less weight and precision are more likely to be published only if a strong treatment effect is noted. No asymmetry was noted when echinocandin as comparator studies were added (e.g., salvage RCT not shown). RCT = clinical trials and not necessarily method of allocation.
Since we found a significant effect of combination therapy among salvage observational studies, which can be prone to selection bias, and noted no such effect among the clinical trials, which are designed to avoid such systematic errors in allocation, we performed meta-regression on ‘high quality’ studies according to the Newcastle–Ottawa components. The adjusted effect measure for salvage 12-week survival remained unchanged (OR 1.80, 95% CI 1.08–3.01, p = 0.02), but the composite success outcome became marginal (OR 1.72, 95% CI 0.96–3.09, p = 0.07). The clinical trials failed to meet ‘high quality’ marks in the salvage setting (Jadad score <3).
Discussion
Our results indicate that there is meta-evidence to support that dual antifungal therapy affords significantly improved 12-week survival and composite success over monotherapy when given as salvage therapy for IA. To control for confounding by indication in the absence of a propensity score-matched analysis, we stratified by primary and salvage therapy, finding that there was an 80% increased odds of 12-week survival among those who received combination therapy compared to those who received single-drug therapy as salvage, and this effect remained when restricted to high-quality observational studies. The global success dropped 39–45% (all vs. high quality studies) after adding similar studies in which echinocandins were the comparator, but remained significant. Moreover, although salvage therapy clinical trials found no benefit, their quality was poor.
Our results also demonstrated that the meta-evidence to support the routine use of combination antifungal therapy for initial target IA treatment is less pronounced. While our fixed effects model showed a benefit of combination therapy for 12-week survival in both study designs, the heterogeneity among the observational studies negated this when random effects were applied. Of note, when we restricted observational studies on primary therapy to high-quality studies, the group became relatively homogeneous (p = 0.16) and the adjusted 12-week survival effect measure became significant (OR 1.50, 95% CI 1.10–2.06, p = 0.01). Overall success was not found to be significant in either study design. This is in line with the recently completed clinical trial by Marr et al., which showed a marginal 12-week survival effect of combination therapy with voriconazole and anidulafungin compared with voriconazole alone (p = 0.08, 95% CI −21.4 to 1.09) for primary IA treatment. Of note, global success favored monotherapy in this trial (p = 0.08, 95% CI −21.6 to 1.15).14,15 Incidentally, when we compared primary to salvage studies, we found insufficient evidence to conclude that any benefit of combination therapy is differential by indication (p = 0.34 and 0.21 for our survival and success endpoints, respectively). This corroborates the findings of Kontoyiannis et al., who demonstrated that L-AmB combined with caspofungin resulted in no significant difference in composite response among those who received it for primary vs. refractory or intolerant to treatment IA (53% vs. 35%, p = 0.36).57
Combination modalities may be useful when the IA species is unknown. Also, if there are concerns for antifungal resistance, combination therapy may expand the armamentarium available until susceptibility testing is back. Thirdly, since voriconazole requires at least 5 days for the achievement of steady-state when a loading dose is not given and its metabolism can be highly influenced by concomitant medications, overlapping a complimentary antifungal may be prudent. Finally, the various amphotericin B formulations, triazoles, and echinocandins exhibit different tissue penetrations based on their pharmacodynamic properties such that the choice of antifungal may depend upon the major site of infection. However, we found no significant difference in site of infection among the salvage treatment group (p = 0.27), whereas ‘pulmonary’ was the predominant location in the primary indication group (p = 0.01). Unfortunately, efficacy by site of infection was not evaluable except in the salvage study by Schwartz et al. on central nervous system IA (Table 1 and Figure 2).43
Our study had several limitations. First, the benefit of combination modalities is highly dependent on the level of immune reconstitution and changes in practice over time – factors that could not be adequately accounted for by a test for trend in our study, since we lacked individualized patient data that captured all necessary parameters, despite much effort on our part to obtain such data through collaboration. For instance, in several of our included studies, patients whose death was expected soon after diagnosis due to their underlying co-morbidities were excluded. Hence, our findings of efficacy may have been diminished if such patients were captured in the primary data, making our conclusions only applicable to settings outside such scenarios. Indeed, other potential confounders such as neutropenia, conditioning or other immunosuppressive regimens, graft versus host disease, and underlying disease were abstracted when possible. We found consistent balance among intervention groups, inferring that the non-differential distribution would only bias towards the null if there were misclassification. Secondly, sources of heterogeneity that were limitations of this meta-analysis included the following: (1) specific drug combination (e.g., OLAT, though this was in the primary indication studies and sensitivity analyses – not affecting the main conclusions of our study); (2) high-risk source populations (e.g., HSCT, SOT, and hematological malignancy) with variable response rates; (3) etiologic Aspergillus species that may have diverse pathogenicity; (4) extent of organs involved (pulmonary vs. extrapulmonary); and (5) variable duration of therapy and ascertainment of response at the end of therapy or follow-up. Regarding the latter, some studies determined the response at EOT and/or the end of the study, which were not always the same, making direct comparisons untenable. However, while the inclusion of studies in which EOT occurred before the end of the study may have diminished the composite success effect measure, we still noted significance among salvage studies. Thirdly, the dose and duration of antifungal treatment, which impact pharmacokinetics, were not consistently and uniformly reported, thus this source of heterogeneity could not be examined. Finally, groups in which patients received salvage therapy could do so if their disease progressed or they developed toxicity to the current regimen. Since all of the included salvage studies in which the reason for salvage was indicated (7 of 10) comprised predominantly the former group (179 (77.5%) of 231 in the combination group and 157 (60.4%) of 260 in the monotherapy group), but reported outcome collectively as ‘refractory or intolerant to treat’, except for one,50 we reasoned that the enhanced treatment effect of combination therapy in this setting was magnified, since the refractory sub-group was in general more ill. Future studies should collect outcome data on these salvage sub-groups if proportions change over time to minimize confounding (by ‘contraindication’ for drug interactions and adverse events, for instance).
Hence, our findings should be interpreted with caution given the inherent limitations of meta-analyses and should be applied only in certain clinical scenarios, such as refractory IA in the setting of host immune recovery. Indeed, time-varying covariates, such as changes in conditioning regimens for HSCT that may ameliorate host immune status, could make our conclusions based on past studies yield uncertain applicability in the future (i.e., a cohort effect).
To conclude, we systematically and quantitatively found the use of either a triazole or lipid amphotericin B formulation in combination with an echinocandin to be beneficial in certain salvage settings. Although the caveat of heterogeneity is inherent with any meta-analysis, the difficulties in completing the clinical trial by Marr et al. (for primary IA treatment comparing modalities), makes the probability of a similar appropriately powered and comparative, double-blinded, multicenter trial for salvage therapy low, reinforcing the importance of our results. Synergism works in refractory disease, perhaps by killing heteroresistant sub-populations, or by killing more rapidly through potentiation. Nonetheless, host immune reconstitution is necessary to afford cure, regardless of modality. Future studies should address this prospectively, correlating host immunological markers and outcomes, particularly since IA outcomes have been improving over time with similar antifungal regimens due to medical advancements.
Acknowledgements
We thank the NIH Library, Dr Jin Qiu, and Dr Guowu Hu for their efforts in providing us translations of non-English articles in order to be as inclusive as possible in this analysis. This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH).
Funding: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: This study was registered at PROSPERO – an international prospective register of systematic reviews (registration number: CRD42013004006; available at: http://www.crd.york.ac.uk/prospero/). The views herein do not reflect the official opinions of the Uniformed Services University or the Department of Defense.
Conflicts of interest: None known.
References
- 1.Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect. 2012;65:453–64. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 50:1091–100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 3.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 50:1101–11. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 4.Dasbach EJ, Davies GM, Teutsch SM. Burden of aspergillosis-related hospitalizations in the United States. Clin Infect Dis. 2000;31:1524–8. doi: 10.1086/317487. [DOI] [PubMed] [Google Scholar]
- 5.Menzin J, Meyers JL, Friedman M, Perfect JR, Langston AA, Danna RP, Papadopoulos G. Mortality, length of hospitalization, and costs associated with invasive fungal infections in high-risk patients. Am J Health Syst Pharm. 2009;66:1711–7. doi: 10.2146/ajhp080325. [DOI] [PubMed] [Google Scholar]
- 6.Ostrosky-Zeichner L, Marr KA, Rex JH, Cohen SH. Amphotericin B: time for a new ‘gold standard’. Clin Infect Dis. 2003;37:415–25. doi: 10.1086/376634. [DOI] [PubMed] [Google Scholar]
- 7.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Invasive Fungal Infections Group of the European Organisation for Research. Treatment of Cancer and the Global Aspergillus Study Group Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–15. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 8.Patterson TF, Boucher HW, Herbrecht R, Denning DW, Lortholary O, Ribaud P, et al. Strategy of following voriconazole versus amphotericin B therapy with other licensed antifungal therapy for primary treatment of invasive aspergillosis: impact of other therapies on outcome. Clin Infect Dis. 2005;41:1448–52. doi: 10.1086/497126. [DOI] [PubMed] [Google Scholar]
- 9.Maertens J, Raad I, Petrikkos G, Boogaerts M, Selleslag D, Petersen FB, et al. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis. 2004;39:1563–71. doi: 10.1086/423381. [DOI] [PubMed] [Google Scholar]
- 10.Maertens J, Egerer G, Shin WS, Reichert D, Stek M, Chandwani S, et al. Study Team C-D Caspofungin use in daily clinical practice for treatment of invasive aspergillosis: results of a prospective observational registry. BMC Infect Dis. 2010;10:182. doi: 10.1186/1471-2334-10-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbrecht R, Maertens J, Baila L, Aoun M, Heinz W, Martino R, et al. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: a European Organisation for Research and Treatment of Cancer study. Bone Marrow Transpl. 2010;45:1227–33. doi: 10.1038/bmt.2009.334. [DOI] [PubMed] [Google Scholar]
- 12.Kirkpatrick WR, Perea S, Coco BJ, Patterson TF. Efficacy of caspofungin alone and in combination with voriconazole in a guinea pig model of invasive aspergillosis. Antimicrob Agents Chemother. 2002;46:2564–8. doi: 10.1128/AAC.46.8.2564-2568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagasaki Y, Eriguchi Y, Uchida Y, Miyake N, Maehara Y, Kadowaki M, et al. Combination therapy with micafungin and amphotericin B for invasive pulmonary aspergillosis in an immunocompromised mouse model. J Antimicrob Chemother. 2009;64:379–82. doi: 10.1093/jac/dkp175. [DOI] [PubMed] [Google Scholar]
- 14.Marr KA, Schlamm H, Rottinghaus ST, Jagannatha S, Bow EJ, Wingard JW, et al. Group atMS . A randomised, double-blind study of combination antifungal therapy with voriconazole and anidulafungin versus voriconazole monotherapy for primary treatment of invasive aspergillosis. ECCMID; London, UK: 2012. [Google Scholar]
- 15.Marr KA, Schlamm H, Rottinghaus ST, Jagannatha S, Bow EJ, Wingard JR, et al. A randomised, double-blind study of combination antifungal therapy with voriconazole and anidulafungin versus voriconazole monotherapy for primary treatment of invasive aspergillosis. Clin Microbiol Infect. 2012;18:713. [Google Scholar]
- 16.Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E, et al. AmBiLoad Trial Study G Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial) Clin Infect Dis. 2007;44:1289–97. doi: 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- 17.Hachem RY, Boktour MR, Hanna HA, Husni RN, Torres HA, Afif C, et al. Amphotericin B lipid complex versus liposomal amphotericin B monotherapy for invasive aspergillosis in patients with hematologic malignancy. Cancer. 2008;112:1282–7. doi: 10.1002/cncr.23311. [DOI] [PubMed] [Google Scholar]
- 18.White MH, Anaissie EJ, Kusne S, Wingard JR, Hiemenz JW, Cantor A, et al. Amphotericin B colloidal dispersion vs. amphotericin B as therapy for invasive aspergillosis. Clin Infect Dis. 1997;24:635–42. [PubMed] [Google Scholar]
- 19.Maertens J, Glasmacher A, Herbrecht R, Thiebaut A, Cordonnier C, Segal BH, et al. Multicenter, noncomparative study of caspofungin in combination with other antifungals as salvage therapy in adults with invasive aspergillosis. Cancer. 2006;107:2888–97. doi: 10.1002/cncr.22348. [DOI] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M, Smith G, Altman D. Systematic reviews in health care: meta-analysis in context. 2nd ed BMJ; London: 2007. [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 24.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wingard JR, Ribaud P, Schlamm HT, Herbrecht R. Changes in causes of death over time after treatment for invasive aspergillosis. Cancer. 2008;112:2309–12. doi: 10.1002/cncr.23441. [DOI] [PubMed] [Google Scholar]
- 26.Segal BH, Herbrecht R, Stevens DA, Ostrosky-Zeichner L, Sobel J, Viscoli C, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008;47:674–83. doi: 10.1086/590566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 28.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. The Ottawa Hospital Research Institute; Ottawa, Ontario: [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 31.Follmann DA, Proschan MA. Valid inference in random effects meta-analysis. Biometrics. 1999;55:732–7. doi: 10.1111/j.0006-341x.1999.00732.x. [DOI] [PubMed] [Google Scholar]
- 32.Rothman KJ, Greenland S. Modern epidemiology. Lippincott Williams & Wilkins; Philadelphia, PA: 1998. [Google Scholar]
- 33.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 34.Munoz LS, Ruthazer R, Boucher H, Loudon S, Skarf L, Hadley S. Combination antifungals for primary treatment of invasive aspergillosis (IA): do they work? Abstract M-1024. ICAAC; Washington DC: 2004. [Google Scholar]
- 35.Steinbach WJ, Benjamin DK, Kontoyiannis DP, Perfect JR, Lutsar I, Marr KA, et al. Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin Infect Dis. 2004;39:192–8. doi: 10.1086/421950. [DOI] [PubMed] [Google Scholar]
- 36.Marr KA, Boeckh M, Carter RA, Hyung WK, Corey L. Combination antifungal therapy for invasive aspergillosis. Clin Infect Dis. 2004;39:797–802. doi: 10.1086/423380. [DOI] [PubMed] [Google Scholar]
- 37.Trullas JC, Cervera C, Benito N, de la Bellacasa JP, Agusti C, Rovira M, et al. Invasive pulmonary aspergillosis in solid organ and bone marrow transplant recipients. Transplant Proc. 2005;37:4091–3. doi: 10.1016/j.transproceed.2005.09.182. [DOI] [PubMed] [Google Scholar]
- 38.Díaz Pedroche C, Cisneros JM, Lumbreras C, Aguado JM. Treatment of invasive fungal infections with voriconazole. Evaluation of experience with compassionate use of voriconazole in Spain. Rev Esp Quimioter. 2005;18:149–58. [PubMed] [Google Scholar]
- 39.Singh N, Limaye AP, Forrest G, Safdar N, Munoz P, Pursell K, et al. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation. 2006;81:320–6. doi: 10.1097/01.tp.0000202421.94822.f7. [DOI] [PubMed] [Google Scholar]
- 40.Waala K, Jain R, Xie H, Fredericks DN, Pottinger PS. Combination antifungal therapy as primary therapy for invasive aspergillosis. IDSA; Philadelphia, PA: 2009. [Google Scholar]
- 41.Pagano L, Caira M, Candoni A, Offidani M, Martino B, Specchia G, et al. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica. 2010;95:644–50. doi: 10.3324/haematol.2009.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mihu CN, Kassis C, Ramos ER, Jiang Y, Hachem RY, Raad II. Does combination of lipid formulation of amphotericin B and echinocandins improve outcome of invasive aspergillosis in hematological malignancy patients? Cancer. 2010;116:5290–6. doi: 10.1002/cncr.25312. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz S, Reisman A, Troke PF. The efficacy of voriconazole in the treatment of 192 fungal central nervous system infections: a retrospective analysis. Infection. 2011;39:201–10. doi: 10.1007/s15010-011-0108-6. [DOI] [PubMed] [Google Scholar]
- 44.Lortholary O, Gangneux JP, Sitbon K, Lebeau B, de Monbrison F, Le Strat Y, et al. French Mycosis Study Group Epidemiological trends in invasive aspergillosis in France: the SAIF network (2005–2007) Clin Microbiol Infect. 2011;17:1882–9. doi: 10.1111/j.1469-0691.2011.03548.x. [DOI] [PubMed] [Google Scholar]
- 45.Jacobs F, Selleslag D, Aoun M, Sonet A, Gadisseur A. An observational efficacy and safety analysis of the treatment of acute invasive aspergillosis using voriconazole. Eur J Clin Microbiol Infect Dis. 2012;31:1173–9. doi: 10.1007/s10096-011-1425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Racil Z, Weinbergerova B, Kocmanova I, Muzik J, Kouba M, Drgona L, et al. Invasive aspergillosis in patients with hematological malignancies in the Czech and Slovak republics: Fungal InfectioN Database (FIND) analysis, 2005–2009. Int J Infect Dis. 2013;17:e101–9. doi: 10.1016/j.ijid.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Baddley JW, Andes DR, Marr KA, Kauffman CA, Kontoyiannis DP, Ito JI, et al. Antifungal therapy and length of hospitalization in transplant patients with invasive aspergillosis. Med Mycol. 2013;51:128–35. doi: 10.3109/13693786.2012.690108. [DOI] [PubMed] [Google Scholar]
- 48.Caillot D, Thiebaut A, Herbrecht R, De Botton S, Pigneux A, Bernard F, et al. Liposomal amphotericin B in combination with caspofungin for invasive aspergillosis in patients with hematologic malignancies: a randomized pilot study (Combistrat trial) Cancer. 2007;110:2740–6. doi: 10.1002/cncr.23109. [DOI] [PubMed] [Google Scholar]
- 49.Izumikawa K, Ohtsu Y, Kawabata M, Takaya H, Miyamoto A, Sakamoto S, et al. Clinical efficacy of micafungin for chronic pulmonary aspergillosis. Med Mycol. 2007;45:273–8. doi: 10.1080/13693780701278386. [DOI] [PubMed] [Google Scholar]
- 50.Kontoyiannis DP, Ratanatharathorn V, Young JA, Raymond J, Laverdiere M, Denning DW, et al. Micafungin alone or in combination with other systemic antifungal therapies in hematopoietic stem cell transplant recipients with invasive aspergillosis: short communication. Transpl Infect Dis. 2009;11:89–93. doi: 10.1111/j.1399-3062.2008.00349.x. [DOI] [PubMed] [Google Scholar]
- 51.Lichtenstern C, Pratschke J, Schulz U, Schmoeckel M, Knitsch W, Kaskel P, et al. [Caspofungin after solid organ transplantation in Germany: observational study on treatment of invasive fungal infections] Der Anaesthesist. 2010;59:1083–90. doi: 10.1007/s00101-010-1795-6. [DOI] [PubMed] [Google Scholar]
- 52.Maertens J, Egerer G, Shin WS, Reichert D, Stek M, Chandwani S, et al. Caspofungin use in daily clinical practice for treatment of invasive aspergillosis: results of a prospective observational registry. BMC Infect Dis. 2009;10 doi: 10.1186/1471-2334-10-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winkler M, Pratschke J, Schulz U, Zheng S, Zhang M, Li W, et al. Caspofungin for post solid organ transplant invasive fungal disease: results of a retrospective observational study. Transpl Infect Dis. 2010;12:230–7. doi: 10.1111/j.1399-3062.2009.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egerer G, Reichert D, Pletz MW, Kaskel P, Krobot KJ, Maertens J. Caspofungin for treatment of invasive aspergillosis in Germany: results of a pre-planned subanalysis of an international registry. Eur J Med Res. 2012;17:7. doi: 10.1186/2047-783X-17-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denning DW, Marr KA, Lau WM, Facklam DP, Ratanatharathorn V, Becker C, et al. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect. 2006;53:337–49. doi: 10.1016/j.jinf.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jarque I, Tormo M, Bello JL, Rovira M, Batlle M, Julia A, et al. Caspofungin for the treatment of invasive fungal disease in hematological patients (ProCAS Study) Med Mycol. 2013;51:150–4. doi: 10.3109/13693786.2012.693213. [DOI] [PubMed] [Google Scholar]
- 57.Kontoyiannis DP, Hachem R, Lewis RE, Rivero GA, Torres HA, Thornby J, et al. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer. 2003;98:292–9. doi: 10.1002/cncr.11479. [DOI] [PubMed] [Google Scholar]