Abstract
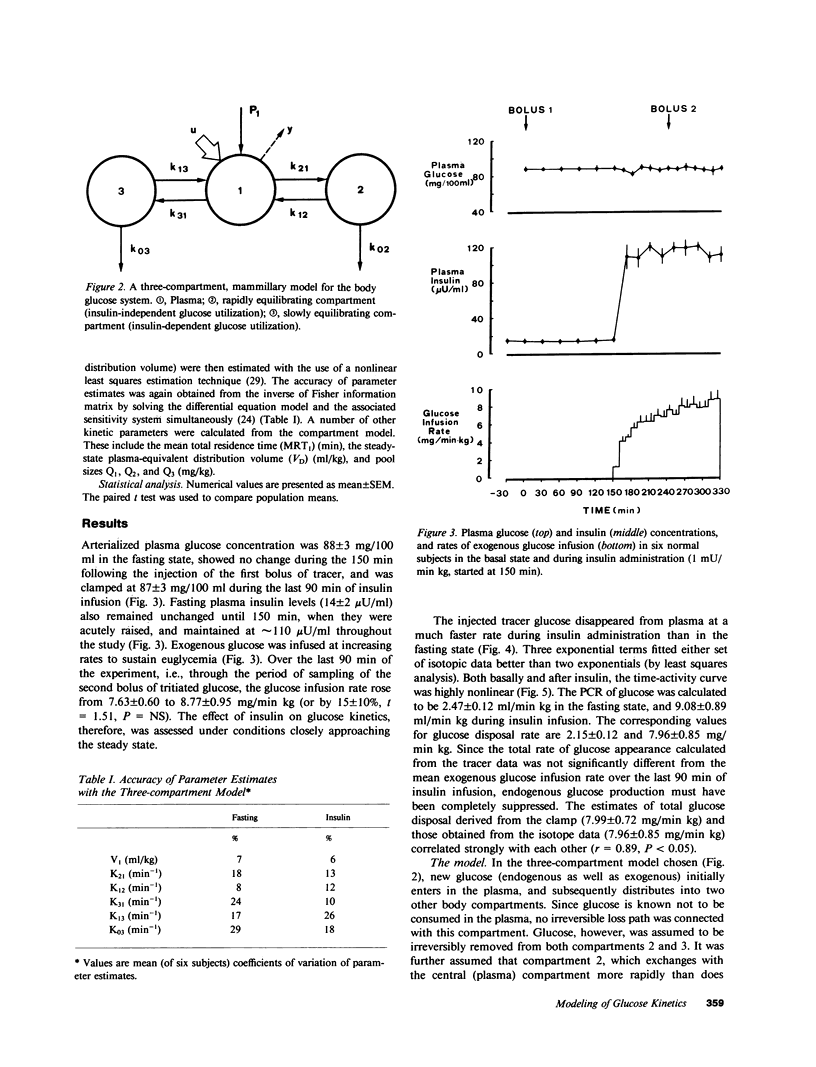
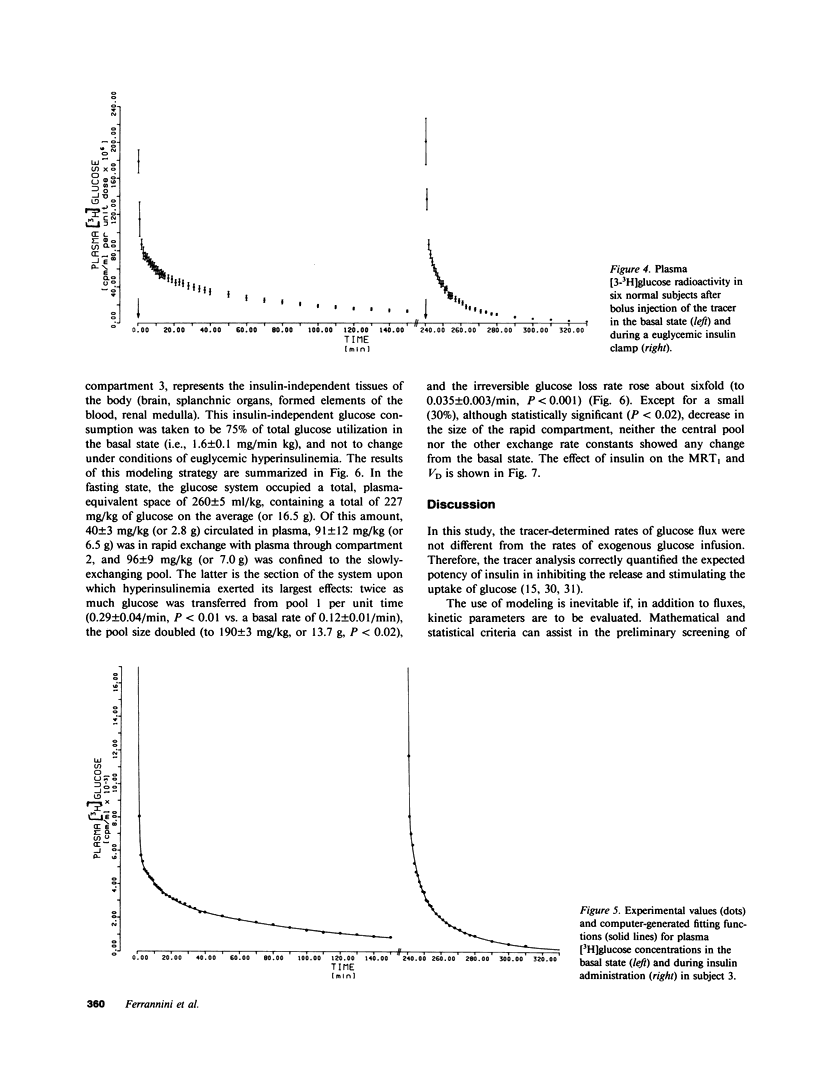
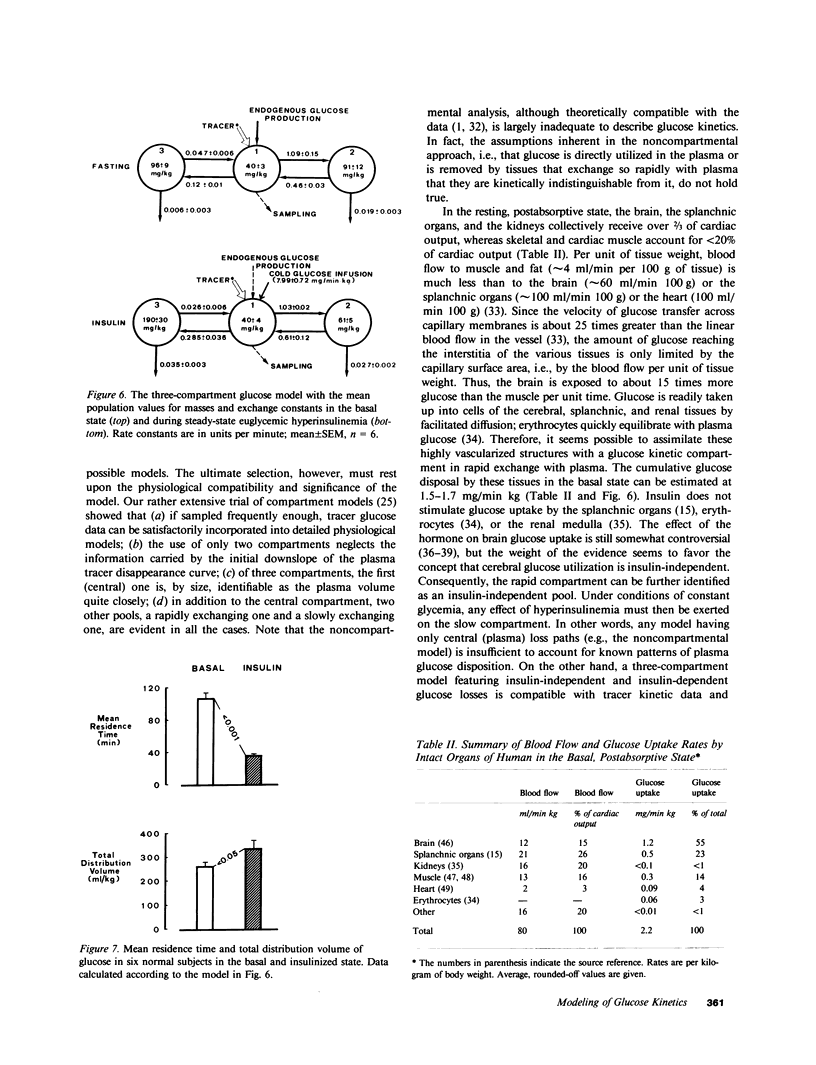
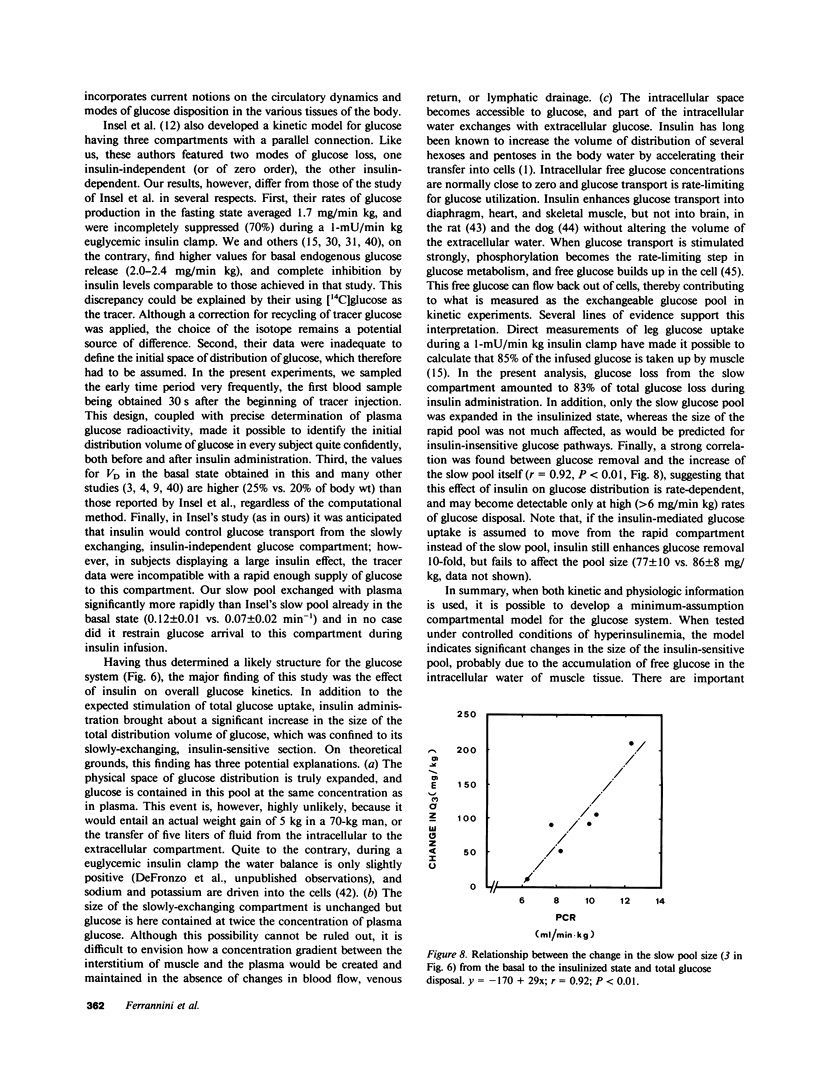
Understanding the influence of insulin on glucose turnover is the key to interpreting a great number of metabolic situations. Little is known, however, about insulin's effect on the distribution and exchange of glucose in body pools. We developed a physiological compartmental model to describe the kinetics of plasma glucose in normal man in the basal state and under steady-state conditions of euglycemic hyperinsulinemia. A bolus of [3-3H]glucose was rapidly injected into a peripheral vein in six healthy volunteers, and the time-course of plasma radioactivity was monitored at very short time intervals for 150 min. A 1-mU/min kg insulin clamp was then started, thereby raising plasma insulin levels to a high physiological plateau (approximately 100 microU/ml). After 90 min of stable euglycemic hyperinsulinemia, a second bolus of [3-3H]glucose was given, and plasma radioactivity was again sampled frequently for 90 min more while the clamp was continued. Three exponential components were clearly identified in the plasma disappearance curves of tracer glucose of each subject studied, both before and after insulin. Based on stringent statistical criteria, the data in the basal state were fitted to a three-compartment model. The compartment of initial distribution was identical to the plasma pool (40 +/- 3 mg/kg); the other two compartments had similar size (91 +/- 12 and 96 +/- 9 mg/kg), but the former was in rapid exchange with plasma (at an average rate of 1.09 +/- 0.15 min-1), whereas the latter exchanged 10 times more slowly (0.12 +/- 0.01 min-1). The basal rate of glucose turnover averaged 2.15 +/- 0.12 mg/min kg, and the total distribution volume of glucose in the postabsorptive state was 26 +/- 1% of body weight. In view of current physiological information, it was assumed that the more rapidly exchanging pool represented the insulin-independent tissues of the body, while the slowly exchanging pool was assimilated to the insulin-dependent tissues. Insulin-independent glucose uptake was estimated (from published data) at 75% of basal glucose uptake, and was constrained not to change with euglycemic hyperinsulinemia. When the kinetic data obtained during insulin administration were fitted to this model, neither the size nor the exchange rates of the plasma or the rapid pool were appreciably changed. In contrast, the slow pool was markedly expanded (from 96 +/- 9 to 190 +/- 30 mg/kg, P less than 0.02) at the same time as total glucose disposal rose fourfold above basal (to 7.96 +/- 0.85 mg/min kg, P less than 0.001). Furthermore, a significant direct correlation was found to exist between the change in size of the slow pool and the insulin-stimulated rate of total glucose turnover (r=0.92, P<0.01). We conclude that hyperinsulinemia, independent of hyperglycemia, markedly increases the exchangeable mass of glucose in the body, presumably reflecting the accumulation of free, intracellular glucose in insulin-dependent tissues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES R., CADER G., ZIERLER K. L. The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state; measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J Clin Invest. 1956 Jun;35(6):671–682. doi: 10.1172/JCI103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins G. L. A new technique for maintaining and monitoring conscious, stress-free rabbits in a steady state: its use in the determination of glucose kinetics. Q J Exp Physiol Cogn Med Sci. 1980 Jan;65(1):63–75. doi: 10.1113/expphysiol.1980.sp002492. [DOI] [PubMed] [Google Scholar]
- Atkins G. L. Investigation of some theoretical models relating the concentrations of glucose and insulin in plasma. J Theor Biol. 1971 Sep;32(3):471–494. doi: 10.1016/0022-5193(71)90152-4. [DOI] [PubMed] [Google Scholar]
- BAKER N., SHIPLEY R. A., CLARK R. E., INCEFY G. E. C14 studies in carbohydrate metabolism: glucose pool size and rate of turnover in the normal rat. Am J Physiol. 1959 Feb;196(2):245–252. doi: 10.1152/ajplegacy.1959.196.2.245. [DOI] [PubMed] [Google Scholar]
- Björntorp P., Sjöström L. Carbohydrate storage in man: speculations and some quantitative considerations. Metabolism. 1978 Dec;27(12 Suppl 2):1853–1865. doi: 10.1016/s0026-0495(78)80004-3. [DOI] [PubMed] [Google Scholar]
- Cobelli C., Ruggeri A., Toffolo G., Avogaro A., Nosadini R. Is the "pool-fraction" paradigm a valid model for assessment of in vivo turnover in non-steady state? Am J Physiol. 1983 Nov;245(5 Pt 1):R624–R632. doi: 10.1152/ajpregu.1983.245.5.R624. [DOI] [PubMed] [Google Scholar]
- Cobelli C., Toffolo G. Compartmental vs. noncompartmental modeling for two accessible pools. Am J Physiol. 1984 Sep;247(3 Pt 2):R488–R496. doi: 10.1152/ajpregu.1984.247.3.R488. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- DeFronzo R. A., Cooke C. R., Andres R., Faloona G. R., Davis P. J. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975 Apr;55(4):845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Wahren J., Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Gatewood L. C., Ackerman E., Rosevear J. W., Molnar G. D. Modeling blood glucose dynamics. Behav Sci. 1970 Jan;15(1):72–87. doi: 10.1002/bs.3830150108. [DOI] [PubMed] [Google Scholar]
- HETENYI G., Jr, WRENSHALL G. A., BEST C. H. Rates of production, utilization, accumulation and apparent distribution space of glucose. Effects of insulin in dogs using a validated tracer method. Diabetes. 1961 Jul-Aug;10:304–311. doi: 10.2337/diab.10.4.304. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Liljenquist J. E., Tobin J. D., Sherwin R. S., Watkins P., Andres R., Berman M. Insulin control of glucose metabolism in man: a new kinetic analysis. J Clin Invest. 1975 May;55(5):1057–1066. doi: 10.1172/JCI108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz B., Jr, Allen M., Borkow I. Estimation of glucose turnover in the dog with glucose-2-T and glucose-U- 14 C. Am J Physiol. 1972 Mar;222(3):710–712. doi: 10.1152/ajplegacy.1972.222.3.710. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Issekutz B., Jr, Elahi D. Estimation of hepatic glucose output in non-steady state. The simultaneous use of 2-3H-glucose and 14C-glucose in the dog. Can J Physiol Pharmacol. 1974 Apr;52(2):215–224. doi: 10.1139/y74-029. [DOI] [PubMed] [Google Scholar]
- Katz J., Dunn A., Chenoweth M., Golden S. Determination of synthesis, recycling and body mass of glucose in rats and rabbits in vivo 3H-and 14C-labelled glucose. Biochem J. 1974 Jul;142(1):171–183. doi: 10.1042/bj1420171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Dunn A. Glucose-2-t as a tracer for glucose metabolism. Biochemistry. 1967 Jan;6(1):1–5. doi: 10.1021/bi00853a001. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfeld D. S., Ramberg C. F., Jr, Shames D. M. Multicompartmental analysis of glucose kinetics in normal and hypoglycemic cows. Am J Physiol. 1971 Apr;220(4):886–893. doi: 10.1152/ajplegacy.1971.220.4.886. [DOI] [PubMed] [Google Scholar]
- LEE J. B., VANCE V. K., CAHILL G. F., Jr Metabolism of C14-labeled substrates by rabbit kidney cortex and medulla. Am J Physiol. 1962 Jul;203:27–36. doi: 10.1152/ajplegacy.1962.203.1.27. [DOI] [PubMed] [Google Scholar]
- LEVINE R., GOLDSTEIN M. S., HUDDLESTUN B., KLEIN S. P. Action of insulin on the 'permeability' of cells to free hexoses, as studied by its effect on the distribution of galactose. Am J Physiol. 1950 Oct;163(1):70–76. doi: 10.1152/ajplegacy.1950.163.1.70. [DOI] [PubMed] [Google Scholar]
- MURPHY J. R. Erythrocyte metabolism. I. The equilibration of glucose-C14 between serum and erythrocytes. J Lab Clin Med. 1960 Feb;55:281–285. [PubMed] [Google Scholar]
- Norwich K. H. Mathematical models of the kinetics of glucose and insulin in plasma. Bull Math Biophys. 1969 Mar;31(1):105–121. doi: 10.1007/BF02478212. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK C. R., JOHNSON L. H. Effect of insulin on transport of glucose and galactose into cells of rat muscle and brain. Am J Physiol. 1955 Jul;182(1):17–23. doi: 10.1152/ajplegacy.1955.182.1.17. [DOI] [PubMed] [Google Scholar]
- PARK C. R., JOHNSON L. H., WRIGHT J. H., Jr, BATSEL H. Effect of insulin on transport of several hexoses and pentoses into cells of muscle and brain. Am J Physiol. 1957 Oct;191(1):13–18. doi: 10.1152/ajplegacy.1957.191.1.13. [DOI] [PubMed] [Google Scholar]
- PARK C. R., REINWEIN D., HENDERSON M. J., CADENAS E., MORGAN H. E. The action of insulin on the transport of glucose through the cell membrane. Am J Med. 1959 May;26(5):674–684. doi: 10.1016/0002-9343(59)90227-x. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ D., ZIERLER K. L. Forearm metabolism in obesity and its response to intra-arterial insulin. Characterization of insulin resistance and evidence for adaptive hyperinsulinism. J Clin Invest. 1962 Dec;41:2173–2181. doi: 10.1172/JCI104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziuk J., Inculet R. The effects of ingested and intravenous glucose on forearm uptake of glucose and glucogenic substrate in normal man. Diabetes. 1983 Nov;32(11):977–981. doi: 10.2337/diab.32.11.977. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978 Jan;234(1):E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- Rescigno A., Gurpide E. Estimation of average times of residence, recycle and interconversion of blood-borne compounds using tracer methods. J Clin Endocrinol Metab. 1973 Feb;36(2):263–276. doi: 10.1210/jcem-36-2-263. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981 Jun;240(6):E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- SCHEINBERG P. OBSERVATIONS ON CEREBRAL CARBOHYDRATE METABOLISM IN MAN. Ann Intern Med. 1965 Feb;62:367–371. doi: 10.7326/0003-4819-62-2-367. [DOI] [PubMed] [Google Scholar]
- SEARLE G. L., MORTIMORE G. E., BUCKLEY R. E., REILLY W. A. Plasma glucose turnover in humans as studied with C14 glucose: influence of insulin and tolbutamide. Diabetes. 1959 May-Jun;8(3):167–173. doi: 10.2337/diab.8.3.167. [DOI] [PubMed] [Google Scholar]
- SEGAL S., BERMAN M., BLAIR A. The metabolism of variously C14-labeled glucose in man and an estimation of the extent of glucose metabolism by the hexose monophosphate pathway. J Clin Invest. 1961 Jul;40:1263–1279. doi: 10.1172/JCI104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg P., Stead E. A. THE CEREBRAL BLOOD FLOW IN MALE SUBJECTS AS MEASURED BY THE NITROUS OXIDE TECHNIQUE. NORMAL VALUES FOR BLOOD FLOW, OXYGEN UTILIZATION, GLUCOSE UTILIZATION, AND PERIPHERAL RESISTANCE, WITH OBSERVATIONS ON THE EFFECT OF TILTING AND ANXIETY. J Clin Invest. 1949 Sep;28(5 Pt 2):1163–1171. doi: 10.1172/JCI102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tserng K. Y., Kalhan S. C. Estimation of glucose carbon recycling and glucose turnover with [U-13C] glucose. Am J Physiol. 1983 Nov;245(5 Pt 1):E476–E482. doi: 10.1152/ajpendo.1983.245.5.E476. [DOI] [PubMed] [Google Scholar]



